Abstract
Introduction
Non-Latina black breast cancer patients experience a shorter survival from breast cancer than their non-Latina white counterparts. We compared breast cancer specific survival for the subset of black and white patients with estrogen and/or progesterone receptor positive tumors that are generally targeted with endocrine therapy.
Methods
Using data collected from a population-based cohort of breast cancer patients from Chicago, IL, Kaplan Meier survival curves and hazard functions were generated and proportional hazards models were estimated to determine the black/white disparity in time to death from breast cancer while adjusting for age at diagnosis, patient characteristics, treatment-related variables, and tumor grade and stage.
Results
In regression models, hazard of breast cancer death among ER/PR positive patients was at least 4 times higher for black than for white patients in all models tested. Notably, even after adjusting for stage at diagnosis, tumor grade, and treatment variables (including initiation of systemic adjuvant therapies), the hazard ratio for death from ER/PR positive breast cancer between black and white women was 4.39 (95% CI: 1.76, 10.9, p=0.001).
Conclusions
We observed a racial disparity in breast cancer survival for patients diagnosed with ER/PR positive tumors that did not appear to be due to differences in tumor stage, grade or therapy initiation in black patients, suggesting that there may be racial differences in the molecular characteristics of hormone receptor positive tumors, such that ER/PR positive tumors in black patients may be less responsive to standard treatments.
Keywords: Breast cancer, Disparities, Race/ethnicity, Survival
Introduction
Non-Latina black (black) women diagnosed with breast cancer experience shorter survival following diagnosis and are more likely to die from the disease compared to their non-Latina white (white) counterparts. This fact can be seen in studies of survival in patients diagnosed with breast cancer [1–3] and from population-based studies that show a corresponding disparity in breast cancer mortality rates within the U.S. population [1, 4–6]. There are many potential explanations for the disparity, including differences in the quality of and access to breast cancer care [7–15], as well as differences in the incidence of aggressive breast cancer subtypes in black versus white patients [2, 10, 16–20]. Particular emphasis has been placed on the increased incidence of triple negative (TN) breast cancers, which lack targetable hormone and growth factor receptors, in black women as a major driver of outcome disparities [21].
However, the majority of breast cancers diagnosed in the United States express the estrogen receptor (ER) and/or progesterone receptor (PR) [18, 19], and this is the case in both black and white women. These tumors generally have a more favorable outcome and frequently respond to endocrine treatments designed to inhibit estrogen receptor-mediated signaling pathways [22–25]. The use of postoperative adjuvant endocrine therapy in patients with hormone-dependent, early breast cancer lowers the rate of recurrence and improves survival [26], and widespread use of these agents are likely to have contributed substantially to the overall decline in breast cancer mortality observed in the U.S. over the past three decades [27]. One hypothesis that has not been thoroughly addressed is whether there are biological mechanism(s) leading to differential sensitivity to available endocrine agents between black and white patients with hormone-dependent breast cancer. With this in mind, we sought to examine whether a survival disparity existed between black and white breast cancer patients with ER/PR positive tumors, and to determine if such a disparity could be explained by differences in socioeconomic status, health care access, treatment-related variables, stage at diagnosis, and tumor grade.
Methods
The Breast Cancer Care in Chicago study was approved by the Institutional Review Board at the University of Illinois at Chicago, and has been described elsewhere [20, 28]. This population-based study performed case finding at all 56 diagnosing facilities in the greater Chicago area that diagnosed patients who were Chicago residents at diagnosis. Briefly, patients were eligible if they resided in Chicago, self-identified as non-Latina white, non-Latina black or Latina, were diagnosed with a primary in situ or invasive breast cancer during 2005– 2008, and were 30–79 years of age at diagnosis. For this particular analysis we restricted the sample to patients who self-identified as white or black and excluded Latina patients (n=181).
Patients provided written informed consent before administration of a 90-minute interview in English or Spanish using computer-assisted personal interview procedures. In all, 989 patients completed the interview (56% response rate); of these, 849 provided written informed consent to medical record reviews to obtain information on pathologic stage, histologic grade, estrogen and progesterone status, and other aspects of diagnosis and treatment. At interview, patients were asked a series of yes/no questions about radiation, chemotherapy and hormone therapy recommendation, acceptance, and initiation (Table 1). Data from medical record abstractions and from linkage to the Illinois State Cancer Registry also provided information on treatment recommendation and initiation. For each treatment type, treatment recommendation was coded “yes” if there was evidence of a recommendation in any of the three data sources; otherwise, recommendation was coded “no” if there was evidence of no recommendation in any of the data sources. Treatment initiation variables were similarly coded (Table 1) [29, 30].
Table 1.
Definition of treatment variables according to source of information in the Breast Cancer Care in Chicago study
| Radiation, Chemotherapy or Hormone Therapy Treatment | Offered/Recommended | Initiated |
|---|---|---|
| In-Person Interviews | Were you offered [treatment] or was it suggested that you accept this treatment? Yes/No | If patient agreed to have [treatment]: Have you begun [this treatment] yet? Yes/No |
| Medical Record Abstraction | Evidence that [this treatment] was recommended (e.g. documented receipt, treatment plan or provider note)? Yes/No | Evidence that [treatment] was initiated (e.g. start date, type, dose)? Yes/No |
| Illinois State Cancer Registry | Recommendation coded as “Yes”: Treatment administered; Not administered (as part of first source) but recommended; Not administered but recommended it and was refused; Recommended, but unknown if administered. Recommendation coded as “No”: Not administered-not part of first course; Not administered-contraindicated. | Treatment initiation coded as “Yes”: Treatment was administered; Initiation coded as “No”: Not administered-not part of first course; Not administered-contraindicated; Not administered-patient died; Not administered (as part of first source) but was recommended; Not administered but physician recommended it and was refused. |
| Final Treatment Variable | “Yes” if yes according to any of the 3 sources, otherwise No. | “Yes” if yes according to any of the 3 sources, otherwise No. |
A National Death Index Plus search was conducted in February 2015 from each patient’s date of diagnosis (during the years 2005–2008) through December 31, 2015. Cause of death was determined using provided International Classification of Disease 10 (ICD-10) codes for matched cases. ICD-10 codes corresponding to malignant neoplasm of breast with the prefix “C50” (C50.0–C50.9) were used to identify breast cancer deaths. A matched patient with breast cancer listed within their top three coded causes of death was considered a breast cancer related death. Observation time was computed as the difference in days from diagnosis date to death of date or censoring. Since the study had a rolling enrollment patient follow-up ranged between five and ten years.
Statistical analysis
The definition of analysis variables corresponding to race, age at diagnosis, income, education, census tract disadvantage and affluence, parity, health insurance status, time since last clinical breast exam, mode of detection have been described previously [20, 31]. Stage at diagnosis, hormone receptor status and histologic grade were defined as abstracted from patient medical records. Stage at diagnosis was categorized using the American Joint Committee on Cancer Version 7.0 categories of 0, 1, 2, 3 and 4. Hormone receptor status was defined by the pathologist’s interpretation at the treating hospital and coded as positive if the tumor contained either estrogen or progesterone receptors. Histologic grade was defined as low, intermediate and high. Kaplan-Meier curves were plotted for breast cancer-specific survival and Log-rank tests were used to compare survival rates between black and white participants, separately for ER/PR positive and ER/PR negative breast cancer. The remainder of the statistical analysis focused on examining factors associated with survival from ER/PR positive breast cancer alone, and on using these factors to explain the disparity in survival from ER/PR positive breast cancer.
Ordinal and continuous variables were categorized into discrete categories for descriptive analyses to compare distributions of risk factors by race and to examine predictors of death from ER/PR positive breast cancer within five years, before accounting for time at risk and censoring. Next, we examined the magnitude of the racial breast cancer mortality disparity by estimating hazard ratios from a series of Cox proportional hazards regression models of time to ER/PR positive breast cancer death. Models included the following independent variables: (1) race and age; (2) race, age and patient characteristics; (3) race, age, tumor stage and grade; (4) race, age, tumor stage and grade, and patient characteristics; and race, age, tumor stage and grade, and treatment variables. Patient factors included a-priori variables for income, education, census tract disadvantage and affluence, parity, health insurance status, recency of clinical breast exam, and mode of detection. When controlling for treatment we used a variable representing all cross-classifications of radiation, chemotherapy and hormone therapy initiation.
Results
There were 397 white and 411 black patients in the study, for whom ER and PR status were available for 299 and 303 patients, respectively. For patients with data, tumors were positive for ER and/ or PR for 88% of white and 72% of black patients. Figure 1 shows that the actuarial risk of death from breast cancer within 5 years of diagnosis was higher among black compared to white women diagnosed with ER/PR positive breast cancer, with 5-year breast cancer death rate of 11% vs. 2%, respectively (p<0.0001). There was no difference in survival between black and white women with ER/PR negative disease (21% vs. 20%, respectively, p=0.89, Figure 1). With respect to mode of detection among ER/PR positive breast cancer, for 163 (white) and 107 (black) screen-detected breast cancers, there were 4 breast cancer deaths, all among black patients (p=0.01). For 101 (white) and 111 (black) symptomatic breast cancers, there were 9 and 26 breast cancer deaths, respectively (p=0.004).
FIGURE 1.
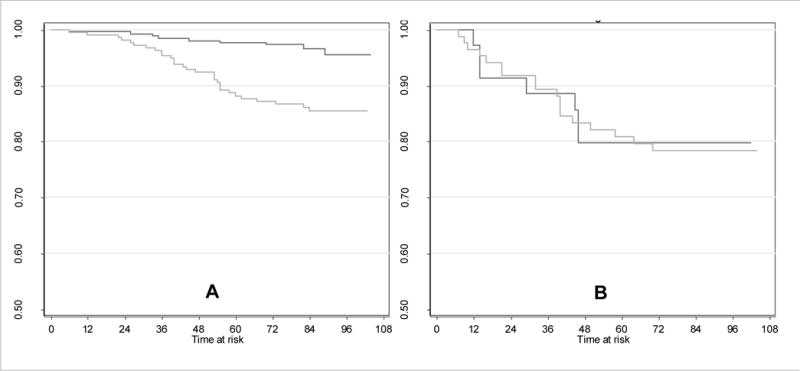
Kaplan Meier estimates for the black, white breast cancer survival disparity, separately for ER/PR positive and ER/PR negative breast cancer patients. Panel A: There were 39 breast cancer deaths among 482 ER/PR positive patients; the p-value (log rank test) for the black-white disparity < 0.0001. Panel B: There were 25 breast cancer deaths among 120 ER/PR negative patients; the p-value (log rank test) for the black-white disparity = 0.89.
Compared to white patients, black patients with ER/PR positive breast cancer were more likely to have less income and education, more likely to live in disadvantaged and less affluent neighborhoods, more likely to be parous, more likely to be overweight and to be obese, less likely to have private insurance or to have had a recent clinical breast exam, more likely to have had their breast cancer detected through symptoms, and slightly less likely to have low grade or in-situ tumors (Table 2). Compared to white patients, black patients with ER/PR positive breast cancer were less likely to have been recommended for or to have initiated either radiation therapy or hormone therapy; in contrast, black patients were more likely to have been recommended for and to have initiated chemotherapy (Table 2).
Table 2.
Differences in patient, clinical, tumor, and treatment-related characteristics by race among 482 patients diagnosed with ER/PR positive breast cancer in the Breast Cancer Care in Chicago study (2005–2008)
| N | White (N=264) % | Black (N=218) % | ||
|---|---|---|---|---|
| Age at diagnosis | ||||
| <50 | 136 | 30 | 26 | |
| 50–59 | 146 | 31 | 30 | |
| 60–79 | 200 | 39 | 44 | |
| Education | <0.0001 | |||
| <12 | 45 | 4 | 16 | |
| 12 | 97 | 13 | 28 | |
| >12 | 338 | 82 | 56 | |
| Income | <0.0001 | |||
| <20,000 | 96 | 10 | 32 | |
| <75,000 | 217 | 36 | 56 | |
| >75,000 | 156 | 49 | 12 | |
| Concentrated affluence | <0.0001 | |||
| <1 SD below mean | 26 | 1 | 11 | |
| Within 1 SD of mean | 332 | 57 | 83 | |
| >1 SD above mean | 124 | 42 | 6 | |
| Concentrated disadvantage | <0.0001 | |||
| <1 SD below mean | 91 | 33 | 1 | |
| Within 1 SD of mean | 295 | 66 | 56 | |
| >1 SD above mean | 96 | 1 | 43 | |
| Family History Breast Cancer | ||||
| None | 360 | 72 | 78 | |
| Weak | 83 | 18 | 17 | |
| Strong | 33 | 8 | 6 | |
| Parity | <0.0001 | |||
| Parous | 354 | 61 | 89 | |
| Nulliparous | 128 | 39 | 11 | |
| Body Mass Index | <0.0001 | |||
| Normal | 166 | 48 | 18 | |
| Over | 136 | 24 | 34 | |
| Obese | 177 | 27 | 48 | |
| Insurance | <0.0001 | |||
| None | 36 | 4 | 11 | |
| Public | 70 | 4 | 27 | |
| Private | 376 | 92 | 61 | |
| Last clinical exam | 0.0002 | |||
| Within two years | 371 | 83 | 69 | |
| Longer/never | 111 | 17 | 31 | |
| Mode of detection | 0.005 | |||
| Screening | 270 | 62 | 49 | |
| Symptoms | 212 | 38 | 51 | |
| Tumor grade | 0.09 | |||
| Low | 114 | 28 | 19 | |
| Moderate | 204 | 41 | 44 | |
| High | 142 | 28 | 31 | |
| Stage at diagnosis | 0.16 | |||
| 0 (in-situ) | 297 | 64 | 58 | |
| 1 | 130 | 26 | 28 | |
| 2–4 | 48 | 9 | 11 | |
| Radiation | 0.05 | |||
| Not recommended | 113 | 22 | 26 | |
| Recommended1 | 79 | 13 | 20 | |
| Initiated2 | 290 | 65 | 54 | |
| Chemotherapy | 0.005 | |||
| Not recommended | 249 | 58 | 44 | |
| Recommended1 | 39 | 7 | 9 | |
| Initiated2 | 194 | 35 | 46 | |
| Hormone Therapy | 0.01 | |||
| Not recommended | 72 | 10 | 21 | |
| Recommended1 | 151 | 33 | 29 | |
| Initiated2 | 259 | 56 | 50 |
P-values >0.2 are suppressed.
Recommended but not initiated by the patient.
Evidence that the treatment was initiated
Among women with ER/PR positive cancers, greater income and education, and residence in a census tract defined as more affluent and less disadvantaged, were each at least marginally associated with reduced risk of death from breast cancer (Table 3). Parity (p=0.02), increasing BMI (p=0.07) and lack of health insurance (p=0.07) were each associated or marginally associated with increased death from breast cancer. Symptomatic detection, later stage at diagnosis, and higher tumor grade were each associated with increased risk of death from breast cancer. For both radiation and chemotherapy, treatment recommendation was associated with more lethal breast cancer, whereas recommendation for hormone therapy was marginally associated with protection from breast cancer-related death (Table 3).
Table 3.
Associations of patient, clinical, and tumor, and treatment-related characteristics with death from breast cancer within 5 years of diagnosis, for 30 deaths among 482 ER/PR positive breast cancer patients.
| N | BC Death % | P-Value | |
|---|---|---|---|
| Race | <0.0001 | ||
| White | 264 | 2 | |
| Black | 218 | 11 | |
| Age at diagnosis | |||
| <50 | 136 | 6 | |
| 50–59 | 146 | 8 | |
| 60–79 | 200 | 5 | |
| Education | 0.08 | ||
| <12 | 45 | 9 | |
| 12 | 97 | 10 | |
| >12 | 338 | 5 | |
| Income | 0.13 | ||
| <20,000 | 96 | 9 | |
| <75,000 | 217 | 6 | |
| >75,000 | 156 | 4 | |
| Concentrated affluence | 0.06 | ||
| <1 SD below mean | 26 | 8 | |
| Within 1 SD of mean | 332 | 8 | |
| >1 SD above mean | 124 | 2 | |
| Concentrated disadvantage | 0.08 | ||
| <1 SD below mean | 91 | 2 | |
| Within 1 SD of mean | 295 | 7 | |
| >1 SD above mean | 96 | 8 | |
| Family History Breast Cancer | |||
| None | 360 | 6 | |
| Weak | 83 | 10 | |
| Strong | 33 | 3 | |
| Parity | 0.02 | ||
| Parous | 354 | 8 | |
| Nulliparous | 128 | 2 | |
| Body Mass Index | 0.07 | ||
| Normal | 166 | 5 | |
| Over | 136 | 9 | |
| Obese | 177 | 10 | |
| Insurance | 0.07 | ||
| None | 36 | 14 | |
| Public | 70 | 7 | |
| Private | 376 | 5 | |
| Last clinical exam | 0.003 | ||
| Within two years | 371 | 4 | |
| Longer/never | 111 | 13 | |
| Mode of detection | <0.0001 | ||
| Screening | 270 | 1 | |
| Symptoms | 212 | 12 | |
| Tumor grade | 0.002 | ||
| Low | 114 | 3 | |
| Moderate | 204 | 4 | |
| High | 142 | 11 | |
| Stage at diagnosis | <0.0001 | ||
| 0 (in-situ) | 297 | 1 | |
| 1 | 130 | 9 | |
| 2–4 | 48 | 25 | |
| Radiation | 0.01 | ||
| Not recommended | 113 | 5 | |
| Recommended1 | 79 | 16 | |
| Initiated2 | 290 | 7 | |
| Chemotherapy | <0.001 | ||
| Not recommended | 249 | 3 | |
| Recommended1 | 39 | 10 | |
| Initiated2 | 194 | 14 | |
| Hormone Therapy | 0.15 | ||
| Not recommended | 72 | 14 | |
| Recommended1 | 151 | 7 | |
| Initiated2 | 259 | 7 |
P-values >0.2 are suppressed
In cox regression models, the hazard of breast cancer death among ER/PR positive patients was at least 4 times higher for black than for white patients in all models tested (Table 4). Notably, even after adjusting for tumor stage and grade and treatment variables (including initiation of systemic adjuvant therapies), the hazard ratio for breast cancer death between black and white women was 4.39 (95% CI: 1.76, 10.9, p=0.001).
Table 4.
Hazard ratios for breast cancer death comparing black to white patients with ER/PR positive tumors after adjusting for patient characteristics and tumor grade.
| N | HR | 95% CI | P-Value | |
|---|---|---|---|---|
| Model adjusted for | ||||
| Age | 482 | 4.29 | (2.03, 9.06) | <0.0001 |
| Age and patient characteristics1 | 461 | 4.84 | (1.81, 12.9) | 0.002 |
| Age, stage and tumor grade | 455 | 4.76 | (1.95, 11.6) | 0.001 |
| Age, stage, grade and patient characteristics | 436 | 7.10 | (2.28, 22.0) | 0.001 |
| Age, stage, grade, and treatment variables2 | 455 | 4.39 | (1.76, 10.9) | 0.001 |
Education, income, tract disadvantage and affluence, family history of breast cancer, mode of detection and history of clinical breast exam.
Controlling for a single variable representing all cross-classifications of radiation, chemotherapy and hormone therapy initiation.
Discussion
We found a substantial black-white disparity in breast cancer survival for patients diagnosed with ER/PR positive tumors, but not for women with ER/PR negative cancers. Others have reported findings consistent with this analysis, confirming the strong association between race and survival for women with subtypes of breast cancer that are normally associated as having a “favorable-prognosis”. In an analysis of 179,414 women with stage 1 breast cancer from the SEER 18-registry database, Iqbal and colleagues reported a hazard ratio of death from breast cancer of 1.57 (95% CI,1.40 to 1.75) for black women compared with white women [32]. The age-adjusted HR for death did not decrease when women with triple negative breast cancer were excluded, suggesting that the disparity was driven by the ER-positive subset. Results from the Carolina Breast Cancer Study, which included 518 black and 631 white women with invasive breast cancer diagnosed between 1993 and 2001 [33], found a black-white difference in breast cancer-specific mortality for women diagnosed with luminal A breast cancer, but not for those diagnosed with basal-like breast cancer (adjusted HR, 1.9; 95% CI, 1.3 to 2.9 and HR, 1.3; HR, 0.8 to 2.3 for luminal A and basal-like breast cancer, respectively). Investigators from the Women’s CARE study [34] observed a difference in the age-adjusted risk of breast cancer-specific mortality among 523 black and 681 white women diagnosed with luminal A breast cancer (HR, 1.52; 95% CI, 1.01 to 2.28), but not among those diagnosed with TN breast cancer (HR, 1.21; 95% CI, 0.81–1.83). While these population-based studies are consistent with our findings, lack of adjustment for tumor grade and treatment-related variables limits the ability to distinguish between racial differences in tumor biology, sensitivity to treatment, and adequacy of treatment as the underlying causes for the worse outcome observed among black women with ER/PR positive breast cancer [35].
Often survival models do not account for lead time bias that may result from differential screening. To account for potential differences in lead time bias by race, we included in our models two measures that were potential markers for lead time bias: mode of detection and history of a recent clinical breast exam. Mode of detection is also a marker for tumor aggressiveness because more aggressive tumors are less likely to be detected by mammography screening. Self-reported mammography use tends to be over-reported due to social desirability issues [36]; history of a clinical breast exam, a measure of access to breast health care that may be less prone to over-reporting, was strongly associated with survival. Having had a clinical breast exam implies that you have a breast health conscious primary care provider which would tend to lead to recommendation and utilization of mammography.
Several studies have shown racial/ethnic differences in the use of adjuvant treatments, which could explain observed differences in survival between black and white patients in the population-based studies cited above [37, 38]. However, data from randomized clinical trials of adjuvant chemotherapy, where treatment is protocol-specified and uniformly delivered, show similar results to population studies [39, 40]. Sparano and colleagues performed a retrospective, secondary survival analysis on a large cohort of women with stage I-III breast cancer who participated in an NCI-sponsored randomized phase III clinical trial of adjuvant chemotherapy with various taxane-containing chemotherapy regimens [40]. Among 2807 women with ER-positive/HER2-negative disease (161 black and 2646 non-black), self-identification of black race was significantly associated with worse breast cancer-specific survival (HR of death from breast cancer = 1.65, 95% CI; 1.11 to 2.46, P = .013). There was no association between race and survival for women with TN or HER2-positive disease. However, the study was not able to separate the effect of race from socioeconomic factors. Albain reported similar findings in a secondary analysis of women participating in a series of randomized trials of adjuvant therapy for breast cancer conducted by the Southwest Oncology Group [39]. The authors reported the largest disparity existed between black and white women with ER-positive breast cancer (HR for death=1.74 and 1.61 for pre-and postmenopausal women, resp.). The association of poor survival with black race persisted after limited adjustment for socioeconomic factors (education and income). There is substantial evidence of differential adherence to oral hormonal treatment by race [8, 41, 42] and the aforementioned studies were not able to account for this potential contributor to survival disparities.
These studies are also consistent with the hypothesis that hormone-dependent breast cancers arising in black women are more resistant to treatment than tumors from their white peers. There is evidence from gene expression profiling studies that black women with ER-positive breast cancer overall have biologically more aggressive disease than white women [43]. With respect to potential biological mechanisms, these studies could not distinguish between differences in tumor aggressiveness vs. resistance to treatment as a mechanism contributing to poor outcomes in black women with ER-positive disease. Our study adds to the existing literature by adjusting for key potential confounders, including a measure of tumor aggressiveness (tumor grade), treatment, and robust adjustment for socioeconomic factors. This analysis provides support for the hypothesis that differential sensitivity to treatment contributes to the survival disparity that exists between black and white women with hormone-dependent breast cancer, and provides strong rationale for laboratory studies to elucidate mechanisms of resistance at the cellular and molecular levels in an effort to understand and ameliorate racial disparities in survival.
There is mounting evidence suggesting that biologic mechanisms are activated in “favorable prognosis” tumors such as ER-positive/Luminal A subtype breast cancers arising in black woman, arming those tumors with greater metastatic potential or intrinsic resistance to endocrine treatment when compared to their white counterparts [39, 40]. Nonetheless, the mechanisms of resistance to endocrine therapy that promote tumor recurrence in ER-positive early breast cancer are poorly understood. Emerging evidence suggests several potential avenues of investigation for exploring the molecular basis of the putative higher rate of endocrine resistance in black breast cancer patients. Gain-of-function mutations in the ligand binding domain of the ESR1 gene, which encodes for ER alpha, are now known to represent a mechanism of acquired resistance to endocrine therapy in metastatic breast cancer [44]. Genomic alterations in ESR1 are an uncommon finding in primary tumors, but are seen frequently in tumors following endocrine treatment [45]. The emergence of ESR1 mutant clones during adjuvant endocrine treatment could promote recurrence and could be explored as a possible biologic mediator of racial disparities.
Evidence suggests that other pathways and mechanisms could contribute to tumors that respond poorly to endocrine therapy. For example, Aurora Kinase B appears to play an important role in tamoxifen resistance [46], raising the possibility that differential activation of the Aurora Kinase B pathway observed between black and white patients may mediate the higher rate of endocrine resistance seen in black patients with ER-positive breast cancer [47]. Likewise, increased expression of the CRYBB2 gene in luminal breast cancers from African American compared to Caucasian patients may also play a role [48, 49]. While the role of CRYBB2 in oncogenesis is unknown, gene knock-out studies in mice have revealed an important role for CRYBB2 in estrogen-regulated pathways [50], making this an attractive candidate for further study. Another gene of interest is SQLE, which encodes for a key enzyme in the cholesterol biosynthetic pathway. SQLE expression is associated with race in Luminal A breast cancers, and high expression is associated with higher mortality in black women [48]. Moreover, there is no differential expression of SQLE transcripts in normal breast tissue, suggesting that this reflects a biologically relevant difference between Luminal tumors arising in black and white women rather than racial variation in gene expression at the population level. Mechanistically, SQLE could contribute to poor therapeutic response by altering local oxysterols levels, which have been shown to promote metastatic breast cancer [51]. Hyperactivation of the FoxM1 transcription factor is a key transcriptional hub in basal breast cancer [52]. Emerging evidence suggests that FoxM1 expression is also a strong predictor of poor survival in hormone-dependent breast cancer [53, 54] and promotes resistance to endocrine treatment. These findings provide rationale for studying FoxM1 as a potential mechanism of resistance to endocrine therapy among black women with ER-positive breast cancer.
Conclusion
While inequities exist along the breast cancer care continuum that have led to disparities in survival for women of color who are diagnosed with breast cancer, it is becoming apparent that biologic factors also contribute to the problem and that cancer biologists should play a more prominent role in cancer disparities research if we are to maximize true equity in breast cancer care. Our findings should stimulate basic and translational scientists to pursue disparities research in order to elucidate the underlying mechanisms of resistance and to develop effective treatment strategies.
Acknowledgments
We thank the Illinois women diagnosed with breast cancer whose information was reported to the Illinois State Cancer Registry thereby making this research possible. The conclusions, opinions, and recommendations expressed are not necessarily the conclusions, opinions, or recommendations of the Illinois State Cancer Registry.
Funding
This work was supported by two Grants from the National Cancer Institute at the National Institutes of Health to the University of Illinois at Chicago (Grants 1P50CA106743, 2P50CA106743).
Footnotes
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Ansell D, Whitman S, Lipton R, Cooper R. Race, income, and survival from breast cancer at two public hospitals. Cancer. 1993;72:2974–8. doi: 10.1002/1097-0142(19931115)72:10<2974::aid-cncr2820721019>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 2.Baquet CR, Mishra SI, Commiskey P, Ellison GL, DeShields M. Breast cancer epidemiology in blacks and whites: disparities in incidence, mortality, survival rates and histology. Journal of the National Medical Association. 2008;100:480–8. doi: 10.1016/s0027-9684(15)31294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curtis E, Quale C, Haggstrom D, Smith-Bindman R. Racial and ethnic differences in breast cancer survival: how much is explained by screening, tumor severity, biology, treatment, comorbidities, and demographics? Cancer. 2008;112:171–80. doi: 10.1002/cncr.23131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitman S, Orsi J, Hurlbert M. The racial disparity in breast cancer mortality in the 25 largest cities in the United States. Cancer Epidemiology. 2012;36:e147–51. doi: 10.1016/j.canep.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Whitman S, Ansell D, Orsi J, Francois T. The racial disparity in breast cancer mortality. Journal of Community Health. 2011;36:588–96. doi: 10.1007/s10900-010-9346-2. [DOI] [PubMed] [Google Scholar]
- 6.Gerend MA, Pai M. Social determinants of Black-White disparities in breast cancer mortality: a review. Cancer Epidemiology, Biomarkers & Prevention. 2008;17:2913–23. doi: 10.1158/1055-9965.EPI-07-0633. [DOI] [PubMed] [Google Scholar]
- 7.Freedman RA, Virgo KS, He Y, Pavluck AL, Winer EP, Ward EM, et al. The association of race/ethnicity, insurance status, and socioeconomic factors with breast cancer care. Cancer. 2011;117:180–9. doi: 10.1002/cncr.25542. [DOI] [PubMed] [Google Scholar]
- 8.Roberts MC, Wheeler SB, Reeder-Hayes K. Racial/Ethnic and socioeconomic disparities in endocrine therapy adherence in breast cancer: a systematic review. American Journal of Public Health. 2015;105(Suppl 3):e4–e15. doi: 10.2105/AJPH.2014.302490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molina Y, Silva A, Rauscher GH. Racial/Ethnic Disparities in Time to a Breast Cancer Diagnosis: The Mediating Effects of Health Care Facility Factors. Med Care. 2015;53:872–8. doi: 10.1097/MLR.0000000000000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mortel M, Rauscher GH, Murphy AM, Hoskins K, Warnecke RB. Racial and Ethnic Disparity in Symptomatic Breast Cancer Awareness despite a Recent Screen: The Role of Tumor Biology and Mammography Facility Characteristics. Cancer Epidemiology, Biomarkers & Prevention. 2015;24:1599–606. doi: 10.1158/1055-9965.EPI-15-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rauscher GH, Allgood KL, Whitman S, Conant E. Disparities in screening mammography services by race/ethnicity and health insurance. Journal of Women’s Health. 2012;21:154–60. doi: 10.1089/jwh.2010.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rauscher GH, Conant EF, Khan JA, Berbaum ML. Mammogram image quality as a potential contributor to disparities in breast cancer stage at diagnosis: an observational study. BMC Cancer. 2013;13:208. doi: 10.1186/1471-2407-13-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rauscher GH, Khan JA, Berbaum ML, Conant EF. Potentially missed detection with screening mammography: does the quality of radiologist’s interpretation vary by patient socioeconomic advantage/disadvantage? Annals of Epidemiology. 2013;23:210–4. doi: 10.1016/j.annepidem.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taplin SH, Yabroff KR, Zapka J. A multilevel research perspective on cancer care delivery: The example of follow-up to an abnormal mammogram. Cancer Epidemiol Biomarkers Prev. 2012;21:1709–15. doi: 10.1158/1055-9965.EPI-12-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zapka J, Taplin SH, Ganz P, Grunfeld E, Sterba K. Multilevel factors affecting quality: examples from the cancer care continuum. Journal of the National Cancer Institute Monographs. 2012;2012:11–9. doi: 10.1093/jncimonographs/lgs005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones BA, Kasl SV, Howe CL, Lachman M, Dubrow R, Curnen MM, et al. African-American/White differences in breast carcinoma: p53 alterations and other tumor characteristics. Cancer. 2004;101:1293–301. doi: 10.1002/cncr.20500. [DOI] [PubMed] [Google Scholar]
- 17.Chlebowski RT, Chen Z, Anderson GL, Rohan T, Aragaki A, Lane D, et al. Ethnicity and breast cancer: factors influencing differences in incidence and outcome. Journal of the National Cancer Institute. 2005;97:439–48. doi: 10.1093/jnci/dji064. [DOI] [PubMed] [Google Scholar]
- 18.Elledge RM, Clark GM, Chamness GC, Osborne CK. Tumor biologic factors and breast cancer prognosis among white, Hispanic, and black women in the United States. Journal of the National Cancer Institute. 1994;86:705–12. doi: 10.1093/jnci/86.9.705. [DOI] [PubMed] [Google Scholar]
- 19.Miller BA, Hankey BF, Thomas TL. Impact of sociodemographic factors, hormone receptor status, and tumor grade on ethnic differences in tumor stage and size for breast cancer in US women. American Journal of Epidemiology. 2002;155:534–45. doi: 10.1093/aje/155.6.534. [DOI] [PubMed] [Google Scholar]
- 20.Rauscher GH, Campbell RT, Wiley EL, Hoskins K, Stolley M, Warnecke RB. Socioeconomic position and reproductive factors mediate racial and ethnic disparities in estrogen/progesterone receptor negative breast cancer. American Journal of Epidemiology. 2015 doi: 10.1093/aje/kwv226. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dietze EC, Sistrunk C, Miranda-Carboni G, O’Regan R, Seewaldt VL. Triple-negative breast cancer in African-American women: disparities versus biology. Nature Reviews Cancer. 2015;15:248–54. doi: 10.1038/nrc3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Althuis MD, Fergenbaum JH, Garcia-Closas M, Brinton LA, Madigan MP, Sherman ME. Etiology of hormone receptor-defined breast cancer: a systematic review of the literature. Cancer Epidemiology, Biomarkers & Prevention. 2004;13:1558–68. [PubMed] [Google Scholar]
- 23.Ballard-Barbash R, Griffin MR, Fisher LD, Covalciuc MA, Jiang NS. Estrogen receptors in breast cancer. Association with epidemiologic risk factors. American Journal of Epidemiology. 1986;124:77–84. doi: 10.1093/oxfordjournals.aje.a114372. [DOI] [PubMed] [Google Scholar]
- 24.Deroo BJ, Korach KS. Estrogen receptors and human disease. Journal of Clinical Investigation. 2006;116:561–70. doi: 10.1172/JCI27987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simon MS, Severson RK. Racial differences in breast cancer survival: the interaction of socioeconomic status and tumor biology. American Journal of Obstetrics & Gynecology. 1997;176:S233–9. doi: 10.1016/s0002-9378(97)70381-8. [DOI] [PubMed] [Google Scholar]
- 26.Early Breast Cancer Clinical Trialists’ Collaborative Group. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–84. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA: a Cancer Journal for Clinicians. 2014;64:52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 28.Mortel M, Rauscher GH, Murphy AM, Hoskins K, Warnecke RB. Racial and Ethnic Disparity in Symptomatic Breast Cancer Awareness despite a Recent Screen: The Role of Tumor Biology and Mammography Facility Characteristics. Cancer Epidemiol Biomarkers Prev. 2015;24:1599–606. doi: 10.1158/1055-9965.EPI-15-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silva A, Rauscher GH, Hoskins K, et al. Assessing racial/ethnic disparities in chemotherapy treatment among breast cancer patients in context of changing treatment guidelines. Breast Cancer Research & Treatment. 2013;142:667–672. doi: 10.1007/s10549-013-2759-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silva A, Rauscher GH, Ferrans CE, et al. Assessing the quality of race/ethnicity, tumor, and breast cancer treatment information in a non-SEER state registry. Journal of Registry Management. 2014;41:24–30. [PubMed] [Google Scholar]
- 31.Dookeran KA, Silva A, Warnecke RB, Rauscher GH. Race/Ethnicity and disparities in mastectomy practice in the breast cancer care in chicago study. Annals of Surgical Oncology. 2015;22:66–74. doi: 10.1245/s10434-014-3945-6. [DOI] [PubMed] [Google Scholar]
- 32.Iqbal J, Ginsburg O, Rochon PA, Sun P, Narod SA. Differences in Breast Cancer Stage at Diagnosis and Cancer-Specific Survival by Race and Ethnicity in the United States. JAMA. 2015;313(2):165–173. doi: 10.1001/jama.2014.17322. [DOI] [PubMed] [Google Scholar]
- 33.O’Brien KM, Cole SR, Tse CK, Perou CM, Carey LA, Foulkes WD, et al. Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina Breast Cancer Study. Clinical Cancer Research. 2010;16:6100–10. doi: 10.1158/1078-0432.CCR-10-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma H, Lu Y, Malone KE, Marchbanks PA, Deapen DM, Spirtas R, Burkman RT, Strom BL, McDonald JA, Folger SG, Simon MS, Sullivan-Halley J, Press MF, Bernstein L. Mortality risk of black women and white women with invasive breast cancer by hormone receptors, HER2, and p53 status. BMC Cancer. 2013;13:225. doi: 10.1186/1471-2407-13-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Warner ET, Tamimi RM, Hughes ME, Ottesen RA, Wong YN, Edge SB, et al. Racial and Ethnic Differences in Breast Cancer Survival: Mediating Effect of Tumor Characteristics and Sociodemographic and Treatment Factors. Journal of Clinical Oncology. 2015;33:2254–61. doi: 10.1200/JCO.2014.57.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rauscher GH, Johnson TP, Cho YI, Walk JA. Accuracy of self-reported cancer-screening histories: a meta-analysis. Cancer Epidemiology, Biomarkers & Prevention. 2008;17:748–757. doi: 10.1158/1055-9965.EPI-07-2629. [DOI] [PubMed] [Google Scholar]
- 37.Wu X, Richardson LC, Kahn AR, Fulton JP, Cress RD, Shen T, et al. Survival difference between non-Hispanic black and non-Hispanic white women with localized breast cancer: the impact of guideline-concordant therapy. [Erratum appears in J Natl Med Assoc 2008 Jun;100(6):659] Journal of the National Medical Association. 2008;100:490–8. doi: 10.1016/s0027-9684(15)31295-5. [DOI] [PubMed] [Google Scholar]
- 38.Li CI, Malone KE, Daling JR. Differences in breast cancer stage, treatment, and survival by race and ethnicity. Archives of Internal Medicine. 2003;163:49–56. doi: 10.1001/archinte.163.1.49. [DOI] [PubMed] [Google Scholar]
- 39.Albain KS, Unger JM, Crowley JJ, Coltman CA, Jr, Hershman DL. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. Journal of the National Cancer Institute. 2009;101:984–92. doi: 10.1093/jnci/djp175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sparano JA, Wang M, Zhao F, Stearns V, Martino S, Ligibel JA, et al. Race and hormone receptor-positive breast cancer outcomes in a randomized chemotherapy trial. Journal of the National Cancer Institute. 2012;104:406–14. doi: 10.1093/jnci/djr543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neugut AI, Subar M, Wilde ET, Stratton S, Brouse CH, Hillyer GC, et al. Association between prescription co-payment amount and compliance with adjuvant hormonal therapy in women with early-stage breast cancer. Journal of Clinical Oncology. 2011;29:2534–42. doi: 10.1200/JCO.2010.33.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Friese CR, Pini TM, Li Y, Abrahamse PH, Graff JJ, Hamilton AS, et al. Adjuvant endocrine therapy initiation and persistence in a diverse sample of patients with breast cancer. Breast Cancer Research & Treatment. 2013;138:931–9. doi: 10.1007/s10549-013-2499-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lund MJ, Mosunjac M, Davis KM, Gabram-Mendola S, Rizzo M, Bumpers HL, et al. 21-Gene recurrence scores: racial differences in testing, scores, treatment, and outcome. Cancer. 2012;118:788–96. doi: 10.1002/cncr.26180. [DOI] [PubMed] [Google Scholar]
- 44.Robinson DR, Wu YM, Vats P, Su F, Lonigro RJ, Cao X, et al. Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nature Genetics. 2013;45:1446–51. doi: 10.1038/ng.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fribbens C, O’Leary B, Kilburn L, Hrebien S, Garcia-Murillas I, Beaney M, et al. Plasma ESR1 Mutations and the Treatment of Estrogen Receptor-Positive Advanced Breast Cancer. Journal of Clinical Oncology. 2016;34:2961–8. doi: 10.1200/JCO.2016.67.3061. [DOI] [PubMed] [Google Scholar]
- 46.Larsen SL, Yde CW, Laenkholm AV, Rasmussen BB, Duun-Henriksen AK, Bak M, et al. Aurora kinase B is important for antiestrogen resistant cell growth and a potential biomarker for tamoxifen resistant breast cancer. BMC Cancer. 2015;15:239. doi: 10.1186/s12885-015-1210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stewart PA, Luks J, Roycik MD, Sang QX, Zhang J. Differentially expressed transcripts and dysregulated signaling pathways and networks in African American breast cancer. PLoS ONE [Electronic Resource] 2013;8:e82460. doi: 10.1371/journal.pone.0082460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.D’Arcy M, Fleming J, Robinson WR, Kirk EL, Perou CM, Troester MA. Race-associated biological differences among Luminal A breast tumors. Breast Cancer Research & Treatment. 2015;152:437–48. doi: 10.1007/s10549-015-3474-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Field LA, Love B, Deyarmin B, Hooke JA, Shriver CD, Ellsworth RE. Identification of differentially expressed genes in breast tumors from African American compared with Caucasian women. Cancer. 2012;118:1334–44. doi: 10.1002/cncr.26405. [DOI] [PubMed] [Google Scholar]
- 50.Gao Q, Patani N, Dunbier AK, Ghazoui Z, Zvelebil M, Martin LA, et al. Effect of aromatase inhibition on functional gene modules in estrogen receptor-positive breast cancer and their relationship with antiproliferative response. Clinical Cancer Research. 2014;20:2485–94. doi: 10.1158/1078-0432.CCR-13-2602. [DOI] [PubMed] [Google Scholar]
- 51.Nelson ER, Wardell SE, Jasper JS, Park S, Suchindran S, Howe MK, Carver NJ, Pillai RV, et al. 27-Hydroxycholesterol Links Hypercholesterolemia and Breast Cancer Pathophysiology. Science. 2013;342(6162):1094–1098. doi: 10.1126/science.1241908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Network CGA. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee JJ, Lee HJ, Son BH, Kim SB, Ahn JH, Ahn SD, et al. Expression of FOXM1 and related proteins in breast cancer molecular subtypes. International Journal of Experimental Pathology. 2016;97:170–7. doi: 10.1111/iep.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ahn H, Sim J, Abdul R, Chung MS, Paik SS, Oh YH, et al. Increased expression of forkhead box M1 is associated with aggressive phenotype and poor prognosis in estrogen receptor-positive breast cancer. Journal of Korean Medical Science. 2015;30:390–7. doi: 10.3346/jkms.2015.30.4.390. [DOI] [PMC free article] [PubMed] [Google Scholar]


