Abstract
Objective and Methods An SGLT2 inhibitor (ipragliflozin, dapagliflozin, luseogliflozin, tofogliflozin, or canagliflozin) was administered to 132 outpatients with type 2 diabetes mellitus with or without other antidiabetic drugs for 6 months to evaluate its efficacy, the incidence of adverse events, and its influence on the renal function.
Results The patient's mean glycated hemoglobin level significantly improved from 7.52±1.16% to 6.95±0.98% (p<0.001). The body weight of the patients was significantly reduced from 78.0±15.3 kg to 75.6±15.1 kg (p<0.001). The estimated visceral fat area was also significantly reduced from 108.4±44.6 cm2 to 94.5±45.3 cm2 (p<0.001). The waist circumference, blood pressure, serum alanine aminotransferase, γ-glutamyl transpeptidase, and uric acid levels also showed a significant decrease. The urinary albumin/creatinine ratio (U-ACR) was significantly reduced in the patients whose U-ACR levels were 30-300 mg/gCr at the baseline. The mean eGFR significantly decreased in the patients with a pre-treatment eGFR value of ≥90 mL/min/1.73 m2 but remained unchanged in the patients with a pre-treatment value of <90 mL/min/1.73 m2. A total of 13 adverse events were noted, including systemic eruption (n=1), cystitis (n=2), pudendal pruritus (n=2), nausea (n=1), malaise (n=1), a strong hunger sensation and increased food ingestion (n=1), and non-serious hypoglycemia (n=5).
Conclusion SGLT2 inhibitors seemed to be useful in the treatment of obese type 2 diabetes mellitus patients. Furthermore, these data suggest that SGLT2 inhibitors may protect the renal function.
Keywords: SGLT2 inhibitor, glycemic control, visceral fat, oral hypoglycemic agent, renal function
Introduction
In April 2014, SGLT2 inhibitors, a new family of antidiabetic drugs, became available for clinical use in Japan. SGLT2 inhibitors are expected to not only improve glycemic control by stimulating urinary glucose excretion but to also enable weight loss, reduce blood pressure, and improve the lipid profile (1-3). Thus, SGLT2 inhibitors are also expected to suppress diabetic complications. However, the history of using SGLT2 inhibitors is still short and -for the most part-their adverse effects remain to be clarified. Since SGLT2 inhibitors were approved for clinical use in Japan, there have been several reports of patients who developed severe ketoacidosis or cerebral infarction, including some fatal cases (4). Thus, SGLT2 inhibitors should only be administered in carefully selected cases. Although there is accumulating evidence on the efficacy and safety of SGLT2 inhibitors in Western countries (where they were marketed earlier), there have been few reports on the large-scale clinical use of SGLT2 inhibitors in Japan, and little is known of their efficacy and safety in clinical cases. In the current study, we evaluated the usefulness and safety of SGLT2 inhibitors in patients with type 2 diabetes mellitus who were treated for 6 months. At the same time, the influence of SGLT2 inhibitors on the renal function was evaluated through the analysis of changes in the estimated glomerular filtration rate (eGFR) and the urinary albumin/creatinine ratio.
Materials and Methods
The present study included 132 type 2 diabetes outpatients with poor glycemic control, who had continued diet therapy, exercise therapy, and/or who were treated with antidiabetic drugs other than SGLT2 inhibitors at our clinic (Table 1). The study excluded patients who were judged as being inappropriate by physicians because of their inability to understand the importance of water intake during treatment or the explanation about the possible adverse effects of SGLT2 inhibitors. This study was performed in compliance with the Declaration of Helsinki, and written informed consent was obtained from each patient. The study received approval from our clinic's ethics committee (Study no. 720901).
Table 1.
The Clinical Background of 132 Type 2 Diabetes Patients Who Were Treated with SGLT2 Inhibitors.
| Age | 51.0 | ± 10.9 | (27-80) | ||
| Sex (male/female) | 75 | / 57 | |||
| Duration | 6.3 | ± 6.1 years | (0-32 years) | ||
| Height | 164.1 | ± 8.8 cm | |||
| Body weight | 77.9 | ± 15.3 kg | (47.7-121.3 kg) | ||
| BMI | 28.8 | ± 4.7 | (20.4-48.8) | ||
| Visceral fat area | 107.5 | ± 44.7 cm2 | (20-220 cm2) | ||
| HbA1c | 7.45 | ± 1.14% |
The values are expressed as the mean ± SD. BMI: body mass index, HbA1c: glycated hemoglobin
One of the SGLT2 inhibitors (Table 2) was administered with or without other oral hypoglycemic drugs, insulin preparations, or glucagon-like peptide-1 receptor (GLP-1R) agonists. The concomitantly used antidiabetic drugs included glimepiride (n=20), metformin (n=79), dipeptidyl peptidase-4 (DPP-4) inhibitors (n=63), glinides (n=9), α-glucosidase inhibitors (n=17), pioglitazone (n=8), insulin (n=15), and GLP-1R agonists (n=4). No concomitant drugs were used in 15 cases. The mean number of concomitant drugs in each case was 1.94. No changes were allowed in the administration of concomitant antidiabetic drugs during the observation period, except in cases where it was necessary in order to prevent hypoglycemia. The dose reductions or discontinuation of glimepiride and the dose reductions of insulin were implemented at the start of SGLT2 inhibitor treatment in the following manner. In the patients whose glimepiride dose was ≥2 mg or 1-1.5 mg, the dose was reduced to 1 mg or 0.5 mg, respectively; while glimepiride was discontinued in patients whose dose was 0.5 mg. Each dose of insulin was reduced by 10% in patients with a glycated hemoglobin (HbA1c) level of <8%. If the blood glucose or HbA1c level was markedly aggravated after the start of SGLT2 inhibitor treatment following a reduction in the dose of insulin, the dose was returned to the original amount. In some cases, we considered reducing or discontinuing the administration of existing diuretics if the patient was of advanced age or if his/her systolic blood pressure in the physician's office was ≤130 mg in order to avoid dehydration or an excessive decrease in blood pressure.
Table 2.
The SGLT2 Inhibitors That Were Administered to the Patients in the Present Study.
| Ipragliflozin (50 mg) | 95 cases |
| Dapagliflozin (5 mg) | 7 cases |
| Luseogliflozin (2.5 mg) | 5 cases |
| Tofogliflozin (20 mg) | 18 cases |
| Canagliflozin (100 mg) | 7 cases |
The administration of SGLT2 inhibitors was continued for 6 months in 111 cases. The details of the 21 cases in which treatment was prematurely discontinued are shown in Table 3.
Table 3.
The Reasons for Treatment Discontinuation in 21 Cases.
| Systemic eruption | 1 case |
| Pollakiuria disturbing the patient’s job | 3 cases |
| Nausea | 1 case |
| Malaise | 1 case |
| Strong hunger/increased food ingestion | 1 case |
| Myalgia | 1 case |
| Chest compression | 1 case |
| Shoulder discomfort | 1 case |
| Difficulty attending visits at 2-week intervals | 1 case |
| Weight loss not desired | 1 case |
| Switched to insulin | 2 cases |
| Admission for hip surgery | 1 case |
| Change of residence | 2 cases |
| Changed to another medical facility | 1 case |
| Discontinued attending visits | 3 cases |
Immediately before and at 3 and 6 months after the start of SGLT2 inhibitor treatment, the following parameters were evaluated: HbA1c, body weight, body mass index (BMI), waist circumference, the estimated visceral fat area, systolic blood pressure, diastolic blood pressure, serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transpeptidase (γ-GTP), blood urea nitrogen, creatinine, uric acid, the estimated glomerular filtration rate (eGFR), the urinary albumin/creatinine ratio (U-ACR) (as determined by casual urine collection in the morning), and the serum levels of low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglyceride.
The visceral fat area was estimated on an OMRON HDS-2000 using the dual impedance method (5). To reduce measurement errors, the data were collected before or ≥2 hours after a meal.
The data were analyzed using a paired t-test. The statistical analyses were performed using the data analysis tool function of Microsoft Excel 2013. p values of <0.05 were considered to indicate statistical significance.
Results
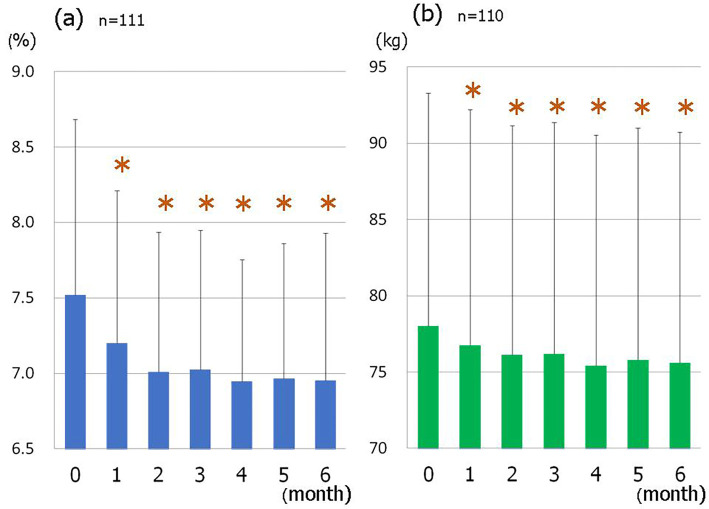
The mean HbA1c level improved over the treatment period (Fig. 1a), and significantly decreased from 7.52±1.16% at the baseline to 6.95±0.98% at 6 months (p<0.001, Table 4). A higher pre-treatment HbA1c level was associated with a significant decrease in the HbA1c level after 6 months of treatment (Fig. 2a).
Figure 1.
The changes in the glycated hemoglobin (HbA1c) level (a) (n=111) and body weight (b) (n=110) over 6 months of SGLT2 inhibitor treatment. *p<0.001 vs. 0 month.
Table 4.
The Clinical Indicators before and after SGLT2 Inhibitor Treatment.
| Before | 3 months | p | 6 months | p | n | |
|---|---|---|---|---|---|---|
| HbA1c (%) | 7.52 ± 1.16 | 7.02 ± 0.92 | <0.001 | 6.95 ± 0.98 | <0.001 | 111 |
| Body weight (kg) | 78.0 ± 15.3 | 76.2 ± 15.7 | <0.001 | 75.6 ± 15.1 | <0.001 | 110 |
| BMI (kg/m2) | 28.9 ± 4.7 | 28.2 ± 4.7 | <0.001 | 28.0 ± 4.6 | <0.001 | 110 |
| Abdominal circumference (cm) | 98.3 ± 10.5 | 96.5 ± 10.5 | 0.002 | 94.9 ± 10.3 | <0.001 | 57 |
| Visceral fat area (cm2) | 108.4 ± 44.6 | 100.1 ± 40.9 | <0.001 | 94.5 ± 45.3 | <0.001 | 64 |
| Subcutaneous fat area (cm2) | 258.3 ± 80.0 | 241.9 ± 75.9 | <0.001 | 229.2 ± 78.0 | <0.001 | 64 |
| Systolic blood pressure (mmHg) | 133.7 ± 15.5 | 128.0 ± 14.6 | <0.001 | 127.4 ± 13.5 | <0.001 | 111 |
| Diastolic blood pressure (mmHg) | 79.6 ± 10.27 | 77.1 ± 9.4 | 0.007 | 75.2 ± 10.4 | <0.001 | 111 |
| AST (IU/L) | 27.7 ± 15.6 | 23.9 ± 11.8 | <0.001 | 23.1 ± 11.8 | 0.001 | 106 |
| ALT (IU/L) | 39.5 ± 31.2 | 32.9 ± 24.2 | 0.001 | 30.7 ± 22.7 | <0.001 | 107 |
| γGTP (IU/L) | 53.1 ± 46.2 | 43.7 ± 33.5 | <0.001 | 41.6 ± 34.1 | <0.001 | 105 |
| BUN (mg/dL) | 14.5 ± 3.6 | 15.2 ± 4.1 | 0.051 | 14.9 ± 3.4 | 0.194 | 104 |
| Uric acid (mg/dL) | 5.3 ± 1.3 | 4.9 ± 1.3 | 0.002 | 4.8 ± 1.3 | <0.001 | 86 |
| Cr (mg/dL) | 0.65 ± 0.15 | 0.68 ± 0.17 | 0.002 | 0.68 ± 0.15 | <0.001 | 106 |
| eGFR (mL/min/1.73m2) | 93.1 ± 20.4 | 89.6 ± 21.0 | 0.010 | 88.7 ± 19.9 | <0.001 | 106 |
| Urinary albumin/creatinine ratio (mg/gCr) | 82.1 ± 227.9 | 62.4 ± 203.1 | <0.001 | 55.5 ± 148.8 | 0.079 | 101 |
| LDL-C (mg/dL) | 107.1 ± 29.7 | 106.0 ± 29.1 | 0.643 | 108.4 ± 32.1 | 0.629 | 106 |
| HDL-C (mg/dL) | 50.7 ± 14.8 | 51.7 ± 15.9 | 0.296 | 51.5 ± 14.9 | 0.329 | 107 |
| Triglyceride (mg/dL) | 228.6 ± 176.8 | 202.0 ± 129.5 | 0.061 | 201.2 ± 125.3 | 0.056 | 107 |
The values are expressed as the mean ± SD. HbA1c: glycated hemoglobin, BMI: body mass index, AST: aspartate aminotransferase, ALT: alanine aminotransferase, γ-GTP: γ-glutamyl transferase, BUN: blood urea nitrogen, Cr: creatinine, eGFR: estimated glomerular filtration rate, LDL-C: low-density lipoprotein cholesterol, HDL-C: high-density lipoprotein cholesterol
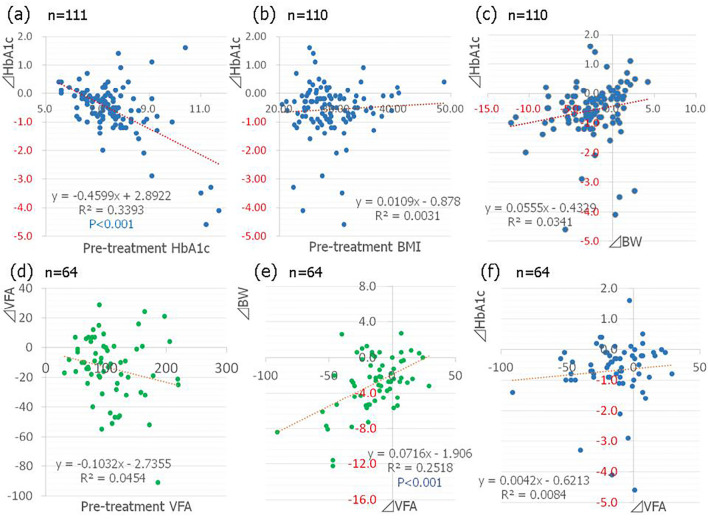
Figure 2.
(a) The correlation between the pre-treatment glycated hemoglobin (HbA1c) level and the changes in the HbA1c level (n=111). (b) The correlation between the pre-treatment body mass index (BMI) and changes in the HbA1c level (n=110). (c) The correlation between changes in body weight (BW) and changes in the HbA1c level (n=110). (d) The correlation between the pre-treatment visceral fat area (VFA) and changes in the VFA (n=64). (e) The correlation between changes in the VFA and changes in BW (n=64). (f) The correlation between changes in the VFA and changes in the HbA1c level (n=64).
The mean body weight also decreased over time after the start of SGLT2 inhibitor treatment (Fig. 1b), with a significant decrease from 78.0±15.3 kg to 75.6±15.1 kg at 6 months (p<0.001, Table 4). The pre-treatment BMI was not correlated with the magnitude of improvement in HbA1c after the start of treatment (Fig. 2b) and there was no significant correlation between the changes in body weight and the magnitude of HbA1c improvement (Fig. 2c).
The estimated visceral fat area, which was measured in 64 cases, showed a significant decrease from 108.4±44.6 cm2 to 94.5±45.3 cm2 (p <0.001, Table 4). The waist circumference also showed a significant decrease from 98.3±10.5 cm to 94.9±10.3 cm (p<0.001, Table 4). A larger pre-treatment visceral fat area was associated with a significant reduction in the visceral fat area at 6 months after the start of treatment (Fig. 2d) and significant weight loss was seen in cases with a decreased visceral fat area (Fig. 2e); however there was no correlation between the reduction in the visceral fat area and the improvement of the HbA1c level (Fig. 2f).
The systolic and diastolic blood pressures significantly decreased during the treatment period (p<0.001, p<0.001; Table 4).
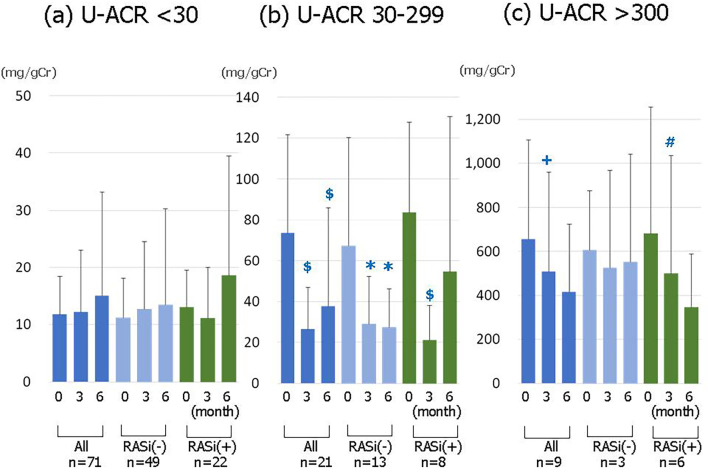
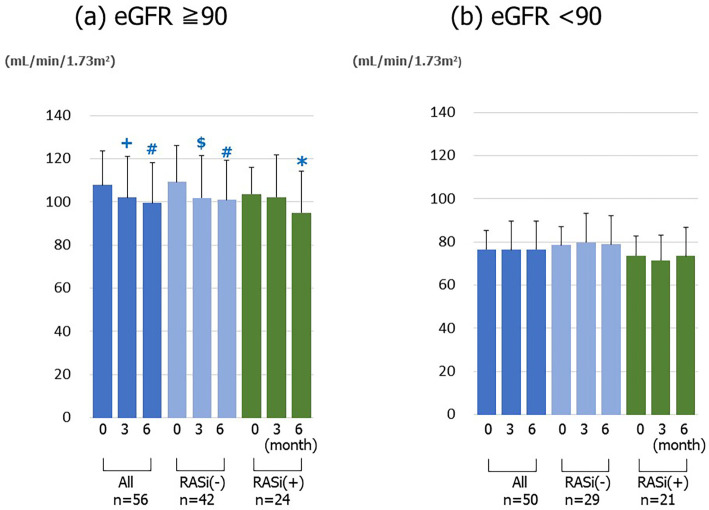
Although the entire U-ACR after the 6-month treatment period was numerically lower -though not to a significant extent- than that at the baseline (Table 4), the ratio in the patients whose U-ACR levels were 30-300 mg/gCr at the baseline was significantly decreased at 6-month timepoint (Fig. 3). In contrast, the eGFR after 6 months of treatment significantly decreased (p<0.001) (Table 4). However, in a subgroup with pre-treatment eGFR values of ≥90 mL/min/1.73 m2, the eGFR significantly decreased (Fig. 4a). On the other hand, the eGFR in a subgroup with pre-treatment values of less than 90 mL/min/1.73 m2 showed no significant change (Fig. 4b).
Figure 3.
The changes in the urinary albumin/creatinine ratio (U-ACR) before and at 3 and 6 months after the start of SGLT2 inhibitor treatment. (a) The patients with a pre-treatment U-ACR of <30 (n=71). (b) The patients with a pre-treatment U-ACR of 30-299 (n=21). (c) The patients with a pre-treatment U-ACR of ≥300 (n=9). * p<0.05, +p<0.01, $ p<0.005, # p<0.001 vs. baseline.
Figure 4.
The changes in the estimated glomerular filtration rate (eGFR) before and at 3 and 6 months after the start of SGLT2 inhibitor treatment. (a) The patients with a pre-treatment eGFR of ≥90 (n=56). (b) The patients with a pre-treatment eGFR of <90 (n=50). *p<0.05, +p<0.01, $ p<0.005, # p<0.001 vs. baseline.
AST, ALT, γ-GTP, and uric acid levels at the end of treatment significantly decreased in comparison to those at the baseline (Table 4). The serum levels of LDL-C and triglyceride tended to decrease, while the serum level of HDL-C tended to increase, but none of these changes was statistically significant (Table 4).
During the 6-month study period, 13 adverse events occurred, namely: systemic eruption (n=1), cystitis (n=2), pudendal pruritus (n=2), nausea (n=1), malaise (n=1), bulimia due to strong sensation of hunger (n=1), and mild to moderate hypoglycemia (n=5) (Table 5).
Table 5.
The Adverse Events Due to the Administration of SGLT2 Inhibitors in 132 Patients.
| Total | 13 cases |
| Systemic eruption | 1 case |
| Cystitis | 2 cases |
| Pudendal pruritus | 2 cases |
| Nausea | 1 case |
| Malaise | 1 case |
| Strong sense of hunger | 1 case |
| Non-severe hypoglycemia | 5 cases |
Discussion
In the present study, the mean HbA1c level significantly decreased by 0.57% at 6 months after the start of SGLT2 inhibitor treatment. The magnitude of reduction in this parameter was smaller than that recorded during the pre-marketing clinical trials of various SGLT2 inhibitors (1,6). This difference may be explained by 1) the relatively low baseline HbA1c level (7.52%) in the present study, and 2) the dose reductions or discontinuance of glimepiride or insulin in some cases. In the 78 cases in which the doses of concomitant drugs were not reduced, the mean HbA1c level decreased by 0.55% from 7.26%. However, in 33 cases in which the doses of concomitant drugs were reduced, the mean HbA1c level decreased by 0.59% from 8.15%. Thus, the doses of concomitant drugs in the latter group were reduced in spite of the higher HbA1c levels. Thus, the latter group would show a further reduction in HbA1c levels without a reduction in the dose of the concomitantly-administered drugs, resulting in a larger improvement of the glycemic control in the whole study population. Instead of a marked decrease in HbA1c levels, our protocol allowed for the safe administration of SGLT2 inhibitors without the induction of severe hypoglycemia.
Higher pre-treatment HbA1c levels were correlated with a significant decrease in the levels at the end of the treatment. This finding is similar to the results obtained with existing antidiabetic drugs (7) and is consistent with the clinical trial data on various SGLT2 inhibitors (8-12). One possible reason for this association is an increase in urinary glucose excretion from the kidneys in patients with higher blood glucose levels.
There was no significant correlation between the pre-treatment BMI or the visceral fat area and the decrease in the HbA1c level at the end of the treatment period. This fact may indicate that SGLT2 inhibitors have the potential to reduce the glucose level in both obese and non-obese patients.
The pre-treatment visceral fat area in the current study averaged 108.4 cm2, exceeding the cut-off point of 100 cm2, which is based on the criterion for visceral obesity proposed by the Japan Society for the Study of Obesity, and decreased by 12.8% at the end of the SGLT2 inhibitor treatment period. Two reports have evaluated the visceral fat-lowering effects of SGLT2 inhibitors in humans: the effects of dapagliflozin [evaluated by dual X-ray absorptiometry (13)], and the effects of canagliflozin [evaluated by X-ray computed tomography (14)]. Both studies also reported that their patients showed body weight loss at the end of the respective treatment periods, primarily due to a decrease in visceral fat. In an animal study, the administration of ipragliflozin to obese rats that were fed high fat diet resulted in a decrease in the visceral and subcutaneous fat mass, indicating that the weight-suppressive activity of this drug is not attributable to a decrease in the lean body mass or bone mass (15). Furthermore, the estimation of energy expenditure using indirect calorimetry revealed that ipragliflozin decreased carbohydrate utilization and elevated fat-derived energy, resulting in a lower respiratory quotient (14)
In the present study, the administration of SGLT2 inhibitors decreased the body weight of the patients by 3.1%. A previous report indicated that a body weight loss of 1-3% through a lifestyle modification program was able to significantly improve the triglyceride, HDL-C, HbA1c and ALT levels in obese Japanese patients (16); thus even a small weight loss 3.1% can contribute to the improvement of overall patient health. In the present study, a larger pre-treatment visceral fat area was associated with a more marked decrease in the visceral fat area after treatment accompanied by weight loss. We may say that appropriate weight loss was achieved by a fat decrease in such cases. Although weight loss was, to a certain extent, also observed in patients with a low pre-treatment BMI or visceral fat area, the weight reduction in such cases was not accompanied by an improvement in the visceral fat area. It is therefore likely to involve a decrease in components other than fat -for example, water, bone, and muscle.
In addition, a decrease in the visceral fat area and a significant improvement in the serum ALT and γ-GTP values were noted after the treatments, suggesting the alleviation of fatty liver. The potential of SGLT2 inhibitors to strongly contribute to the treatment of obese diabetic patients through their visceral fat-lowering effects is therefore considered to be high. If the improved condition can be maintained for a long period of time, blood glucose-improving effects can be expected via reductions in insulin resistance in addition to the blood glucose-lowering effects through the promotion of urinary glucose excretion by SGLT2 inhibitory activity. Furthermore, considering the reports that ipragliflozin improved insulin sensitivity (as measured by a glucose tolerance test) at 4 weeks after the start of treatment (17) and that dapagliflozin improved the insulin sensitivity in the muscles (18), the treatment can also be expected to have protective effects on β-cells over the long term.
An increase in dietary consumption was reported in a study involving the 38-day repeated oral administration of dapagliflozin to rats with diet-induced obesity (19). In the current study, most patients whose body weights showed no decrease confessed to increases in their food intake during the treatment period. Thus, it might be important for patients to adhere to their diet therapies during SGLT2 inhibitor treatment.
Although the urinary albumin/creatinine ratio in the patients with a pre-treatment ratio of <30 mg/gCr was not significantly different after 6 months of treatment; the ratio at the end of the treatment was significantly lower than the ratio at the start of treatment in the patients with a pre-treatment ratio of 30-299 mg/gCr. The ratio after 6-months of treatment was slightly -but not significantly- lower than the pre-treatment ratio in the patients with a baseline ratio of ≥300 mg/gCr. We divided the patients into two groups, those who were treated with or without renin-angiotensin system (RAS) inhibitors. In the patients with a ratio of 30-299 mg/gCr who were treated without RAS inhibitors, the ratio at 3 months and 6 months after the start of treatment was also significantly decreased. This effect, which is probably attributable to the influence of improvements in the HbA1c level and body weight, could indicate the possibility that SGLT2 inhibitor treatment improves the urinary albumin levels in patients with the early stages of diabetic nephropathy.
After SGLT2 inhibitor treatment, although the eGFR remained unchanged in the patients with a pre-treatment eGFR of <90 mL/min/1.73 m2, the eGFR significantly decreased in the patients with a pre-treatment eGFR of ≥90 mL/min/1.73 m2. Among the patients with a pre-treatment eGFR of ≥90 mL/min/1.73 m2, the eGFR was also found to be significantly decreased in the patients who were treated without RAS inhibitors. In a previous report, the administration of empagliflozin to patients with type 1 diabetes mellitus alleviated excessive glomerular filtration by improving tubule-glomerular feedback (20). The results of the present study were consistent with this recent finding. Given that excessive glomerular filtration is one of the most important pathophysiological characteristics at the early stage of diabetic nephropathy, SGLT2 inhibitors might modify the progression of diabetic nephropathy. The decrease in blood pressure derived from treatments with SGLT2 inhibitors may also contribute to the suppression of renal damage. Furthermore, dapagliflozin has been reported to significantly suppress interstitial fibrosis in db/db mice (21). These findings suggest the ability of SGLT2 inhibitors to protect the renal function.
In the present study, there was a significant decrease in the mean uric acid level. This result is consistent with the tendency for the reduction in the uric acid level that was seen in a phase II clinical trial of ipragliflozin (22). The decrease in the uric acid levels might be attributable to osmotic diuresis or the direct suppression of uric acid reabsorption at the tubular level by SGLT2 inhibitors (23).
During the current study, no patients developed diabetic ketoacidosis. However, the risk of SGLT2 inhibitor-induced ketoacidosis was indicated in an FDA Safety Announcement (5-15-2015 (24)), and a case has been reported in Japan (4); however this was confined to cases in which the assessment of the patient's disease status by the physician was considered to be insufficient, such as cases in which all oral hypoglycemic drugs (including SU) were suddenly discontinued despite compromised insulin secretion, and/or patients with a low carbohydrate diet.
In the present study, patients were advised to consume a sufficient amount water from before the treatment period in order to avoid excessive dehydration through the diuretic activity of SGLT2 inhibitors. After the start of treatment, the patients were repeated provided with a concrete explanation (using different expressions) during each visit at 2-week intervals. It seems that a sufficient prior explanation about the increase in the urine volume that can be expected after the start of treatment will help to prevent patients from deciding to discontinue the drug after the onset of polyuria.
Conclusion
SGLT2 inhibitors, a new class of antidiabetic drugs, were administered to Japanese patients with type 2 diabetes mellitus to evaluate their usefulness and safety in clinical practice. Although the 6-months treatment period was short, the results suggest that SGLT2 inhibitors may be useful in the treatment of type 2 diabetes mellitus and that they may influence the renal function. However, care is needed in many aspects of treatment and prior explanation to patients seems to strongly affect the usefulness of the drug as well as the onset of adverse events. It seems necessary to evaluate the usefulness and safety of SGLT2 inhibitors over a longer period of time in the future.
Author's disclosure of potential Conflicts of Interest (COI).
Takahiro Tosaki: Honoraria, Eli Lilly, Astellas, AstraZeneca and Mitsubishi Tanabe; Research funding, Mitsubishi Tanabe, Takeda and Daiichi Sankyo. Hideki Kamiya: Honoraria, Eli Lilly, Ono, Novartis Pharma, Astellas, MSD, Sanofi and Mitsubishi Tanabe; Research funding, Ono, Mitsubishi Tanabe, Taisho Toyama, Japan Tobacco, Astellas, Takeda, Sanofi and Novo Nordisk. Tatsuhito Himeno: Research funding, Ono, Mitsubishi Tanabe, Taisho Toyama, Japan Tobacco, Astellas, Takeda, Sanofi and Novo Nordisk. Yoshiro Kato: Research funding, Ono, Mitsubishi Tanabe, Taisho Toyama, Japan Tobacco, Astellas, Takeda, Sanofi and Novo Nordisk. Masaki Kondo: Research funding, Ono, Mitsubishi Tanabe, Taisho Toyama, Japan Tobacco, Astellas, Takeda, Sanofi and Novo Nordisk. Jiro Nakamura: Honoraria, Kyowa Hakko Kirin, Ono, Pfizer, Eli Lilly, Sanofi, MSD, Taisho Toyama, Mitsubishi Tanabe, Astellas and Shionogi; Research funding, Ono, Mitsubishi Tanabe, Taisho Toyama, Japan Tobacco, Astellas, Takeda, Sanofi and Novo Nordisk.
References
- 1.Kashiwagi A, Kazuta K, Yoshida S, Nagase I. Randomized, placebo-controlled, double-blind glycemic control trial of novel sodium-dependent glucose cotransporter 2 inhibitor ipragliflozin in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig 5: 382-391, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishimura R, Tanaka Y, Koiwai K, et al. . Effect of empagliflozin monotherapy on postprandial glucose and 24-hour glucose variability in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled, 4-week study. Cardiovasc Diabetol 14: 11, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaku K, Watada H, Iwamoto Y, et al. . Efficacy and safety of monotherapy with the novel sodium/glucose cotransporter-2 inhibitor tofogliflozin in Japanese patients with type 2 diabetes mellitus: a combined Phase 2 and 3 randomized, placebo-controlled, double-blind, parallel-group comparative study. Cardiovasc Diabetol 13: 65, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayami T, Kato Y, Kamiya H, et al. . Case of ketoacidosis by a sodium-glucose cotransporter 2 inhibitor in a diabetic patient with a low-carbohydrate diet. J Diabetes Investig 6: 587-590, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ida M, Hirata M, Odori S, et al. . Early changes of abdominal adiposity detected with weekly dual bioelectrical impedance analysis during calorie restriction. Obesity 21: 350-353, 2013. [DOI] [PubMed] [Google Scholar]
- 6.Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet 375: 2223-2233, 2010. [DOI] [PubMed] [Google Scholar]
- 7.Bloomgarden ZT, Dodis R, Viscoli CM, Holmboe ES, Inzucchi SE. Lower baseline glycemia reduces apparent oral agent glucose-lowering efficacy: a meta-regression analysis. Diabetes Care 29: 2137-2139, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Roden M, Weng J, Eilbracht J, et al. ; EMPA-REG MONO trial investigators. . Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol 1: 208-219, 2013. [DOI] [PubMed] [Google Scholar]
- 9.Kaku K, Kiyosue A, Inoue S, et al. . Efficacy and safety of dapagliflozin monotherapy in Japanese patients with type 2 diabetes inadequately controlled by diet and exercise. Diabetes Obes Metab 16: 1102-1110, 2014. [DOI] [PubMed] [Google Scholar]
- 10.Wilding JP, Blonde L, Leiter LA, et al. . Efficacy and safety of canagliflozin by baseline HbA1c and known duration of type 2 diabetes mellitus. J Diabetes Complications 29: 438-444, 2015. [DOI] [PubMed] [Google Scholar]
- 11.Monami M, Nardini C, Mannucci E. Efficacy and safety of sodium glucose co-transport-2 inhibitors in type 2 diabetes: a meta-analysis of randomized clinical trials. Diabetes Obes Metab 16: 457-466, 2014. [DOI] [PubMed] [Google Scholar]
- 12.Kashiwagi A, Kazuta K, Takinami Y, Yoshida S, Utsuno A, Nagase I. Ipragliflozin improves glycemic control in Japanese patients with type 2 diabetes mellitus: the BRIGHTEN study. Diabetology International 6: 8-18, 2015. [Google Scholar]
- 13.Bolinder J, Ljunggren Ö, Kullberg J, et al. . Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab 97: 1020-1031, 2012. [DOI] [PubMed] [Google Scholar]
- 14.Cefalu WT, Leiter LA, Yoon KH, et al. . Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet 382: 941-950, 2013. [DOI] [PubMed] [Google Scholar]
- 15.Yokono M, Takasu T, Hayashizaki Y, et al. . SGLT2 selective inhibitor ipragliflozin reduces body fat mass by increasing fatty acid oxidation in high-fat diet-induced obese rats. Eur J Pharmacol 727: 66-74, 2014. [DOI] [PubMed] [Google Scholar]
- 16.Muramoto A, Matsushita M, Kato A, et al. . Three percent weight reduction is the minimum requirement to improve health hazards in obese and overweight people in Japan. Obes Res Clin Pract 8: 466-475, 2013. [DOI] [PubMed] [Google Scholar]
- 17.Takahara M, Shiraiwa T, Matsuoka TA, Katakami N, Shimomura I. Ameliorated pancreatic β cell dysfunction in type 2 diabetic patients treated with a sodium-glucose cotransporter 2 inhibitor ipragliflozin. Endocr J 62: 77-86, 2015. [DOI] [PubMed] [Google Scholar]
- 18.Merovci A, Solis-Herrera C, Daniele G, et al. . Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest 124: 509-514, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Devenny JJ, Godonis HE, Harvey SJ, Rooney S, Cullen MJ, Pelleymounter MA. Weight loss induced by chronic dapagliflozin treatment is attenuated by compensatory hyperphagia in diet-induced obese (DIO) rats. Obesity 20: 1645-1652, 2012. [DOI] [PubMed] [Google Scholar]
- 20.Cherney DZ, Perkins BA, Soleymanlou N, et al. . Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 129: 587-597, 2014. [DOI] [PubMed] [Google Scholar]
- 21.Terami N, Ogawa D, Tachibana H, et al. . Long-term treatment with the sodium glucose cotransporter 2 inhibitor, dapagliflozin, ameliorates glucose homeostasis and diabetic nephropathy in db/db mice. PLoS One 9: e100777, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilding JP, Ferrannini E, Fonseca VA, Wilpshaar W, Dhanjal P, Houzer A. Efficacy and safety of ipragliflozin in patients with type 2 diabetes inadequately controlled on metformin: a dose-finding study. Diabetes Obes Metab 15: 403-409, 2013. [DOI] [PubMed] [Google Scholar]
- 23.Chino Y, Samukawa Y, Sakai S, et al. . SGLT2 inhibitor lowers serum uric acid through alteration of uric acid transport activity in renal tubule by increased glycosuria. Biopharm Drug Dispos 35: 391-404, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenstock J, Ferrannini E. Euglycemic diabetic ketoacidosis: a predictable, detectable, and preventable safety concern with SGLT2 inhibitors. Diabetes Care 38: 1638-1642, 2015. [DOI] [PubMed] [Google Scholar]