Abstract
Objective We performed a prospective study to determine the efficacy and safety of denosumab on bone metabolic indices and bone mineral density (BMD) in 29 patients receiving long-term glucocorticoids (GCs) who had clinical risk factors for fracture.
Methods Among these patients, 16 had systemic lupus erythematosus (SLE), 6 RA, 4 other autoimmune diseases, and 3 renal diseases. All patients received donosumab 60 mg at baseline and 6 months. Serum N-terminal cross-linked telopeptide of type I collagen (NTX) and bone-specific alkaline phosphatase (BAP) levels were measured as bone metabolic indices. BMD at the lumbar spine (LSBMD) and femoral neck (FNBMD) were measured using dual energy X-ray absorptiometry and expressed as a percentage of the young adult mean (%YAM).
Results Denosumab therapy significantly reduced serum NTX and BAP levels from baseline after 12 months (from 19.2 to 13.9 nmol BCE/L; from 11.9 to 9.2 U/L, respectively). In 18 patients treated with bisphosphonates before the start of denosumab therapy, the improvements in the LSBMD and FNBMD values were 1.5%YAM/year and 1.1%YAM/year, respectively. The LSBMD and FNBMD values were both significantly higher 12 months after denosumab therapy (3.5%YAM/year and 3.0%YAM/year, respectively). The LSBMD gain was significantly higher after denosumab therapy than during bisphosphonate therapy. No fractures were observed in any patients during denosumab therapy.
Conlusion Denosumab is effective and safe in preventing bone resorption and BMD loss in patients treated with long-term GCs for inflammatory diseases. This is the first study showing a significant increase in not only LSBMD but also FNBMD in GC-induced osteoporosis after denosumab therapy.
Keywords: bone mineral density, bone metabolic markers, denosumab, glucocorticoid-induced osteoporosis
Introduction
Glucocorticoids (GCs) are widely used to treat various inflammatory disorders, including rheumatic and renal diseases (1), and improve the outcomes of these diseases. However, they also have adverse effects on bone (glucocorticoid-induced osteoporosis: [GIO]). Bone loss progresses rapidly during the first 3 to 6 months of GC therapy, and fractures occur in 30-50% of adult patients receiving long-term GCs (2,3).
The Japanese Society for Bone and Mineral Research (JSBMR) established a Committee for the Revision of Guidelines on the Management and Treatment of GIO-using a scoring system of risk factors for fracture, including prior fragility fractures, age, GC dose, and lumbar bone mineral density (BMD) (4). This Committee recommends GIO treatments for patients administered GCs for more than 3 months who have a score ≥3 (the optimal cut-off score for pharmacological intervention). Prior fragility fracture, age ≥65 years, prednisolone ≥7.5 mg/day (or its equivalent), and lumbar BMD <70% of the young adult means (YAM) are all assigned a score ≥3 as single risk factors. Therefore, the Committee proposes that drug therapy be started for subjects with any of these factors.
The Committee has limited the pharmacological interventions recommended in the updated guidelines to agents that are currently approved for the treatment of osteoporosis in Japan (4). Bisphosphonates, such as alendronate and risedronate, are recommended as first-line drugs to prevent a decrease in BMD at the lumbar spine (LSBMD) and femoral neck (FNBMD). Other agents, including active vitamin D3 analogs, such as alfacalcidol and calcitol as well as recombinant human parathyroid hormone (teriparatide), are recommended as alternative treatments. Vitamin K2, selective estrogen-receptor modulators, and humanized monoclonal antibodies against the receptor activator of nuclear factor-κB (RANK) ligand (RANKL) are not recommended, due to either insufficient or limited evidence of fracture prevention.
Denosumab is a fully human monoclonal antibody against RANKL, and prevents its binding and activation of RANK receptors on osteoclasts, thereby suppressing the survival and functions of osteoclasts (5). A randomized controlled trial showed the efficacy of denosumab in reducing vertebral and non-vertebral fractures in postmenopausal women (6). However, few studies have examined the use of denosumab in patients with GIO (7-10). In the present prospective study, we investigated the effects of denosumab on bone turnover and BMD in patients receiving long-term treatment with GCs.
Materials and Methods
Ethical statements and patients
The protocol of this study was approved by the Ethics Committee of the institutions involved (approval numbers: 1102 and 1105), and informed consent for the studies was obtained from all subjects.
We selected 29 patients receiving long-term treatment with GCs in Akita University Hospital to participate in this study (Table 1). These patients had a score ≥3 proposed in the JSBMR guidelines (4). The underlying diseases in these patients were systemic lupus erythematosus (SLE) (n=16), rheumatoid arthritis (RA) (n=6), other autoimmune diseases (n=4), and renal diseases (n=3). All patients received denosumab 60 mg via a subcutaneous injection at baseline and 6 months.
Table 1.
Baseline Characteristics of Enrolled Patients.
| Male:Female | 7:22 | |
| Age (years) | 50.4 ± 15.9 | |
| <50 | 15 (51.7 %) | |
| 50- 65 | 8 (27.6 %) | |
| ≥65 | 6 (20.7 %) | |
| Risk factor scores for fracture* | 5.7 ± 2.8 | |
| Past history of fracture | 5 (17.2 %) | |
| fracture site | all lumbar spine | |
| fracture fragility | all | |
| Number of postmenopausal patients | 9 (31.0 %) | |
| Underlying disease (n) | ||
| systemic lupus erythematosus | 16 (55.2 %) | |
| rheumatoid arthritis | 6 (20.7 %) | |
| dermetomyoisitis/polymyositis | 2 (6.9 %) | |
| Behçet disease | 1 (3.4 %) | |
| Sjögren syndrome | 1 (3.4 %) | |
| renal diseases | 3 (10.4 %) | |
| Disease duration (years) | 17.4 ± 9.3 | |
| Initial GC dose (mg/day) | 147.2 ± 235.6 | |
| Current GC dose (mg/day) | 7.4 ± 5.4 | |
| <5 | 6 (20.7 %) | |
| 5- 7.5 | 10 (34.5 %) | |
| ≥7.5 | 13 (44.8 %) | |
| GC duration (years) | 17.4 ± 9.3 | |
| Pre-denosumab treatment | ||
| Bisphosphonate | 18 (62.1 %) | |
| Vitamin D | 20 (69.0 %) | |
| Vitamin K | 2 (6.9 %) | |
| Fracture risk factor score | 6.4 ± 2.2 | |
| BMD, YAM (%) | ||
| Lumbar spine | 91.5 ± 23.9 | |
| <70 | 2 (6.9 %) | |
| 70- 80 | 10 (34.5 %) | |
| ≥80 | 17 (58.6 %) | |
| Femoral neck | 86.4 ± 22.2 | |
| <70 | 4 (13.8 %) | |
| 70-80 | 5 (17.2 %) | |
| ≥80 | 20 (29.0 %) | |
| Bone turnover markers | ||
| serum NTX (nmol BCE/L) | 19.2 ± 10.6 | |
| serum BAP (U/L) | 12.1 ± 6.5 |
Data are the mean ± standard deviation.
*Scores for the categories of each fracture predictor [4].
Prior fragility fractures: No = 0, Yes = 7
Age (years): <50 = 0, 50- 65 = 2, ≥65 = 4
GC dose (PSL equivalent mg/day): <5 = 0, 5-7.5 = 1, ≥7.5 = 4
Lumbar BMD (%YAM): ≥80 = 0, 70-80 = 2, <70 = 4
BAP: bone-specific alkaline phosphatase, BMD: bone mineral density, GC: glucocorticoid, NTX: N-terminal cross-linked telopeptide of type 1 collagen, PSL: prednisolone, YAM: young adult mean
Assay of bone turnover markers
Serum biomarkers of bone resorption (N-terminal cross-linked telopeptide of type 1 collagen: NTX) and bone formation (bone-specific alkaline phosphatase: BAP) were examined at baseline and 12 months after the initiation of denosumab therapy. Serum NTX and BAP levels were measured using an enzyme-linked immunosolvent assay kit (SRL, Tokyo, Japan) and chemiluminescent enzyme immunoassay kit (SRL), respectively.
Measurement of BMD
LSBMD at L1-4 and FNBMD were measured using dual energy X-ray absorptiometry (DXA; Toyo Medic, Tokyo, Japan) at baseline and 12 months after the initiation of denosumab therapy. BMD values were automatically calculated from the bone area and bone mineral content, and were expressed as %YAM. Spine X-ray examinations were also performed before and after denosumab therapy.
Safety
The safety of denosumab therapy was assessed based on the reports of clinical adverse events, including clinical symptoms/signs and changes in laboratory parameters.
Statistical analysis
The laboratory parameters and BMD values were evaluated using the Wilcoxon t-test. All analyses were performed using the Excel Statistical Software Program (Igakutosho Shuppan Corp, Tokyo, Japan). The data are expressed as the mean ± standard deviation. P values <0.05 were regarded as significant.
Results
Patients and clinical assessment
The characteristics of 29 patients treated with GCs (7 males and 22 females) are shown in Table 1. The mean age was 50.4 years old. The numbers of patients aged <50, 50-65, and >65 were 15, 8, and 6, respectively. There were 9 postmenopausal patients. All patients were considered to have risk factors for fracture because they had scores ≥3 (mean: 5.7) according to the JSBMR guidelines (4). Spine compression fragility fractures were observed at the lumbar spine in 5 patients before denosumab therapy. The underlying diseases were SLE (n=16), RA (n=6), dermatomyositis/polymyositis (n=2), Behçet's disease (n=1), Sjögren's syndrome (n=1), and renal diseases (n=3), including myeloperoxidase anti-neutrophil cytoplasmic antibody (MPO-ANCA) related glomerulonephritis, minimal change nephrotic syndrome, and IgA nephropathy. The mean disease duration was 17.4 years. All patients had been treated with prednisolone (PSL) of 2 to 23 mg/day (mean: 7.8 mg/day) at the initiation of denosumab therapy. The numbers of patients taking PSL of <5, 5-7.5, and ≥7.5 mg/day were 6, 10, and 13, respectively. Prior to the initiation of denosumab therapy, bisphosphonates, vitamin D3, and vitamin K2 had been prescribed to 18, 20, and 2 patients, respectively. The mean duration of bisphosphonate therapy was 56.8 months (12-132 months). Bisphosphonates were discontinued after the initiation of denosumab therapy. Regarding the concomitant use of vitamin D3, alfacalcidol and eldecalcitol had been prescribed to 13 and 7 patients, respectively. Three patients were not treated with anti-osteoporotic drugs. After denosumab therapy, alfacalcidol, eldecalcitol, and denotas chewable combination tablets were prescribed to 12, 5, and 5 patients, respectively. The mean LSBMD and FNBMD were 91.5 and 86.4%YAM, respectively. The numbers of patients having LSBMD of <70, 70-80, and ≥80%YAM were 2, 10 and 17, respectively. The numbers of patients having FNBMD of <70, 70-80, and ≥80%YAM were 4, 5 and 20, respectively. The mean serum levels of NTX and BAP were 19.2 nmol BCE/L and 12.1 U/L, respectively.
Effects of denosumab on bone metabolic indices and BMD
Overall, the serum NTX levels from baseline (19.2 nmol BCE/L) to 12 months after denosumab therapy (13.9 nmol BCE/L) were significantly reduced (p=0.0003) (Fig. 1A). In 5 patients, the serum NTX levels from baseline to 12 months after denosumab therapy were increased. Serum BAP levels from baseline (11.9 U/L) to 12 months after denosumab therapy (9.2 U/L) were also significantly reduced (p=0.001) (Fig. 1B). In 1 patient (Fig. 1), both the serum NTX and BAP levels from baseline to 12 months after denosumab therapy were increased.
Figure 1.
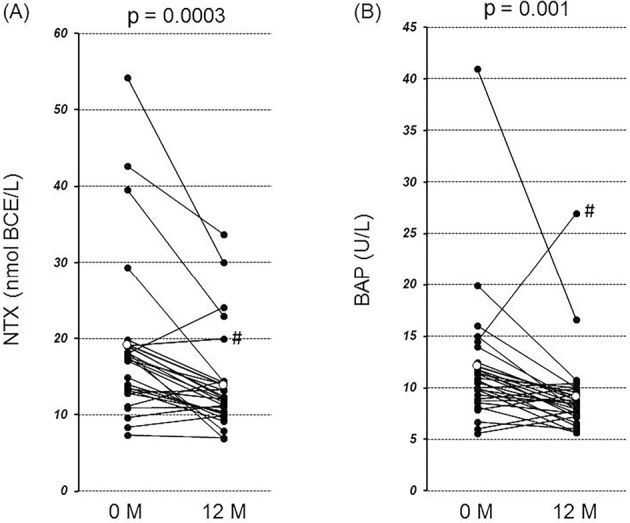
The changes in the levels of the serum markers for bone turnover from baseline to 12 months after denosumab therapy. (A) The serum NTX levels were significantly lower 12 months after denosumab therapy than at baseline (from 19.2 to 13.9 nmol BCE/L) (p=0.0003). (B) The serum BAP levels were significantly lower 12 months after denosumab therapy than at baseline (from 11.9 to 9.2 U/L) (p=0.001). Open circles indicate means. #The serum levels of both NTX and BAP were increased in 1 patient.
Fig. 2 shows the changes in the LSBMD and FNBMD before and after denosumab therapy. Significant BMD gains were observed 12 months after denosumab therapy (LSBMD: from 91.5 to 95.0%YAM, 3.5%YAM/year, p=0.00064; FNBMD: from 86.4 to 89.4%YAM, 3.0%YAM/year, p=0.0002).
Figure 2.
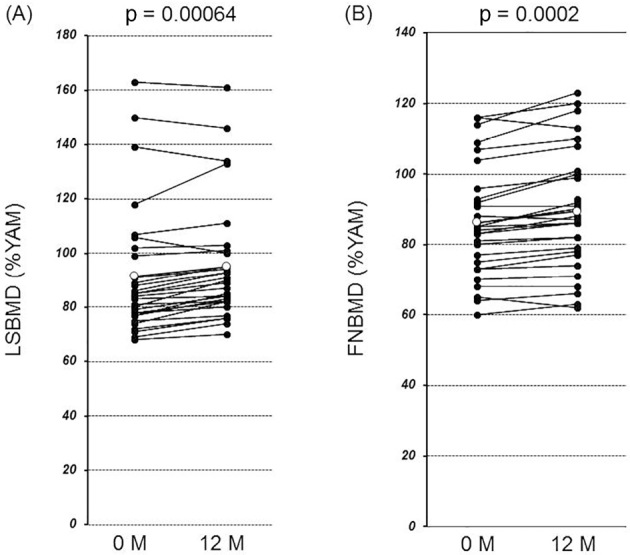
The changes in bone mineral density (BMD) from baseline to 12 months after denosumab therapy. The BMD data expressed as a percentage of the young adult means (%YAM) were compared between baseline and 12 months after denosumab therapy. (A) A significant gain occurred in BMD at the lumbar spine (LSBMD) (from 91.5 to 95.0 %YAM) (p=0.00064). (B) A significant gain occurred in BMD at the femoral neck (FNBMD) (from 86.4 to 89.4 %YAM) (p=0.0002). Open circles indicate means.
In 18 patients treated with bisphosphonates before the initiation of denosumab therapy, changes in the LSBMD and FNBMD during bisphosphonate therapy were able to be evaluated in 11 and 8 patients, respectively. The mean LSBMD gain was 1.5%YAM/year in 11 patients while the mean FNBMD gain was 1.2%YAM/year in 8 patients. The LSBMD gain was significantly higher after the initiation of denosumab therapy (4.5%YAM/year) than during bisphosphonate therapy (p=0.01), while the FNBMD gain was slightly but not significantly higher after the initiation of denosumab therapy (2.1%YAM/year) than during bisphosphonate therapy (p=0.40).
No fractures were observed in any patients during the denosumab therapy.
Adverse events
Upper respiratory tract infection occurred in 2 patients (6.9 %), but no serious adverse events, including infections, neoplasms, and hypocalcemia, were observed during the study period.
Discussion
The present study showed that denosumab is effective for reducing the serum levels of bone turnover markers and increasing BMD in patients receiving long-term GCs with risk factors for fracture. Increases in the LSBMD were greater after denosumab therapy than during bisphosphonate therapy. No adverse effects were observed during the study period.
Our results are consistent with previous findings showing that patients treated with GCs achieved increases in BMD with denosumab administration (7-10) (Table 2). Dore et al. (7) reported that a subcutaneous injection of 60 mg of denosumab at baseline and 6 months reduced serum levels of the bone turnover markers, serum type I C-telopeptide (CTX: bone-reabsorption marker) and serum procollagen 1N-terminal peptide (P1NP: bone-formation marker), and increased the LSBMD and BMD of the total hip (THBMD) in 28 RA patients treated with prednisolone (median dose 5 mg/day at baseline). In our study, the serum levels of NTX (bone-reabsorption marker) and BAP (bone-formation marker) were also reduced during denosumab therapy. These results are similar to those reported by Eastell et al. (11). They examined effects of denosumab on bone turnover markers in postmenopausal osteoporosis, and observed early and late changes in bone-resorption markers compared with bone-formation markers. Denosumab is regarded as a potent inhibitor of bone turnover with a pattern of response that differs from other agents used for the treatment of osteoporosis (11).
Table 2.
Summary of Effects of Denosumab on Bone Turnover and Bone Mineral Density in Patients Receiving Glucocorticoids.
| Reference | No. of patients | Serum levels of biomarkers | Bone mineral density | Others | ||||
|---|---|---|---|---|---|---|---|---|
| CTX or NTX | P1NP | LS | TH | FN | ||||
| 7 | 28* | approx. - 61% | approx. - 47% | approx. 3.0% | approx.1.3% | ND | ||
| 8 | 30* | ND | ND | 5.8% | 2.3% | ND | ||
| 9 | 14 | ND | ND | ND | ND | ND | BMSi: 15%** | |
| 10 | 21* | - 30% | -15% | 3.4% | 1.4% | - 0.1% | ||
| Present study | 29* | - 28% | ND | 3.5% | ND | 3.0% | BAP: - 23% | |
Values of biomarkers and bone mineral density are percentages vs. baseline.
*Treated with denosumab 60 mg once every 6 months for 2 doses
**At 20 weeks
BAP: bone type-specific alkaline phosphatase, BMSi: Bone material strength index, CTX: C-terminal cross-linked telopeptide of type 1 collagen, FN: femoral neck, LS: lumbar spine, ND: not determined, NTX: N-terminal cross-linked telopeptide of type 1 collagen, P1NP: procollagen type 1 N-terminal propeptide, TH: total hip.
Petranova et al. (8) showed that 12-month treatment with denosumab increased the LSBMD and THBMD from baseline (5.8 % and 2.3 %, respectively) in 30 patients receiving concomitant GC therapy. Notably, however, denosumab therapy also increased the LSBMD and THBMD from baseline (6.1% and 2.8%, respectively) in 30 patients who were not treated with GCs. Mellibovsky et al. (9) examined bone toughness, as shown by a bone material strength index using an Osteoprobe instrument in patients enrolled within 4 weeks of initiating GC treatment (at least 5 mg/day of prednisolone). Fifty-two patients were enrolled, and 14 received denosumab. The bone material strength index significantly increased from 76.2 at baseline to 84.0 and 87.3 units at the 7- and 20-week follow-up visits, respectively, in denosumab-treated patients; however, no changes were observed in BMD. Furthermore, Mok et al. (10) performed a 12-month randomized controlled trial in order to evaluate the effects of switching from oral bisphosphonates to denosumab on BMD in 21 patients with long-term GC therapy (mean dose of prednisolone, 4.6 mg/day). The underlying diseases of their patients were SLE (81%) and RA (19%). Significant LSBMD and THBMD gains from baseline (3.4% and 1.4%, respectively) were observed 12 months after denosumab therapy. The report from Mok et al. (10) and our results may suggest that patients treated with GCs can achieve greater increases in BMD with denosumab than with bisphosphonates.
Although recent randomized control studies in postmenopausal women have demonstrated that denosumab is more effective than oral bisphosphonates at increasing BMD in various body sites after a 12-month treatment (12), there has only been 1 study on the comparative effects of denosumab and bisphosphonates in GIO (10). As discussed above, Mok et al. (10) showed significant LSBMD and THBMD gains from baseline (3.4 %/year and 1.4 %/year, respectively) in denosumab-treated patients. They also found significant LSBMD gains from baseline (1.5 %/year), but no significant THBMD gains (0.8 %/year) in bisphosphonate-treated patients. Furthermore, no significant changes were observed in FNBMD in the denosumab- or bisphosphonate-treated groups in their study. It appears that our study is the first study showing a significant increase in BMD not only in the lumbar spine but also the femoral neck in GIO after denosumab therapy. In the present study, the LSBMD gain was significantly higher after denosumab therapy (4.5 %YAM/year) than during bisphosphonate therapy (1.5 %YAM/year), whereas the FNBMD gain was slightly but not significantly higher after denosumab therapy (2.1 %YAM/year) than during bisphosphonate therapy (1.2 %YAM/year).
One of the advantages of denosumab therapy over oral bisphosphonate therapy is its lower frequency of administration, which may lead to a better compliance rate (10). Since bisphosphonates have a strong affinity for bone and may persist in the bone matrix for years, even after therapy is discontinued, potentially resulting in fetal bisphosphonate exposure during pregnancy, many clinicians may have concerns about prescribing bisphosphonates to women of childbearing age (13). In contrast, denosumab is not embedded within the bone tissue (10). This may be another advantage of denosumab when used in younger women with childbearing potential, as younger patients are encountered more often in patients with rheumatic diseases requiring long-term GC therapy.
Denosumab has been shown to be safe (12), and the only serious adverse event that was found to be significantly more common than that in the placebo group in a pivotal registration trial was skin infection (cellulitis including erysipelas) (14). Our patients did not develop serious adverse events during the study period.
Several limitations associated with the present study warrant mention. The sample size was not large enough and the duration of study was too short to address the efficacy of denosumab in reducing fragility fractures. Furthermore, the present study was not a randomized control study to compare the effects between bisphosphonates and denosumab. However, we showed for the first time that denosumab was more effective at suppressing bone turnover markers and increasing BMD than bisphosphonates in Japanese patients treated with long-term GCs for inflammatory diseases.
In conclusion, we demonstrated that denosumab significantly reduced serum levels of bone turnover markers (NTX and BAP) and increased the LSBMD and FNBMD after 12 months of therapy. Denosumab therapy is regarded as an effective and safe therapeutic option for patients receiving long-term treatment with GCs.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids―new mechanisms for old drugs. N Engl J Med 353: 1711-1723, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int 13: 777-787, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Weinstein RS. Glucocorticoid-induced bone disease. N Engl J Med 365: 62-70, 2011. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki Y, Nawata H, Soen S, et al. . Guidelines on the management and treatment of glucocorticoid-induced osteoporosis of the Japanese Society for Bone and Mineral Research: 2014 update. J Bone Miner Metab 32: 337-350, 2014. [DOI] [PubMed] [Google Scholar]
- 5.Bekker PJ, Holloway DL, Rasmussen AS, et al. . A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J Bone Miner Res 19: 1059-1066, 2004. [DOI] [PubMed] [Google Scholar]
- 6.McClung MR, Lewiecki EM, Cohen SB, et al. . Denosumab in postmenopausal women with low bone mineral density. N Engl J Med 354: 821-831, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Dore RK, Cohen SB, Lane NE, et al. . Effects of denosumab on bone mineral density and bone turnover in patients with RA receiving concurrent glucocorticoids or bisphosphonates. Ann Rheum Dis 69: 872-875, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petranova T, Sheytanov I, Monov S, Nestorova R, Rashkov R. Denosumab improves bone mineral density and microarchitecture and reduces bone pain in women with osteoporosis with and without glucocorticoid treatment. Biotechnol Biotechnol Equip 28: 1127-1137, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mellibovsky L, Prieto-Alhambra D, Mellibovsky F, et al. . Bone tissue properties measurement by reference point indentation in glucocorticoid-induced osteoporosis. J Bone Miner Res 30: 1651-1656, 2015. [DOI] [PubMed] [Google Scholar]
- 10.Mok CC, Ho LY, Ma KM. Switching of oral bisphosphonates to denosumab in chronic glucocorticoid users: a 12-month randomized controlled trial. Bone 75: 222-228, 2015. [DOI] [PubMed] [Google Scholar]
- 11.Eastell R, Christiansen C, Grauer A, et al. . Effects of denosumub on bone turnover markers in postmenopausal osteoporosis. J Bone Miner Res 26: 530-537, 2011. [DOI] [PubMed] [Google Scholar]
- 12.Miller PD. A review of the efficacy and safety of denosumab in postmenopausal women with osteoporosis. Ther Adv Musculoskelet Dis 3: 271-282, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suresh E, Pazianas M, Abrahamsen B. Safety issues with bisphosphonate therapy for osteoporosis. Rheumatology (Oxford) 53: 19-31, 2014. [DOI] [PubMed] [Google Scholar]
- 14.Cummings SR, San Martin J, McClung MR, et al. . Denosumab foe prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 361: 756-765, 2009. [DOI] [PubMed] [Google Scholar]


