Abstract
A 55-year-old man was transported to our hospital after a sudden onset of left lower abdominal pain while driving. Computed tomography (CT) of the abdominal region revealed an extensive iso-intense signal region that had a maximum area of 14×15 cm, which we treated conservatively. A series of follow-up CT images showed the gradual decrease of the left peritoneal mass, while continuity with the left adrenal gland became apparent. He was diagnosed with idiopathic adrenal hemorrhage. Adrenal hemorrhage presenting with huge retroperitoneal tumors is rare, and most cases are treated surgically. Therefore, CT images with conservative treatment are rare, holding both clinical interest and significance.
Keywords: acute adrenal hemorrhage, idiopathic adrenal hemorrhage, computed tomography (CT)
Introduction
Adrenal hemorrhage presenting with huge retroperitoneal tumors is rare, with few published reports in the literature (1). In addition, surgical treatment is selected for most cases; therefore, reports of the course of conservative treatment tracked by computed tomography (CT) images are few. We herein report a case presenting with a huge left retroperitoneal mass that was later complicated with idiopathic unilateral adrenal hemorrhage, which was treated conservatively; the course of treatment was followed by a series of CT images.
Case Report
The patient, a 55-year-old man, was transported to our hospital after becoming aware of sudden left lower abdominal quadrant pain while driving a car. He had a history of hypertension and was taking antihypertensive drugs (imidapril, amlodipine, and indapamide). He had no prior history of surgery or any trauma. He had a height of 176.5 cm, weight of 87.5 kg (body mass index, 28.1), and performed very little exercise. The patient also had a habit of drinking a large amount of beer (1-2 L) each day and was fond of greasy foods. Upon arrival, he was lucid with a body temperature of 37℃, blood pressure of 97/70 mmHg, and heart rate of 90 bpm. Gradually worsening tenderness was noted in the left lower abdominal quadrant; however, no symptoms of peritoneal irritation were observed. Bowel sounds were slightly decreased, and abdominal eruption was absent. Respiratory difficulties, nausea, and vomiting were absent. Blood testing results were as follows: white blood cell (WBC) count, 11,300 /μL (neutrophils, 69.2%); red blood cell (RBC) count, 47.7×104/μL; hemoglobin (Hb), 15.4 g/dL; hematocrit, 44.0%; platelet count, 21.3×104/μL; C-reactive protein (CRP), 0.18 mg/dL; amylase (AMY), 39 IU/L; prothrombin time (PT), 13.8 s; partial thromboplastin time, 21.8 s; PT-international normalized ratio, 1.06; fibrinogen, 303 mg/dL; and fibrin degradation product, 2 μg/mL. Thus, CRP, AMY, coagulation, and fibrinolytic parameters were all within normal ranges, although the WBC count was elevated.
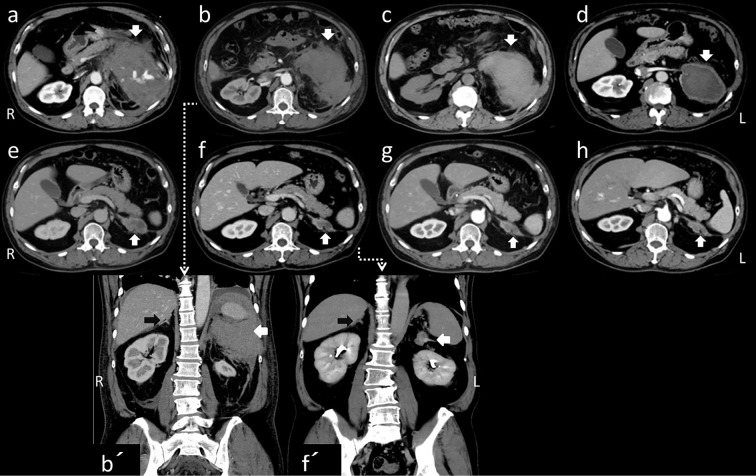
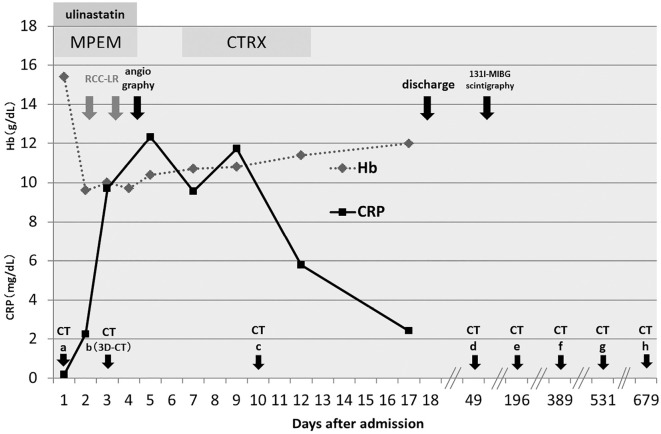
Contrast-enhanced CT of the abdominal region revealed an extensive iso-intense signal portion that had a maximum area of 14×15 cm and irregular borders on the left side of the peritoneal cavity from the body of the pancreas to the kidney; this was accompanied by an internal hyperintense signal that was contrasted and appeared to be a blood vessel (Fig. 1a, white arrow, transverse section). We suspected the presence of intraperitoneal hemorrhage but also considered the possibility of acute pancreatitis because of his drinking history. Therefore, ulinastatin, meropenem and gabexate mesilate were administered, and treatment was begun with bed rest and a drip infusion. The pain could be controlled with pentazocine hydrochloride; however, the Hb level dropped to 9.6 g/dL on the following day, and so RBC transfusion was performed (red cell concentrate, leukocyte-reduced [RCC-LR] 1 pack/day for 2 days) (Fig. 2). CT images (Fig. 1b, with coronal sections in 1b') on the 3rd day of illness showed that the right adrenal gland had normal morphology on coronal sections (black arrow), with no increasing trend in the left peritoneal mass. However, the left adrenal gland could not be identified. Magnetic resonance cholangiopancreatography revealed no abnormalities in the pancreatic or bile ducts (data not shown). No subsequent elevation of the AMY level was observed, and the serum lipase and elastase-1 levels remained within normal ranges. The possibility of pancreatitis was disregarded, and the administration of ulinastatin and meropenem was stopped on the 4th day of illness. Elevation of the CRP level was detected on the 5th day, and ceftriaxone (CTRX) was administered for 6 days in case of an infection associated with necrosis occurring after hemorrhage.
Figure 1.
Abdominal CT scans of the axial lesion, taken on the 1st (a), 3rd (b), 10th (c), 49th (d), 196th (e), 389th (f), 531st (g), and 679th (h) days [on the 3rd (b) and 389th (f) days, with coronal sections (b’) (f’) ]. Except on the 10th day (c), all of the images are enhanced. The left peritoneal mass gradually decreased, and continuity with the left adrenal gland became apparent (white arrows). The right adrenal gland had a normal shape (black arrows in b’, f’).
Figure 2.
Clinical course after admission.
Three-dimensional CT angiography on the 3rd day (Fig. 3) failed to reveal any clear blood vessel abnormalities, such as an aneurysm in the abdominal region. Abdominal angiography performed on the 4th day also revealed no blood vessel abnormalities or hemorrhage in the abdominal region; therefore, conservative treatment was continued. Because it was difficult to identify the left adrenal gland on CT, we also considered the possibility of adrenal hemorrhage from that region. The basal adrenal hormone values measured on the 6th and 9th day of illness were as follows: serum adrenocorticotropic hormone (ACTH), 41.1 pg/mL (normal range: 7.4-55.7); cortisol, 18.49 μg/dL (6.0-20.0); active renin concentration (ARC), 3.3 pg/mL (2.5-21.4); aldosterone, 87.7 pg/mL (35.7-240.0); dehydroepiandrosterone sulfate (DHEA-S), 2,325 ng/mL (380-3,130); urinary cortisol, 270.0 μg/day (11.2-80.3); adrenaline, 13.0 μg/day (1.0-23.0); noradrenaline, 407.0 μg/day (29.0-120.0); dopamine, 1,000.0 μg/day (100-1,000); and vanillylmandelic acid, 6.7 mg/day (1.5-4.3). Thus, urinary cortisol and catecholamines were elevated. We suspected that these elevated values were due to stress and performed precautionary 131I-MIBG scintigraphy because it has been reported that adrenal hemorrhage might develop as a complication of pheochromocytoma (2,3). However, no clear uptake was observed in the adrenal gland, and re-testing performed approximately two months later revealed that all of the adrenal hormones had normalized (data not shown).
Figure 3.
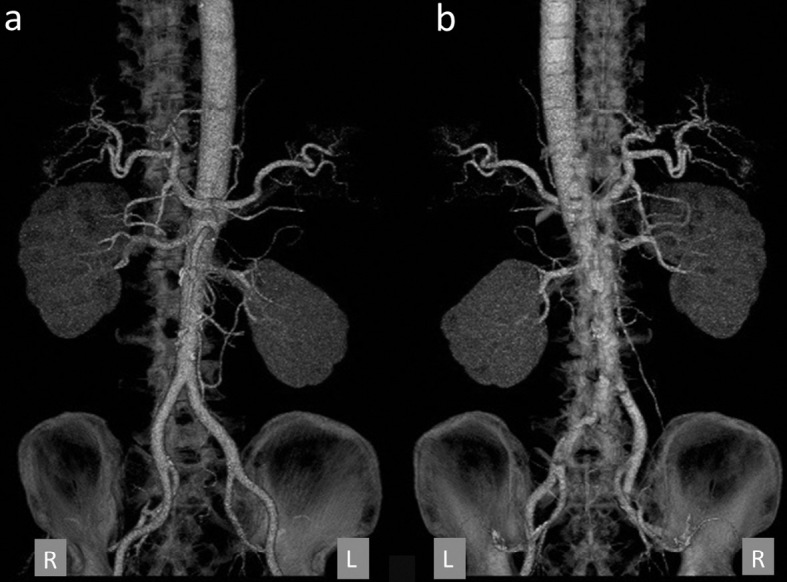
Three-dimensional CT angiography on the 3rd day. (a) Anterior-posterior, (b) posterior-anterior images. There were no obvious blood vessel abnormalities such as aneurysms in the abdominal region.
A CT scan performed on the 10th day of illness (Fig. 1c) showed no increase in the hemorrhage. In addition, no subsequent anemia progression was observed, and the patient's abdominal pain improved. Although surgical treatment was considered, a non-surgical follow-up was selected following consultation with the patient. Therefore, he was discharged on the 18th day of illness. Follow-up examinations involving CT scans taken on the 49th, 389th, 531st, and 679th days (Fig. 1d-h, 1f with coronal sections 1f') showed that the fluid volume gradually decreased, and continuity with the left adrenal gland gradually became apparent. The right adrenal grand had assumed a normal shape (1b' 1f' black arrows). A CT scan taken approximately 1 year after hospitalization (Fig. 1f) revealed a mildly swollen left adrenal gland with post-hemorrhage scarring continuing from it. Therefore, the patient was diagnosed with adrenal hemorrhage. No recurrence has been observed in the four years since the initial presentation.
Discussion
Adrenal hemorrhage is rare and thought to be most commonly caused by external injury (4). Other reported causes include adrenal cancer, metastatic tumor (5), adrenal cyst (6), pheochromocytoma (2,3), neoplastic lesions such as myelolipoma, hemorrhagic diathesis due to coagulation disorders such as antiphospholipid syndrome (7), or use of oral anticoagulant agents, accompanying stress, sepsis, and idiopathic adrenal hemorrhage of unknown cause (1).
The adrenal gland receives a rich blood supply from the aorta and inferior phrenic and renal arteries, but only the adrenal vein permits the drainage of blood (7). The unique anatomy of the adrenal gland vascular supply contributes to its vulnerability to hemorrhage. ACTH increases the vascular flow to the adrenal gland; thus, increased ACTH secretion due to stress could result in adrenal hemorrhage. Streeten reported that the administration of high-dose ACTH caused necrosis and bleeding in the adrenal cortex in animal models (8). Sepsis also represents a cause of adrenal hemorrhage. Waterhouse-Friderichsen syndrome is defined as the failure of the adrenal gland due to bleeding into the glands as a result of disseminated intravascular coagulation (DIC). The condition commonly occurs in childhood (9). Some patients experience sudden onset with severe abdominal pain, as seen in the present case, while in others, illness develops slowly and asymptomatically without abdominal pain and/or distension. In addition, the other diagnostic criterion is “chronic expanding hematoma” of the adrenal gland, which is characterized by a rapid increase in size over a month (10).
In the present case, at the outset of the hemorrhage, contrast-enhanced CT of the abdominal region also failed to identify a clear source of bleeding. Subsequent CT follow-up gradually demonstrated the morphology as the hematoma decreased, and a diagnosis of left idiopathic adrenal hemorrhage was ultimately made. The hematoma scar continued from a somewhat swollen left adrenal gland and had a cystoid shape. Cystic changes after adrenal hemorrhage have been reported (11,12); we therefore suspected that a cystic change was associated with the hemorrhage in this case. We could not exclude the possibility that it was a scar from bleeding into an adrenal cyst that had previously existed; however, because no clear blood vessel or coagulation abnormalities were noted, no vessel abnormality such as aneurysm was suspected, and the patient did not take anticoagulant agents, the cause of the bleeding was unclear. In most previous reports of adrenal hemorrhage with a huge tumor or hematoma, surgical treatment has been selected because of the size of the mass. Therefore, the report of a series of CT images that describe a course of absorption of huge hematoma is exceptionally rare. However, our case improved with conservative treatment, and the source of bleeding, which was unclear at the time, was identified as hemorrhage from the adrenal gland based on imaging follow-up over the patient's clinical course. We believe that these findings make the present case of interest and clinically significant.
Of note: it was impossible to exclude the presence of an adrenal tumor, not only as the source of bleeding, but also as a potential re-bleeding problem. Surgical resection was considered even when the hematoma began to shrink in size and when there were no further symptoms of re-bleeding and tumor enlargement. Although we believe the possibility of a functional/malignant adrenal tumor to be very low because of the presence of only basal levels of adrenal hormones and DHEA-S, careful and continuous observation of the patient remains essential. Despite the present findings, surgical intervention should still be carefully considered in similar cases, due to the possibility of re-bleeding and the presence of a functional/malignant adrenal tumor.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Christoforides C, Petrou A, Loizou M. Idiopathic unilateral adrenal haemorrhage and adrenal mass: a case report and review of the literature. Case Rep Surg 2013: 567186, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okutur K, Küçükler K, Öztekın E, Borlu F, Erdem L, Demır G. A rare cause of acute abdomen: ruptured adrenal pheochromocytoma. Turk J Gastroenterol 21: 467-469, 2010. [DOI] [PubMed] [Google Scholar]
- 3.Souiki T, Tekni Z, Laachach H, et al. Catastrophic hemorrhage of adrenal pheochromocytoma following thrombolysis for acute myocardial infarction: case report and literature review. World J Emerg Surg 9: 50, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker H, Cesnik H, Tscherne H. Adrenal gland hemorrhage: clinical aspects and pathology. Munch Med Wochenschr 109: 2646-2650, 1967. [PubMed] [Google Scholar]
- 5.Akiyama S, Imamura T, Koyama R, Tamura T, Koizumi Y, Takeuchi K. Adrenal metastasis and hemorrhage secondary to hepatocellular carcinoma. Intern Med 54: 1513-1517, 2015. [DOI] [PubMed] [Google Scholar]
- 6.da Silva EC, Viamontez F, Silva VS, et al. Hemorrhagic adrenal cyst. Einstein (Sao Paulo) 10: 96-99, 2012. [DOI] [PubMed] [Google Scholar]
- 7.Presotto F, Fornasini F, Betterle C, Federspil G, Rossato M. Acute adrenal failure as the heralding symptom of primary antiphospholipid syndrome: report of a case and review of the literature. Eur J Endocrinol 153: 507-514, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Streeten DHP. Adrenal hemorrhage. Endocrinologist 6: 227-284, 1996. [Google Scholar]
- 9.Adem PV, Montgomery CP, Husain AN, et al. Staphylococcus aureus sepsis and the Waterhouse-Friderichsen syndrome in children. N Engl J Med 353: 1245-1251, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Chu T, Ma K, Wong CS, Cheng LF, Yung WT, Chan KW. Chronic expanding haematoma of the adrenal gland mimicking malignancy. J HK Coll Radiol 11: 89-91, 2008. [Google Scholar]
- 11.Foster DG. Adrenal cysts. Review of literature and report of case. Arch Surg 92: 131-143, 1966. [DOI] [PubMed] [Google Scholar]
- 12.Takeuchi Y, Ozaki Y, Suyama T, et al. A case of giant hemorrhagic adrenal pseudocystectomy with a flank incision. Hinyokika Kiyo 57: 315-318, 2011. (in Japanese, Abstract in English). [PubMed] [Google Scholar]