Abstract
Pulmonary mucormycosis (PM) is a life-threatening fungal infection in patients with hematologic malignancies, and early and accurate diagnostic modalities are urgently needed. We conducted a polymerase chain reaction (PCR) assay targeting these fungi in peripheral blood from four patients with hematologic malignancies who were strongly suspected of having PM. In these four patients, the Rhizopus species was identified in two patients, and the Cunninghamella and Absidia species in one each. Based on these molecular findings, all of the patients were successfully treated via targeted therapy with liposomal amphotericin B. In this report, a PCR analysis proved very useful for managing PM in patients with hematologic malignancies.
Keywords: pulmonary mucormycosis, polymerase chain reaction, hematologic malignancy
Introduction
Pulmonary mucormycosis (PM) is an invasive fungal infection caused by members of the Mucorales order that can occur in severely immunocompromised hosts (1,2). Over the past two decades, PM has been increasingly recognized as a fatal infectious complication in patients with hematologic malignancies, particularly those receiving intensive chemotherapy or undergoing allogeneic hematopoietic stem cell transplantation (1,3).
However, making an early and precise diagnosis of PM is quite challenging, because the positive rate of blood cultures is low and there are no specific clinical signs or available serological markers such as galactomannan antigen (GM) or (1-3)-β-D glucan (BG) (2,3). Although a diagnosis requires a histopathological examination of the infected tissue, obtaining deep tissue samples through procedures such as transbronchial lung biopsies (TBLBs) from hematologic patients is extremely difficult because of the serious condition (e.g. severe thrombocytopenia) of most of these patients (4). Therefore, antemortem diagnosis is rare, and instead, the diagnosis is usually made by postmortem autopsy (1,3). Accordingly, culture- and histopathology-independent diagnostic tests that can accurately detect this pathogen in its early stages are urgently needed. Such tests will allow for appropriate therapeutic intervention and consequently improve the survival rate of patients with PM.
We used a polymerase chain reaction (PCR) to detect circulating DNA from Mucorales in peripheral blood (PB) samples from four patients with hematologic malignancies who were strongly suspected of having PM. All four patients were successfully treated via targeted therapy with liposomal amphotericin B (L-AmB) based on the molecular evidence for Mucorales. Only the PCR data showing the presence of Mucorales in three patients (Case 1, first episode of Case 2, and Case 3) were published previously (4). In this report, we added additional cases and described their detailed clinical courses and management.
Case Reports
From April 2007 to March 2015, we encountered four patients with hematologic malignancies (acute myeloid leukemia [AML] in three patients and non-Hodgkin's lymphoma [NHL] in one patient): all patients were strongly suspected of having PM and were treated at Mie University Hospital. The management courses of these four cases were retrospectively evaluated. We obtained informed consent from either the patients or their families when the patients had died or had been hospitalized at another hospital at the start of this study. The institutional review board of the Mie University Hospital approved the present study as well as this consent procedure, due to the retrospective nature of the study.
As previously described (4), when a patient was suggested of having an invasive fungal disease, PCR was performed to detect circulating fungal DNA in EDTA-anticoagulated PB samples (1 mL). To prevent contamination, the PB sample was taken only for this PCR analysis, and all procedures were performed inside a laminar flow clean bench. The extraction of the DNA and the subsequent PCR analysis were performed within 24 h after collection of the PB samples. We performed a broad-range PCR targeting the highly conserved sequence of the 18S ribosomal RNA genes in fungal DNA shared by most fungi during the first PCR. Nested PCR was performed to detect small amounts of fungal DNA that could not be detected during the first PCR, and the sequence of the DNA was determined. If a patient was suspected of having PM, genus-specific PCR for mucormycosis was also performed using specific primers for Mucorales in addition to the broad-range (18S ribosomal RNA gene) PCR. The primers for Mucorales-specific PCR were designed based on the sequences in the GenBank database. We used the following genus-specific forward primers for Mucorales: Rhizopus (5'-TGATCTACGTGACAAATTCT-3'), Rhizomucor (5'-TGATCTACGCGAGCGAACAA-3'), Mucor (5'-TGATCTACACGGCATCAAAT-3'), Absidia (5'-TGATCTACACGGCATCAAAT-3') and Cunninghamella (5'-GGATTGTAAACTAAAGTTTTC-3'), and the following reverse primers: Rhizopus, Rhizomucor, Mucor, and Absidia (5'-AGTAGTTTGTGTTCGGKCAA-3') and Cunninghamella (5'-AAATTCTCTAATTATTCCCTC-3').
Case 1
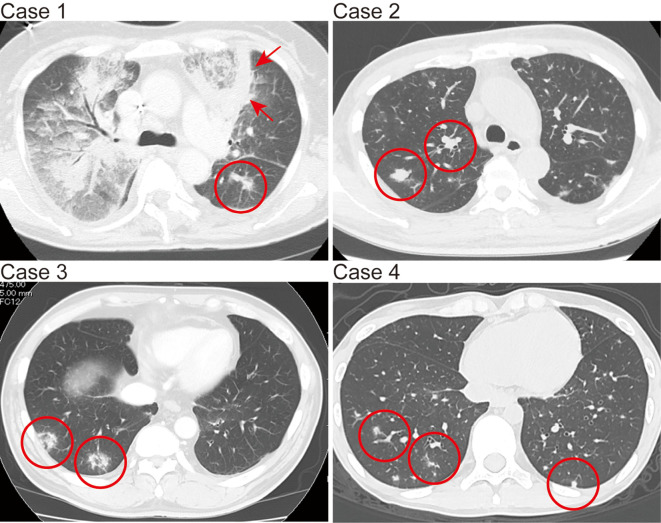
A 53-year-old woman was referred to our hospital because of leukocytosis and paralysis of the legs. The patient was diagnosed with AML based on the hematologic findings. Magnetic resonance imaging of the thoracic and lumber vertebrae showed multiple masses in Th5, Th11, and L2 that pressed on the spinal cord. These lesions were considered to be extramedullary myeloid sarcoma. After induction chemotherapy consisting of daunorubicin (DNR) and cytarabine (Ara-C), the patient achieved complete remission (CR), with a reduction in size of the extramedullary tumors, followed by consolidation chemotherapy with high-dose Ara-C (2 g/m2 twice daily for 5 days). During the second course of the consolidation chemotherapy, the patient developed febrile neutropenia (FN) and was treated with doripenem (DRPM), amikacin, and teicoplanin (TEIC). Oral voriconazole (VRCZ, 200 mg twice daily) had been given as anti-fungal prophylaxis for 5 weeks. A chest CT scan revealed bilateral small nodules and diffuse infiltration partly surrounded by a thick wall-like consolidation in the left anterior upper lung (Figure, Case 1). The patient's ferritin level increased to 3,110 ng/mL. The GM test, Cryptococcus antigen, and DNA of Pneumocystis jirovecii were negative. The BG level increased only in one instance (12.8 pg/mL). As there was suspicion of PM because the pulmonary nodules appeared after receiving VRCZ, we performed a PB PCR assay for Mucorales, which revealed the presence of DNA from the Cunninghamella spp. At this time, the antifungal agent was switched to L-AmB (2.5 mg/kg daily), and the doses were gradually increased to 4.0 mg/kg daily. After four months of this treatment, the PM-suspected shadows improved in chest CT scans. However, the patient later died due to a relapse of leukemia. Autopsy was not performed.
Figure.
Chest computed tomography images of pulmonary mucormycosis-suspected findings in Cases 1-4. The red circles in the images of Cases 1, 2, and 4 show multiple small nodules. The red arrows in the image of Case 1 show diffuse infiltration partly surrounded by a thick, wall-like consolidation. The red circles in the image of Case 3 show two nodules with the reversed halo sign.
Case 2
A 48-year-old man was referred to our hospital because of leukocytosis and thrombocytopenia. The patient was diagnosed with AML based on the hematologic findings. Induction chemotherapy with DNR and Ara-C was performed. After achieving CR, three courses of consolidation chemotherapy (mitoxantrone + Ara-C, DNR + Ara-C, and aclarubicin + Ara-C) were administered. Cord blood transplantation (CBT) was then performed after preconditioning (total body irradiation, 12 Gy; Ara-C, 2 g/m2 twice daily for 2 days; cyclophosphamide, 60 mg/kg for 2 days). After CBT, he developed FN and was given meropenem, vancomycin, and intravenous (IV) VRCZ (200 mg twice daily). Although the BG test and Cryptococcus antigen findings were negative, the GM index transiently increased to 5.0. A chest CT scan showed a small nodule in the left lower lung. The patient also developed acute graft-versus-host disease (GVHD, Grade II), which required treatment with IV prednisolone (PRD, 1 mg/kg daily) with tapering in addition to prophylactic administration of IV cyclosporine. At this time, Rhizopus spp. DNA was detected via a PB PCR for Mucorales. We changed the antifungal agent from VRCZ, which had been given for 6 weeks, to L-AmB (2.5 mg/kg daily); this dose was later reduced to 2 mg/kg daily because of renal dysfunction. L-AmB therapy was continued for one month, at which time a chest CT scan showed no abnormalities, thus allowing the restarting of oral VRCZ (200 mg twice daily) treatment as anti-Aspergillus prophylaxis. The patient's condition improved, and he was discharged. However, two months later, he was readmitted to our hospital because of a high fever and general fatigue. A chest CT scan revealed multiple small nodular shadows in both lungs (Figure, Case 2). At that time, the BG and GM tests were negative. The patient's ferritin level increased to 3,550 ng/mL, and he had poorly controlled diabetes mellitus (DM). We therefore changed the antifungal therapy from oral VRCZ to L-AmB (2.5 mg/kg daily) owing to strong suspicion of PM; this dose was gradually increased to 4.0 mg/kg daily and continued for 2 months. A PB PCR assay for Mucorales showed the DNA from Rhizopus spp. The patient was successfully treated with L-AmB and discharged from our hospital.
Case 3
A 67-year-old man suspected of having NHL was referred to our hospital. Angio-immunoblastic T cell lymphoma (stage IIA) was diagnosed based on the histopathological findings for the left inguinal lymph node, a whole-body CT scan, and a bone marrow examination. He received oral chemotherapy with etoposide and PRD while in the hospital and later in the hospital outpatient clinic. He did not receive any anti-fungal prophylaxis. He was readmitted to our hospital because of a hoarse voice. A chest CT scan revealed several small nodular shadows with reversed halo sign in both lungs (Figure, Case 3). He also suffered from uncontrolled DM at this time. Because Aspergillus infection was suspected at first, oral VRCZ (200 mg twice daily) was commenced. However, the BG, GM, and Cryptococcus antigen test findings were all negative. Given the possibility of PM, we performed a PB PCR assay for Mucorales, and Rhizopus spp. DNA was detected. We stopped VRCZ within 3 days and changed the antifungal medication to L-AmB; the initial dose was 2.5 mg/kg daily, which was later increased to 3.0 mg/kg daily and continued for 7 weeks. We could not increase the dose any further because the patient developed hypokalemia and an acute renal injury. He gradually recovered from the infection while receiving L-AmB and was discharged from our hospital.
Case 4
A 20-year-old woman was referred to our hospital because of a fever and general fatigue. The patient was diagnosed with AML based on the hematologic findings and received induction chemotherapy with DNR and Ara-C. Oral fluconazole (200 mg daily) was given as an anti-fungal prophylaxis. After achieving CR, 3 courses of consolidation chemotherapy with high-dose Ara-C (2 g/m2 twice daily for 5 days) were administered. During the third course of this therapy, she developed FN and was treated with DRPM and TEIC. Despite neutrophil recovery, the fever persisted, and micafungin (MCFG) (150 mg daily) was added. Although the level of BG increased to 28.2 pg/mL, it decreased gradually to normal after treatment with MCFG. At that time, the GM test findings and DNA of Pneumocystis jirovecii in the PB were negative. However, the chest CT scan revealed multiple small nodular shadows in both lungs (Figure, Case 4). We switched the antifungal agent to oral VRCZ (100 mg twice daily), which was continued for 2 weeks. Nevertheless, the CT findings did not improve. We therefore suspected PM and performed a PB PCR assay for Mucorales, which revealed DNA from Absidia spp. In addition, a broad-range PCR analysis also detected Candida krusei. We switched the antifungal medication to L-AmB (3.0 mg/kg daily) and continued it for 6 weeks. Although the development of hypokalemia and angialgia prevented us from increasing the dose of L-AmB, the chest CT findings gradually improved, and the patient was discharged from our hospital.
Discussion
In clinical settings among patients with hematologic malignancies, it is critical to accurately distinguish PM from pulmonary aspergillosis (PA) because the treatments are different, and delays in administering therapy to patients with PM can significantly increase mortality (5). However, a rapid and precise diagnosis of PM is quite difficult (2,4). In radiological imaging, including chest X-ray and CT, the characteristics of PM are >10 nodular shadows, pleural effusion (6), and the reversed halo sign (7). However, these findings are also observed in pulmonary infections other than PM, albeit less frequently (6). Additionally, because serological markers such as BG and GM are lacking in some cases of PA (8), their absence does not necessarily rule out this mycosis. Furthermore, invasive pathological examinations are mostly precluded by the presence of severe neutropenia and/or thrombocytopenia (4). Thus, the detection of circulating Mucorales DNA in the PB via PCR is considered the only noninvasive method for obtaining evidence of mucormycosis at present. In our series, when the clinical symptoms strongly suggested mucormycosis, genus-specific PCR for Mucorales was performed as well as a broad-range PCR analysis. In the present study, all cases strongly suggested PM (Table) because of multiple nodular shadows in the chest CT scan, iron overload (9), and prior use of VRCZ (10). In addition, Cases 2 and 3 had poorly controlled DM (1). Accordingly, we performed PCR assays on PB samples from these cases and identified Mucorales DNA. We then administered targeted therapy with L-AmB on the basis of the molecular findings and were able to successfully treat all of the episodes in the four patients.
Table.
Clinical Characteristics of 4 Cases with Pulmonary Mucormycosis.
| Case no. |
Age / Sex |
Underlying disease |
Prior antifungal |
Documented DNA of Mucorale |
Chest CT imaging |
BG (pg/mL) |
GM index | Ferritin (ng/mL) |
DM | Neutropenia | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 54/F | AML | VRCZ | Cunninghamella species | Small nodules, diffuse infiltration with thick wall | 12.8 | Negative | 3,110 | No | Yes | L-AmB | bDied of AML |
| a2 (1) | 48/M | AML | VRCZ | Rhizopus species | A small nodule | Negative | 5.0' | 3,500 | Yes | No | L-AmB | Improved |
| a2 (2) | 49/M | AML | VRCZ | Rhizopus species | Multiple small nodules | Negative | Negative | 3,550 | Yes | No | L-AmB | Improved |
| 3 | 67/M | NHL | VRCZ | Rhizopus species | Small nodules with reversed hallo sign | Negative | Negative | 1,080 | Yes | No | L-AmB | Improved |
| 4 | 20/F | AML | FLCZ→MCFG → VRCZ |
Absidia species | Multiple small nodules | 28.2 | Negative | 818 | No | Yes | L-AmB | Improved |
AML:acute myeloid leukemia, NHL: non-Hodgkin's lymphoma, VRCZ: voriconazole, FLCZ: fluconazole, MCFG: micafungin, CT: computed tomography, BG: (1-3)-β-D glucan, GM: galactomannan antigen, DM: diabetes mellitus, L-AmB: liposomal amphotericin B
aTwo episodes were included in case 2.
bThis case died of AML recurrence though mucormycosis was improved.
As for the serological findings, BG was slightly positive in Cases 1 and 4, and GM was transiently elevated in the first episode in Case 2. However, the BG elevation in Case 1 was transient and might have been related to the frequent blood transfusions at that time. In Case 4, the DNA of Candida krusei was also detected via broad-range PCR at the same time as that of Absidia spp. via Mucorales PCR, indicating that the BG positivity was due to the Candida infection. This case seemed to have been successfully treated with L-AmB owing to its potent activity against both fungi. The elevation of GM in Case 2 was likely a GVHD-related false positive result (11).
In terms of the DNA analysis of fungal infections, many PCR-based approaches have been reported, but they are predominantly limited to the evaluation of the Candida and Aspergillus species (12). It is not yet clear whether this technique can be applied to the analysis of other fungi such as Mucorales. Some investigators have reported the detection of Mucorales DNA via PCR performed on DNA extracted from tissue specimens (13,14). Although this method can allow guided antifungal treatment, invasive biopsies of organs are unsuitable for hematologic patients. Millon et al. reported a PCR analysis detecting circulating DNA in serum for the diagnosis of mucormycosis (15). However, their study retrospectively analyzed stored serum samples from patients with previously confirmed mucormycosis, and they did not use their technique in decision-making for the treatment of this mycosis. In contrast, using our PB PCR strategy, we were able to rapidly obtain molecular evidence for Mucorales using only 1 mL of the PB sample. Based on the molecular results, we were able to promptly select an appropriate antifungal agent for targeted therapy and treated patients with PM successfully.
In conclusion, the PCR-based approach used in this study allows for the highly sensitive and specific detection of Mucorales and can provide indispensable information that can be used to manage PM in high-risk patients with hematologic malignancies, particularly those who cannot undergo histopathological examinations. In such patients, conducting an early examination using a PCR assay upon the suspicion of mucormycosis is very important if there is radiographic evidence of pulmonary nodules, irrespective of negative findings for serological biomarkers (GM and BG). The presence of prior use of VRCZ, hyperferritinemia, or hyperglycemia seems to strengthen the basis of performing this PCR technique. However, as the main limitation of this study was the small number of cases examined, large prospective studies concerning the clinical validation and standardization of this approach are therefore required before such a strategy can become widely accepted for routine use in the future.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We thank all of the clinicians in the Department of Hematology and Oncology at Mie University Hospital who provided the data for this study.
References
- 1.Roden MM, Zaoutis TE, Buchanan WL, et al. . Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis 41: 634-653, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Pagano L, Offidani M, Fianchi L, et al. . Mucormycosis in hematologic patients. Haematologica 89: 207-214, 2004. [PubMed] [Google Scholar]
- 3.Yano S, Minami J, Nishiwaki K, et al. . Rapid progression and unusual premortal diagnosis of mucormycosis in patients with hematologic malignancies: analysis of eight patients. Int J Hematol 93: 344-350, 2011. [DOI] [PubMed] [Google Scholar]
- 4.Sugawara Y, Nakase K, Nakamura A, et al. . Clinical utility of a panfungal polymerase chain reaction assay for invasive fungal diseases in patients with haematologic disorders. Eur J Haematol 90: 331-339, 2013. [DOI] [PubMed] [Google Scholar]
- 5.Chamilos G, Lewis RE, Kontoyiannis DP. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis 47: 503-509, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Chamilos G, Marom EM, Lewis RE, Lionakis MS, Kontoyiannis DP. Predictors of pulmonary zygomycosis versus invasive pulmonary aspergillosis in patients with cancer. Clin Infect Dis 41: 60-66, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Wahba H, Truong MT, Lei X, Kontoyiannis DP, Marom EM. Reversed halo sign in invasive pulmonary fungal infections. Clin Infect Dis 46: 1733-1737, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Mclintock LA, Jones BL. Advances in the molecular and serological diagnosis of invasive fungal infection in haemato-oncology patients. Br J Haematol 126: 289-297, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Ibrahim AS, Spellberg B, Edwards JJr. Iron acquisition: a novel perspective on mucormycosis pathogenesis and treatment. Curr Opin Infect Dis 21: 620-625, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trifilio SM, Bennett CL, Yarnold PR, et al. . Breakthrough zygomycosis after voriconazole administration among patients with hematologic malignancies who receive hematopoietic stem-cell transplants or intensive chemotherapy. Bone Marrow Transpl 39: 425-429, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Asano-Mori Y, Kanda Y, Oshima K, et al. . False-positive Aspergillus galactomannan antigenaemia after haematopoietic stem cell transplantation. J Antimicrob Chemother 61: 411-416, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Khot PD, Fredricks DN. PCR-based diagnosis of human fungal infections. Expert Rev Anti infect Ther 7: 1201-1221, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bialek R, Konrad F, Kern J, et al. . PCR based identification and discrimination of agents of mucormycosis and aspergillosis in paraffin wax embedded tissue. J Clin Pathol 58: 1180-1184, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dannaoui E, Schwarz P, Slany M, et al. . Molecular detection and identification of zygomycetes species from paraffin-embedded tissues in a murine model of disseminated zygomycosis: a collaborative European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Fungal Infection Study Group (EFISG) evaluation. J Clin Microbiol 48: 2043-2046, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Millon L, Larosa F, Lepiller Q, et al. . Quantitative polymerase chain reaction detection of circulating DNA in serum for early diagnosis of mucormycosis in immunocompromised patients. Clin Infect Dis 56: e95-e101, 2013. [DOI] [PubMed] [Google Scholar]