The use of a flexible open-loop anterior chamber intraocular lens (AC-IOL) (e.g., Kelman Multiflex™ style) in the absence of capsular support during cataract surgery is well established, and the overall incidence of post-operative complications associated with their implantation is favorable when compared to techniques of implanting posterior chamber intraocular lenses via fixation of haptics to the iris or sclera. 1–3 The Multiflex™ lens is designed to provide semi-compressible, four-point haptic fixation, in order to reduce lens rotation in the anterior chamber, as well as forward vaulting of the optic, in order to maintain an adequate degree of clearance between the optic and the corneal endothelium anteriorly as well as the iris posteriorly. If inadvertently placed in an inverted configuration, such a lens would be predicted to have an abnormally anterior and unstable haptic placement and an undesirable, posterior vault of the optic against the iris. We present the clinical outcomes of four cases of complicated cataract surgery in which a flexible open-loop polymethylmethacrylate (PMMA) Kelman Multiflex™-style AC-IOL was placed in an inverted configuration, resulting in a variety of early and late intraocular complications. All cases were referred to one of the authors (DGH) of the Cornea Service, Department of Ophthalmology at the University of California, San Francisco (UCSF).
Report of Cases
Case 1
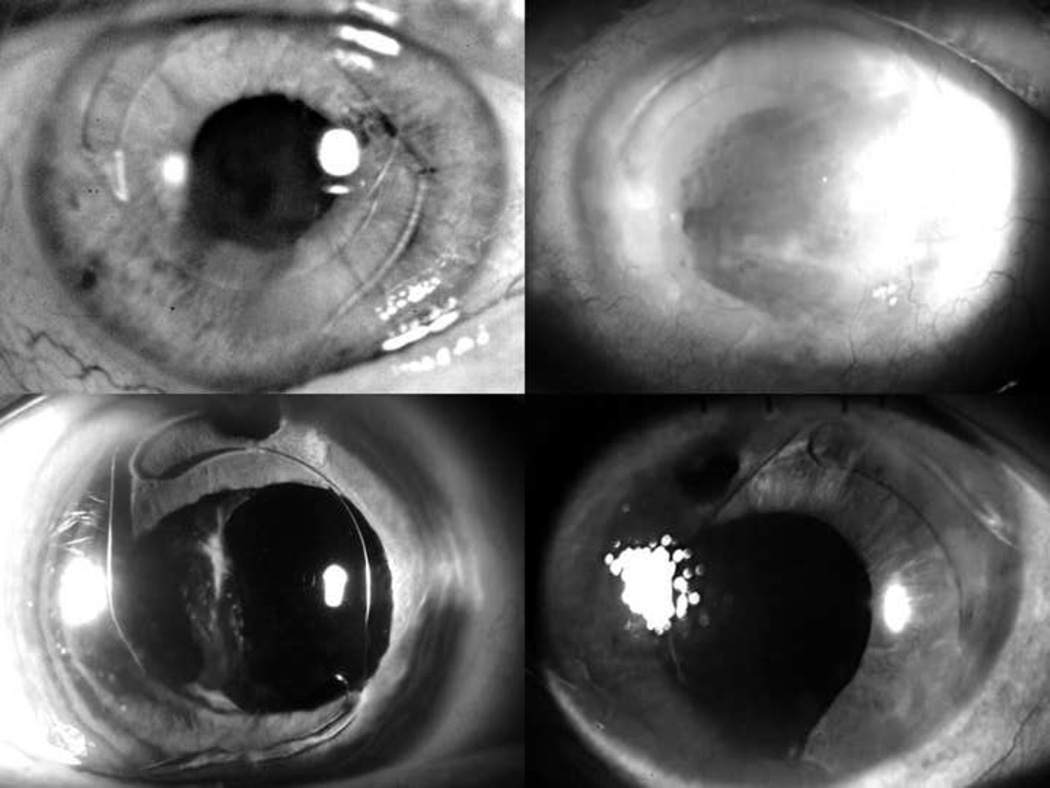
A 70-year-old man had cataract surgery with posterior capsular rupture followed by anterior vitrectomy and AC-IOL placement in his right eye. Three weeks after surgery, the patient developed a retinal detachment in the operated eye, and a scleral buckle was placed. The patient presented to us nine years later complaining of decreasing vision. His best corrected visual acuity (BCVA) was 20/25, intraocular pressure was normal, and corneal pachymetry measured 730 micrometers (mcm). Slit lamp examination revealed corneal edema and an inverted Kelman Multiflex™-style AC-IOL, which was bowed posteriorly into the iris (Figure 1 top left). Ultrasound biomicroscopy revealed that the inferior haptic was in contact with the peripheral corneal endothelium. Specular microscopy showed significant loss of endothelial cells with an average cell density of 397 cells per square millimeter.
Figure 1.
Slit lamp photographs of Cases 1 (top left), 2 (top right), 3 (bottom left), and 4 (bottom right) demonstrating the reversed configuration of the haptics of the Kelman Multiflex™ anterior chamber intraocular lens, accompanied by varying degrees of corneal edema and iris adhesions.
Because of the patient’s good visual acuity he was initially observed, but nine months after initial presentation, the BCVA decreased to count fingers at one foot. Slit lamp examination revealed diffuse corneal edema with epithelial bullae. Penetrating keratoplasty was performed along with AC-IOL explantation followed by iris-sutured PCIOL implantation. Two years after penetrating keratoplasty, the BCVA remained stable at 20/30.
Case 2
A 76-year-old woman with a history of glaucoma and Ahmed valve placement in the left eye underwent complicated cataract surgery with posterior capsular tear, anterior vitrectomy and AC-IOL placement. The patient’s post-operative course was complicated by cystoid macular edema (CME) and iris capture of the optic with formation of synechiae. One year later, repositioning of the AC-IOL and synechiolysis was performed along with a sub-Tenon’s injection of 40mg of triamcinolone acetonide. Post-operatively, the patient’s BCVA was 20/80 with persistent CME and peripheral corneal edema corresponding to the area overlying the Ahmed valve tube tip. The patient’s corneal edema progressively worsened with drop in vision to count fingers at three feet.
The patient was referred to us five years after her initial cataract surgery for worsening pain in the left eye. On ocular examination, her BCVA was count fingers at 6 inches. Central corneal thickness was 950 microns, and slit lamp examination revealed diffuse corneal edema with large epithelial bullae. The tip of the Ahmed valve in the anterior chamber appeared to be in close proximity to the corneal endothelium. The Kelman Multiflex™ AC-IOL was in an inverted configuration with the haptics and optic compressed posteriorly against the iris (Figure 1 top right).
The patient first underwent repositioning of the Ahmed tube given the presumption that the tube location may have been contributing at least in part to the corneal decompensation. Although a subsequent penetrating keratoplasty with intraocular lens exchange was planned for the patient, she was unable to obtain medical clearance for this procedure due to a decline in her health. Because of pain secondary to pseudophakic corneal edema and questionable visual potential in the affected eye, the patient elected to undergo a Salleras procedure (corneal cautery), which resulted in good relief of her pain.
Case 3
A 78-year-old woman underwent cataract extraction with posterior capsular rupture, zonular dehiscence, anterior vitrectomy and AC-IOL placement in her right eye in 2005. She was seen by us four months after surgery for a second opinion. Her BCVA was 20/50 with a normal intraocular pressure. Slit lamp examination showed a clear cornea with an upside-down Kelman Multiflex™ AC-IOL vaulting posteriorly against the iris (Figure 1 bottom left, 2). Optical coherence tomography (OCT) revealed evidence of CME.
Figure 2.
Gonioscopic view of inverted Kelman Multiflex™ anterior chamber intraocular lens with iris chafing
The patient underwent AC-IOL explantation and implantation of a PCIOL using a McCannel fixation technique, accompanied by intravitreal injection of triamcinolone acetonide. At the one-month follow-up visit, the patient’s BCVA was 20/50 and the sutured PCIOL appeared to be in good position. There was some persistence of CME, and treatment for this was initiated. The patient was then lost to follow-up.
Case 4
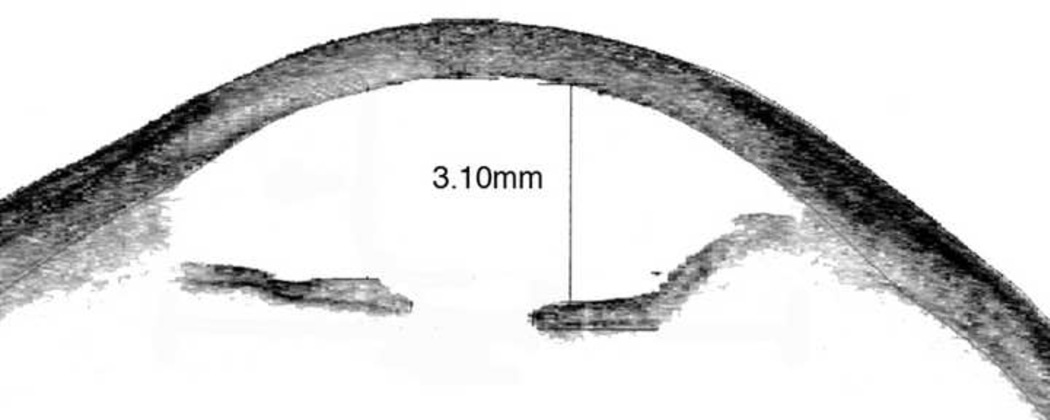
A 66-year-old woman had undergone cataract extraction with posterior capsular tear, anterior vitrectomy and AC-IOL placement in her left eye the week prior to her presentation in 2007. Her BCVA was counting fingers at two feet, and central corneal thickness measured 903 microns. On follow-up 2 months later slit lamp examination revealed an inverted Kelman Multiflex™ AC-IOL with the haptics resting on the cornea and the iris in a posteriorly convex position (Figure 1 bottom right) as evidenced on anterior segment OCT (Figure 3). Three months after her initial presentation, the patient underwent IOL exchange with a scleral-sutured PCIOL. Descemet’s stripping automated endothelial keratoplasty (DSAEK) was performed two months later because of nonclearing corneal edema. Three months following endothelial keratoplasty, her vision had improved to 20/30, a level which was maintained two years later.
Figure 3.
Anterior segment ocular coherence tomography showing posterior bowing of iris due to posteriorly vaulting inverted Kelman Multiflex™ anterior chamber intraocular lens
Discussion
The Kelman Multiflex™-style, semi-flexible, four-point fixation, open-loop, PMMA AC-IOL is a common lens choice for secondary or primary intraocular lens implantation in the absence of capsular support. This lens is specifically designed to be placed in the correct orientation (each haptic forming the bottom half of a reverse “Z”), as diagrammed on the lens packaging. The optic is vaulted forward so that iris chafing is minimized, adequate clearance away from the corneal endothelium is provided, and good fixation stability is achieved with the haptics resting in the angle, adjacent to the iris root and away from the peripheral cornea.
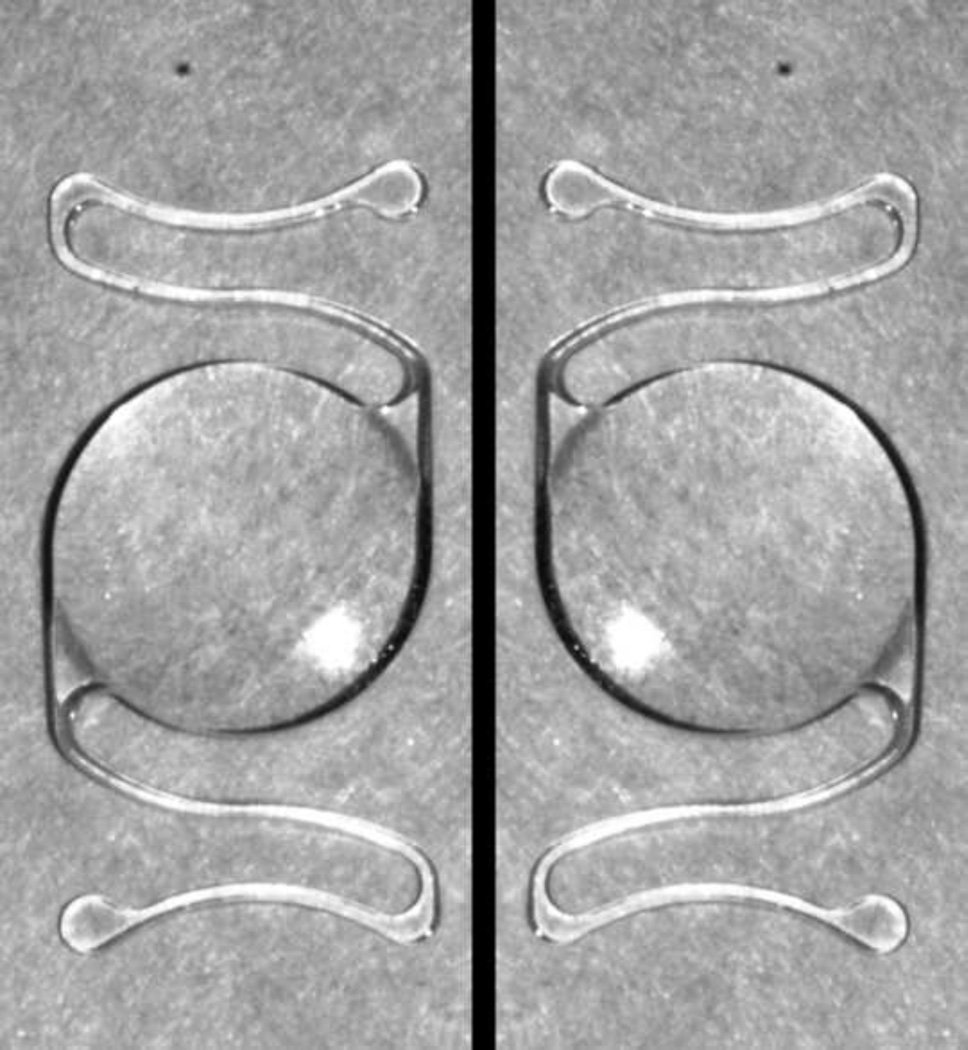
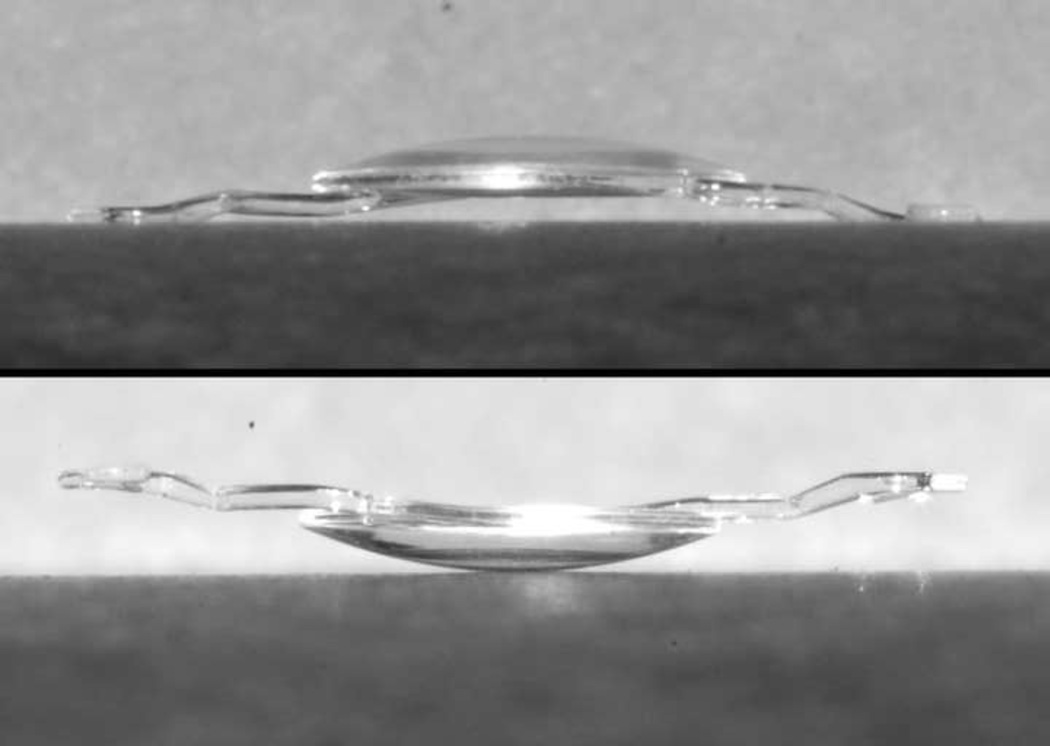
There are several measures that can be taken to avoid inadvertent upside-down placement of the Kelman Multiflex™ AC-IOL, including inspection of the haptic configuration to verify that it matches the diagram indicated on the exterior packaging or in the package insert (Figure 4); confirmation of the forward vault of the lens optic when viewed in side profile (Figure 5); and finally, compression of the haptics and observation of the further forward vaulting of the optic when viewed in side profile.
Figure 4.
En face view of Kelman Multiflex™ anterior chamber intraocular lens in left: Correct configuration right: Incorrect, inverted configuration
Figure 5.
Side view of Kelman Multiflex™ anterior chamber intraocular lens demonstrating top: anterior vault of correct configuration; bottom: posterior vault of inverted configuration
When this lens is placed in an inverted configuration, the optic is vaulted posteriorly causing the optic to press against the iris. Pupil capture and formation of synechiae can result (Case # 2). The chronic chafing of the iris against the AC-IOL can lead to chronic uveitis and may precipitate or exacerbate CME. Furthermore, the haptics in an inverted AC-IOL are unable to properly rest on the iris root and can migrate anteriorly and contact the peripheral corneal endothelium, resulting in endothelial cell loss and corneal edema (Case #1, 4).
The hallmark findings of this constellation of findings, which we have termed “upside down lens syndrome,” is the reversed haptic configuration accompanied by posterior vaulting of the AC-IOL optic against the iris, causing the iris to bow backwards. Peripheral iridectomies had been performed in all of our cases, and no cases had evidence of pupillary block. In two of our cases, CME was documented by OCT. In our second patient, iris capture of the optic occurred which prompted an additional surgical procedure to reposition the implant and to lyse iris synechiae; however the inverted orientation of the AC-IOL was not recognized or addressed, which led to eventual corneal decompensation. Pseudophakic bullous keratopathy (PBK) occurred in Cases 1, 2, and 4. Surgical intervention in each case, consisting of penetrating keratoplasty, corneal cautery, and endothelial keratoplasty, respectively.
It is conceivable that our patients may have developed ocular complications even if the AC-IOL was placed in the proper orientation, since AC-IOL placement was associated in each case with complicated cataract surgery. On the other hand, the use of flexible, open-loop AC-IOLs is associated with relatively low rates of PBK and CME. In a retrospective study of AC-IOL placement in eyes with poor capsular support at the time of surgery, 5 of AC-IOL 83 patients developed corneal edema and 12 of 83 AC-IOL patients developed CME. 1 The median follow-up was 18.8 months (range 1 to 80.6). Reported rates of PBK and CME are somewhat lower in cases of secondary, elective AC-IOL implantation for aphakia. In a study looking at complications after secondary, flexible, open-loop PMMA AC-IOL placement, 2/73 eyes developed PBK and 5/73 developed CME. 4
A smaller study from the University of Utah concluded that AC-IOLs are as safe as sutured PCIOLs. 5
Correct placement of a flexible-loop AC-IOL in properly selected patients has a good long-term outcome. 6 Inverted placement of AC-IOLs, however can lead to poor clinical outcomes including the development of one or more of the following complications: cystoid macular edema, chronic iritis, pupil capture, iris synechiae, and corneal edema. To our knowledge, this is the first series of patients reported with inverted configuration AC-IOL implantation, which we have termed “upside-down lens syndrome.” In each case, complications of varying severity developed, which we believe were directly attributable at least in part to the broad posterior contact of the optic against the iris and the abnormally anterior placement of the haptics against the peripheral cornea. The relatively low frequency of use of anterior chamber intraocular lenses and resultant surgeon unfamiliarity with their use may predispose to their incorrect placement. Verification of correct orientation before and after implantation is crucial to avoid possible complications. Early recognition of this entity is vital, and timely intraocular lens exchange or proper repositioning of the existing lens should be considered to prevent adverse clinical outcomes.
Acknowledgments
Funding:
This study was supported in part by unrestricted grants from That Man May See and from Research to Prevent Blindness.
This study was approved by the Committee on Human Research at University of California, San Francisco.
Biographies
Robert E. Fintelmann, MD received his medical degree from the University of Ulm, Germany. His post graduate work included a residency in ophthalmology at the Wills Eye Institute and a fellowship in Cornea and External disease a the Proctor I Francis foundation at the University of California, San Francisco.
Dr Fintelmann is currently in private practice in Phoenix, Arizona.

David G. Hwang, MD, FACS, Professor, University of California, San Francisco
David G. Hwang, MD, FACS obtained his B.S. and M.D. degrees from the University of California, San Francisco (UCSF). Following ophthalmology residency at UCSF, he completed fellowships in cornea, refractive surgery, and uveitis at the Doheny Eye Institute. In 1990 he joined the full-time faculty at UCSF, where he is Professor, Co- Director of the Cornea Service, and Director of the Refractive Surgery Service.

Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: Neither Dr Fintelmann nor Dr Kim have any financial disclosures. Dr Hwang is consultant for Inspire and Santen Pharmaceuticals, and has received lecture fees from Bausch&Lomb.
Contributions of Authors: Design of the study (DH); Conduct of the study (RF, SK, DH); Writing the article (RF, DH); Critical revision of the article (RF, DH); Final approval of the article (RF, SK, DH); Data collection (RF, SK); Literature search (RF)
References
- 1.Donaldson KE, Gorscak JJ, Budenz DL, Feuer WJ, Benz MS, Forster RK. Anterior chamber and sutured posterior chamber intraocular lenses in eyes with poor capsular support. J Cataract Refract Surg. 2005;31(5):903–909. doi: 10.1016/j.jcrs.2004.10.061. [DOI] [PubMed] [Google Scholar]
- 2.Wagoner MD, Cox TA, Ariyasu RG, Jacobs DS, Karp CL. Intraocular lens implantation in the absence of capsular support: a report by the American Academy of Ophthalmology. Ophthalmology. 2003;110(4):840–859. doi: 10.1016/s0161-6420(02)02000-6. [DOI] [PubMed] [Google Scholar]
- 3.Ellerton CR, Rattigan SM, Chapman FM, Chitkara DK, Smerdon DL. Secondary implantation of open-loop, flexible, anterior chamber intraocular lenses. J Cataract Refract Surg. 1996;22(7):951–954. doi: 10.1016/s0886-3350(96)80197-0. [DOI] [PubMed] [Google Scholar]
- 4.Evereklioglu C, Er H, Bekir NA, Borazan M, Zorlu F. Comparison of secondary implantation of flexible open-loop anterior chamber and scleral-fixated posterior chamber intraocular lenses. J Cataract Refract Surg. 2003;29(2):301–308. doi: 10.1016/s0886-3350(02)01526-2. [DOI] [PubMed] [Google Scholar]
- 5.Jin GJ, Crandall AS, Jones JJ. Changing indications for and improving outcomes of intraocular lens exchange. Am J Ophthalmol. 2005;140(4):688–694. doi: 10.1016/j.ajo.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 6.Drolsum L. Long-term follow-up of secondary flexible, open-loop, anterior chamber intraocular lenses. J Cataract Refract Surg. 2003;29(3):498–503. doi: 10.1016/s0886-3350(02)01614-0. [DOI] [PubMed] [Google Scholar]