Abstract
Purpose
Tuberculosis (TB) remains a problem in the community. TB patients usually experience malnutrition, which is characterized by both decreased body weight (BW) and body fat percentage (BFP). Leptin, an important regulator of BW, also plays an important role in cellular immunity, which is integral to defense against Mycobacterium tuberculosis infection. We analyzed the effect of an anti-TB treatment regimen on the leptin level, BW, and BFP of children with TB.
Methods
The design of this study was a group interrupted time series. The subjects were children with probable TB according to clinical criteria based on an Indonesian scoring system adopted from the Consensus of Expert Panel. BW; BFP; energy intake; fat and protein intake; and leptin levels before, 2 months after (intensive phase), and 6 months after (continuation phase) anti-TB treatment, were measured. About 40 children, aged 5–14 years, participated in this study.
Results
The BW, BFP and leptin level increased from before treatment to after completion of the intensive phase and still showed an increased during the continuation phase: BW 18.65 kg, 19.75 kg, and 20.85 kg; BFP 18.3%, 19.5%, and 20.2%; and leptin level 1.9 mg/dL, 3.07 mg/dL, and 3.4 mg/dL, respectively (P<0.01).
Conclusion
Leptin level, BW, and BFP increased throughout the course of anti-TB treatment, compared with pretreatment values. Further research is needed to compare the results with data for healthy children.
Keywords: Leptin, Tuberculosis treatment, Weight gain, Child
Introduction
Tuberculosis (TB) remains one of the major causes of morbidity and mortality worldwide, especially in Asia and Africa. According to the World Health Organization, 9.2 million incident TB cases occurred globally in 2006, resulting in around 1.7 million deaths1). The majority of TB cases in children younger than 15 years occur in Southeast Asia and Africa2). Indonesia has the third highest prevalence and incidence of TB (after India and China) in the world. In 2006, an estimated 578,000 people had TB in Indonesia, with 88,000 TB-related deaths1). In Indonesia, malnutrition is highly prevalent among TB patients3) and moderate to severe malnutrition is correlated with early mortality4).
Wasting is a systemic clinical manifestation of TB, which may affect both the severity and outcome of the disease5,6). The pathogenesis of wasting due to TB remains unclear6). However, it is hypothesized that microbial products can stimulate the production of proinflammatory mediators and cytokines. This, in turn, stimulates the acute phase of the host response, which leads to anorexia7,8). Leptin is thought to be a mediator in the complex process between TB, nutrition status, and host immune response; leptin may play an important role in regulating nutrition intake, energy consumption, and body weight (BW)9). Leptin is a 16-kDa protein coded by obese genes, especially in adipocytes10). Leptin levels are correlated with body mass index, increasing in overweight cases and decreasing in wasting cases5,11,12). Leptin regulates appetite and energy consumption at a hypothalamic level by binding with its specific receptor9). Circulating leptin levels are correlated with fat mass and can be decreased by hunger13). However, leptin not only serves to suppress appetite and regulate weight, it is a multifunctional hormone. In addition to regulating food intake and energy homeostasis, it has a role in neuroendocrine processes, angiogenesis, bone formation, reproduction, hematopoiesis, and immunity3,14).
Leptin plasma concentrations in TB patients can be affected by two opposing mechanisms, namely: chronic inflammation—which causes loss of body fat mass, thereby reducing the production of leptin5,11) and the host's acute inflammatory response—which increases levels of leptin, theoretically leading to appetite suppression, anorexia, and reduced body mass8,10). Low levels of leptin can worsen the prognosis of TB because leptin plays an important role in cellular immunity, the means by which the body attacks Mycobacterium tuberculosis5,10,12).
This study aimed to investigate the effect of anti-TB drugs on leptin levels, changes in BW, and body fat percentage (BFP), by analyzing leptin level, BW, height, nutritional status, and food intake before treatment and after the 2-month intensive phase and the 4-month continuation phase of TB treatment.
Materials and methods
This was a quasi-experimental study, with a group interrupted time series design. The study participants were children with TB treated at the outpatient clinic of the Pediatric Division of Kayen District Hospital in Pati, Central Java. Inclusion criteria were children aged 5–14 years who were diagnosed as Probable TB based on the Concensus of Expert Panel15), which are (1) showing signs and symptoms suggestive of TB, (2) chest radiography is consistent with intrathoracic disease due to M. tuberculosis, and (3) at least having one of the following: (a) a positive clinical response to anti-TB treatments, (b) documented exposure to M. tuberculosis, or (c) immunological evidence of M. tuberculosis infection. This consensus adopted in Indonesia by adding scoring system, which noted more on persistent cough, weight loss/failure to thrive, persistent unexplained fever, and persistent unexplained lethargy or reduce playfulness; chest radiography; and positive tuberculin test by using 0,1 mL PPD RT 23 (2 TU) (Statens Serum Institut, Copenhagen, Denmark). The scoring above 6 is to be treated as TB in children16). The exclusion criteria were pulmonary TB with chronic diarrhea and extra-pulmonary. Dropout criteria were discontinuation of anti-TB drugs for at least 1 continuous week, experiencing side effects that resulted in the drugs being (temporarily or permanently) discontinued, and not returning for follow-up examinations.
Participants were recruited using a consecutive sampling method during the last 6 months of 2014. BW; BFP; energy, fat, and protein intake; and leptin levels were examined before, and after 2 (end of the intensive phase) and 6 months (end of the continuation phase) of TB treatment.
BW and BFP were measured using a Tanita Body Composition Analyzer-515 (Tanita, Japan); height was measured using a Tanita stadiometer (Tanita, Japan); and energy, fat, and protein intake were evaluated using 24-hour food recall (on 3 nonconsecutive days). Data were analyzed using NutriSoft software. About 3 mL of venous blood, taken using a disposable plastic syringe, were immediately transferred to a vacutainer for leptin measurement. Leptin levels were analyzed using a DSL-10-23100 active Human Leptin ELISA (enzyme-linked immunosorbent assay), which is a sandwich-type immunoassay amplified using a 2-step enzyme process (Diagnostic Systems Laboratories, Beckman Coulter Co., Webster, TX, USA).
Data were analyzed using SPSS ver. 11.0 (SPSS Inc., Chicago, IL, USA). The Shapiro-Wilk test was used to determine the distribution of continuous data. Data are presented as the mean and standard deviation, or as the median and interquartile range for normally and nonnormally distributed data, respectively. The difference between the levels of leptin, BW, and BFP before and after the intensive phase as well as after the continuation phase of TB treatment, was tested using the paired t test or the Wilcoxon rank sum test, for normally and nonnormally distributed data, respectively. A P value <0.05 was considered significant.
The study protocol was approved by the Institutional Review Board of the Faculty of Medicine - Diponegoro University and the Dr. Kariadi Hospital (No. 509/EC/FK-RSDK/2014). Informed consent was obtained from the parents of the participating children before the study began.
Results
Initially, 43 children aged 5–14 years with TB—who met the study's inclusion criteria—were recruited from the pediatric division of Kayen Hospital. At the end of the 2-month intensive phase of treatment, 2 children had dropped out: 1 had stopped taking anti-TB drugs for more than 1 consecutive week and 1 was excluded because of side effects (jaundice) related to the treatment. Another child was excluded because his parents refused to continue his participation in the study. By the end of the 4-month continuation phase of treatment, 40 children had completed this study.
The mean age of the children before TB treatment was 6.8 years, with a mean BW of 19.8 kg, and mean height of 116.3 cm. Twenty-seven of the participants (67.5%) were boys. The education level of the mothers was categorized, with 55% having graduated high school.
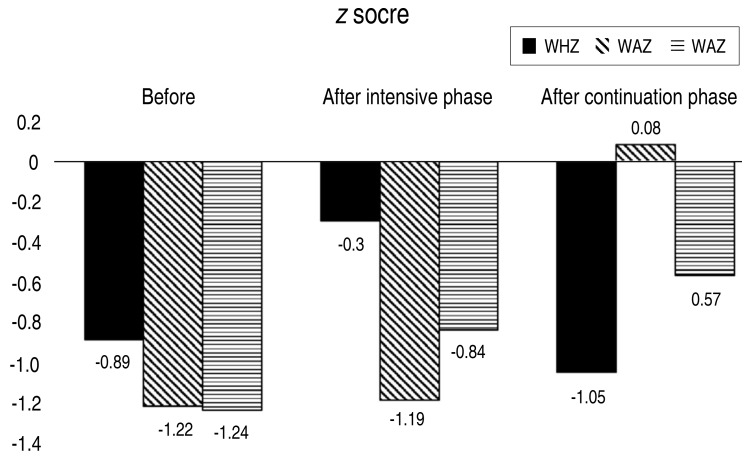
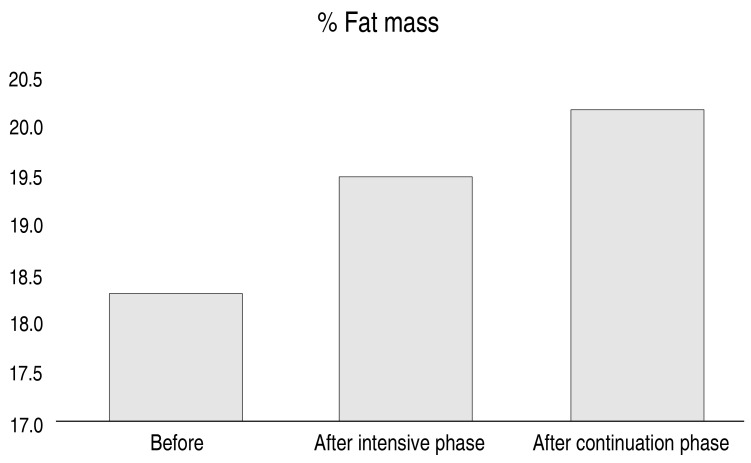
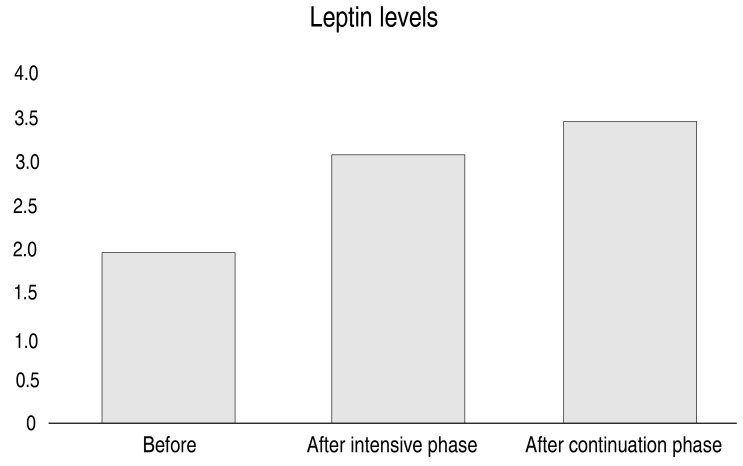
Table 1 shows that, except for height-for-age z scores before and after the intensive phase of treatment, all variables increased significantly (P<0.01). Additionally, we found significant increases in all variables when comparing data before treatment and after completion of the continuation phase of TB treatment (P<0.01).
Table 1. Comparison of variables before and after tuberculosis treatment.
| Variable | Before treatment | After intensive phase | P value* | After continuation phase | P value† |
|---|---|---|---|---|---|
| Weight (kg) | 18.65 (14–45) | 19.75 (15–46.3) | <0.001b | 20.85 (16.3–47) | <0.001b |
| Height (cm) | 114.4 (93–151) | 115.6 (95–153) | <0.001b | 116 (97–156) | <0.001b |
| WAZ | −1.24 (−2.71–0.89) | −0.84 (−2.32–1.14) | <0.001b | −0,57 (−1,95–1,26) | <0.001b |
| HAZ | −1.22 (−3.51–1.76) | −1.19 (−3.48–1.56) | 0.097b | −1.05 (−3.7–1.15) | <0.001b |
| WHZ | -0.89 (−4.34–1.55) | -0.30 (−3.55–1.76) | <0.001b | 0.08 (−2.56–1.71) | <0.001b |
| Body fat (%) | 18.3 (9.9–27) | 19.5 (9.8–27.6) | <0.001b | 20.2 (10.3–28.9) | <0.001b |
| Energy intake (kcal) | 1,133±214.1 | 1,451±167.0 | <0.001a | 1,501±193.7 | <0.001a |
| Fat intake (g) | 39.9±10.88 | 55.3±11.51 | <0.001a | 59.6±12.75 | <0.001a |
| Protein intake (g) | 37.4±8.27 | 45.9±9.09 | <0.001a | 45.07±10.18 | <0.001a |
| Leptin level (mg/dL) | 1.90±0.87 | 3.07±0.93 | <0.001a | 3.40±0.88 | <0.001b |
Values are presented as median (range) or mean±standard deviation.
WHZ, weight for height z score; HAZ, height for age z score; WAZ, weight for age z score.
*P value between “before treatment” and “after intensive phase.” †P value between “before treatment” and “after continuation phase.” aPaired t test. bWilcoxon test.
Fig. 1 depicts the effect of TB treatment on anthropometric indices, Fig. 2 demonstrates increases in the mean BFP observed from before treatment to after completion of the continuation phase of TB intervention, and Fig. 3 displays the marked increases in mean leptin level observed before treatment to after the intensive phase of intervention.
Fig. 1. Anthropometric measurement during tuberculosis treatment. Graphical summary of changes in anthropometric measures over the course of tuberculosis treatment, at 3-time points (before, at the end of the intensive phase, and at the end of the continuation phase of treatment). WHZ, weight for height z score; HAZ, height for age z score; WAZ, weight for age z score.
Fig. 2. Body fat percentage measurement during tuberculosis treatment. Graphical summary of changes in body fat percentage measures over the course of tuberculosis treatment, at three time points (before, at the end of the intensive phase, and at the end of the continuation phase of treatment).
Fig. 3. Body fat percentage measurement during tuberculosis treatment. Graphical summary of changes in body fat percentage measures over the course of tuberculosis treatment, at 3-time points (before, at the end of the intensive phase, and at the end of the continuation phase of treatment).
Discussion
Participants showed increases in weight, fat, and leptin levels over the course of their TB treatment. A cross-sectional study conducted in Indonesia found that serum leptin levels in patients with TB were nearly 5 times lower than in the controls3); therefore, it was concluded that leptin levels were significantly increased after TB treatment. Low leptin levels increased the incidence of wasting in children with TB. Another study reported that serum C-reactive protein levels were higher in children with TB compared with children without TB3). Other studies comparing leptin and tumor necrosis factor alpha (TNF-α) levels before and after the administration of anti-TB drugs found that leptin levels were low in patients with TB, but increased after treatment 3,11,12). Additionally, low leptin levels may contribute to increased susceptibility to infection3,12).
TB often leads to weight loss, affects the inflammatory response, and suppresses cellular immunity. Leptin is predicted to be a mediator in the complex relationship between TB, nutritional status, and immune response5,17). A decrease in body fat mass is not the only reason for the reduction in plasma leptin concentrations in patients with TB. Although body fat mass was the major determinant of plasma leptin concentrations, starvation, hormones (such as insulin and cortisol), and various mediators of inflammation appear to modulate the production of leptin. Research has shown that lipopolysaccharides, TNF-α, and interleukin-1β may all increase concentrations of leptin in the serum and leptin mRNA in adipose tissue18). Besides, leptin levels have also been associated with outcomes in critically ill patients with acute respiratory distress syndrome due to pneumonia. The in vitro mechanism showed that leptin enhanced immune cell function, such as monocyte and macrophage activation, phagocytosis, and cytokine secretion, has roles as a neutrophil chemoattractant and an antiapoptotic19). After antiretroviral therapy, leptin level is lower at the poor immune response human immunodeficiency virus (HIV) patients as indicated by CD4 than those with better immune response. It might be justified that leptin may be involved in the link between the energy and nutritional status to the T helper immune response20). In a study of patients with TB, the production of C-reactive protein and TNF-α was inversely related to plasma leptin concentrations. Reduction of the acute phase response and the production of proinflammatory cytokines during TB treatment appear to be accompanied by increased plasma leptin concentrations. The pattern of plasma leptin concentrations in the few weeks or months preceding diagnosis is still unknown, but it is estimated that the long-term inflammatory responses associated with TB reduce its production or cause fatigue6).
Leptin regulates appetite and energy consumption at the level of the hypothalamus by binding to specific receptors9). Study on hormonal status of HIV children showed that leptin, adiponectin, insulin and insulin-like growth factor-1 level of HIV children are lower compared with non-HIV ones. This profile indicates a state that glucose production and fat catabolism—associated to lypodystrophy—are more prioritized than energy storage and growth21). In patients with TB who have not received treatment, a decrease in body fat mass, reduced energy intake, and host immune response, will reduce the production of leptin3,5).
Because leptin plays an important role for cellular immunity against M. tuberculosis, suppression of the concentration of leptin may play a role in worse outcomes, especially in patients with cachexia3,5). Once on TB treatment, the reduction of the acute phase response and the production of proinflammatory cytokines, are expected to play a role in increasing plasma concentrations of leptin5).
This study found that the increases in leptin concentrations were greater after administration of anti-TB drugs between the intensive and continuation phase of treatment (P<0.01). The intensive phase consisting of 3 drugs (isoniazid, rifampicin, and pyrazinamide) administered for 2 months aims to eliminate the actively replicating bacterial population to reduce the bacterial load. The continuation phase, which consists of 2 drugs (isoniazid and rifampicin) administered for 4 months, aims to remove bacteria persisting in the dormant state and bacteria that managed to escape the intensive phase, which show intermittent activity22). We hypothesized that the increase in leptin level was greater in the intensive phase because of the decrease in the number of bacteria coupled with the decrease in the acute phase response, although the production of proinflammatory cytokines was greater in the continuation phase of treatment23).
Our study found that leptin levels and energy, protein, and fat intake increased after administration of anti-TB drugs. We concluded that the increased concentrations of leptin may play a role in stimulating appetite in patients, leading to enhanced dietary intake (energy, protein, and fat). Our results are supported by another study conducted in Thailand, which found that leptin concentration was positively correlated with BW and BFP. However, in contrast with our study, it found no significant correlation between leptin and energy intake18).
Leptin is an important mediator of energy metabolism. It plays a role in communicating the status of the body's energy reserves to the appetite center in the hypothalamus. Decreased leptin levels produce a distinctive response to starvation, while increased levels of leptin are associated with weight gain11,23). The largest component of energy expenditure is resting energy expenditure (REE)24). Leptin concentration has been found to be correlated with REE, although the results are inconsistent11). A study in adults with pulmonary TB showed a weak negative correlation between leptin concentration and REE, and no association with energy intake11). In our study, we did not measure the REE or the physical activity of participants. However, we found data from another study that measured energy expenditure in Indonesian children, and showed no significant difference in REE between children of varying nutritional statuses25).
Because leptin plays an important role in cellular immunity against M. tuberculosis, suppression of leptin concentrations may play a role in obtaining worse outcomes from TB, especially in patients with cachexia. Metreleptin is a form of leptin that has a substantial effect on the metabolic regulation of food intake, BW, energy expenditure, glucose and lipid metabolism, hypothalamic-pituitary axis immunity, and the structure and function of the brain. Theoretically, administration of metreleptin may be useful for patients with TB, but this has not been implemented in many countries as yet3,5,24).
Some limitations of this study should be noted. First, the diagnosis of TB was based on Indonesian scoring system adopted from Consensus of Expert Panel, thus we did not investigate bacterial load or virulence. Second, plasma concentrations may not always reveal the biologic activity of leptin as diurnal rhythms and pulsatile release occur26). Third, we did not recruit a healthy control group for comparison. Finally, dietary intake measurement using 24-hour recall methods are often biased toward over- or underreporting27).
In conclusion, we found that there were increases in BW, BFP, and leptin level during both the intensive and continuation phase of treatment for TB, compared with pretreatment measures. Further research is needed to compare the results with healthy children.
Acknowledgment
This study was supported by research grant from Dr. Kariadi Hospital, Ministry of Health.
Footnotes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Mustafa T. Does leptin have a role in immunity to tuberculosis? Indian J Med Res. 2008;128:691–693. [PubMed] [Google Scholar]
- 2.Nelson LJ, Wells CD. Global epidemiology of childhood tuberculosis. Int J Tuberc Lung Dis. 2004;8:636–647. [PubMed] [Google Scholar]
- 3.Herlina M, Nataprawira HM, Garna H. Association of serum C-reactive protein and leptin levels with wasting in childhood tuberculosis. Singapore Med J. 2011;52:446–450. [PubMed] [Google Scholar]
- 4.Zachariah R, Spielmann MP, Harries AD, Salaniponi FM. Moderate to severe malnutrition in patients with tuberculosis is a risk factor associated with early death. Trans R Soc Trop Med Hyg. 2002;96:291–294. doi: 10.1016/s0035-9203(02)90103-3. [DOI] [PubMed] [Google Scholar]
- 5.van Crevel R, Karyadi E, Netea MG, Verhoef H, Nelwan RH, West CE, et al. Decreased plasma leptin concentrations in tuberculosis patients are associated with wasting and inflammation. J Clin Endocrinol Metab. 2002;87:758–763. doi: 10.1210/jcem.87.2.8228. [DOI] [PubMed] [Google Scholar]
- 6.van Lettow M, van der Meer JW, West CE, van Crevel R, Semba RD. Interleukin-6 and human immunodeficiency virus load, but not plasma leptin concentration, predict anorexia and wasting in adults with pulmonary tuberculosis in Malawi. J Clin Endocrinol Metab. 2005;90:4771–4776. doi: 10.1210/jc.2004-2539. [DOI] [PubMed] [Google Scholar]
- 7.Pavan Kumar N, Anuradha R, Andrade BB, Suresh N, Ganesh R, Shankar J, et al. Circulating biomarkers of pulmonary and extrapulmonary tuberculosis in children. Clin Vaccine Immunol. 2013;20:704–711. doi: 10.1128/CVI.00038-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng Y, Ma A, Wang Q, Han X, Cai J, Schouten EG, et al. Relation of leptin, ghrelin and inflammatory cytokines with body mass index in pulmonary tuberculosis patients with and without type 2 diabetes mellitus. PLoS One. 2013;8:e80122. doi: 10.1371/journal.pone.0080122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Margetic S, Gazzola C, Pegg GG, Hill RA. Leptin: a review of its peripheral actions and interactions. Int J Obes Relat Metab Disord. 2002;26:1407–1433. doi: 10.1038/sj.ijo.0802142. [DOI] [PubMed] [Google Scholar]
- 10.Wieland CW, Florquin S, Chan ED, Leemans JC, Weijer S, Verbon A, et al. Pulmonary Mycobacterium tuberculosis infection in leptin-deficient ob/ob mice. Int Immunol. 2005;17:1399–1408. doi: 10.1093/intimm/dxh317. [DOI] [PubMed] [Google Scholar]
- 11.Schwenk A, Hodgson L, Rayner CF, Griffin GE, Macallan DC. Leptin and energy metabolism in pulmonary tuberculosis. Am J Clin Nutr. 2003;77:392–398. doi: 10.1093/ajcn/77.2.392. [DOI] [PubMed] [Google Scholar]
- 12.Buyukoglan H, Gulmez I, Kelestimur F, Kart L, Oymak FS, Demir R, et al. Leptin levels in various manifestations of pulmonary tuberculosis. Mediators Inflamm. 2007;2007:64859. doi: 10.1155/2007/64859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang R, Barouch LA. Leptin signaling and obesity: cardiovascular consequences. Circ Res. 2007;101:545–559. doi: 10.1161/CIRCRESAHA.107.156596. [DOI] [PubMed] [Google Scholar]
- 14.Park HK, Ahima RS. Physiology of leptin: energy homeostasis, neuroendocrine function and metabolism. Metabolism. 2015;64:24–34. doi: 10.1016/j.metabol.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graham SM, Ahmed T, Amanullah F, Browning R, Cardenas V, Casenghi M, et al. Evaluation of tuberculosis diagnostics in children: 1. Proposed clinical case definitions for classification of intrathoracic tuberculosis disease. Consensus from an expert panel. J Infect Dis. 2012;205(Suppl 2):S199–S208. doi: 10.1093/infdis/jis008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Indonesian Ministry of Health. Petunjuk teknis manajemen TB anak (Technical guidelines for TB in children) Jakarta: Kemenkes RI; 2013. pp. 7–26. [Google Scholar]
- 17.Malli F, Papaioannou AI, Gourgoulianis KI, Daniil Z. The role of leptin in the respiratory system: an overview. Respir Res. 2010;11:152. doi: 10.1186/1465-9921-11-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamborisut U, Riabroy N, Phonrat B, Tungtrongchitr R. Serum leptin levels and body composition in obese Thai children. Southeast Asian J Trop Med Public Health. 2009;40:544–552. [PubMed] [Google Scholar]
- 19.Ubags ND, Stapleton RD, Vernooy JH, Burg E, Bement J, Hayes CM, et al. Hyperleptinemia is associated with impaired pulmonary host defense. JCI Insight. 2016;1(8) doi: 10.1172/jci.insight.82101. pii: e82101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tiliscan C, Aramă V, Mihăilescu R, Munteanu DI, Streinu-Cercel A, Ion DA, et al. Leptin expression in HIV-infected patients during antiretroviral therapy. Germs. 2015;5:92–98. doi: 10.11599/germs.2015.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mody A, Bartz S, Hornik CP, Kiyimba T, Bain J, Muehlbauer M, et al. Effects of HIV infection on the metabolic and hormonal status of children with severe acute malnutrition. PLoS One. 2014;9:e102233. doi: 10.1371/journal.pone.0102233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joshi JM. Tuberculosis chemotherapy in the 21 century: back to the basics. Lung India. 2011;28:193–200. doi: 10.4103/0970-2113.83977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benoit SC, Clegg DJ, Seeley RJ, Woods SC. Insulin and leptin as adiposity signals. Recent Prog Horm Res. 2004;59:267–285. doi: 10.1210/rp.59.1.267. [DOI] [PubMed] [Google Scholar]
- 24.Paz-Filho G, Mastronardi CA, Licinio J. Leptin treatment: facts and expectations. Metabolism. 2015;64:146–156. doi: 10.1016/j.metabol.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 25.Mexitalia M, Yamauchi T, Utari A, Sjarif DR, Subagio HW, Soemantri A, et al. The role of uncoupling protein 2 and 3 genes polymorphism and energy expenditure in obese Indonesian children. J Pediatr Endocrinol Metab. 2013;26:441–447. doi: 10.1515/jpem-2012-0311. [DOI] [PubMed] [Google Scholar]
- 26.Steyn FJ, Xie TY, Huang L, Ngo ST, Veldhuis JD, Waters MJ, et al. Increased adiposity and insulin correlates with the progressive suppression of pulsatile GH secretion during weight gain. J Endocrinol. 2013;218:233–244. doi: 10.1530/JOE-13-0084. [DOI] [PubMed] [Google Scholar]
- 27.Hill RJ, Davies PS. The validity of self-reported energy intake as determined using the doubly labelled water technique. Br J Nutr. 2001;85:415–430. doi: 10.1079/bjn2000281. [DOI] [PubMed] [Google Scholar]