Abstract
Autoimmune hepatitis (AIH) is a complex autoimmune disease characterized by immune-mediated destruction of hepatic parenchyma which can result in cirrhosis, liver failure, and death. Current AASLD and EASL guidelines recommend corticosteroids alone or in combination with azathioprine as first-line treatment strategies. However, a significant proportion of patients may not be able to tolerate or achieve complete biochemical response with these options. In this article, we discuss approaches to these patients and other challenging AIH patient groups such as the asymptomatic, pregnant, elderly and liver transplant recipients.
Keywords: autoimmune hepatitis, pregnancy, cirrhosis, nonstandard treatment, liver transplantation, refractory
INTRODUCTION
Autoimmune hepatitis (AIH) is a female predominant condition characterized by immune-mediated destruction of liver parenchyma and presence of peripheral autoantibodies (1,2). Waldenström first described this disease in a group of young females with hypergammaglobulinemia over 60 years ago (3). Despite forward progress in diagnosis and therapeutic strategies, variable clinical and phenotypic presentations have prevented the formation of standardized algorithmic treatment for all patients. Similar to other autoimmune liver diseases (4,5) all AIH is not the same; high risk populations such as African Americans (6), or those with early disease onset (7,8), incomplete normalization of liver tests(7), and advanced disease at diagnosis (8,9) have worse overall survival.
AIH was the first chronic liver disease in which medical treatment was associated with improved survival (10), yet an individualized therapeutic approach has not yet been established. Management principles even among experts in this evolving field remain heterogeneous especially beyond accepted first-line therapies. Much like any rare disease, the variation in therapeutic approaches are the result of small retrospective studies, poor understanding of disease associated immunologic mechanisms, and wide knowledge gaps in disease pathogenesis. The clarification of evidence-based strategies is paramount, as recent epidemiologic data suggest a rising incidence of AIH (11).
Strategic AIH goals of normalization of liver inflammation, prevention of subsequent parenchymal insult, and inhibition of fibrosis progression or reversal of existing scar are similar to those of any chronic liver disease. This review will highlight these therapeutic aims while clarifying the approach to challenging groups of adult AIH patients.
BEYOND GUIDELINE RECOMMENDED FIRST-LINE TREATMENT STRATEGIES
The current American Association for the Study of Liver Diseases (AASLD) and European Association for the Study of Liver (EASL) recommend treatment of disease-related inflammation with either high-dose corticosteroids alone or in combination with Azathioprine (AZA) (1,12). Therapeutic endpoints have become more stringent in updated guidelines, and treating clinicians should now target normalization of both aspartate aminotransferase (AST) and alanine aminotransferase (ALT) as well as immunoglobulin G (IgG) in order to optimize transplant-free survival (7). Unfortunately, not all AIH patients will have favorable biochemical responses to first-line regimens because of medication intolerance (10%) (12), incomplete response (15%) (12,13), and treatment failure (9%) (14).
TREATMENT INTOLERANT
The inability to normalize liver transaminases and IgG due to intolerance (side effects) of medication requires urgent exploration of other treatment agents given the increased risk of fibrosis progression and worse survival (15,16). Fortunately, AIH maintenance armaments have expanded in recent years, and multiple reports of nonstandard therapies in challenging patient groups are available. Intolerance to recommended first-line therapy, AZA and prednisone, is a significant cause for cessation of therapy in up to 10% of patients related to physical, somatic, or hematologic findings (12).
There is currently no consensus on the optimal second-line choice for AZA intolerance, but mycophenolate mofetil (MMF)has been the most studied second-line agent, and observational data suggests it is tolerated in 54–74% of patients in this group (17,18) (20,21). However, 6-mercaptopurine (6-MP), a molecule formed from non-enzymatic degradation of the nitroimidazol group from AZA, could also be a viable treatment strategy subsequent to AZA because of retained immunosuppressive properties (Table 1). In studies of inflammatory bowel disease, up to 60% of patients intolerant to AZA are able to tolerate 6-MP (19,20). A recent study by Hübener et al (13) retrospectively examined 20 AZA-intolerant AIH patients, largely from gastrointestinal side effects, from two large European referral centers. 6-MP was tolerated well by 15 (75%) patients, and resulted in complete and partial biochemical response in 8 and 7 patients respectively. Therefore, 6-MP may have tolerance rates similar to MMF, and could be considered as an option for this group of patients (1,12). We prefer challenging AZA-intolerant patients with 6-MP (25mg daily and increasing to 50mg daily if tolerated), as it remains an alternative that could help avoid risk of teratogenicity in females that are pregnant or become pregnant while taking MMF as well as provide cost-savings. Furthermore, as a downstream immunologically active product of the AZA, 6-MP may provide much of the survival benefits as its well-studied parent compound.
Table 1.
Clinical Considerations and Management Options for Difficult AIH Cases
| Challenge | Considerations | Management Options |
|---|---|---|
| Intolerance, contraindications or complications from AZA | - Compliance - Contributing axis disorder - 6-TG and 6-MMP levels - Medication side effect |
- 6-MP or MMF - Consider allopurinol in fast metabolizers - Increase AZA dose if 6-TG levels low |
| Intolerance, contraindications or complications from corticosteroids | - Avoid in patients with poorly controlled diabetes or hypertension, unstable mental illness or osteoporosis | - Budesonide in patients without cirrhosis or portosystemic collaterals |
| Incomplete or no response to prednisone and AZA | - Compliance - 6-TG and 6-MMP levels - Overlap conditions (PSC, PBC, NASH) - Drug induced liver injury - Viral hepatitis |
- MMF, cyclosporine, tacrolimus, sirolimus, or
everolimus - Overlap condition specific therapy - Stop offending drug |
| Acute severe presentation | - Assess for liver failure - Exclude concomitant liver conditions |
- Early evaluation and listing for liver
transplant - Intravenous corticosteroids |
| Pregnancy | - Maintenance of remission - No MMF |
- Continue therapy with AZA or prednisone |
Patients with intolerance or drug-induced complications from systemic corticosteroids therapy (21) are also a challenging cohort of patients with difficult to treat AIH (Table 1). Corticosteroids have shown survival benefit with or without AZA since the 1960s, yet AZA alone for induction therapy was associated with an excess of mortality in early clinical trials (10,22). Budesonide, a next-generation corticosteroid, may have a critical role in those with systemic corticosteroid contraindications such as patient with osteoporosis, poorly controlled diabetes or hypertension, or unstable mental illness. The results from a 6-month, blinded, phase IIb trial including AIH patients without cirrhosis on budesonide (3mg, either 3 times or 2 times daily) and AZA was published in 2010 (23). In 6 months, the budesonide and AZA combination resulted in both higher frequency of normalized liver tests (60% vs 38.8%) and less steroid related side effects (28% vs 54.4%) compared to standard therapy with prednisone and AZA. This only prospective randomized control trial with budesonide was criticized because of lower than expected remission rates on prednisone that may have been due to scheduled prednisone weaning, a relatively low dose of prednisone in the control arm, short term follow-up, and no histologic comparison of outcomes. Mindful application of budesonide in AIH requires consideration of no defined long-term outcomes, unclear dose-scheduling, and contraindication in patients with cirrhosis and those with portosystemic collaterals (1). We have observed good response rates in some AIH patients treated with combination therapy including budesonide in place of prednisone. However, we have also witnessed a few incomplete responses despite optimization of the maintenance agent, and agree with the most recent EASL guidelines that a change to systemic corticosteroids is commonly beneficial in this group.
INCOMPLETE AND NON-RESPONDERS
Incomplete response to a treatment regimen is defined by incomplete recovery of clinical symptoms, biochemical data (AST/ALT and IgG), and possibly histologic findings. Current guidelines (1,12) suggest normalization of aminotransferases and IgG levels as a key therapeutic aim, as the clinical impact of incomplete response has been linked to fibrosis progression, liver-related death or requirement of liver transplantation (7,24,25).
Patients are identified as incomplete responders if they fail to normalize liver tests and IgG within 3 years according to the AASLD guidelines (12) (Table 1). It is to be determined if fulfillment of AALSD and EASL treatment goals have dramatic impact on long-term outcomes prospectively. In retrospective reviews, it seems meeting more stringent response criteria may better predict those with excellent outcomes (26). In fact, utilization of early biochemical response may have merit in this arena, as Kanzler et al observed that patients exhibiting a biochemical response in only 3 months have excellent long-term survival (27). Furthermore, incomplete normalization of ALT within 6 months of therapy in a study of 133 AIH patients from New Zealand was independently associated with poor outcomes (7). However, it must be observed that normal liver tests may not be the best surrogate for hepatic inflammation, thus disagreement with histologic activity is relatively common according to study by Dhaliwal et al (28). In that study of 120 AIH patients with normal ALT and globulin levels at 6 months, persistent histologic inflammation was observed in 46% of patient biopsy specimens. Furthermore, those with continued inflammation had less frequent regression of fibrosis and excess mortality compared to patients with histologic normalization.
The demarcation of incomplete response requires further management considerations beyond that of biochemical follow-up. In fact, the failure to meet treatment goals, at treatment initiation or in follow-up, should prompt examination for concurrent liver disease (including overlap phenomenon, Figure 1), drug induced liver injury, treatment compliance, inadequatepharmacologic therapy, and accuracy of diagnosis. Consideration of co-existent autoimmune liver diseases, primary sclerosing cholangitis (PSC) and primary biliary cholangitis (PBC), is necessary, particularly among individuals with evolution of cholestasis. Overlap phenomenon are not rare in AIH groups, as a recent cross-sectional study of over 1300 AIH patients from the Netherlands identified PBC and PSC in 9% and 6% of AIH patients respectively (29). Liver biopsy plays an important role not only initially at the time of establishing the diagnosis of AIH, but also in cases with incomplete response to optimal therapy. Typical (interface hepatitis, lymphoplasmacytic infiltrates, hepatic rosette formation, and emperipolesis) and compatible histologic findings on liver biopsy are critical to confirming the diagnosis AIH (1) and clarifying the presence or absence of alternative or coexisting hepatic disease. However, there are no pathognomonic AIH features on biopsy, and histologic findings should lend support or opposition to diagnosis.
Figure 1.
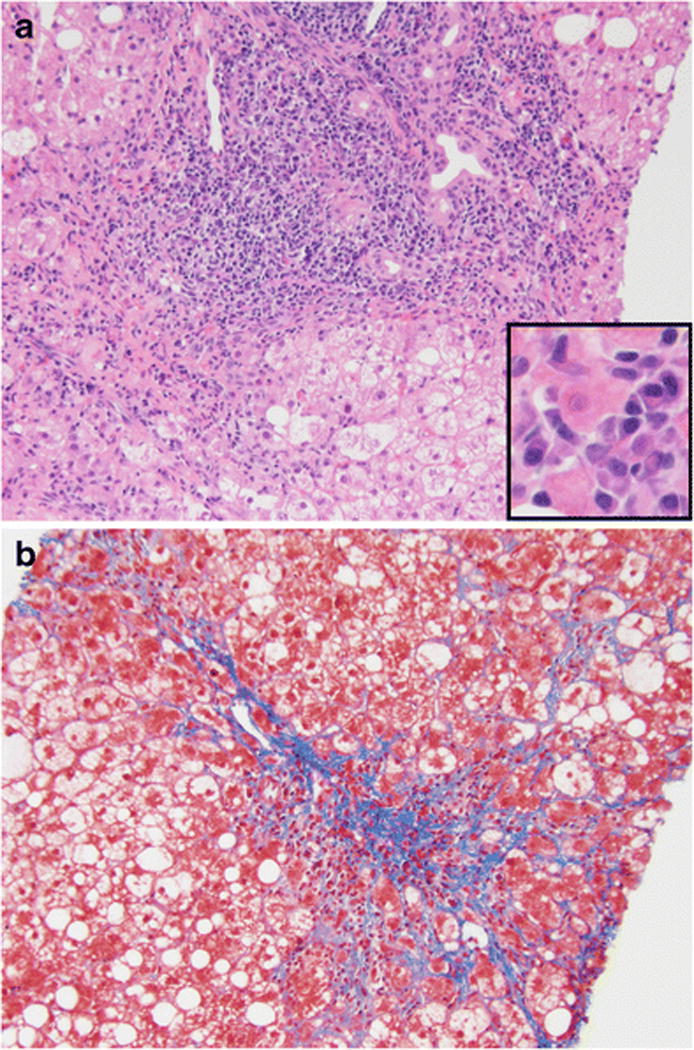
Overlap syndrome in a patient with autoimmune hepatitis, primary biliary cholangitis, and non-alcoholic steatohepatitis
Liver biopsy showing findings of autoimmune hepatitis (portal and lobular inflammation with interface activity and abundant plasma cells [Figure 1A: Small window highlights plasma cells]), primary biliary cholangitis (bile duct injury with lymphocytic infiltrate [Figure 1A]), and steatohepatitis (macrovesicular steatosis, ballooned hepatocytes with Mallory bodies, and pericellular fibrosis [Figure 1A and 1B]) with bridging fibrosis (Figure 1B).
Furthermore, careful exposure and drug histories (including AZA), as well as pinpointing other contributing comorbidities such as the metabolic syndrome should raise suspicions for additive processes (Table 1). Coexisting non-hepatic conditions should also be examined and optimized in order to provide best care, as incomplete responders have more anxiety, depression, and avoidant relationship styles (30). Thus, aggressive identification and treatment of anxiety and depression, as well as the education of medical compliance is a critical therapeutic step in this distinct group.
Monitoring of AZA metabolites (6-thioguanine nucleotide (6-TGN) and 6-methyl mercaptopurine (6-MMP)) metabolites in AZA-treated patients could also identify a proportion of patients that may be rescued from incomplete response with dose adjustment. 6-TGN concentrations more than 220 pmol per 8×108 red blood cells have been associated with biochemical remission (31). Elevated 6-MMP levels (>5700 pmol per 8×108) can also contribute to associated symptoms of nausea, anorexia, and influenza-like symptoms (32), thereby impacting compliance. Allopurinol, through inhibition of xanthine oxidase, represents a therapeutic approach in patients with increased 6-MMP and low 6-TGN, as it produces preferential AZA metabolism by the thiopurine methyltransferase enzymatic pathway towards 6-TGN (33).
Despite elimination of contributing hepatic disease or insults, optimization of first-line therapies, and assurance of medical adherence, abnormal liver tests will be present in 9–34% of treated patients at 2 years (14,27). These patients, along with those intolerant and failing (minimal clinical and laboratory improvement in several weeks without liver failure with standard first-line therapy(1)) standard therapy are candidates for alternative immunosuppressive treatments. The more common strategies including MMF, sirolimus/everolimus, tacrolimus, and cyclosporine have encouraging results in regard to biochemical improvement. However, extrapolation of these agents to practice must be cautiously undertaken as they are founded primarily on small retrospective case series with heterogeneous endpoints.
MMF, a purine antagonist widely used in the setting of liver transplantation, has been utilized in a number of small retrospective studies including patients with AIH with AZA-intolerance, incomplete response, and failure (17,34,35). MMF (goal 1,500 – 3,000 mg in divided doses per day) seems to be effective as a second-line agent for patients with AZA-intolerance. A small retrospective study showed that complete response rate was observed in 8 out of 9 patients who were intolerant to AZA (34). In the same study, patients switched to MMF after treatment failure with AZA were only able to gain biochemical improvement, but not complete response. A similar observation was made by Hennes et al, as 75% of patients with AZA failure did not respond to MMF (17). Only recently has MMF been considered for first-line AIH therapy, and results suggest it may be an effective and well tolerated medication with 88% of patients obtaining biochemical normalization within the first few months (36). However, there is no long-term survival data for MMF, nor are the implications of its role as a potential teratogen during pregnancy commonly considered. Utilization of MMF among women of child bearing age necessitates the documented discussion of increased risk of spontaneous abortion and major birth defects (37) associated with its use in pregnancy. We require at risk patients to use two forms of birth control and periodic urine pregnancy tests. GI symptoms (e.g., nausea, dyspepsia and diarrhea), headache, and bone marrow suppression are among the common side effects seen with MMF use. If supportive measures do not alleviate these symptoms, MMF dose reduction should be considered next.
Mammalian target of rapamycin (mTOR) inhibitors, such as sirolimus and everolimus, work to modulate the expansion and survival of activated lymphocytes. These agents were initially reported in the post-transplant experience with AIH (38), yet this experience led to their introduction in challenging AIH patients. A recent small US report included 5 AIH patients with first-line (3 patients with second-line MMF as well) failure treated with sirolimus (2 mg per day) and titrated trough levels of 10–20 ng/dL. Four (80%) of these patients showed an improvement in liver tests and 2 (40%) patients had normalization (39). Similarly, everolimus showed some efficacy for AIH patients with treatment nonresponse and intolerance. In one study, 43% of patients had normal ALT levels and 57% had ALT levels less than 55 international units after 5 months of therapy (40). While experience with mTOR inhibitors in AIH is currently limited, they may represent a treatment option in AIH patients with recent history of malignancy based on their anti-proliferative effect (41),.
Calcineurin inhibitors, such as cyclosporine and tacrolimus, have been used longest in the treatment of refractory cases, yet these experiences are marked by small treatment numbers and limited follow-up data. The literature contains 10 reports of 133 patients utilizing cyclosporine as initial and second-line agent for incomplete response and failure, and has been commonly effective in over 90% of patients (42). In one study, 5 AIH patients with poor response to AZA and corticosteroids were treated with cyclosporine at 2–3 mg/kg/day which resulted in biochemical remission of 80% of patients in 3 months(43). Tacrolimus has also shown some benefit in this hard to treat AIH group, as studies have supported improvement by any measure in 98% of patients(42). The most recent experience with tacrolimus in this setting included 13 patients with incomplete response or failure at a single large center, where 12 obtained normalization of liver enzymes (mean trough 6.0 ng/mL) (44).
ACUTE SEVERE PRESENTATION
Arguably one of the most challenging groups of AIH is that with acute severe hepatitis, with or without liver failure. There is limited literature and a common association with outcomes of death or transplant in these patients (45,46). The decision to pursue a course of corticosteroids remains complex, particularly as the subsequent determination of clinical response to treatment and the timing of listing for liver transplantation are unclear. Most recently, Heneghan et al published the outcomes of group of 32 acute severe AIH patients from the United Kingdom (no cirrhosis, but INR >=1.5 at presentation) of which, 23 (72%) were treated with steroids (47). Approximately half of treated and all of the untreated patients required liver transplantation, yet there was no difference in sepsis episodes or mortality between the groups. Prognostic classifications are still not available for this high risk group, yet are key as severity of liver failure may play a role in steroid responsiveness (48). In fact, a study of 40 South American patients with a fulminant AIH revealed that corticosteroid failure was much more likely among those with higher MELD scores and encephalopathy grade 3 or higher (49). Utilization of corticosteroids in an acute severe presentation, preferably at high dose intravenously, requires close observation of clinical improvement or deterioration and infection. Early evaluation and listing for liver transplantation should be done for patients presenting with acute liver failure while response to therapy is assessed.
PREGNANCY
The approach to pregnancy in AIH patients requires close attention, as disease development or flare during or after pregnancy can pose a significant risk to both mother and baby. A recent report of 83 pregnancies in 53 women with AIH revealed maternal complications and disease flares in 38% and 33% respectively. AIH flares (worsening liver inflammation) were more likely to occur in patients who were not on therapy or had a flare in the year prior to conception (50). An earlier study of 22 women with a total of 44 pregnancies found that over half had disease flares after delivery and almost a quarter flared during pregnancy (51). A variety of approaches have been utilized in pregnant AIH patients prior to or at conception including discontinuation of all immunosuppressants or modification of long term maintenance medications. Pharmacologic alterations have been focused on minimizing risk to baby, however, the aforementioned data suggests that maternal disease control remains important throughout pregnancy and after delivery. AZA remains a US Food and Drug Association category D medication in pregnancy. Yet, multiple retrospective studies have shown no increase in birth defects, stillbirths, or fetal malformations with use of AZA (51–53). A similar safety profile of AZA in pregnancy has been shown among inflammatory bowel disease patients (54). Mindful consideration of calcineurin inhibitor use in AIH during pregnancy should also be exercised, as post-transplant data has suggested favorable pregnancy outcomes in this group including patients treated with tacrolimus (60%) and cyclosporine A (38%) (55).
Despite the theoretical increase in anti-inflammatory cytokines secondary to rising estrogen levels (56), we routinely counsel pregnant or about to get pregnant patients with AIH about the risk and benefits of maintaining remission with AZA or corticosteroids throughout pregnancy in accordance with above data and current EASL guidelines (1). Optimal disease control in the year leading up to pregnancy and careful monitoring for disease activity after delivery are also suggested. MMF should be withdrawn prior to conception and should not be used during pregnancy as it has been associated with increased teratogenicity(57). We try to avoid MMF use in child bearing patients, yet if necessary, we carefully counsel patients on the risk of this drug in pregnancy, ensure two forms of birth control, and engage in frequent urine pregnancy testing.
ASYMPTOMATIC and ELDERLY
Approximately one third of AIH patients will present without complaints (11,26,58), commonly with findings of slightly abnormal liver tests from routine laboratory work completed for other indications. Despite the range of clinical variability between asymptomatic and symptomatic patients at presentation, similar degrees of lobular hepatitis and bridging fibrosis have been observed at diagnosis. Furthermore, many with a mild asymptomatic presentation will become symptomatic and develop a variety of symptoms such as malaise, nausea, abdominal pain, pruritus, or jaundice. Left untreated, a lower overall survival can be expected for these patients (59). Improvement of hepatic fibrosis, including cirrhosis, in both groups is achievable, as observed in 57% of treated AIH patients with paired liver biopsies (60). Among those diagnosed and treated early, approximately 80% can expect fibrosis resulting from hepatic inflammation to be prevented or delayed (61). In fact, in this study from the Mayo Clinic with 87 patients, fibrosis scores improved in 53% and remained stable in 26%. As expected, improvement of fibrosis scores were related to improvement in histological activity indices during the approximately 4-year follow-up. Therefore, we recommend aggressively treating patients with mild asymptomatic disease in order to minimize symptomatic disease, improve overall-survival, and prevent fibrosis progression.
The decision to treat older patients with asymptomatic disease and mild inflammation is still debatable, as the medication risks may outweigh the theoretical benefit of treatment. One study showed 67% overall 10-year survival of untreated mild asymptomatic patients (62). An uncontrolled study of 31 asymptomatic patients (half did not receive therapy) showed no difference in survival among the non-treated patients and the rest of the cohort (9). Elderly patients commonly constitute a large proportion of the asymptomatic patients at presentation, but have been shown to present with increased frequency of advanced fibrosis (62,63). We offer treatment to those with advanced fibrosis and evidence of significant inflammatory activity on liver biopsy. However, in the elderly with mild activity and early fibrosis, the decision to treat should be carefully considered, and exercising pharmaceutical reluctance, especially among patients with significant co-morbidities, remains reasonable.
CIRRHOSIS
Treatment of AIH patients with advanced fibrosis and cirrhosis remains critical if not contraindicated by associated comorbidities. Cirrhosis at diagnosis has been observed to be a predictor of reduced survival, and is associated with need for liver transplantation (8). Findings of inflammatory activity on biopsy among patients with cirrhosis necessitates treatment, as failure to normalize histologic inflammation is associated with less fibrosis regression and also worsened overall-survival (28). In fact, improvement of fibrosis may explain previous findings of similar survival rates between patients with and without cirrhosis at diagnosis (64). However, we commonly withhold therapy in cirrhotic AIH patients without histologic inflammation on biopsy (burned out cirrhosis), as the impact in overall outcome is likely to be minimal at best and may even increase risk of drug-related side effects (65,66).
RECURRENT AUTOIMMUNE HEPATITIS AFTER OTHOTROPIC LIVER TRANSPLANTATION
Liver transplantation should be considered in patients with AIH when signs of fulminant failure, hepatic decompensation, or liver cancer occur. The recurrence of AIH (rAIH) after transplantation is common and ranges from 8 to 12 percent at one year after transplantation (67). The 5-year risk of recurrence is 36–68%. Despite high rate of recurrence, graft failure requiring re-transplantation occurs in only 13–23% and the 5-year survival of adults with recurrent autoimmune hepatitis is excellent at 89–100% (12). Given these statistics, the possibility of recurrence should not preclude the prospect of liver transplantation for a suitable candidate.
Particular patient populations may be at higher risk for recurrent autoimmune hepatitis after transplantation. Studies show recurrent AIH tends to be more frequent in HLA-DR3–positive transplant recipients (68,69). HLA mismatching may be a significant factor in rAIH (70); however given the scarcity of organ donors, we do not recommend HLA matching for liver transplantation. A recent study demonstrated that severity of original disease correlates with risk of aggressive rAIH (70,71). Patients with higher IgG, AST and ALT are more likely to have recurrent AIH, suggesting incomplete suppression at the time of transplant may contribute to rAIH.
Diagnosis of rAIH can be difficult, as this entity may be found with normal liver tests (72). Some authors suggest protocol liver biopsies may be used to identify clinically silent remission(72); however this is not our common practice. When a biopsy is performed, histology should demonstrate interface hepatitis with plasma cells and lymphocytes but without endotheliaitis or ductilitis. One might expect less recurrent autoimmune disease since transplant patients are maintained on immunosuppressive therapy; however, rAIH can be more aggressive than prior to transplantation. Many patients will require multi-drug immunosuppression long-term including a calcineurion inhibitor, mycophenolate and corticosteroids with or without an mTOR inhibitor (73).
CONCLUSIONS
AZA and corticosteroids remain a well-established and guideline driven approach to AIH treatment (1,12). However, second-line treatment strategies for AIH and distinctive patient groups at risk for disease related complications remain a major challenge. Beyond second-line therapy considerations, AIH patient populations such as those with an acute severe or asymptomatic presentation, pregnancy, advanced age, cirrhosis, and rAIH also represent special cohorts where limited study numbers have been unable to clarify an algorithmic approach. We propose an individualized strategy based on current literature and guidelines to address these difficult to manage cases.
AIH represents a dynamic field of study with a breadth of unmet research needs. Insight into the nuances of AIH management may become transparent with further dissection of key genetic underpinnings and environmental risk factors. Until then, AIH will require continued study, collaboration of investigators, and access to large populations with AIH to accumulate the best clinical evidence. Our center has formed the Autoimmune Hepatitis Research Network (www.facebook.com/groups/autoimmunehep) and Autoimmune Hepatitis Association (www.facebook.com/autoimmunehepatititsassociation and www.aihep.org) in 2014 with the hope that social media use as a research tool will make research opportunities for AIH easily accessible to proactive patients and interested academic centers (74).
Acknowledgments
Grant Support: This publication was made possible with support provided to Craig Lammert from Grant Numbers, KL2TR001106, and UL1TR001108 (A. Shekhar, PI) from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award.
Abbreviations
- AIH
autoimmune hepatitis
- AALSD
American Association for the Study of Liver Diseases
- EASL
European Association for the Study of Liver
- AZA
azathioprine
- AST
aspartate aminotransferase
- ALT
alanine aminotransferase
- IgG
immunoglobulin G
- 6-MP
6-mercaptopurine
- MMF
mycophenolate mofetil
- PSC
primary sclerosing cholangitis
- PBC
primary biliary cholangitis
- 6-TGN
6-thioguanine nucleotide
- 6-MMP
6-methyl mecaptopurine
- mTOR
mammalian target of rapamycin
- INR
international normalized ratio
- MELD
model for end-stage liver disease
- rAIH
recurrent autoimmune hepatitis
- HLA
human leukocyte antigen
Footnotes
Conflicts of Interest
Craig Lammert reports grants from NIH, during the conduct of the study; Veronica M. Loy, Kiyoko Oshima, and Samer Gawrieh declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
With regard to the authors’ research cited in this paper, all procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. In addition, all applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1••.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Autoimmune hepatitis. J Hepatol. 2015 Oct;63(4):971–1004. doi: 10.1016/j.jhep.2015.06.030. Electronic address: easloffice@easloffice.eu. Most up to date, recently released national society AIH consensus statement aimed at providing diagnostic and therapeutic guidance for clinicians. Includes a wide collection of small observational AIH studies, few randomized controlled trails, and a large degree of expert opinion. [DOI] [PubMed] [Google Scholar]
- 2.Heneghan MA, Yeoman AD, Verma S, Smith AD, Longhi MS. Autoimmune hepatitis. Lancet Lond Engl. 2013 Oct 26;382(9902):1433–44. doi: 10.1016/S0140-6736(12)62163-1. [DOI] [PubMed] [Google Scholar]
- 3.Waldenstrom J. Liver, blood proteins and nutritive protein. Dtsch Z Für Verdau- Stoffwechselkrankheiten. 1953;9:113–9. [PubMed] [Google Scholar]
- 4.de Vries AB, Janse M, Blokzijl H, Weersma RK. Distinctive inflammatory bowel disease phenotype in primary sclerosing cholangitis. World J Gastroenterol. 2015 Feb 14;21(6):1956–71. doi: 10.3748/wjg.v21.i6.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carbone M, Mells GF, Pells G, Dawwas MF, Newton JL, Heneghan MA, et al. Sex and age are determinants of the clinical phenotype of primary biliary cirrhosis and response to ursodeoxycholic acid. Gastroenterology. 2013 Mar;144(3):560–9.e7. doi: 10.1053/j.gastro.2012.12.005. quiz e13–4. [DOI] [PubMed] [Google Scholar]
- 6.Verma S, Torbenson M, Thuluvath PJ. The impact of ethnicity on the natural history of autoimmune hepatitis. Hepatol Baltim Md. 2007 Dec;46(6):1828–35. doi: 10.1002/hep.21884. [DOI] [PubMed] [Google Scholar]
- 7•.Ngu JH, Gearry RB, Frampton CM, Stedman CAM. Predictors of poor outcome in patients w ith autoimmune hepatitis: a population-based study. Hepatol Baltim Md. 2013 Jun;57(6):2399–406. doi: 10.1002/hep.26290. A large population based evaluation of 133 AIH patients and predictors of outcome with median follow-up of 9 years. Model indicated that early normalization of liver tests are associated with improved outcomes, yet cirrhosis, was not a poor predictor. [DOI] [PubMed] [Google Scholar]
- 8•.Kirstein MM, Metzler F, Geiger E, Heinrich E, Hallensleben M, Manns MP, et al. Prediction of short- and long-term outcome in patients with autoimmune hepatitis. Hepatol Baltim Md. 2015 Nov;62(5):1524–35. doi: 10.1002/hep.27983. A large European single center outcome experience with 354 AIH patients. Early diagnosis (<18 years) and + anti-SLA/LP antibodies were significant risk factors for worse outcomes. [DOI] [PubMed] [Google Scholar]
- 9.Feld JJ, Dinh H, Arenovich T, Marcus VA, Wanless IR, Heathcote EJ. Autoimmune hepatitis: effect of symptoms and cirrhosis on natural history and outcome. Hepatol Baltim Md. 2005 Jul;42(1):53–62. doi: 10.1002/hep.20732. [DOI] [PubMed] [Google Scholar]
- 10.Cook GC, Mulligan R, Sherlock S. Controlled prospective trial of corticosteroid therapy in active chronic hepatitis. Q J Med. 1971 Apr;40(158):159–85. doi: 10.1093/oxfordjournals.qjmed.a067264. [DOI] [PubMed] [Google Scholar]
- 11••.Grønbæk L, Vilstrup H, Jepsen P. Autoimmune hepatitis in Denmark: incidence, prevalence, prognosis, and causes of death. A nationwide registry-based cohort study. J Hepatol. 2014 Mar;60(3):612–7. doi: 10.1016/j.jhep.2013.10.020. A Danish nationwide assessment of over 1700 AIH patients indicating that AIH incidence doubled during the study period (1994–2012). During the first year of AIH diagnosis, mortality was 6 times higher than that of the general population, and 2 times higher thereafter. [DOI] [PubMed] [Google Scholar]
- 12.Manns MP, Czaja AJ, Gorham JD, Krawitt EL, Mieli-Vergani G, Vergani D, et al. Diagnosis and management of autoimmune hepatitis. Hepatol Baltim Md. 2010 Jun;51(6):2193–213. doi: 10.1002/hep.23584. [DOI] [PubMed] [Google Scholar]
- 13•.Hübener S, Oo YH, Than NN, Hübener P, Weiler-Normann C, Lohse AW, et al. Efficacy of 6-Mercaptopurine as Second-Line Treatment for Patients With Autoimmune Hepatitis and Azathioprine Intolerance. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2015 Oct 19; doi: 10.1016/j.cgh.2015.09.037. Largest assessment of 6-MP in AIH patients that are intolerant to AZA therapy. 75% (15) of patients tolerated 6-MP well even after significant (mostly gastrointestinal) side effects of AZA. [DOI] [PubMed] [Google Scholar]
- 14.Montano-Loza AJ, Carpenter HA, Czaja AJ. Features associated with treatment failure in type 1 autoimmune hepatitis and predictive value of the model of end-stage liver disease. Hepatol Baltim Md. 2007 Oct;46(4):1138–45. doi: 10.1002/hep.21787. [DOI] [PubMed] [Google Scholar]
- 15.Werner M, Wallerstedt S, Lindgren S, Almer S, Björnsson E, Bergquist A, et al. Characteristics and long-term outcome of patients with autoimmune hepatitis related to the initial treatment response. Scand J Gastroenterol. 2010 Apr;45(4):457–67. doi: 10.3109/00365520903555861. [DOI] [PubMed] [Google Scholar]
- 16.Lüth S, Herkel J, Kanzler S, Frenzel C, Galle PR, Dienes HP, et al. Serologic markers compared with liver biopsy for monitoring disease activity in autoimmune hepatitis. J Clin Gastroenterol. 2008 Sep;42(8):926–30. doi: 10.1097/MCG.0b013e318154af74. [DOI] [PubMed] [Google Scholar]
- 17.Hennes EM, Oo YH, Schramm C, Denzer U, Buggisch P, Wiegard C, et al. Mycophenolate mofetil as second line therapy in autoimmune hepatitis? Am J Gastroenterol. 2008 Dec;103(12):3063–70. doi: 10.1111/j.1572-0241.2008.02180.x. [DOI] [PubMed] [Google Scholar]
- 18•.Jothimani D, Cramp ME, Cross TJS. Role of mycophenolate mofetil for the treatment of autoimmune hepatitis-an observational study. J Clin Exp Hepatol. 2014 Sep;4(3):221–5. doi: 10.1016/j.jceh.2014.05.003. MMF is well tolerated in 74% of AIH patients with AZA intolerance. In a small subgroup, it is unlikely to provide liver test normalization in AZA treatment failure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lees CW, Maan AK, Hansoti B, Satsangi J, Arnott IDR. Tolerability and safety of mercaptopurine in azathioprine-intolerant patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2008 Feb 1;27(3):220–7. doi: 10.1111/j.1365-2036.2007.03570.x. [DOI] [PubMed] [Google Scholar]
- 20.Hindorf U, Johansson M, Eriksson A, Kvifors E, Almer SHC. Mercaptopurine treatment should be considered in azathioprine intolerant patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2009 Mar 15;29(6):654–61. doi: 10.1111/j.1365-2036.2008.03925.x. [DOI] [PubMed] [Google Scholar]
- 21.Czaja AJ. Drug choices in autoimmune hepatitis: part A–Steroids. Expert Rev Gastroenterol Hepatol. 2012 Sep;6(5):603–15. doi: 10.1586/egh.12.40. [DOI] [PubMed] [Google Scholar]
- 22.Soloway RD, Summerskill WH, Baggenstoss AH, Geall MG, Gitnićk GL, Elveback IR, et al. Clinical, biochemical, and histological remission of severe chronic active liver disease: a controlled study of treatments and early prognosis. Gastroenterology. 1972 Nov;63(5):820–33. [PubMed] [Google Scholar]
- 23.Manns MP, Woynarowski M, Kreisel W, Lurie Y, Rust C, Zuckerman E, et al. Budesonide induces remission more effectively than prednisone in a controlled trial of patients with autoimmune hepatitis. Gastroenterology. 2010 Oct;139(4):1198–206. doi: 10.1053/j.gastro.2010.06.046. [DOI] [PubMed] [Google Scholar]
- 24.Miyake Y, Iwasaki Y, Terada R, Okamaoto R, Ikeda H, Makino Y, et al. Persistent elevation of serum alanine aminotransferase levels leads to poor survival and hepatocellular carcinoma development in type 1 autoimmune hepatitis. Aliment Pharmacol Ther. 2006 Oct 15;24(8):1197–205. doi: 10.1111/j.1365-2036.2006.03113.x. [DOI] [PubMed] [Google Scholar]
- 25.Verma S, Gunuwan B, Mendler M, Govindrajan S, Redeker A. Factors predicting relapse and poor outcome in type I autoimmune hepatitis: role of cirrhosis development, patterns of transaminases during remission and plasma cell activity in the liver biopsy. Am J Gastroenterol. 2004 Aug;99(8):1510–6. doi: 10.1111/j.1572-0241.2004.30457.x. [DOI] [PubMed] [Google Scholar]
- 26.Muratori L, Muratori P, Lanzoni G, Ferri S, Lenzi M. Application of the 2010 American Association for the study of liver diseases criteria of remission to a cohort of Italian patients with autoimmune hepatitis. Hepatology. 2010 Nov 1;52(5):1857–1857. doi: 10.1002/hep.23924. [DOI] [PubMed] [Google Scholar]
- 27.Kanzler S, Löhr H, Gerken G, Galle PR, Lohse AW. Long-term management and prognosis of autoimmune hepatitis (AIH): A single center experience. Z Für Gastroenterol. 2001 May;39(5):339–48. doi: 10.1055/s-2001-13708. [DOI] [PubMed] [Google Scholar]
- 28•.Dhaliwal HK, Hoeroldt BS, Dube AK, McFarlane E, Underwood JCE, Karajeh MA, et al. Long-Term Prognostic Significance of Persisting Histological Activity Despite Biochemical Remission in Autoimmune Hepatitis. Am J Gastroenterol. 2015 Jul;110(7):993–9. doi: 10.1038/ajg.2015.139. A small paired liver biopsy study revealing that only young age at diagnosis and continued disease activity on biopsy (despite normal liver tests) are associated with reduced transplant-free survival. [DOI] [PubMed] [Google Scholar]
- 29•.van Gerven NMF, Verwer BJ, Witte BI, van Erpecum KJ, van Buuren HR, Maijers I, et al. Epidemiology and clinical characteristics of autoimmune hepatitis in the Netherlands. Scand J Gastroenterol. 2014 Oct;49(10):1245–54. doi: 10.3109/00365521.2014.946083. This large Scandinavian AIH epidemiology study revealed AIH prevalence is 18.3 per 100,000 and concurrent autoimmune diseases were found in a quarter of those with AIH. [DOI] [PubMed] [Google Scholar]
- 30.Sockalingam S, Blank D, Abdelhamid N, Abbey SE, Hirschfield GM. Identifying opportunities to improve management of autoimmune hepatitis: Evaluation of drug adherence and psychosocial factors. J Hepatol. 2012;57(6):1299–304. doi: 10.1016/j.jhep.2012.07.032. [DOI] [PubMed] [Google Scholar]
- 31.Dhaliwal HK, Anderson R, Thornhill EL, Schneider S, McFarlane E, Gleeson D, et al. Clinical significance of azathioprine metabolites for the maintenance of remission in autoimmune hepatitis. Hepatol Baltim Md. 2012 Oct;56(4):1401–8. doi: 10.1002/hep.25760. [DOI] [PubMed] [Google Scholar]
- 32.Ansari A, Elliott T, Baburajan B, Mayhead P, O’Donohue J, Chocair P, et al. Long-term outcome of using allopurinol co-therapy as a strategy for overcoming thiopurine hepatotoxicity in treating inflammatory bowel disease. Aliment Pharmacol Ther. 2008 Sep 15;28(6):734–41. doi: 10.1111/j.1365-2036.2008.03782.x. [DOI] [PubMed] [Google Scholar]
- 33.Al-Shamma S, Eross B, Mclaughlin S. Use of a xanthine oxidase inhibitor in autoimmune hepatitis. Hepatology. 2013 Mar 1;57(3):1281–2. doi: 10.1002/hep.26198. [DOI] [PubMed] [Google Scholar]
- 34.Sharzehi K, Huang MA, Schreibman IR, Brown KA. Mycophenolate mofetil for the treatment of autoimmune hepatitis in patients refractory or intolerant to conventional therapy. Can J Gastroenterol J Can Gastroenterol. 2010 Oct;24(10):588–92. doi: 10.1155/2010/891252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jothimani D, Cramp ME, Cross TJS. Role of mycophenolate mofetil for the treatment of autoimmune hepatitis-an observational study. J Clin Exp Hepatol. 2014 Sep;4(3):221–5. doi: 10.1016/j.jceh.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zachou K, Gatselis N, Papadamou G, Rigopoulou EI, Dalekos GN. Mycophenolate for the treatment of autoimmune hepatitis: prospective assessment of its efficacy and safety for induction and maintenance of remission in a large cohort of treatment-naïve patients. J Hepatol. 2011 Sep;55(3):636–46. doi: 10.1016/j.jhep.2010.12.032. [DOI] [PubMed] [Google Scholar]
- 37.Hoeltzenbein M, Elefant E, Vial T, Finkel-Pekarsky V, Stephens S, Clementi M, et al. Teratogenicity of mycophenolate confirmed in a prospective study of the European Network of Teratology Information Services. Am J Med Genet A. 2012 Mar;158A(3):588–96. doi: 10.1002/ajmg.a.35223. [DOI] [PubMed] [Google Scholar]
- 38.Kerkar N, Dugan C, Rumbo C, Morotti RA, Gondolesi G, Shneider BL, et al. Rapamycin successfully treats post-transplant autoimmune hepatitis. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2005 May;5(5):1085–9. doi: 10.1111/j.1600-6143.2005.00801.x. [DOI] [PubMed] [Google Scholar]
- 39.Chatrath H, Allen L, Boyer TD. Use of sirolimus in the treatment of refractory autoimmune hepatitis. Am J Med. 2014 Nov;127(11):1128–31. doi: 10.1016/j.amjmed.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 40.Ytting H, Larsen FS. Everolimus treatment for patients with autoimmune hepatitis and poor response to standard therapy and drug alternatives in use. Scand J Gastroenterol. 2015 Aug;50(8):1025–31. doi: 10.3109/00365521.2014.998271. [DOI] [PubMed] [Google Scholar]
- 41.Porcelli L, Quatrale AE, Mantuano P, Silvestris N, Rolland JF, Biancolillo L, et al. Synergistic antiproliferative and antiangiogenic effects of EGFR and mTOR inhibitors. Curr Pharm Des. 2013;19(5):918–26. [PubMed] [Google Scholar]
- 42.Czaja AJ. Review article: the management of autoimmune hepatitis beyond consensus guidelines. Aliment Pharmacol Ther. 2013 Aug;38(4):343–64. doi: 10.1111/apt.12381. [DOI] [PubMed] [Google Scholar]
- 43.Fernandes NF, Redeker AG, Vierling JM, Villamil FG, Fong TL. Cyclosporine therapy in patients with steroid resistant autoimmune hepatitis. Am J Gastroenterol. 1999 Jan;94(1):241–8. doi: 10.1111/j.1572-0241.1999.00807.x. [DOI] [PubMed] [Google Scholar]
- 44.Tannous MM, Cheng J, Muniyappa K, Farooq I, Bharara A, Kappus M, et al. Use of tacrolimus in the treatment of autoimmune hepatitis: a single centre experience. Aliment Pharmacol Ther. 2011 Aug;34(3):405–7. doi: 10.1111/j.1365-2036.2011.04749.x. [DOI] [PubMed] [Google Scholar]
- 45.Ichai P, Duclos-Vallée J-C, Guettier C, Hamida SB, Antonini T, Delvart V, et al. Usefulness of corticosteroids for the treatment of severe and fulminant forms of autoimmune hepatitis. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2007 Jul;13(7):996–1003. doi: 10.1002/lt.21036. [DOI] [PubMed] [Google Scholar]
- 46.Miyake Y, Iwasaki Y, Terada R, Onishi T, Okamoto R, Sakai N, et al. Clinical characteristics of fulminant-type autoimmune hepatitis: an analysis of eleven cases. Aliment Pharmacol Ther. 2006 May 1;23(9):1347–53. doi: 10.1111/j.1365-2036.2006.02894.x. [DOI] [PubMed] [Google Scholar]
- 47•.Yeoman AD, Westbrook RH, Zen Y, Bernal W, Al-Chalabi T, Wendon JA, et al. Prognosis of acute severe autoimmune hepatitis (AS-AIH): The role of corticosteroids in modifying outcome. J Hepatol. 2014 Oct 1;61(4):876–82. doi: 10.1016/j.jhep.2014.05.021. Well phenotyped, acute severe hepatitis carries a high risk of progression to transplant or death. Treatment with high-dose corticosteroids did not increase risk for poor outcomes. Approximately half of patients treated with steroids recovered, yet there was no difference in MELD scores for responders versus nonresponders in this group. [DOI] [PubMed] [Google Scholar]
- 48.De Martin E, Coilly A, Ichai P, Samuel D, Duclos-Vallée J-C. The role of corticosteroids in acute-severe autoimmune hepatitis is still highly debatable. J Hepatol. 2015 Oct;63(4):1041–2. doi: 10.1016/j.jhep.2015.04.032. [DOI] [PubMed] [Google Scholar]
- 49••.Mendizabal M, Marciano S, Videla MG, Anders M, Zerega A, Balderramo DC, et al. Fulminant presentation of autoimmune hepatitis: clinical features and early predictors of corticosteroid treatment failure. Eur J Gastroenterol Hepatol. 2015 Jun;27(6):644–8. doi: 10.1097/MEG.0000000000000353. Approximately half of fulminant AIH patients are rescued from transplant with corticosteroids. Advanced hepatic encephalopathy and a higher MELD (>27) score seemed to be associated with corticosteroid failure. [DOI] [PubMed] [Google Scholar]
- 50.Westbrook RH, Yeoman AD, Kriese S, Heneghan MA. Outcomes of pregnancy in women with autoimmune hepatitis. J Autoimmun. 2012 May;38(2–3):J239–44. doi: 10.1016/j.jaut.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 51.Werner M, Björnsson E, Prytz H, Lindgren S, Almer S, Broomé U, et al. Autoimmune hepatitis among fertile women: strategies during pregnancy and breastfeeding? Scand J Gastroenterol. 2007 Aug;42(8):986–91. doi: 10.1080/00365520601155266. [DOI] [PubMed] [Google Scholar]
- 52.Heneghan MA, Norris SM, O’Grady JG, Harrison PM, McFarlane IG. Management and outcome of pregnancy in autoimmune hepatitis. Gut. 2001 Jan;48(1):97–102. doi: 10.1136/gut.48.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schramm C, Herkel J, Beuers U, Kanzler S, Galle PR, Lohse AW. Pregnancy in autoimmune hepatitis: outcome and risk factors. Am J Gastroenterol. 2006 Mar;101(3):556–60. doi: 10.1111/j.1572-0241.2006.00479.x. [DOI] [PubMed] [Google Scholar]
- 54.Francella A, Dyan A, Bodian C, Rubin P, Chapman M, Present DH. The safety of 6-mercaptopurine for childbearing patients with inflammatory bowel disease: a retrospective cohort study. Gastroenterology. 2003 Jan;124(1):9–17. doi: 10.1053/gast.2003.50014. [DOI] [PubMed] [Google Scholar]
- 55.Christopher V, Al-Chalabi T, Richardson PD, Muiesan P, Rela M, Heaton ND, et al. Pregnancy outcome after liver transplantation: a single-center experience of 71 pregnancies in 45 recipients. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2006 Jul;12(7):1138–43. doi: 10.1002/lt.20810. [DOI] [PubMed] [Google Scholar]
- 56.Whitacre CC, Reingold SC, O’Looney PA. A gender gap in autoimmunity. Science. 1999 Feb 26;283(5406):1277–8. doi: 10.1126/science.283.5406.1277. [DOI] [PubMed] [Google Scholar]
- 57.Kim M, Rostas S, Gabardi S. Mycophenolate fetal toxicity and risk evaluation and mitigation strategies. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2013 Jun;13(6):1383–9. doi: 10.1111/ajt.12238. [DOI] [PubMed] [Google Scholar]
- 58.van Gerven NMF, Verwer BJ, Witte BI, van Erpecum KJ, van Buuren HR, Maijers I, et al. Epidemiology and clinical characteristics of autoimmune hepatitis in the Netherlands. Scand J Gastroenterol. 2014 Oct;49(10):1245–54. doi: 10.3109/00365521.2014.946083. [DOI] [PubMed] [Google Scholar]
- 59.Kogan J, Safadi R, Ashur Y, Shouval D, Ilan Y. Prognosis of symptomatic versus asymptomatic autoimmune hepatitis: a study of 68 patients. J Clin Gastroenterol. 2002 Jul;35(1):75–81. doi: 10.1097/00004836-200207000-00016. [DOI] [PubMed] [Google Scholar]
- 60.Schvarcz R, Glaumann H, Weiland O. Survival and histological resolution of fibrosis in patients with autoimmune chronic active hepatitis. J Hepatol. 1993 Apr;18(1):15–23. doi: 10.1016/s0168-8278(05)80005-4. [DOI] [PubMed] [Google Scholar]
- 61.Czaja AJ, Carpenter HA. Decreased fibrosis during corticosteroid therapy of autoimmune hepatitis. J Hepatol. 2004 Apr;40(4):646–52. doi: 10.1016/j.jhep.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 62.Czaja AJ. Features and consequences of untreated type 1 autoimmune hepatitis. Liver Int Off J Int Assoc Study Liver. 2009 Jul;29(6):816–23. doi: 10.1111/j.1478-3231.2008.01904.x. [DOI] [PubMed] [Google Scholar]
- 63.Chen J, Eslick GD, Weltman M. Systematic review with meta-analysis: clinical manifestations and management of autoimmune hepatitis in the elderly. Aliment Pharmacol Ther. 2014 Jan;39(2):117–24. doi: 10.1111/apt.12563. [DOI] [PubMed] [Google Scholar]
- 64.Roberts SK, Therneau TM, Czaja AJ. Prognosis of histological cirrhosis in type 1 autoimmune hepatitis. Gastroenterology. 1996 Mar;110(3):848–57. doi: 10.1053/gast.1996.v110.pm8608895. [DOI] [PubMed] [Google Scholar]
- 65.Feld JJ, Dinh H, Arenovich T, Marcus VA, Wanless IR, Heathcote EJ. Autoimmune hepatitis: effect of symptoms and cirrhosis on natural history and outcome. Hepatol Baltim Md. 2005 Jul;42(1):53–62. doi: 10.1002/hep.20732. [DOI] [PubMed] [Google Scholar]
- 66.Uribe M, Go VL, Kluge D. Prednisone for chronic active hepatitis: pharmacokinetics and serum binding in patients with chronic active hepatitis and steroid major side effects. J Clin Gastroenterol. 1984 Aug;6(4):331–5. [PubMed] [Google Scholar]
- 67.Czaja AJ. Diagnosis, pathogenesis, and treatment of autoimmune hepatitis after liver transplantation. Dig Dis Sci. 2012 Sep;57(9):2248–66. doi: 10.1007/s10620-012-2179-3. [DOI] [PubMed] [Google Scholar]
- 68.Czaja AJ. Autoimmune hepatitis after liver transplantation and other lessons of self-intolerance. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2002 Jun;8(6):505–13. doi: 10.1053/jlts.2002.33485. [DOI] [PubMed] [Google Scholar]
- 69.González-Koch A, Czaja AJ, Carpenter HA, Roberts SK, Charlton MR, Porayko MK, et al. Recurrent autoimmune hepatitis after orthotopic liver transplantation. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2001 Apr;7(4):302–10. doi: 10.1053/jlts.2001.21449. [DOI] [PubMed] [Google Scholar]
- 70.Ayata G, Gordon FD, Lewis WD, Pomfret E, Pomposelli JJ, Jenkins RL, et al. Liver transplantation for autoimmune hepatitis: a long-term pathologic study. Hepatol Baltim Md. 2000 Aug;32(2):185–92. doi: 10.1053/jhep.2000.9077. [DOI] [PubMed] [Google Scholar]
- 71.Montano-Loza AJ, Mason AL, Ma M, Bastiampillai RJ, Bain VG, Tandon P. Risk factors for recurrence of autoimmune hepatitis after liver transplantation. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2009 Oct;15(10):1254–61. doi: 10.1002/lt.21796. [DOI] [PubMed] [Google Scholar]
- 72.Duclos-Vallee J-C, Sebagh M. Recurrence of autoimmune disease, primary sclerosing cholangitis, primary biliary cirrhosis, and autoimmune hepatitis after liver transplantation. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2009 Nov;15(Suppl 2):S25–34. doi: 10.1002/lt.21916. [DOI] [PubMed] [Google Scholar]
- 73.Tencate V, Komorowski R, Cronin D, Hong J, Gawrieh S. A case study: refractory recurrent autoimmune hepatitis following liver transplantation in two male patients. Transplant Proc. 2014 Feb;46(1):298–300. doi: 10.1016/j.transproceed.2013.09.028. [DOI] [PubMed] [Google Scholar]
- 74•.Lammert C, Comerford M, Love J, Bailey JR. Investigation Gone Viral: Application of the Social Mediasphere in Research. Gast. 2015 Oct;149(4):839–43. doi: 10.1053/j.gastro.2015.08.042. The rarity of AIH and challenge of geographic barriers represent major limitations to development of large observational and prospective studies. Social media overcomes these limitations, and provides patient access to support and disease engagement. [DOI] [PMC free article] [PubMed] [Google Scholar]


