Abstract
Infections are a devastating complication of titanium alloy orthopedic implants. Current therapies include antibiotic-impregnated bone cement and antibiotic-containing coatings. Daptomycin (DAP) (1) is a novel peptide antibiotic that penetrates the cell membranes of Gram-positive bacteria. Few DAP-resistant strains have appeared so far. We hypothesized that when DAP covalently bonded via a flexible, hydrophilic spacer it could prevent bacterial colonization of titanium alloy surfaces. We designed and synthesized a series of DAP conjugates for bonding to the surface of Ti6Al4V foils through tetra(ethylene glycol) spacers via thioether linkages. The stability and antimicrobial activity of the attached conjugates were evaluated using Staphylococcus aureus ATCC 25923. Colonization of the Ti6Al4V foils was inhibited by 72% at 8 h and 54% at 24 h. The strategy described in this report provides a new, more facile way to prepare bactericidal Ti6Al4V implants.
1. Introduction
Orthopedic implants, oral implants, heart valves, and stents have extended the life span and improved the quality of life of patients.1 However, implant-associated infections are serious problems in clinical practice.2 Implant-associated infections may be introduced at the time of surgery by contiguous spread from overlying wound infections or hematogenous spread from systemic infections. Bacterial biofilms substantially raise minimum inhibitory concentrations (MICs) of rifampicin, vancomycin, and tigecycline.3 The measures taken for surgery, such as the prophylactic use of antibiotics, ultraclean air in the operating room, and careful patient selection, have decreased the rate of infection.4 The overall incidence of implant infection is in the range of 1–3%.4 Once the implant is determined to be infected, the standard protocol requires complete removal of all foreign material, identification of the infecting organism, prolonged systemic application of antibiotics, and finally reimplantation of the prosthesis.5,6
Antibacterial modifications of the implant surface have been developed as additional preventive measures to avoid the side effect of a prolonged systemic antibiotic application and decrease the incidence of implant-associated infections. Antibiotics, antiseptics, and silver have been used in local delivery systems, such as antimicrobial coatings, cements, and membranes.7,8 The antimicrobials are released from the implant surface to provide a local level exceeding usual systemic antibiotic concentrations by several orders of magnitude. Nevertheless, the antimicrobial coating, cement, and membrane systems can release antimicrobials only for a limited time, and at some point in time, these delivery systems must be removed by surgery.2
The covalent attachments of the antimicrobials to the surfaces of implants have been investigated as a strategy to provide permanent self-protection from infection to the implants. Quaternary ammonium salts, such as poly(4-vinyl-N-alkylpyridinium bromide) and 3-(trimethoxysilyl)-propyldimethyloctadecyl ammonium chloride, were covalently bounded to the biomaterial surface to provide bactericidal activity.9,10 Ampicillin, a broad spectrum suicide inhibitor, has been used to modify the surface of poly(tetrafluoroethylene) to establish surface antibiotic activity.11,12
The antimicrobial peptide, LL-37, has also been attached to the surfaces of the titanium alloy implants to prevent infections.13,14 Such peptides display broad-spectrum activity against bacteria and fungi through peptide–membrane interactions, leading to membrane permeation.
We previously reported the covalent modification of titanium powder and Ti6Al4V alloy pins by vancomycin, a Gram-positive peptide antibiotic that blocks peptidoglycan cross-linking15 via a flexible, hydrophilic spacer.16,17 The surfaces of the Ti6Al4V alloy pins were first uniformly oxidized with a hydrogen peroxide/sulfuric acid mixture, then functionalized with aminopropyltriethyoxysilane. After amide bonding of the two aminoethoxyethoxyacetates to surface aminopropyl groups, vancomycin was coupled to the Ti6Al4V alloy surface via a third amide bond. The covalently attached vancomycin prevented Staphylococcus aureus(18) and Staphylococcus epidermidis(19) colonization and biofilm formation on the Ti6Al4V alloy surfaces. The covalently attached vancomycin synergized with rifampicin in solution and blocked the selection for rifampicin-resistant mutants.20 Nevertheless, resistance to vancomycin is becoming more prevalent.21,22
Treatment with daptomycin (DAP) (1) (Scheme 1), a recently discovered antibacterial cyclic lipopeptide,23 might be more efficacious due to lack of resistant organisms so far. DAP appears to function by penetrating the bacterial cell membrane and causing rapid depolarization, resulting in a loss of membrane potential, leading to the inhibition of protein, DNA and RNA synthesis, thereby killing the bacteria.24 We demonstrated that when DAP covalently attached via a bisphosphonate linker to tetra(ethylene glycol) (TEG) extended from the Ti6Al4V surface it killed 53% of a high-challenge dose of 3 × 105 cfu Staphylococcus aureus.(25) TEG served as a flexible, hydrophilic spacer to increase freedom of motion and elevate DAP off the titanium alloy surface. In this report, we covalently attached DAP via a thioether linker to TEG extended from the Ti6Al4V surface, taking a simpler route than before. The DAP-thioether-modified Ti6Al4V foils were protected against S. aureus colonization by 72% at 8 h.
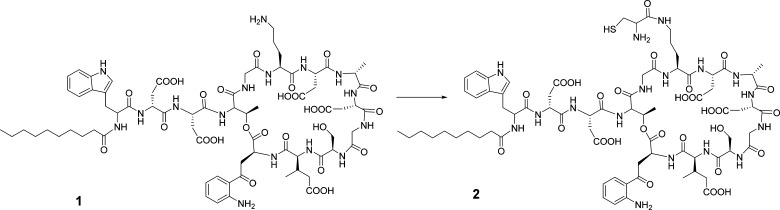
Scheme 1. Conversion of DAP (1) to N-Cys-DAP (2).
Reagents: (i) t-Butyloxycarbonyl (Boc)-Cys(Trt)-N-hydroxysuccinimide (NHS) ester, N,N-dimethylformamide (DMF), room temperature (rt), overnight. (ii) Trifluoroacetyl (TFA)/ethane dithiol (EDT)/water (90:5:5).
2. Materials and Methods
2.1. Materials
All reagents and solvents were purchased from Sigma-Aldrich (St. Louis, MO), except for DAP (1) (Cubist Pharmaceuticals, Lexington, MA), 2-(7-aza-1H-benzotriazole-1-yl)-1,1,3,3-tetramethyl-uronium hexafluorophosphate (HATU) (Novabiochem, San Diego, CA), Mueller–Hinton broth (Becton-Dickinson, Sparks, MD), maleimidyl-dPEG-succinimidyl ester (Quantum Biodesign, Powell, OH), and Ti6Al4V foils (Goodfellow, Oakdale, PA).
2.2. Apparatus
Mass spectra were recorded on an Ettan matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometer (GE Healthcare, Piscataway, NJ) and a SciEx 4800 MALDI-TOF/TOF mass spectrometer (Applied Biosystems, Foster City, CA). Lyophilization was performed using a Sentry freeze-dryer (Virtis, Gardiner, NY) equipped with a Savant Vacuum Pump (Holbrook, NY). Fluorescence readings were recorded on a PTI fluorimeter (Photon Technology International, Birmingham, NJ). Altima C18 5 μm reversed phase columns of 10 × 250 mm2 and 22 × 250 mm2 (Alltech, Deerfield, IL) were used as analytical and semipreparative columns, respectively. Columns were eluted with linear gradients delivered by a Waters 600E dual pump liquid chromatograph monitored by a Waters 486 absorbance detector (Waters, Waltham, MA).
2.3. Methods
2.3.1. N-Cys-DAP (2) (Scheme 1)
To a solution of 231.8 mg (0.5 mmol) of Boc-Cys(Trt)-OH in 5 mL of EtOAc were added 1 equiv of NHS and N,N′-dicyclohexylcarbodiimide (DCC). The mixture was stirred at rt for 2 h, and the reaction was monitored by thin-layer chromatography (TLC) on Merck 60 F254 silica gel plates developed with dichloromethane (DCM)/MeOH as the development solvent. After filtering out the N,N′-dicyclohexylurea precipitate, the filtrate was concentrated using a rotary evaporator. The residual solid was used for the next reaction step without purification.
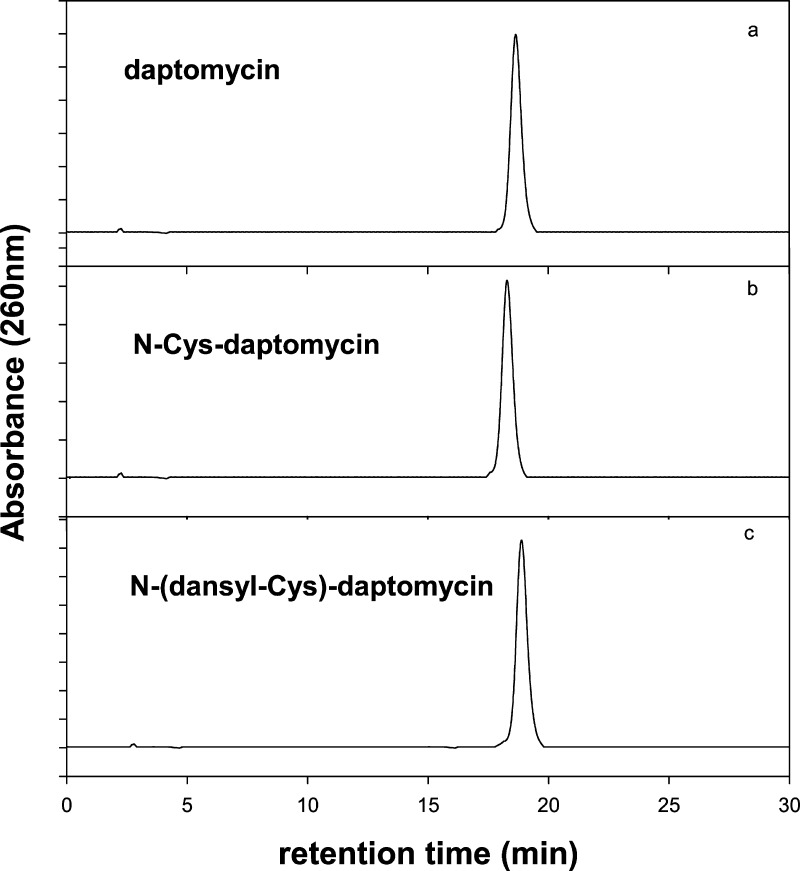
To a solution of 100 mg (62 μmol) of DAP in 1 mL of DMF was added 1.1 equiv of Boc-Cys(Trt)-succinimidyl ester (38 mg, 68 μmol). The mixture was stirred at rt overnight. The product was precipitated with 1 vol of Et2O. The precipitate was deprotected with 4 mL of CF3CO2H/H2O/EDT (90:5:5) at rt for 15 min. The deprotected product was collected by precipitation with Et2O and then purified by high-performance liquid chromatography (HPLC), with a 30 min linear gradient from 30 to 75% CH3CN in aqueous 0.1% CF3CO2H on a 22 × 250 mm2 C18 column, and detected at 254 nm. The eluent corresponding to the product was pooled, concentrated, and lyophilized. The purified sample was characterized by analytical HPLC (Figure 1A) and MALDI-TOF mass spectroscopy (Figure 2).
Figure 1.
Analytical HPLC of DAP derivatives on a 10 × 250 mm2 C18 column eluted with a 30 min gradient from 10 to 45% CH3CN in aqueous 0.1% CF3CO2H, at 1 mL/min, monitored at 260 nm. (a) DAP (1); (b) N-Cys-DAP (2); (c) purified N-(dansyl-Cys)-DAP (4).
Figure 2.
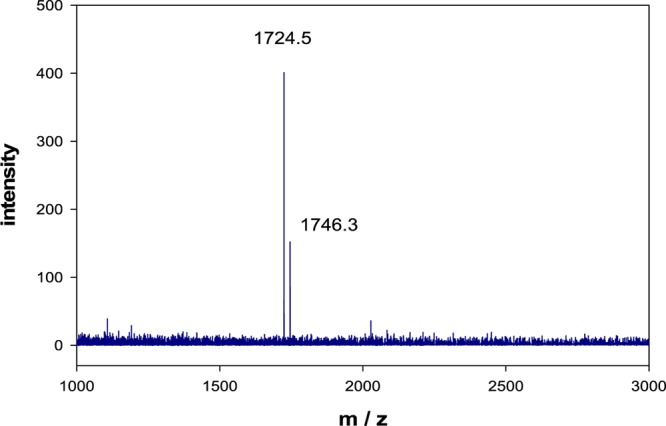
MALDI-TOF mass spectrum of purified N-Cys-DAP (2). Calculated for C75H105N17O28S: 1724.8 Da, observed 1724.5 Da.
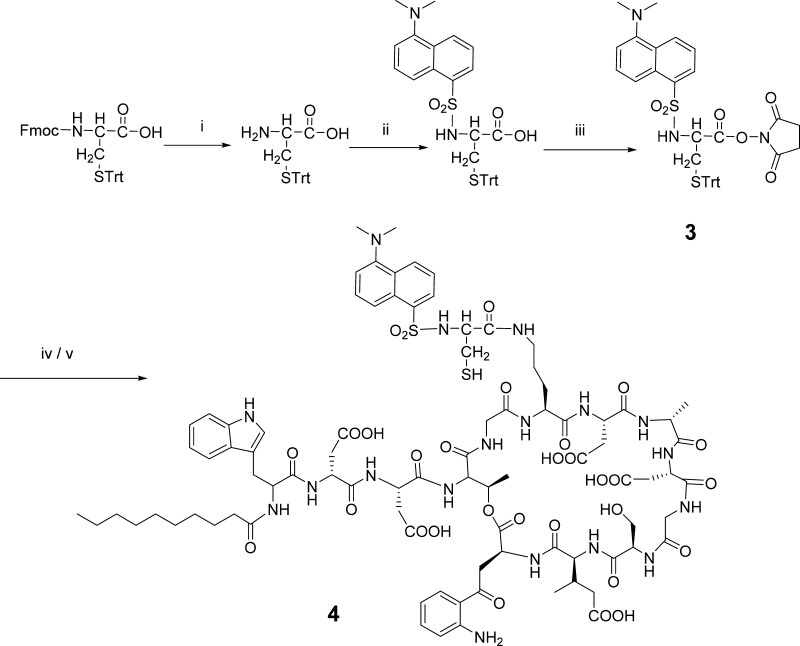
2.3.2. N-Dansyl-Cys(Trt)-OH (3) (Scheme 2)
Scheme 2. Synthesis of N-Dansyl-Cys-DAP (4).
(i) Piperidine (20%) in DMF, rt, 15 min; (ii) dansyl chloride, DCM, DIPEA, rt, 3 h; (iii) DCC, ethyl acetate, rt, 2 h; (iv) DAP, DMF, rt, overnight; and (v) TFA/TIS (95:5), rt, 30 min.
To a solution of fluorenylmethoxycarbonyl (Fmoc)-Cys(Trt)-OH (585.7 mg, 1 mmol) in 3.2 mL of DMF was added 0.8 mL of piperidine to remove the Fmoc group. The mixture was stirred at rt for 15 min, and the solvent was removed. The residue was dissolved in 4 mL of DCM containing 2 mmol of iPr2NEt, followed by the dropwise addition of dansyl chloride (405 mg, 1.5 mmol) dissolved in 4 mL of DCM. The reaction mixture was stirred at rt for 3 h, and the reaction was monitored by TLC on Merck 60 F254 silica gel plates developed with 3% MeOH/97% DCM. Once the starting material disappeared, the solvent was removed by rotary evaporation.
The residue was dissolved in 25 mL of DCM, and the resulting solution was washed three times with water. The organic phase was dried with Na2SO4, then purified by flash chromatography on silica gel 60 (70–230 mesh) eluted with 3% MeOH/97% DCM. The eluent corresponding to the product was pooled, the solvent was removed by rotary evaporation, and the solid product was dried under vacuum overnight. The product was characterized by analytical HPLC (Figure 1B) and MALDI-TOF mass spectroscopy (Figure 2).
2.3.3. N-(Dansyl-Cys)-DAP (4) (Scheme 2)
N-Dansyl-Cys(Trt)-OH (55.2 mg, 92 μmol) and NHS (10.7 mg, 93 μmol) were dissolved in 2 mL of EtOAc, followed by the addition of DCC (19.1 mg, 93 μmol). The reaction was carried out at rt and monitored by TLC until the starting material disappeared. The precipitate was filtered off, and the filtrate was concentrated to dryness. The active ester was used without further purification to couple with DAP.
The active ester was dissolved in 2 mL of DMF followed by the addition of 100 mg (62 μmol) of DAP. The reaction was performed at rt overnight. The product was collected by ether precipitation. The precipitate was dried under vacuum, and the dry sample was incubated with CF3CO2H/H2O/EDT (90:5:5) for 15 min. The product was precipitated by Et2O and purified by reversed phase C18 HPLC. The purified sample was pooled, concentrated, and lyophilized. The dry sample was characterized by analytical HPLC (Figure 1C) and MALDI-TOF mass spectroscopy (Figure 3).
Figure 3.
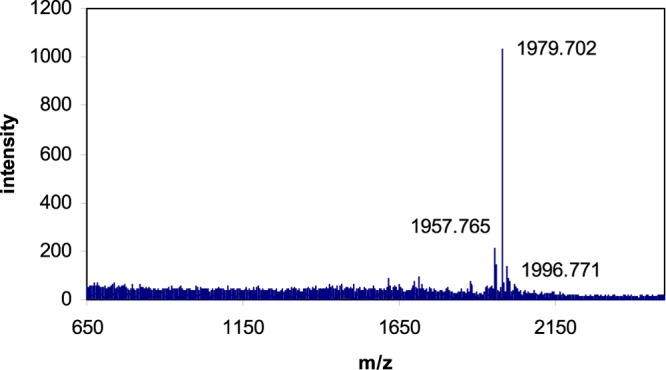
MALDI-TOF mass spectrum of purified N-(dansyl-Cys)-DAP (4). Calculated, 1958.1 Da; observed, 1957.8 Da; and (M + Na), 1979.7 Da.
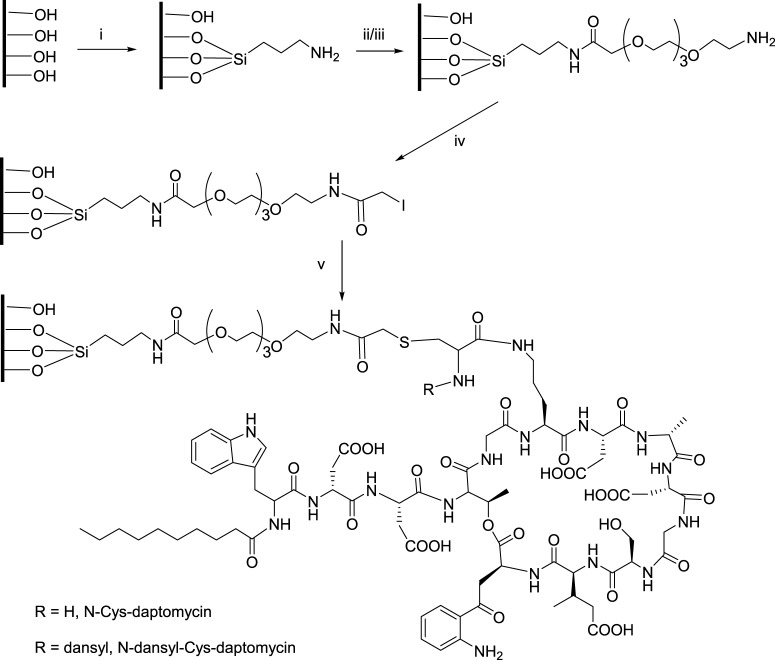
2.3.4. Passivation of Ti6Al4V Foils (Scheme 3)
Scheme 3. Preparation of DAP-Modified Foils.
Reagents: (i) APTS (5%) in toluene, 100°C, 4 h; (ii) Fmoc-TEG-COOH, HATU, DIPEA in DMF, rt, 2 h; (iii) piperidine (20%) in DMF, 20 min; (iv) iodoacetic anhydride, DMF, rt, 2 h; and (v) DAP derivative, DMF, rt, 2 h.
Ti6Al4V foils (10 × 10 × 0.06 cm3) were cut into 1 × 1 cm2 pieces. The foils were cleaned with (a) 1 M NaOH at rt for 5 min, then with (b) MeOH/concentrated HCl (1:1) at rt for 30 min with intermittent sonication. The foils were then washed with (c) distilled water five times and passivated with (d) 30% H2O2/concentrated H2SO4 (1:1) for 4 h with intermittent shaking at 0 °C. Finally, the foils were washed with (e) double-deionized water, DMF, then double-deionized water. The passivated material was dried under vacuum overnight.
2.3.5. Silanization of the Surface of Ti6Al4V (Scheme 3)
The passivated material was incubated with 5% (v/v) aminopropyltriethoxysilane (APTS) in anhydrous toluene (under argon) for 4 h at 100 °C. After removal of the reaction mixture by filtration, Ti6Al4V was washed with toluene, then DMF, followed by water (5 times); the foils were dried overnight under vacuum and then baked at 110 °C for 30 min under the protection of argon.
2.3.6. Quantification of Amino Groups on the Modified Ti6Al4V Surface
The amino groups on the surface of Ti6Al4V foils were estimated by reaction with dansylglycine in the presence of HATU and diisopropylethylamine (DIPEA) in DMF. The attached dansylglycine was cleaved by incubating the modified foils in 0.01 M aqueous sodium hydroxide at rt for 3 h. The fluorescence released into the solution was recorded with a PTI fluorimeter at λex = 337 nm and λem = 520 nm. The amount of dansylglycine in the solution was calculated using a standard curve of dansylglycine at λex = 337 nm and λem = 520 nm (Figure 4).
Figure 4.
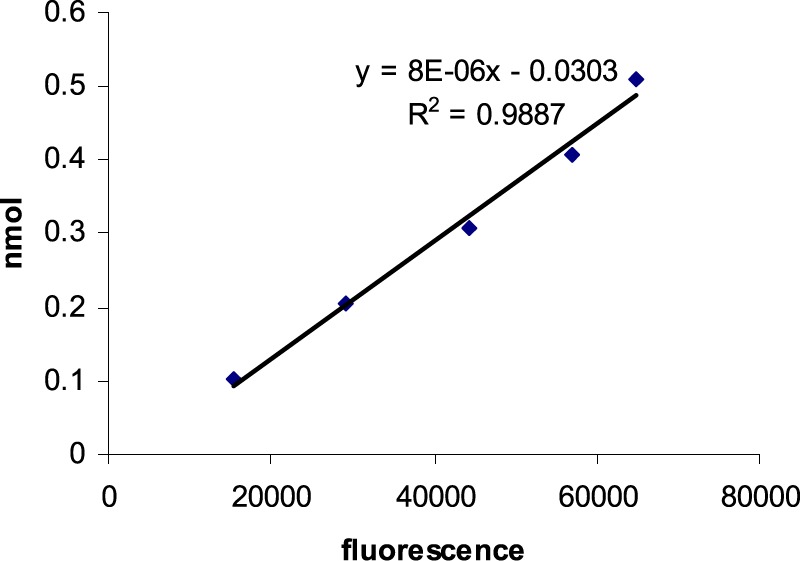
Standard curve of dansyl-Cys-DAP fluorescence in 0.01 M NaOH, with λex = 337 nm, λem = 520 nm.
2.3.7. Functionalization of the Silanized Surface of Ti6Al4V with TEG (Scheme 3)
The amino groups on the surface of Ti6Al4V foils were coupled twice with 0.2 M Fmoc-TEG-COOH in anhydrous DMF with HATU and DIPEA to activate the coupling. The reaction was carried out at rt for 2 h with intermittent shaking. The foils were deprotected in 20% piperidine in DMF for 20 min and washed extensively with DMF, then dried under vacuum and stored in a freezer.
2.3.8. Iodoacetylation (Scheme 3)
Iodoacetic anhydride was prepared from a solution of iodoacetic acid in ethyl acetate in the presence of DCC. After stirring at rt for 2 h, the precipitate was removed by filtration and the solvent was removed using a rotary evaporator. The resulting iodoacetic anhydride was used in the next reaction without purification. Ti6AlV4 foils functionalized with TEG were incubated with 0.1 M iodoacetic anhydride in DMF at rt for 2 h with intermittent shaking. The foils were washed three times with DMF and water, respectively, then dried under vacuum and stored in a freezer.
2.3.9. Attachment of N-Dansyl Cysteine to the Iodoacetylated Titanium Surface (Scheme 3)
Iodoacetylated titanium foils were incubated with N-dansyl cysteine in DMF (2 mg/mL) at rt for 2 h with intermittent shaking. After incubation, the foils were collected by filtration, washed with DMF and water, then stored in the freezer for use in the next step.
2.3.10. Attachment of N-Cys-DAP (2) to the Iodoacetylated Ti6Al4V Surface (Scheme 3)
The foils with iodoacetyl groups were incubated with N-Cys-DAP (2 mg/mL) at rt in DMF for 2 h under the protection of argon. The foils were then washed with DMF and water, dried under vacuum, and stored in the freezer. N-Dansyl-Cys-DAP (4) was coupled to iodoacetylated Ti6Al4V foils using the same protocol used for N-Cys-DAP (2). The loading was investigated using the same protocol described in Quantification of amino groups on the modified Ti6Al4V surface. The standard fluorescence plot of N-dansyl-Cys-DAP (4) is shown in Figure 4.
2.3.11. MIC of DAP Derivatives
The MIC of N-Cys-DAP (2) versus DAP was investigated using the methodology described by CLSI for broth microdilution testing of aerobic Gram-positive organisms,26 using Staphylococcus aureus strain 25923 (ATCC, Germantown, MD). The experiment was carried out in Mueller–Hinton broth supplemented with 50 mg/L calcium chloride, according to the Cubist Pharmaceuticals protocol, at a final S. aureus titer of 5 × 105 cfu/mL, using DAP (1) as the positive control.
2.3.12. Inhibition of Bacterial Colonization on the Modified Ti6Al4V Surface
The modified and nonmodified control Ti6Al4V foils were sterilized in 75% EtOH at rt for 30 min. After drying in a biological safety hood, the foils were incubated in 1 mL of calcium-supplemented (50 mg/L) Mueller–Hinton broth containing 5 × 105S. aureus cfu, a large challenge dose, in a 24-well plate. The foils were incubated at 37 °C for 2, 4, 8, and 24 h. Following incubation, the foils were washed seven times with phosphate-buffered saline (PBS), then stained for 20 min at rt with a Live/Dead BacLight Kit (Invitrogen, Eugene, Oregon) to visualize live bacteria through green fluorescence. The stained foils were washed three times with PBS to remove the nonabsorbed dye, then imaged on a CK40 fluorescence microscope (Olympus, Japan) interfaced with an RT digital color camera (Diagnostic Instruments Ltd, MI). Live green fluorescent bacterial colonies on the surface of Ti6Al4V foils were processed to colorized three-dimensional (3D) images and counted using Image Pro Plus 4.5 (Media Cybernetics, Inc., Bethesda, MD). Three independent batches of foils were incubated and analyzed.
3. Results and Discussion
3.1. Preparation of DAP Derivatives
DAP is a lipopeptide antibiotic with five free carboxylic acid groups and one amino group. Acylation of the amino group did not significantly reduce the bioactivity of DAP.27 Because DAP exerts its bactericidal activity at the bacterial membrane, it is important to maintain the mobility of DAP and its accessibility after attachment to the surface of Ti6Al4V. On the basis of our experience with attaching vancomycin to Ti6Al4V surfaces functionalized with APTS and a TEG spacer,16 we designed a route for coupling N-Cys-DAP to iodoacetylated Ti6Al4V (Scheme 1). Under this protocol, modification began with the amino group of DAP introducing a sulfhydryl group, which enabled specific coupling with the iodoacetylated Ti6Al4V surface in the presence of several other functional groups, such as amines or carboxylates.
Boc-Cys(Trt)-OH was first reacted with NHS to form an active ester, which was coupled to DAP in DMF. The reaction was complete after 4 h at rt; no significant side reaction was found after the overnight reaction. Precipitation with Et2O removed most of the small-molecule impurities. The precipitate was redissolved in CF3CO2H/H2O/EDT (90:5:5) under anhydrous argon to remove the Boc-protecting group and give N-Cys-DAP (2) a free amino and sulfhydryl group (Scheme 2).
3.2. Ti6Al4V Surface Modification and Characterization
Most metallic implant materials are made of titanium and its alloys because of their biocompatibility and osseointegrating ability.28 To covalently attach antibiotics, the Ti6Al4V surfaces were functionalized with APTS to introduce amino groups. A flexible, hydrophilic TEG spacer was coupled to the amino group to extend DAP away from the metal surface. Iodoacetylation then provided the specific functional group necessary to conjugate with N-Cys-DAP. The density of amino groups on the surface was estimated by the attachment of N-dansyl-Cys to the surface, followed by hydrolysis with 0.01 M NaOH.
The released dansyl fluorescence was recorded, and the density of surface amines was calculated from a standard curve (Figure 4). The surface density of amino groups fell in the range of 0.3–0.6 nmol/cm2. Covalently bonded N-dansyl-Cys-DAP (4) was stripped with 0.01 M NaOH, as above. The density of N-dansyl-Cys-DAP (4) on the Ti6Al4V surfaces varied over a range of 0.05–0.2 nmol/cm2 (Table 1). The foil loading results imply that 2 mg/mL dansyl-Cys-DAP is sufficient to obtain maximum bonding.
Table 1. N-Dansyl-Cys-DAP (4) (DCD) Bound to the Surface of Titanium Foils.
| batch number | DCD coupling concentration (mg/mL) | fluorescence (arbitrary units, λex = 337 nm, λem = 520 nm) | DCD released from foil (nmol/cm2) |
|---|---|---|---|
| 1 | 2 | 24407.7 | 0.0736 |
| 4 | 19790.7 | 0.0572 | |
| 8 | 49947.7 | 0.1649 | |
| 2 | 2 | 31285.5 | 0.0982 |
| 4 | 59242.5 | 0.1981 | |
| 8 | 29916.9 | 0.0933 | |
| 3 | 2 | 45939.0 | 0.1505 |
| 4 | 40111.0 | 0.1297 | |
| 8 | 19203.6 | 0.0551 | |
| 4 | 2 | 24770.7 | 0.0749 |
| 4 | 49160.0 | 0.1620 | |
| 8 | 20837.6 | 0.0609 |
These results agree with other reports of Ti6Al4V surface modification.29 X-ray photoelectron spectroscopy is regarded as the standard method to estimate the surface density of functional groups.30 Radiolabeling was also reported to modify the APTS-functionalized Ti6Al4V surface for estimation of the amino groups attached.29 Fluorescence labeling does not need special instruments and is environment-friendly, so it has been used by several research groups to check the density of amine after silanization.17
The titanium–oxygen–silicon bond is stable at rt under physiological conditions. However, the bond is labile to 0.01 M NaOH. The covalently bonded fluorescence could be released under this basic condition. There are several factors influencing the results of amino density estimations. The efficiency of fluorescence coupling, nonspecific binding, and low quantum efficiency of dansyl contributed to experimental variability. The variations suggest that passivation, aminopropylation, or TEG attachment are not yet optimized, perhaps due to the biphasic system of Ti6Al4V foil and organic solvents.
3.3. Inhibitory Activity of DAP Immobilized on the Ti6Al4V Surface
The MICs of DAP and N-Cys-DAP (2) were investigated using a standard protocol26 modified by Cubist Pharmaceuticals by the addition of 50 mg/L calcium chloride, with pure DAP (1) as the positive control, displaying an MIC of 0.2 μg/mL. N-Cys-DAP (2) yielded an MIC of 2.0 μg/mL, illustrating some loss of activity upon modification.
The target of DAP is the cell membrane of bacteria. Although the chemically modified N-Cys-DAP (2) lost a log of activity in solution, the simultaneous binding of thousands of N-Cys-DAPs to the surface of each docking bacterium could enable cooperative inhibition of bound bacterium. Considering the high local concentration of N-Cys-DAP bonded to the surface of modified Ti6Al4V via a long spacer, the DAP derivative might still have sufficient affinity to inhibit the colonization of bacteria on the surface because the attached antibiotics have a high density on the two-dimensional surface of Ti6Al4V, enabling interaction with multiple sites on the surfaces of each bacterial cell.
N-Cys-DAP (2) attached to the Ti6Al4V surface visibly inhibited colonization due to the large challenge dose of 5 × 105S. aureus at 8 and 24 h (Figure 5). Quantitation and integration of green fluorescence (Table 2) revealed 72% inhibition at 8 h and 54% inhibition at 24 h. In clinical practice, S. aureus challenges to implants fall in the range of 100–10 000 cfu.31 The high challenge dose used here was a demanding test of the system.
Figure 5.
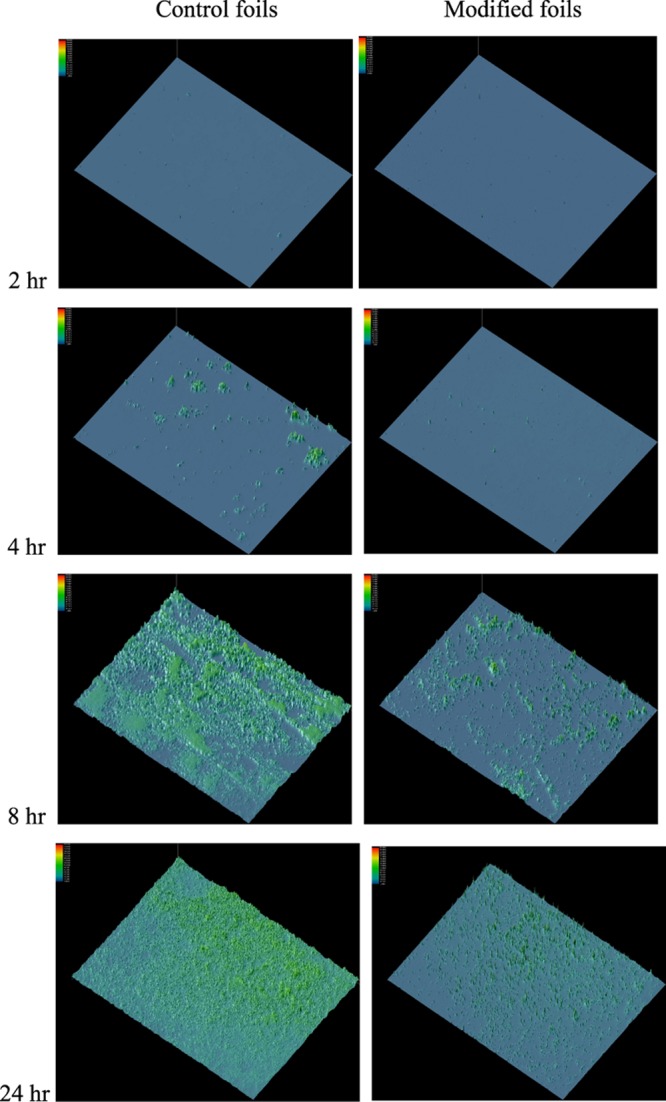
Inhibition of colony formation on the DAP-Cys-TEG-NPrSi-O-Ti6Al4V surfaces. The foils were incubated at 37 °C. Colonization of bacteria on the surface of Ti6Al4V foils was recorded after 2, 4, 8, and 24 h incubation. Following incubation, the foils were washed seven times with PBS, then stained for 20 min at rt with a live/dead BacLight kit to visualize live bacteria by green fluorescence, with nonmodified Ti6Al4V foils as the controls. Three independent sets of foils were analyzed. Representative fluorescence surface plots of colonies, processed to appear as 3D colorized peaks, are shown at 40× magnification.
Table 2. Inhibition of Colonization on DAP–Ti6Al4V Surfacea.
| time | 2 h | 4 h | 8 h | 24 h |
|---|---|---|---|---|
| control Ti6Al4V | 2.14 ± 0.58 | 7.55 ± 2.81 | 61.80 ± 37.64 | 90.34 ± 31.60 |
| DAP-Ti6Al4V | 5.06 ± 1.33 | 5.38 ± 1.09 | 17.49 ± 20.04 | 41.20 ± 22.27 |
Colonies from all three independent batches were stained with a live/dead BacLight kit. Live green fluorescent bacterial colonies on the surface of the Ti6Al4V foils were imaged on a CK40 fluorescence microscope and counted using Image Pro Plus 4.5.
Even in the unlikely event that all DAP attached to the surface were released into the broth, for which no mechanism is apparent, the concentration of N-Cys-DAP in solution would be far below the MIC. Thus, the observed inhibitory effect can be attributed to the surface-immobilized N-Cys-DAP. Future studies with an animal implant infection model will provide insight into the potential effectiveness of DAP-modified titanium alloy implants in clinical practice.
4. Conclusions
We designed and prepared DAP derivatives for modification of the Ti6Al4V foil surfaces to create self-protecting bactericidal implants. Fluorescently labeled DAP was also synthesized and used to quantify the amount of DAP bonded to the Ti6Al4V surface. The MIC data showed that cysteine-modified DAP maintained antibiotic activity, lowered by 1 log. The antibiotic activity of DAP immobilized on the metal was visualized by fluorescence microscopy after vital staining, revealing 72% inhibition at 8 h and 54% inhibition at 24 h. The strategy described in this report provides a new way to prepare bactericidal implants.
Acknowledgments
We thank Dr. Richard Wassell and Zhixian Lu of the Thomas Jefferson University Proteomics Center for assistance in mass spectrometry. This work was supported by a grant from Cubist Pharmaceuticals to E.W. and NIH grant AR051303 to Dr. Irving Shapiro.
Glossary
Abbreviations
- Boc
t-butyloxycarbonyl
- DAP
daptomycin
- DCC
N,N′-dicyclohexylcarbodiimide
- DCM
dichloromethane
- DMF
N,N-dimethylformamide
- DMSO
dimethylsulfoxide
- EDT
ethane dithiol
- Fmoc
fluorenylmethoxycarbonyl
- HATU
2-(7-aza-1H-benzotriazole-1-yl)-1,1,3,3-tetramethyl-uronium hexafluorophosphate
- HPLC
high-performance liquid chromatography
- MIC
minimum inhibitory concentration
- mp
melting point
- NHS
N-hydroxysuccinimide
- rt
room temperature
- PBS
phosphate-buffered saline
- TEG
tetra(ethylene glycol)
The authors declare the following competing financial interest(s): E.W. is a co-founder of SecureImplant LLC, which might ultimately benefit from the results of this investigation but did not support the work.
References
- Moss A. J.; Hamburger S.; Moore R. M. Jr.; Jeng L. L.; Howie L. J. Adv. Data 1991, 1. [PubMed] [Google Scholar]
- Garvin K. L.; Hanssen A. D. J. Bone Jt. Surg. 1995, 77, 1576. 10.2106/00004623-199510000-00015. [DOI] [PubMed] [Google Scholar]
- Rose W. E.; Poppens P. T. J. Antimicrob. Chemother. 2009, 63, 485. 10.1093/jac/dkn513. [DOI] [PubMed] [Google Scholar]
- Mahan J.; Seligson D.; Henry S. L.; Hynes P.; Dobbins J. Orthopedics 1991, 14, 305. 10.3928/0147-7447-19910301-12. [DOI] [PubMed] [Google Scholar]
- Duggan J. M.; Georgiadis G. M.; Kleshinski J. F. Infect. Med. 2001, 18, 534. [Google Scholar]
- Zimmerli W.; Trampuz A.; Ochsner P. E. N. Engl. J. Med. 2004, 351, 1645. 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]
- Huang Y. Y.; Chung T. W. J. Microencapsulation 2001, 18, 457. 10.1080/02652040010019479. [DOI] [PubMed] [Google Scholar]
- Mi F. L.; Shyu S. S.; Lin Y. M.; Wu Y. B.; Peng C. K.; Tsai Y. H. Biomaterials 2003, 24, 5023. 10.1016/S0142-9612(03)00413-7. [DOI] [PubMed] [Google Scholar]
- Isquith A. J.; Abbott E. A.; Walters P. A. Appl. Environ. Microbiol. 1972, 24, 859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiller J. C.; Liao C. J.; Lewis K.; Klibanov A. M. Proc. Natl. Acad. Sci. U.S.A. 2001, 98, 5981. 10.1073/pnas.111143098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aumsuwan N.; Heinhorst S.; Urban M. W. Biomacromolecules 2007, 8, 3525. 10.1021/bm700803e. [DOI] [PubMed] [Google Scholar]
- Aumsuwan N.; Danyus R. C.; Heinhorst S.; Urban M. W. Biomacromolecules 2008, 9, 1712. 10.1021/bm800176t. [DOI] [PubMed] [Google Scholar]
- Boman H. G. J. Intern. Med. 2003, 254, 197. 10.1046/j.1365-2796.2003.01228.x. [DOI] [PubMed] [Google Scholar]
- Gabriel M.; Nazmi K.; Veerman E. C.; NieuwAmerongen A. V.; Zentner A. Bioconjugate Chem. 2006, 17, 548. 10.1021/bc050091v. [DOI] [PubMed] [Google Scholar]
- Kahne D.; Leimkuhler C.; Lu W.; Walsh C. Chem. Rev. 2005, 105, 425. 10.1021/cr030103a. [DOI] [PubMed] [Google Scholar]
- Jose B.; Antoci V. Jr.; Zeiger A. R.; Wickstrom E.; Hickok N. J. Chem. Biol. 2005, 12, 1041. 10.1016/j.chembiol.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Edupuganti O. P.; Antoci V. Jr.; King S. B.; Jose B.; Adams C. S.; Parvizi J.; Shapiro I. M.; Zeiger A. R.; Hickok N. J.; Wickstrom E. Bioorg. Med. Chem. Lett. 2007, 17, 2692. 10.1016/j.bmcl.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Antoci V. Jr.; King S. B.; Jose B.; Parvizi J.; Zeiger A. R.; Wickstrom E.; Freeman T. A.; Composto R. J.; Ducheyne P.; Shapiro I. M.; Hickok N. J.; Adams C. S. J. Orthop. Res. 2007, 25, 858. 10.1002/jor.20348. [DOI] [PubMed] [Google Scholar]
- Antoci V. Jr.; Adams C. S.; Parvizi J.; Davidson H. M.; Composto R. J.; Freeman T. A.; Wickstrom E.; Ducheyne P.; Jungkind D.; Shapiro I. M.; Hickok N. J. Biomaterials 2008, 29, 4684. 10.1016/j.biomaterials.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottman M.; Goldberg J.; Hacking S. A. PLoS One 2012, 7, e52883 10.1371/journal.pone.0052883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel L. M.; Clewell D. B.; Gill S. R.; Clark N. C.; McDougal L. K.; Flannagan S. E.; Kolonay J. F.; Shetty J.; Killgore G. E.; Tenover F. C. Science 2003, 302, 1569. 10.1126/science.1090956. [DOI] [PubMed] [Google Scholar]
- Brandl K.; Plitas G.; Mihu C. N.; Ubeda C.; Jia T.; Fleisher M.; Schnabl B.; DeMatteo R. P.; Pamer E. G. Nature 2008, 455, 804. 10.1038/nature07250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E. W. Lancet Infect. Dis. 2005, 5, 209. 10.1016/S1473-3099(05)70051-7. [DOI] [PubMed] [Google Scholar]
- Steenbergen J. N.; Alder J.; Thorne G. M.; Tally F. P. J. Antimicrob. Chemother. 2005, 55, 283. 10.1093/jac/dkh546. [DOI] [PubMed] [Google Scholar]
- Chen C.-P.; Wickstrom E. Bioconjugate Chem. 2010, 21, 1978. 10.1021/bc100136e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen J. H.; Cleeland W. A.; Craig G.; Doern M.; Ferraro J.; Finegold C. M.; Hansen S. L.; Jenkins S. G.; Novick W. J.; Pfaller M. A.. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: NCCLS Document M7-A3; National Committee for Clinical Laboratory Standards, 1993; Vol. 13, p 1. [Google Scholar]
- Hill J.; Siedlecki J.; Parr I.; Morytko M.; Yu X.; Zhang Y.; Silverman J.; Controneo N.; Laganas V.; Li T.; et al. Bioorg. Med. Chem. Lett. 2003, 13, 4187. 10.1016/j.bmcl.2003.07.019. [DOI] [PubMed] [Google Scholar]
- Liu X.; Chu P. K.; Ding C. Mater. Sci. Eng., R 2004, 47, 49. 10.1016/j.mser.2004.11.001. [DOI] [Google Scholar]
- Xiao S. J.; Textor M.; Spencer N. D.; Wieland M.; Keller B.; Sigrist H. J. Mater. Sci.: Mater. Med. 1997, 8, 867. 10.1023/A:1018501804943. [DOI] [PubMed] [Google Scholar]
- Watts J. F.; Wolstenholme J.. An Introduction to Surface Analysis by XPS and AES; John Wiley & Sons: Chichester, England, 2003. [Google Scholar]
- Lucke M.; Schmidmaier G.; Sadoni S.; Wildemann B.; Schiller R.; Stemberger A.; Haas N. P.; Raschke M. J. Biomed. Mater. Res., Part B 2003, 67, 593. 10.1002/jbm.b.10051. [DOI] [PubMed] [Google Scholar]