Abstract
Background
Immune dysfunction and a higher risk of uterine infections are characteristics of the transition into lactation in dairy cows. The supply of complexed trace minerals, which are more bioavailable, could help overcome the greater needs of these nutrients in tissues around parturition and early lactation.
Results
Twenty Holstein cows received an oral bolus with a mix of inorganic trace minerals (INO) or complexed trace minerals (AAC) to achieve 75, 65, 11, and 1 ppm supplemental Zn, Mn, Cu, and Co, respectively, in the total diet dry matter from -30 d through +30 d relative to parturition. Blood for polymorphonuclear leukocyte (PMNL) isolation was collected at -30, -15, +10, and + 30 d relative to parturition, whereas endometrium biopsies were performed at +14 and +30 d. Feeding AAC led to greater PMNL expression of genes related with inflammation response (DDX58), oxidative stress response (MPO), eicosanoid metabolism (PLA2G4A and ALOX5AP), transcription regulation (PPARG), and cellular adhesion (TLN1). The upregulation by AAC in endometrium of genes related with inflammation response (TLR2, TLR4, NFKB1, TNF, IL6, IL1B, IL10, IL8), prostaglandin synthesis (PTGS2, PTGES), and antioxidant responses (NFE2L2, SOD1) indicated a faster remodeling of uterine tissue and potentially greater capacity to control a local bacterial invasion.
Conclusions
Data indicate that trace mineral supplementation from amino acid complexes improves PMNL activity and allows the prompt recovery of uterine tissue during early lactation. As such, the benefits of complexed trace minerals extend beyond an improvement of liver function and productive performance.
Electronic supplementary material
The online version of this article (doi:10.1186/s40104-017-0163-7) contains supplementary material, which is available to authorized users.
Keywords: Inflammation, Oxidative stress, Trace minerals, Transition period
Background
The transition to lactation is a challenging period for dairy cows in large part because the immune system, e.g., neutrophil migration and phagocytosis, is generally dysfunctional [1–4]. Besides the hormonal and metabolic changes that contribute to a dysfunctional immune system, during parturition the physical barriers in the cervix, vagina and vulva also are compromised providing the opportunity for bacteria from the environment as well as the animal’s skin and feces to ascend the genital tract, hence, predisposing the cow to uterine diseases [5]. In the first 2 weeks after calving, 80–100% of cows present uterine colonization by bacteria, and an optimal response by the immune system is essential to rapidly eliminate the pathogens [5].
Neutrophils account for ca. 25% of leukocytes in bovine peripheral blood of healthy animals and they are the first line of innate immune defense against invading pathogens [2]. During uterine infection, toll-like receptors on endometrial cells recognize pathogen-associated molecules, leading to secretion of cytokines, antimicrobial peptides, and chemokines [6]. Chemokines recruit polymorphonuclear leukocytes (PMNL) into the site of infection within minutes and promote direct action against the microbes and attract lymphocytes; however, persistent infiltration is detrimental because the site of infection is continually exposed to pro-inflammatory cytokines and reactive oxygen metabolites (ROM) leading to chronic inflammation and oxidative stress and consequently subclinical endometritis and infertility [6].
Trace minerals are key components of antioxidant systems, metabolic reactions, protein synthesis pathways, and membrane integrity (physical barrier to pathogens) [4]. In postpartum dairy cows, supplementation of trace minerals (e.g., Zn, Se and Cu) benefits the immune system, and PMNL adhesion and superoxide production [7, 8]. While the demand of trace minerals increases around parturition, the blood and liver concentrations of trace minerals decreases [7, 9]. Thus, we hypothesized that supplementation of trace minerals through more bioavailable forms, e.g., amino acid complexes, would benefit recovery of the endometrium and the innate immune response at least in part by altering the expression of genes associated with PMNL activity and inflammation. Therefore, the objective of the present study was to evaluate the effects of organic trace mineral supplementation on expression of key genes associated with inflammation, oxidative stress, and eicosanoids in PMNL and endometrium tissue. Production responses and biomarkers of energy balance have been reported elsewhere [10].
Methods
All the procedures for this study were conducted in accordance with the protocol approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Illinois (Protocol #12097).
Animals, experimental design, and dietary treatments
Details of the experiment design have been published previously [10]. Briefly, 44 multiparous Holstein cows were blocked (6 cows per block) according to parity, previous lactation milk yield, and expected day of parturition. All cows received a common diet from -110 to -30 d relative to parturition and were supplemented at 100% of the National Research Council [11] requirements with Zn, Mn, Cu, and Co in the form of an inorganic trace mineral mix (INO). From -30 d relative to expected day of parturition, cows received a common prepartal diet (close-up diet), and from calving to 30 d in milk (DIM) a common postpartal diet (fresh diet). Both close-up and fresh diet were partially supplemented with an INO mix of Zn, Mn, and Cu to supply 35, 45, and 6 ppm, respectively, of the total dietary minerals. The diets and chemical composition are presented in Table 1. At -30 d relative to parturition, cows were randomly assigned to an oral administration of a bolus once daily at the time of feeding the TMR. This contained a mix of either inorganic (INO) or complexed (AAC) Zn, Mn, Cu, and Co to achieve 75, 65, 11, and 1 ppm supplemental, respectively, in the total diet dry matter intake (DMI). The complexed trace minerals were provided as Availa®Zn (Zn AA complex), Availa®Mn (Mn AA complex), Availa®Cu (Cu AA complex), and CoPro® (Co glucoheptonate) (Zinpro Corp, Eden Prairie, MN) and the inorganic trace minerals in sulfate form. IACUC approved uterine biopsies in a maximum of 12 cows per group, which was deemed appropriate to detect statistical significance based on previous research [12–14]. However, only 20 (AAC = 9; INO = 11) out of 44 cows used for this study had a complete set of uterine endometrial biopsies and PMNL isolations. Per IACUC guidelines, cows with a clinical disorder could not continue on experiment; thus, a total of 7 cows had to be removed from the experiment due to clinical ketosis, clinical mastitis, retained placenta, displaced abomasum, or leg fracture [10]. All cows used for PMNL and endometrium gene expression were clinically-healthy.
Table 1.
Ingredient and analyzed chemical composition of diets fed during close-up (-30 d to calving) and early lactation (1 to 30 d in milk)
| Componenta | Far-off | Close-up | Early lactation |
|---|---|---|---|
| Ingredient, % of DM | |||
| Alfalfa silage | 12.2 | 7.6 | 4.9 |
| Alfalfa hay | - | 3.5 | 3.9 |
| Corn silage | 33.6 | 38.9 | 33.1 |
| Wheat straw | 34.8 | 8.4 | 2.6 |
| Cottonseed | - | - | 3.9 |
| Wet brewers grains | - | 6.1 | 9.4 |
| Ground shelled corn | 4.9 | 18.8 | 22.6 |
| Soy hulls | 2.0 | 4.1 | 3.9 |
| Soybean meal, 48% CP | 8.9 | 3.0 | 5.6 |
| Expeller soybean mealb | - | 0.7 | 0.2 |
| SoyChlorc | 0.2 | 2.3 | - |
| Blood meal 85% CP | 1.0 | 0.6 | 0.3 |
| Molasses | - | 0.4 | - |
| Urea | 0.3 | - | 0.7 |
| Rumen-inert fatd | - | - | 2.0 |
| Limestone | 0.8 | 2.2 | 1.6 |
| Salt (plain) | 0.3 | - | 0.3 |
| Ammonium chloride | - | 1.14 | - |
| Dicalcium phosphate | 0.1 | 0.3 | 0.4 |
| Magnesium oxide | - | 0.1 | 0.1 |
| Magnesium sulfate | 0.2 | 1.4 | 0.3 |
| Sodium bicarbonate | - | - | 0.7 |
| Calcium sulfate | - | - | 0.1 |
| Mineral-vitamin mixe | 0.2 | 0.2 | 0.2 |
| Vitamin Af | 0.02 | 0.03 | 0.04 |
| Vitamin Dg | 0.01 | 0.02 | 0.02 |
| Vitamin Eh | 0.36 | 0.36 | 0.20 |
| Chemical analysis | |||
| NEL, Mcal/kg DM | 1.25 | 1.59 | 1.67 |
| CP, % DM | 14.4 | 14.3 | 18.7 |
| NDF, % DM | 53.0 | 39.1 | 35.9 |
| ADF, % DM | 34.5 | 23.9 | 22.2 |
| Zn, mg/kg of DM | 103 | 83 | 69 |
| Mn, mg/kg of DM | 84 | 76 | 70 |
| Cu, mg/kg of DM | 15.5 | 14.4 | 12.3 |
| CO, mg/kg of DM | 0.83 | 0.72 | 0.19 |
aBasal close up and lactation diets were considered as basal diet plus
inorganic trace minerals, or basal diet plus organic trace minerals
bSoyPLUS (West Central Soy, Ralston, IA)
cSoyChlor (West Central Soy)
dEnergy Booster 100 (MSC, Carpentersville, IL)
eContained a minimum of 4.3% Mg, 8% S, 6.1% K, 2.0% Fe, 3.0% Zn,
3.0% Mn, 5,000 mg/kg of Cu, 250 mg/kg of I, 40 mg/kg of Co, 150
mg/kg of Se, 2,200 kIU/kg of vitamin A, 660 kIU/kg of vitamin D3,
and 7,700 IU/kg of vitamin E
fContained 30,000 kIU/kg
gContained 5,009 kIU/kg
hContained 44,000 IU/kg
Sample collection
Blood samples (120 mL) were collected from the tail vein using 20-gauge BD Vacutainer needles (Becton Dickinson, Franklin Lakes, NJ) and vacutainers (8 mL, Becton Dickinson, Franklin Lakes, NJ) containing solution A of trisodium citrate, citric acid and dextrose (ACD) at -30, -15, +10 and +30 d relative to parturition. After blood collection, the tubes were mixed well by inversion and placed on ice until PMNL isolation (~30 min).
Endometrial biopsies were collected by a single individual at +14 and +30 d relative to calving following similar procedures described previously [15]. Briefly, an epidural was performed (4 mL of 2% lidocaine) prior to introducing a Hauptner biopsy instrument protected with a sanitary chemise into the vagina. Manipulation per rectum allowed the biopsy tool to pass through the cervix, after which the biopsy instrument alone was introduced into the uterus subsequent to rupturing the sanitary chemise at the external cervical orifice. The tool was guided into the uterine horn approximately 5 cm past the uterine bifurcation. The tip of the biopsy instrument inside the uterus was carefully identified using the non-operating hand per rectum. This approach should have allowed the reproducible procurement of tissue. With the help of the hand in the rectum, the medial uterine wall was gently pressed into the open instrument jaws prior to closing the jaws and withdrawing the instrument. No attempt was made to determine the relative contribution of caruncular and non-caruncular tissue in the biopsies, even though there is some evidence for differences in transcriptome profiles [16]. The tissue clipped off was immediately placed in liquid nitrogen and frozen at -80 °C until RNA extraction.
Polymorphonuclear leukocyte (PMNL) isolation and viability analysis
Complete details of PMNL isolation and viability analysis are included in the Additional file 1. Briefly, PMNL were isolated from whole blood collected in ACD-containing vacutainers. An aliquot (20 μL) obtained during the isolation process was used for PMNL quantification and viability using a granulocyte primary antibody (CH138A, Veterinary Microbiology and Pathology, Washington State University, Pullman, WA) followed by a second antibody (Goat Anti-Mouse IgM, Human ads-PE, Southern Biotech, Birmingham, AL). Cells were fixed with 150 μL of 4% paraformaldehyde (Sigma-Aldrich, St. Louis, MO) and preserved at 4 °C until flow cytometry reading (LSR II, Becton Dickinson, San Jose, CA). All samples harvested and used for analysis contained more than 80% PMNL and had at least 90% viability.
RNA extraction, primer design and evaluation, and quantitative PCR
Methods for RNA extraction from PMNL and endometrium, primer design and evaluation, cDNA synthesis, quantitative reverse transcription PCR and gene function are presented in the Additional files 2, 3 and 4. Briefly, RNA samples were extracted using Qiazol reagent in combination with the miRNeasy® Mini Kit (Cat. #217004, Qiagen). Thirty-two target genes involved in inflammation response, oxidative stress, eicosanoid metabolism, cellular receptors, transcription regulation and glucose metabolism were evaluated in the PMNL, while 30 target genes related to inflammation, oxidative stress, eicosanoid metabolism, transcription regulation and antimicrobial peptides were assessed in the endometrium. Primers were designed via Primer Express 3.0.1 software (Applied Biosystems). Quantitative PCR (qPCR) was performed in an ABI Prism 7900 HT SDS instrument (Applied Biosystems). Details of primer sequences and amplicon size, primer product sequencing information, and qPCR performance are presented in the Additional file 5, 6, 7 and 8. For PMNL, the internal controls were GOLGA5, SMUG1, and OSBPL2 [17, 18], while for endometrium were GAPDH, RPS9, and UXT. The geometric mean of the internal control genes was used to normalize the expression data.
Statistical analysis
Data were analyzed using the MIXED procedure of SAS 9.3 (SAS Institute Inc., Cary, NC) according to the following model:
Where Y ijkl represent the dependent variable; μ is the overall mean; D i is the fixed effect of treatment (i = 1, 2); b j is the random effect of block (j = 1, …9); c k is the random effect of cow within treatment and block (l = 1…, nij); T l is the fixed effect of time (day or week) of the experiment (m = 1,… n); DT il is the fixed effect of treatment by time interaction; and e ijkl is the residual error. Endometrium gene expression results were log2-scale transformed in order to comply with normal distribution of residuals. For PMNL, the gene expression data at -15, +10, and +30 d relative to parturition was expressed as fold-change relative to -30 d. Statistical differences were declared significant at P ≤ 0.05 and tendencies at P ≤ 0.10.
Results
PMNL
Inflammation response
The cell surface receptors TLR2 (P = 0.85) and TLR4 (P = 0.48), which are involved in the inflammation-response were not affected by treatments (Table 1). The transcription factors STAT3 (P = 0.62), TNF (P = 0.14) and NFKB1 (P = 0.75) also were not affected by treatments. Among the proteins that recognize foreign DNA, DDX58 had greater expression (P = 0.05; Table 2 and Fig. 1) in the AAC compared with INO cows, while there was a tendency (P = 0.09) for the opposite effect for ZBP1; however, IPS1 (P = 0.23) was not affected by treatments (Table 2). There was an overall decrease in expression of NFKB1 (P = 0.03) from -15 to +10 d regardless of treatment (Fig. 1).
Table 2.
Effects of supplementing cows with inorganic (INO, n = 11) or complexed (AAC, n = 9) trace minerals during the peripartal period on mRNA expression (fold-change relative to -30 d prepartum) of genes related with inflammation response, oxidative stress, eicosanoids, transcription factors, receptors and glucose metabolism in polymorphonuclear leukocytes (PMNL)
| Gene | Treatments | SEMa | P value1 | |||
|---|---|---|---|---|---|---|
| INO | AAC | Treatment | Time | T × Tb | ||
| Inflammation | ||||||
| DDX58 | 1.02 | 1.59 | 0.21 | 0.05 | 0.01 | 0.40 |
| IPS1 | 0.80 | 1.00 | 0.13 | 0.23 | 0.41 | 0.22 |
| NFKB1 | 0.89 | 0.91 | 0.06 | 0.75 | 0.03 | 0.74 |
| STAT3 | 1.17 | 1.09 | 0.12 | 0.62 | 0.62 | 0.84 |
| TLR2 | 1.27 | 1.21 | 0.25 | 0.85 | 0.84 | 0.41 |
| TLR4 | 1.14 | 1.43 | 0.30 | 0.48 | 0.11 | 0.49 |
| TNF | 1.33 | 0.82 | 0.25 | 0.14 | 0.99 | 0.68 |
| ZBP1 | 0.97 | 0.66 | 0.13 | 0.09 | 0.15 | 0.62 |
| Oxidative stress | ||||||
| MPO | 0.66 | 0.83 | 0.18 | 0.48 | <0.01 | 0.03 |
| NFE2L2 | 1.32 | 1.57 | 0.30 | 0.54 | 0.15 | 0.70 |
| NOX1 | 0.97 | 0.99 | 0.26 | 0.94 | 0.61 | 0.12 |
| S100A8 | 2.05 | 1.90 | 0.40 | 0.78 | 0.08 | 0.98 |
| SOD1 | 0.73 | 0.91 | 0.09 | 0.14 | 0.02 | 0.94 |
| SOD2 | 1.38 | 1.72 | 0.22 | 0.27 | 0.70 | 0.99 |
| SOD3 | 0.66 | 0.06 | 0.31 | 0.15 | 0.32 | 0.70 |
| Eicosanoids | ||||||
| ALOX5AP | 0.98 | 1.28 | 0.12 | 0.09 | 0.10 | 0.35 |
| LTA4H | 0.71 | 0.67 | 0.10 | 0.76 | <0.01 | 0.18 |
| PLA2G4A | 0.84 | 1.23 | 0.15 | 0.06 | 0.22 | 0.23 |
| PTGS2 | 0.93 | 1.30 | 0.46 | 0.53 | 0.04 | 0.93 |
| Transcription factors | ||||||
| PPARA | 1.03 | 0.63 | 0.14 | 0.04 | 0.01 | 0.21 |
| PPARD | 1.14 | 1.23 | 0.16 | 0.71 | 0.30 | 0.74 |
| PPARG | 0.84 | 1.68 | 0.33 | 0.09 | 0.80 | 0.79 |
| RXRA | 1.21 | 1.03 | 0.11 | 0.22 | 0.05 | 0.78 |
| Receptors | ||||||
| ADORA1 | 1.08 | 1.55 | 0.14 | 0.01 | 0.08 | 0.15 |
| ENTPD1 | 1.14 | 1.64 | 0.23 | 0.12 | 0.34 | 0.80 |
| IL10 | 1.37 | 1.36 | 0.20 | 0.95 | 0.16 | 0.85 |
| IL1B | 1.22 | 1.76 | 0.45 | 0.38 | 0.51 | 0.77 |
| ITGAM | 0.75 | 0.78 | 0.07 | 0.74 | 0.10 | 0.32 |
| ITGB2 | 0.99 | 0.86 | 0.08 | 0.22 | 0.34 | 0.29 |
| P2RY11 | 1.02 | 0.89 | 0.18 | 0.60 | 0.14 | 0.44 |
| PANX1 | 0.86 | 0.95 | 0.13 | 0.61 | 0.02 | 0.15 |
| SELL | 1.41 | 1.66 | 0.41 | 0.65 | 0.27 | 0.80 |
| TLN1 | 0.93 | 1.04 | 0.03 | 0.01 | 0.60 | 0.74 |
| VCL | 0.74 | 0.83 | 0.06 | 0.25 | <0.01 | 0.76 |
| Glucose metabolism | ||||||
| LDHA | 1.37 | 0.84 | 0.20 | 0.06 | 0.04 | 0.95 |
| SLC2A1 | 0.83 | 0.56 | 0.06 | <0.01 | <0.01 | <0.01 |
1 P values represents the probability of statistical significance for the fixed effects (treatment, time, treatment × time). Statistical differences were declared significant at P ≤ 0.05 and tendencies at P ≤ 0.10
aLargest standard error of the mean is shown
bInteraction of treatment × time
Fig. 1.
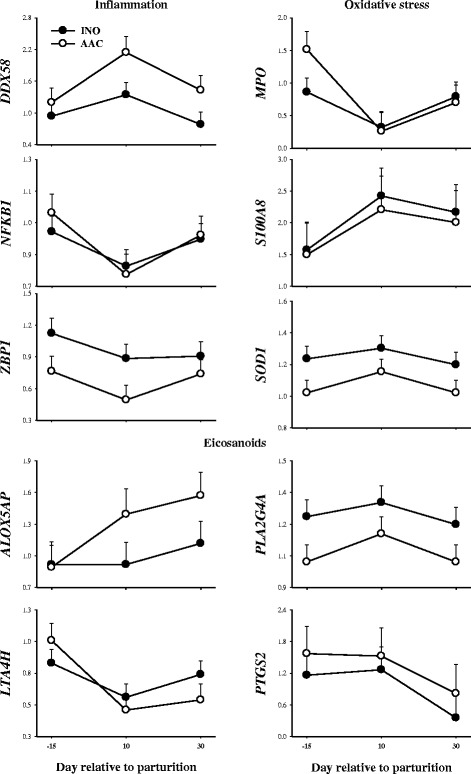
mRNA expression (fold-change relative to -30 d prepartum) of genes associated with inflammation (DDX58, NFKB1 and ZBP1), oxidative stress (MPO, S100A8 and SOD1) and eicosanoids (ALOX5AP, LTA4H, PLA2G4A and PTGS2) in polymorphonuclear leukocytes (PMNL) of cows supplemented with inorganic (INO; n = 11) or complexed (AAC; n = 9) trace minerals during the pre- and postpartal period
Oxidative stress
An interaction T × T (treatment × time; P = 0.03) was detected for MPO due to its upregulation at -15 d in the AAC cows (Table 2, Fig. 1). The expression of the superoxide dismutase enzymes SOD1 (P = 0.14), SOD2 (P = 0.27) and SOD3 (P = 0.15) was not affected by treatment. However, the expression of SOD1 increased from -15 to +10 d regardless of treatment (Table 2, Fig. 1). The oxidant scavenger proteins S100A8 (P = 0.78) and NOX1 (P = 0.94) as well as the transcription factor NFE2L2 (P = 0.54) were not affected by treatments (Table 2, Fig. 1). However, S100A8 was upregulated (P = 0.08) from -15 to +10 d in both treatments.
Eicosanoids
Among the genes related with arachidonic acid, PLA2G4A (P = 0.06) and ALOX5AP (P = 0.09) tended to have greater expression in cows fed AAC (Table 2, Fig. 1). In contrast, the mRNA expression of LTA4H (P = 0.76) and PTGS2 (P = 0.53) were not affected by treatments (Table 2, Fig. 1). A marked decrease (P < 0.01) in expression of LTA4H was observed between -15 and +10 d regardless of treatment, whereas PTGS2 expression gradually decreased (P = 0.04) between -15 and +30 d regardless of treatment (Fig. 1).
Transcription factors
PPARA had lower overall mRNA expression (P = 0.04) in AAC cows, whereas PPARG tended to have greater mRNA expression compared with INO (P = 0.09; Table 2 and Fig. 2). The mRNA expression of PPARD (P = 0.71) and RXRA (P = 0.22) were not affected by treatments (Table 2). However, expression of RXRA increased (P = 0.05) from -15 to +10 d and then decreased to prepartum values in both treatments (Fig. 2).
Fig. 2.
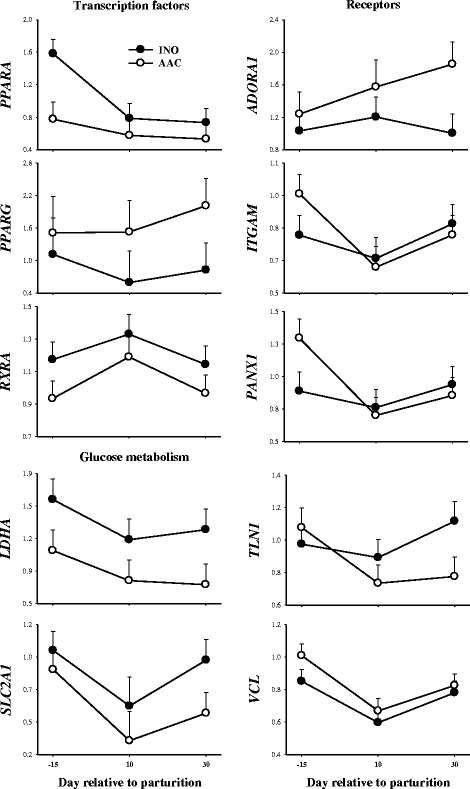
mRNA expression (fold-change relative to -30 d prepartum) of genes associated with transcription factors (PPARA, PPARG and RXRA), receptors (ADORA1, ITGAM, PANX1, TLN1 and VCL) and glucose metabolism (LDHA and SLC1A1) in polymorphonuclear leukocytes (PMNL) of cows supplemented with inorganic (INO; n = 11) or complexed (AAC; n = 9) trace minerals during the pre- and postpartal period
Receptors
The expression of the receptors TLN1 (P = 0.01) and ADORA1 (P = 0.01) was greater in the cows receiving AAC treatment (Table 2, Fig. 2). However, the treatments did not alter (all P > 0.10) expression of other receptors measured (SELL, ITGAM, ITGB2, VCL, PANX1, ENTPD1, P2RY11, IL1B, and IL10) (Table 2, Fig. 2).
Glucose metabolism
An interaction T × T (P < 0.01) was detected for the glucose transporter SLC2A1 due to the marked decrease in expression between -15 and +10 d. Whereas, the mRNA expression of LDHA (P = 0.06) tended to be lower in the AAC compared with INO cows (Table 2, Fig. 2).
Endometrium
Inflammation response
A tendency for a T × T interaction was detected for TLR2 (P = 0.08), TLR4 (P = 0.08), TNF (P = 0.10), and NFKB1 (P = 0.06) due to greater mRNA expression in AAC cows at +14 d, whereas lower expression was observed at +30 d (Table 3, Fig. 3). Furthermore, a T × T was observed for IL6 (P = 0.03), IL1B (P < 0.01), IL8 (P < 0.01), and IL10 (P < 0.01) because all these genes had greater mRNA expression in the AAC cows at +14 d but no treatment effect was detected at +30 d. In addition, AAC treatment increased STAT3 (P = 0.05) and tended to increase MYD88 (P = 0.06) mRNA expression (Table 3). The expression of MYD88 decreased (P = 0.05) regardless of treatment from +14 to +30 d postpartum.
Table 3.
Effects of supplementing cows with inorganic (INO; n = 11) or complexed (AAC; n = 9) trace minerals during the peripartal period on mRNA expression (log-2 scale) of genes related with inflammation response, oxidative stress, eicosanoids, transcription factors and antimicrobial peptides in endometrium tissue at +14 and +30 d after parturition
| Gene | Treatments | SEMa | P value1 | |||||
|---|---|---|---|---|---|---|---|---|
| INO | AAC | |||||||
| +14 | +30 | +14 | +30 | Treatment | Time | T × Tb | ||
| Inflammation | ||||||||
| IL10 | 4.13 | 3.83 | 5.13 | 3.72 | 0.19 | 0.04 | <0.01 | <0.01 |
| IL1B | 0.65 | -0.37 | 3.51 | -0.98 | 0.84 | 0.26 | <0.01 | <0.01 |
| IL6 | 2.12 | 2.91 | 2.88 | 2.70 | 0.23 | 0.24 | 0.15 | 0.03 |
| IL8 | 1.26 | -0.01 | 4.51 | -1.52 | 0.84 | 0.37 | <0.01 | <0.01 |
| MYD88 | 4.98 | 4.86 | 5.46 | 4.93 | 0.15 | 0.06 | 0.05 | 0.20 |
| NFKB1 | 5.06 | 5.36 | 5.41 | 5.06 | 0.16 | 0.87 | 0.90 | 0.06 |
| SAA3 | 3.10 | 2.77 | 4.88 | 2.06 | 1.00 | 0.46 | 0.15 | 0.25 |
| STAT3 | 5.05 | 5.22 | 5.69 | 5.16 | 0.19 | 0.05 | 0.40 | 0.11 |
| TLR2 | 5.05 | 4.58 | 5.87 | 4.39 | 0.28 | 0.19 | <0.01 | 0.10 |
| TLR4 | 5.24 | 4.49 | 5.69 | 3.34 | 0.18 | 0.40 | <0.01 | 0.08 |
| TNF | 4.21 | 4.60 | 5.67 | 4.32 | 0.36 | 0.11 | 0.12 | 0.01 |
| Oxidative stress | ||||||||
| NFE2L2 | 4.85 | 5.11 | 5.20 | 5.26 | 0.13 | 0.06 | 0.22 | 0.43 |
| NOS3 | 4.94 | 5.16 | 5.11 | 5.07 | 0.36 | 0.91 | 0.76 | 0.66 |
| NRROS | 5.18 | 4.62 | 5.20 | 4.87 | 0.32 | 0.68 | 0.13 | 0.70 |
| SOD1 | 5.19 | 5.04 | 5.26 | 5.28 | 0.09 | 0.09 | 0.39 | 0.30 |
| SOD2 | 4.83 | 4.13 | 5.57 | 3.83 | 0.32 | 0.44 | <0.01 | 0.12 |
| SOD3 | 4.87 | 5.27 | 5.08 | 5.16 | 0.26 | 0.85 | 0.28 | 0.45 |
| Eicosanoid synthesis | ||||||||
| ALOX5 | 4.89 | 4.83 | 5.08 | 5.14 | 0.41 | 0.51 | 0.99 | 0.88 |
| ALOX5AP | 4.86 | 4.92 | 5.33 | 4.95 | 0.38 | 0.51 | 0.60 | 0.48 |
| LTA4H | 5.07 | 5.30 | 5.24 | 5.23 | 0.10 | 0.51 | 0.30 | 0.24 |
| LTC4S | 3.91 | 3.87 | 4.35 | 3.98 | 0.42 | 0.45 | 0.62 | 0.70 |
| PLA2G4A | 4.92 | 5.39 | 5.44 | 4.96 | 0.29 | 0.86 | 0.99 | 0.13 |
| PTGDS | 5.26 | 4.94 | 5.05 | 4.87 | 0.29 | 0.63 | 0.34 | 0.77 |
| PTGES | 4.50 | 3.68 | 5.53 | 3.92 | 0.34 | 0.10 | <0.01 | 0.15 |
| PTGS2 | 3.17 | 4.32 | 4.94 | 2.85 | 0.77 | 0.83 | 0.52 | 0.04 |
| Transcription regulation | ||||||||
| PPARA | 5.09 | 5.48 | 5.20 | 5.68 | 0.21 | 0.48 | 0.02 | 0.79 |
| PPARD | 5.47 | 5.13 | 5.46 | 5.07 | 0.15 | 0.81 | 0.01 | 0.89 |
| PPARG | 4.72 | 5.17 | 4.64 | 6.03 | 0.31 | 0.21 | <0.01 | 0.11 |
| RXRA | 5.28 | 5.24 | 5.24 | 5.09 | 0.13 | 0.49 | 0.38 | 0.60 |
| Antimicrobial peptides | ||||||||
| MUC1 | 2.84 | 3.78 | 4.29 | 4.05 | 0.46 | 0.04 | 0.49 | 0.25 |
1 P values represents the probability of statistical significance for the fixed effects (treatment, time, treatment × time). Statistical differences were declared significant at P ≤ 0.05 and tendencies at P ≤ 0.10
aLargest standard error of the mean is shown
bInteraction of treatment × time
Fig. 3.
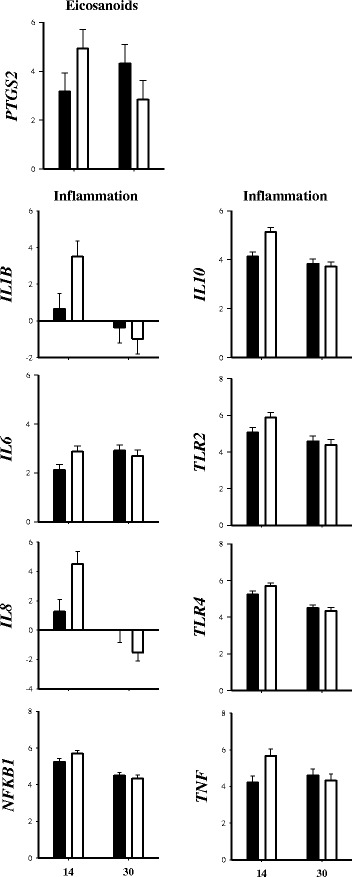
mRNA expression (log 2-scale) of genes associated with immune-related receptors (TLR2 and TLR4), pro-inflammatory response (NFKB1, TNF, IL6, IL1B and IL8), anti-inflammatory response (IL10) and eicosanoids (PTGS2) in endometrium of cows supplemented with inorganic (INO; n = 11) or complexed (AAC; n = 9) trace minerals during the pre- and postpartal period
Oxidative stress
No T × T was observed (P > 0.10) for genes related with oxidative stress (Table 3). However, mRNA expression of SOD1 (P = 0.09) and NEF2L2 (P = 0.06) tended to be greater in AAC compared with INO cows (Table 3). In addition, expression of SOD2 decreased (P < 0.01) regardless of treatment from +14 to +30 d postpartum.
Eicosanoids
A T × T (P = 0.04) was observed for PTGS2 because its expression became greater over time with INO while it decreased with AAC (Table 3, Fig. 3). A tendency (P = 0.10) for a greater overall PTGES mRNA expression was observed for AAC cows (Table 3). In addition, expression of PTGES decreased (P < 0.01) from +14 to +30 d postpartum regardless of treatment (Table 3).
Transcription factors
No T × T or overall treatment effect (P > 0.10) was observed for PPARA, PPARD, PPARG and RXRA. However, expression of PPARA and PPARG increased and PPARD decreased from +14 to +30 d postpartum (Table 3).
Antimicrobial peptides
MUC1 mRNA expression was upregulated (P = 0.04) overall in the AAC compared with INO cows (Table 3).
Discussion
Neutrophil function around calving
Neutrophil function and bactericidal efficiency is compromised during the transition period [1]. A lower capacity for trafficking, phagocytosis, and pathogen killing during this period is partly associated with changes in hormones and metabolites and with immune or stress-like conditions [19]. Some studies also have reported that neutrophils have impaired generation of ROM during the transition period [20, 21], a feature that may contribute to greater susceptibility to disorders in early lactation. The general hypothesis of this study was that feeding trace minerals through more bioavailable forms would improve immune health, enhance PMNL activity, and help overcome the challenges of the transition period [10]. In the companion paper [10] we reported production and biomarker data indicating that better DMI and milk production in cows fed AAC was partly due to better liver function and PMNL phagocytosis.
Inflammation response
The activation of innate immune responses are regulated by several DNA sensors including toll-like receptors, e.g., TLR2, TLR4, DDX58 and ZBP1 [22]. Subsequent to pathogen detection, these receptors activate signaling pathways (e.g., STAT3 and NF-κB), which trigger the synthesis of pro-inflammatory cytokines and chemokines [23]. Despite the fact that cows in the AAC treatment had greater expression of DDX58 and lower expression of ZBP1, those responses did not seem to activate the pro-inflammatory pathways as indicated by the lack of change in expression of the cytokines TNF and IL1B.
Oxidative stress
Myeloperoxidase (MPO) is the main peroxidase enzyme released upon neutrophil activation, and catalyzes the formation of hypochlorous acid, a potent oxidant that displays bactericidal activity [24]. Furthermore, MPO has traditional cytokine-like function and acts as an autocrine modulator of neutrophil activation [25]. In steers, a Cu-deficient diet impaired neutrophil killing capacity without altering phagocytosis [26]. Similarly, Cu-depleted calves exhibited impaired phagocytic killing activity, which was restored by Cu supplementation [27]. Therefore, the greater mRNA expression of MPO indicated that AAC cows were more likely to have greater PMNL activation, hence, superior capacity to kill invading pathogens.
When ROM production elicits a metabolic imbalance in cells, the release of endogenous neutralizing agents helps to minimize their potential deleterious effects. The protein S100A8 comprises ~20% of the PMN cytoplasm [28], and exerts an important protective mechanism during inflammation because it scavenges intracellular ROM produced by activated PMN and attenuates nitric oxide production [29]. Similarly, the cytoplasmic enzyme SOD1 transforms the harmful superoxide radicals to molecular oxygen and hydrogen peroxide [30]. Therefore, the tendency for upregulation of SOD1 and S100A8 at +10 d regardless of treatment could be taken as an indication of PMNL attempting to neutralize the greater oxidative stress experienced after parturition [31].
Eicosanoid metabolism
Neutrophil stimulation produces oxygen-derived reactive species, lysosomal enzymes, nitric oxide as well as pro-inflammatory and anti-inflammatory mediators which include bioactive lipids such as the eicosanoids (e.g., prostaglandins and leukotrienes) [32]. The tendency for upregulation of PLA2G4A in AAC cows could have resulted in an increase in the hydrolysis of cell membrane phospholipids to release arachidonic acid, which subsequently could be used for leukotriene (via ALOX5AP) synthesis. Leukotrienes, such as leukotriene B4, are essential components of the inflammatory response because they act as chemoattractants for mature neutrophils, and promote neutrophil activation [33]. Furthermore, leukotriene B4 enhances cytokine production and the presence of these fatty acids seems to determine the duration and magnitude of the inflammatory response [34]. In vitro, Zn, Cu, and Ni enhanced PMN motility by chemotactic activation indicating that the inflammatory response can be partly modulated through the availability of those metals [35].
Transcription regulation
In non-ruminants, the family of transcription factors termed peroxisome proliferator-activated receptors (PPAR) is involved in the control of inflammation [34]. Eicosanoids are PPARα activators [36] that can inhibit arachidonic acid-induced inflammation in part by enhancing degradation of leukotriene B4 [37]. It is noteworthy that the expression of ALOX5AP (leukotriene synthesis) and PPARA followed opposite patterns of expression with the AAC treatment indicating that the inflammatory response in those cows likely was of a greater magnitude but of brief duration. The absence of change in the expression of the pro-inflammatory cytokine IL1B may be due to the upregulation of PPARG in the AAC treatment. Prior research in non-ruminant cells indicated an inhibitory effect of PPARγ on cytokine production [34].
Receptors
Neutrophil recruitment and migration to inflamed tissues are critical for proper immune function. Cytoskeletal proteins, such as talin-1 (TLN1), facilitate the transition from neutrophil rolling to arrest [38]. Furthermore, modulation of neutrophil function by adenosine (ADORA1) promotes neutrophil chemotaxis and phagocytosis [39]. Therefore, the upregulation of TLN1 and ADORA1 in AAC cows indicated that PMNL were better equipped to be deployed into the inflamed sites. Despite these unique effects of AAC, the overall downregulation of PANX1 and VCL from -15 to +10 d regardless of treatment seemed to indicate a degree of impairment in the recruitment of PMNL and their ability to adhere to endothelium. Some evidence indicates that PANX1 channels are activated by ATP [40], which may explain the gradual upregulation of ADORA1 over time, i.e., a counter regulatory mechanism to help regulate PMNL activity. In addition, the downregulation of VCL could partly be explained by gradual degradation of PMNL plasma membrane phosphatidylinositol 4,5-bisphosphate, which is essential for activation of VCL [41]. Whether such effect is directly related to catabolic enzymes (e.g., phospholipases) or greater turnover of PMNL is unknown.
Glucose metabolism
At least in non-ruminants, neutrophils rely on glycolysis as the main source of energy; however, the extra energy required for phagocytosis is usually derived from metabolism of lactate [42]. Both SCL2A1 and LDHA are important regulators of energy metabolism in neutrophils. The first facilitates the transport of glucose across the plasma membrane, whereas the second is involved in the interconversion of pyruvate to lactate after glycolysis [43]. The parallel downregulation of SLC2A1 and LDHA regardless of treatment to a nadir at +10 d postpartum was most likely a result of the shortfall in circulating glucose commonly observed after parturition [10]. The numerically-greater expression of SLC2A1 and LDHA in INO cows during the study could indicate that these cows were more immuno-compromised because a previous study detected marked upregulation of LDHA in PMNL after a mastitis challenge [14]. If such an effect existed it could help to partly explain the lower phagocytic activity in whole blood that was measured on +30 d in INO cows [10]. Because cows in INO had greater plasma concentrations of ketones, the upregulation of LDHA in these cows could have been a mechanism induced by ketone body (e.g., hydroxybutyrate) metabolism to decrease glucose oxidation by the PMNL [44].
Endometrium
Bacterial contamination and consequent inflammatory response of the uterine tissue after parturition are common and are associated with lower conception rates, longer interval periods from calving to first service or conception, and more animals culled for failure to conceive [45]. Considering that trace minerals play important roles in the health and immunity of peripartal dairy cows [46] and complexed trace mineral supplementation in partial substitution of sulfate sources elicited an improvement in immune function [10, 47], it was important to ascertain if complexed trace minerals also elicited a local response in the endometrium.
Inflammation response
Although the inflammatory response is a natural defense mechanism that could be initiated by tissue injury [22], it can be beneficial or deleterious. After calving, the inflammatory and immune response in the endometrium attempts to eliminate any pathogenic bacterial contamination as well as initiate tissue repair as part of the involution process [45]. However, prolonged inflammation and cytokine production within the reproductive tract impair immune status and reproductive performance [45]. Therefore, the upregulation of genes related with the pro-inflammatory cascade (TLR2, TLR4, NFKB1, TNF, IL6 and IL1B) in response to AAC at +14 d compared with +30 d indicated that pathogen elimination and tissue remodeling processes occurred earlier than in INO cows. In addition, the concentrations of blood biomarkers of inflammation [47] in these cows indicated a lower systemic inflammation status in AAC than INO cows.
Oxidative stress response
Essential trace minerals such as Zn and Cu play a central role in metabolism and have the potential to reduce oxidative stress through several mechanisms. Evidence from human studies suggest that Zn is essential for expression and function of the transcription factor NFE2L2 [48]. This transcription factor helps control oxidative damage through its control of some antioxidant defense systems such as SOD1 activity [49] which requires Zn and Cu as co-factors. Therefore, the overall greater expression of NFE2L2 and SOD1 indicated that feeding AAC reduced oxidative stress within the endometrium and potentially help alleviate an overt inflammatory response. It is noteworthy that upregulation of NFE2L2 also occurred in the PMNL and hoof corium (unpublished results) in the cows fed AAC, which strongly indicates a consistent effect of trace minerals on cellular stress through this transcription regulator.
Eicosanoid metabolism
Among several biological functions, prostaglandins play a central role in the generation of an inflammatory response. They have pro-inflammatory properties and are responsible for typical signs of inflammation including redness, swelling and pain [50]. The synthesis of prostaglandins is partly dependent on Zn, hence, this trace mineral could play an indirect role in regulating enzymes involved in the arachidonic acid cascade that result in production of prostaglandins [51, 52]. Therefore, the upregulation of PTGS2 and PTGES in cows fed AAC indicated that supplemental complexed Zn was more bioavailable for prostaglandin synthesis.
Antimicrobial peptides
The upregulation of MUC1, a transmembrane glycoprotein abundantly expressed at the surface of the uterine epithelial tissue, in AAC cows indicated a greater ability to eliminate invading pathogenic bacteria [53]. This idea is supported by previous work demonstrating that MUC1-null mice were susceptible to chronic infections and inflammation, and had a markedly reduced fertility [54].
Conclusions
Taken together, our findings reveal that supplementation with Zn, Mn, and Cu from AA complexes and Co from Co glucoheptonate during the transition period improved PMNL function and likely confer these cells a greater capacity to control invading pathogens. The robust inflammatory response coupled with the anti-oxidant response discerned from the transcriptome data in the uterine samples of cows fed complexed trace minerals likely allowed for a faster uterine recovery. These data indicate that the benefits of trace minerals from AA complexes extend beyond an improvement of liver function and productive performance [10]. Although our findings suggest that peripheral and uterine immune function was improved in cows supplemented with more bioavailable forms of trace minerals, further research to evaluate the clinical impact of that supplementation is warranted. Such research also will help to better define complexed trace mineral requirements beyond productive purposes.
Additional files
PMNL Isolation: Blood (120 mL), collected in ACD solution A vacutainer tubes, was mixed well by inversion and placed on ice until PMN isolation (within ~30 min). Tubes were combined into three 50-mL conical tubes (Fisher Scientific, Pittsburgh, PA) and centrifuged at 918 × g for 30 min at 4 °C. The plasma, buffy coat, and approximately one-third of the red blood cells (RBC) were removed and discarded. Cells were lysed with 25 mL of deionized water at 4 °C, homogenized gently by inversion, and then 5 mL of 5 × PBS (pH 7.4) at 4 °C was added, in order to restore an iso-osmotic environment. The cell suspension was centrifuged at 330 × g for 10 min at 4 °C and the supernatants were decanted. Ten milliliters of 1 × PBS at 4 °C was added in each tube, homogenized until there was nothing attached to the bottom of the tube, and then the three tubes were combined in one. The cell suspension was centrifuged at 663 × g for 5 min at 4 °C and the supernatants were discarded. The remaining RBC were lysed with 8 mL of deionized water at 4 °C, homogenized gently by inversion and 2 mL of 5 × PBS at 4 °C was added. The samples were centrifuged at 663 × g for 5 min at 4 °C and the supernatant was discarded. Two subsequent washings using 10 mL of 1 × PBS at 4 °C were performed, centrifuged at 663 × g for 5 min at 4 °C and supernatant discarded. Prior to the last centrifugation, 100 μL of the cell suspension were aliquoted for further PMN concentration and cell viability analysis. (DOC 43 kb)
RNA extraction: Approximately 40 mg of frozen tissue was weighed and immediately placed in ice-cold 1 mL Qiazol reagent (Qiagen 75842; Qiagen Inc., Valencia, CA) for homogenization. After homogenization, the samples were centrifuged for 10 min at 12,000 × g at 4 °C to remove the insoluble material. The supernatant was transferred to a collection tube and incubated for 5 min on ice. Chloroform (200 µL) was added to each tube and the sample incubated at room temperature for 3 min. Subsequently, samples were centrifuged for 15 min at 12,000 × g at 4 °C, and the upper phase was transferred to a new collection tube without disturbing the mid and lower phases. A second wash was performed with 100% ethanol; 750 µL was added and transferred to a miRNeasy Mini Kit columns (Cat. No: 217004, Qiagen). Genomic DNA was removed on column from RNA samples with RNase-free DNase I, using the recommended protocol provided with the miRNeasy Mini Kit. RNA concentration was measured with a NanoDrop ND-1000 spectrophotometer (Thermo Fischer Scientific; Wilmington, DE), while the RNA quality was assessed using the Agilent 2100 Bioanalyzer system (Agilent Technologies, Santa Clara, CA). Samples of RNA used for analysis had an RNA integrity number ≥7.0. (DOC 44 kb)
Function of the genes measured in the PMNL. (DOC 67 kb)
Function of the genes measured in the endometrium. (DOC 61 kb)
Features of used primers for qPCR analysis. Hybridization position, sequence, and amplicon size of primers for Bos taurus used to analyze gene expression. (DOC 107 kb)
Sequencing results of PCR products from primers of genes used for this experiment. (DOC 62 kb)
qPCR performance among the genes measured in PMNL. (DOC 77 kb)
qPCR performance among the genes measured in the endometrium tissue. (DOC 71 kb)
Acknowledgments
Fernanda Batistel (FB) was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). Juan Loor (JL) was supported by National Institute of Food and Agriculture (Grant: ILLU-538-914). Zinpro Corporation provided support to Juan J. Loor and Michael T. Socha. Zinpro Corporation had a role in the study design and provided financial support to cover costs of animal use, data collection, and sample analyses. The specific roles of the authors are articulated in the ‘author’s contributions’ section.
Funding
Not applicable.
Availability of data and materials
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
FB, JSO, MRT, CL, and JC performed analyses and analyzed data. JJL, JSO, and MTS conceived the animal experiments. FB wrote the manuscript. All authors approved the final version of the manuscript.
Authors’ information
F. Batistel is PhD candidate, University of Illinois, Urbana, Illinois, 61801, USA. J. S. Osorio is Assistant Professor in the Department of Dairy Science, South Dakota State University, Brookings, South Dakota, USA. M. R. Tariq is Assistant Professor in the Department of Food Science and Technology, University College of Agriculture & Environmental Sciences, The Islamia University of Bahawalpur, Bahawalpur, Punjab, Pakistan. C. Li, PhD, College of Animal Science and Technology, Key Laboratory of Animal Genetics and Breeding of Ministry of Agriculture, National Engineering Laboratory for Animal Breeding, China Agricultural University, Beijing 100193, China. J. Caputo, PhD, University of Illinois, Urbana, Illinois, 61801, USA. M. T. Socha, PhD, is Regional RNS Manager-North America, Research and Nutritional Services, Zinpro Corporation, 10400 Viking Dr., Suite 240, Eden Prairie, Minnesota 55344, USA. J. J. Loor is Associate Professor in the Department of Animal Sciences, University of Illinois, Urbana, Illinois, 61801, USA.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval
All procedures for this study (protocol no. 12097) were approved by the Institutional Animal Care and Use Committee of the University of Illinois.
Abbreviations
- AAC
Amino acid-complexed Zn, Mn, Cu, and Co
- ACD
Citric acid and dextrose
- DIM
Days in milk
- DMI
Dry matter intake
- IACUC
Institutional Animal Care and Use Committee
- INO
Inorganic trace mineral mix
- PMNL
Polymorphonuclear leukocyte
- PPAR
Peroxisome proliferator-activated receptors
- ROM
Reactive oxygen metabolites
Contributor Information
Fernanda Batistel, Email: batiste2@illinois.edu.
Johan S. Osorio, Email: johan.osorio@sdstate.edu
Muhammad Rizwan Tariq, Email: rizwan_choudary143@yahoo.com.
Cong Li, Email: li10020902@163.com.
Jessica Caputo, Email: Jessica_caputo@hotmail.it.
Michael T. Socha, Email: msocha@zinpro.com
Juan J. Loor, Email: jloor@illinois.edu
References
- 1.Burvenich C, Van Merris V, Mehrzad J, Diez-Fraile A, Duchateau L. Severity of E. coli mastitis is mainly determined by cow factors. Vet Res. 2003;34(5):521–564. doi: 10.1051/vetres:2003023. [DOI] [PubMed] [Google Scholar]
- 2.Rinaldi M, Morono P, Paape MJ, Bannerman DD. Differential alterations in the ability of bovine neutrophils to generate extracellular and intracellular reactive oxygen species during the periparturient period. Vet J. 2008;178(2):208–213. doi: 10.1016/j.tvjl.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 3.Kehrli ME, Nonnecke BJ, Roth JA. Alterations in bovine neutrophil function during the periparturient period. Am J Vet Res. 1989;50(2):207–214. [PubMed] [Google Scholar]
- 4.Overton TR, Yasui T. Practical applications of trace minerals for dairy cattle. J Anim Sci. 2014;92(2):416–426. doi: 10.2527/jas.2013-7145. [DOI] [PubMed] [Google Scholar]
- 5.Sheldon IM, Willians EJ, MIller ANA, Nash DM, Herath S. Uterine diseases in cattle after parturition. Vet J. 2008;176(1):115–121. doi: 10.1016/j.tvjl.2007.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheldon IM, Cronin J, Goetze L, Donofrio G, Schuberth HJ. Defining postpartum uterine disease and the mechanisms of infection and immunity in the female reproductive tract in cattle. Biol Reprod. 2009;81(6):1025–1032. doi: 10.1095/biolreprod.109.077370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meglia GE, Johannisson A, Petersson L, Walker KP. Changes in some blood micronutrients, leukocytes and neutrophil expression of adhesion molecules in periparturient dairy cows. Acta Vet Scand. 2001;42(1):139–150. doi: 10.1186/1751-0147-42-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cebra CK, Heidel JR, Crisman RO, Stang BV. The relationship between endogenous cortisol, blood micronutrients, and neutrophil function in postparturient Holstein cows. J Vet Intern Med. 2003;17(6):902–907. doi: 10.1111/j.1939-1676.2003.tb02531.x. [DOI] [PubMed] [Google Scholar]
- 9.Xin Z, Waterman DF, Hemken RW, Harmon RJ. Copper status and requirement during the dry period and early lactation in multiparous Holstein cows. J Dairy Sci. 1993;76(9):2711–2716. doi: 10.3168/jds.S0022-0302(93)77607-9. [DOI] [PubMed] [Google Scholar]
- 10.Osorio JS, Trevisi E, Li C, Drackley JK, Socha MT, Loor JJ. Supplementing Zn, Mn, and Cu from amino acid complexes and Co from cobalt glucoheptonate during the peripartal period benefits postpartal cow performance and blood neutrophil function. J Dairy Sci. 2016;99(3):1868–1883. doi: 10.3168/jds.2015-10040. [DOI] [PubMed] [Google Scholar]
- 11.NRC, Nutient requirement of dairy cattle. 7th rev. ed. 2001, Washington: National Academy Press. xxi, p 381.
- 12.Rhoads ML, Meyer JP, Lamberson WR, Keisler DH, Lucy MC. Uterine and hepatic gene expression in relation to days postpartum, estrus, and pregnancy in postpartum dairy cows. J Dairy Sci. 2008;91(1):140–150. doi: 10.3168/jds.2007-0439. [DOI] [PubMed] [Google Scholar]
- 13.Sakumoto R, Hayashi KG, Saito S, Kanahara H, Kizaki K, Iga K. Comparison of the global gene expression profiles in the bovine endometrium between summer and autumn. J Reprod Dev. 2015;61(4):297–303. doi: 10.1262/jrd.2015-024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moyes KM, Graugnard DE, Khan MJ, Mukesh M, Loor JJ. Postpartal immunometabolic gene network expression and function in blood neutrophils are altered in response to prepartal energy intake and postpartal intramammary inflammatory challenge. J Dairy Sci. 2014;97(4):2165–2177. doi: 10.3168/jds.2013-7433. [DOI] [PubMed] [Google Scholar]
- 15.Chapwanya A, Meade KG, Narciandi F, Stanley P, Mee JF, Doherty M, et al. Endometrial biopsy: a valuable clinical and research tool in bovine reproduction. Theriogenology. 2010;73(7):988–994. doi: 10.1016/j.theriogenology.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Mansouri-Attia N, Aubert J, Reinaud P, Giraud-Delville C, Taghouti G, Galio L, et al. Gene expression profiles of bovine caruncular and intercaruncular endometrium at implantation. Physiol Genomics. 2009;39(1):14–27. doi: 10.1152/physiolgenomics.90404.2008. [DOI] [PubMed] [Google Scholar]
- 17.Moyes KM, Drackley JK, Morin DE, Loor JJ. Greater expression of TLR2, TLR4, and IL6 due to negative energy balance is associated with lower expression of HLA-DRA and HLA-A in bovine blood neutrophils after intramammary mastitis challenge with Streptococcus uberis. Funct Integr Genomics. 2010;10(1):53–61. doi: 10.1007/s10142-009-0154-7. [DOI] [PubMed] [Google Scholar]
- 18.Seo J, Osorio JS, Loor JJ. Purinergic signaling gene network expression in bovine polymorphonuclear neutrophils during the peripartal period. J Dairy Sci. 2013;96(12):7675–7683. doi: 10.3168/jds.2013-6952. [DOI] [PubMed] [Google Scholar]
- 19.Mallard BA, Dekkers JC, Ireland MJ, Leslie KE, Sharif S, Lacey Vankampen C, et al. Alteration in immune responsiveness during the peripartum period and its ramification on dairy cow and calf health. J Dairy Sci. 1998;81(2):585–595. doi: 10.3168/jds.S0022-0302(98)75612-7. [DOI] [PubMed] [Google Scholar]
- 20.Revelo XS, Waldron MR. Effects of in vitro insulin and 2,4-thiazolidinedione on the function of neutrophils harvested from blood of cows in different physiological states. J Dairy Sci. 2010;93(9):3990–4005. doi: 10.3168/jds.2009-2922. [DOI] [PubMed] [Google Scholar]
- 21.Stevens MG, Peelman LJ, De Spiegeleer B, Pezeshki A, Van De Walle GR, Duchateau L, et al. Differential gene expression of the toll-like receptor-4 cascade and neutrophil function in early- and mid-lactating dairy cows. J Dairy Sci. 2011;94(3):1277–1288. doi: 10.3168/jds.2010-3563. [DOI] [PubMed] [Google Scholar]
- 22.Cui J, Chen Y, Wanf HY, Wang RF. Mechanisms and pathways of innate immune activation and regulation in health and cancer. Hum Vaccin Immunother. 2014;10(11):3270–3285. doi: 10.4161/21645515.2014.979640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5(10):987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 24.Winterbourn CC. Biological reactivity and biomarkers of the neutrophil oxidant, hypochlorous acid. Toxicology. 2002;181–182:223–227. doi: 10.1016/S0300-483X(02)00286-X. [DOI] [PubMed] [Google Scholar]
- 25.Lau D, Mollnau H, Eiserich JP, Freeman BA, Daiber A, Gehling UM, et al. Myeloperoxidase mediates neutrophil activation by association with CD11b/CD18 integrins. Proc Natl Acad Sci U S A. 2005;102(2):431–436. doi: 10.1073/pnas.0405193102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xin Z, Waterman DF, Hemken RW, Harmon RJ. Effects of copper status on neutrophil function, superoxide dismutase, and copper distribution in steers. J Dairy Sci. 1991;74(9):3078–3085. doi: 10.3168/jds.S0022-0302(91)78493-2. [DOI] [PubMed] [Google Scholar]
- 27.Jones DG, Suttle NF. Some effects of copper deficiency on leucocyte function in sheep and cattle. Res Vet Sci. 1981;31(2):151–156. [PubMed] [Google Scholar]
- 28.Donato R, Cannon BR, Sorci G, Riuzzi F, Hsu K, Weber DJ, et al. Functions of S100 proteins. Curr Mol Med. 2013;13(1):24–57. doi: 10.2174/156652413804486214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sy L, Raftery M, Cai H, Hsu K, Yan WX, Hseih HL, et al. S-nitrosylated S100A8: novel anti-inflammatory properties. J Immunol. 2008;181(8):5627–5636. doi: 10.4049/jimmunol.181.8.5627. [DOI] [PubMed] [Google Scholar]
- 30.Milani P, Gagliard S, Cova E, Cereda C. SOD1 Transcriptional and Posttranscriptional Regulation and Its Potential Implications in ALS. Neurol Res Int. 2011;2011:458427. doi: 10.1155/2011/458427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bionaz M, Trevisi E, Calamari L, Librandi F, Ferrari A, Bertoni G. Plasma paraoxonase, health, inflammatory conditions, and liver function in transition dairy cows. J Dairy Sci. 2007;90(4):1740–1750. doi: 10.3168/jds.2006-445. [DOI] [PubMed] [Google Scholar]
- 32.Bates EJ. Eicosanoids, fatty acids and neutrophils: Their relevance to the pathophysiology of disease. Prostaglandins Leukot Essent Fatty Acids. 1995;53(2):75-86. [DOI] [PubMed]
- 33.Busse WW. Leukotrienes and inflammation. Am J Respir Crit Care Med. 1998;157(6 Pt 1):S210–S213. doi: 10.1164/ajrccm.157.6.mar-1. [DOI] [PubMed] [Google Scholar]
- 34.Daynes RA, Jones DC. Emerging roles of PPARs in inflammation and immunity. Nat Rev Immunol. 2002;2(10):748–759. doi: 10.1038/nri912. [DOI] [PubMed] [Google Scholar]
- 35.Hujanen ES, Seppä ST, Virtanen K. Polymorphonuclear leukocyte chemotaxis induced by zinc, copper and nickel in vitro. Biochim Biophys Acta. 1995;1245(2):145–152. doi: 10.1016/0304-4165(95)00082-M. [DOI] [PubMed] [Google Scholar]
- 36.Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS, et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc Natl Acad Sci U S A. 1997;94(9):4318–4323. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colville-Nash P, Willis D, Papworth J, Freemantle C, Lam C, Andrews G, et al. The peroxisome proliferator-activated receptor alpha activator, Wy14,643, is anti-inflammatory in vivo. Inflammopharmacology. 2005;12(5–6):493–504. doi: 10.1163/156856005774382724. [DOI] [PubMed] [Google Scholar]
- 38.Dixit N, Kim MH, Rossaint J, Yamayoshi I, Zarbock A, Simon SI. Leukocyte function antigen-1, kindlin-3, and calcium flux orchestrate neutrophil recruitment during inflammation. J Immunol. 2012;189(12):5954–5964. doi: 10.4049/jimmunol.1201638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barletta KE, Ley K, Mehrad B. Regulation of neutrophil function by adenosine. Arterioscler Thromb Vasc Biol. 2012;32(4):856–864. doi: 10.1161/ATVBAHA.111.226845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maslieieva V, Thompson RJ. A critical role for pannexin-1 in activation of innate immune cells of the choroid plexus. Channels (Austin) 2014;8(2):131–141. doi: 10.4161/chan.27653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Metzner B, Heger M, Hofmann C, Czech W, Norgauer J. Evidence for the involvement of phosphatidylinositol 4,5-bisphosphate 3-kinase in CD18-mediated adhesion of human neutrophils to fibrinogen. Biochem Biophys Res Commun. 1997;232(3):719–723. doi: 10.1006/bbrc.1997.6350. [DOI] [PubMed] [Google Scholar]
- 42.Borregaard N, Herlin T. Energy metabolism of human neutrophils during phagocytosis. J Clin Invest. 1982;70(3):550–557. doi: 10.1172/JCI110647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ganapathy-Kanniappan S, Geschwind JF, Kunjithapatham R, Buijs M, Vossen JA, Tchernysyov I, et al. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is pyruvylated during 3-bromopyruvate mediated cancer cell death. Anticancer Res. 2009;29(12):4909–4918. [PMC free article] [PubMed] [Google Scholar]
- 44.Rolleston FS, Newsholme EA. Effects of fatty acids, ketone bodies, lactate and pyruvate on glucose utilization by guinea-pig cerebral cortex slices. Biochem J. 1967;104(2):519–523. doi: 10.1042/bj1040519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.LeBlanc SJ, Duffield TF, Leslie KE, Bateman KG, Keefe GP, Walton JS, et al. Defining and diagnosing postpartum clinical endometritis and its impact on reproductive performance in dairy cows. J Dairy Sci. 2002;85(9):2223–2236. doi: 10.3168/jds.S0022-0302(02)74302-6. [DOI] [PubMed] [Google Scholar]
- 46.Spears JW, Weiss WP. Role of antioxidants and trace elements in health and immunity of transition dairy cows. Vet J. 2008;176(1):70–76. doi: 10.1016/j.tvjl.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 47.Batistel F, Osorio JS, Ferrari A, Trevisi E, Socha MT, Loor JJ. Better immunometabolic Status in Peripartal Cows Supplemented with Zn, Mn, and Cu from Amino Acid Complexes and Co from Co Glucoheptonate. PLoS One. 2016;11(5) doi: 10.1371/journal.pone.0155804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li B, Cui W, Tan Y, Luo P, Chen Q, Zhang C, et al. Zinc is essential for the transcription function of Nrf2 in human renal tubule cells in vitro and mouse kidney in vivo under the diabetic condition. J Cell Mol Med. 2014;18(5):895–906. doi: 10.1111/jcmm.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294(5548):1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 51.Sn M, Dupont J. Effect of zinc deficiency on prostaglandin synthesis in different organs of the rat. J Nutr. 1982;112(6):1098–1104. doi: 10.1093/jn/112.6.1098. [DOI] [PubMed] [Google Scholar]
- 52.Chanmugam P, Wheeler C, Hwang DH. The effect of zinc deficiency on prostaglandin synthesis in rat testes. J Nutr. 1984;114(2):2066–2072. doi: 10.1093/jn/114.11.2066. [DOI] [PubMed] [Google Scholar]
- 53.Kasimanickam R, Kasimanickam V, Kastelic PK. Mucin 1 and cytokines mRNA in endometrium of dairy cows with postpartum uterine disease or repeat breeding. Theriogenology. 2014;81(7):952–958. doi: 10.1016/j.theriogenology.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 54.DeSouza MM, Surveyor GA, Price RE, Julian J, Kardon R, Zhou X, et al. MUC1/episialin: a critical barrier in the female reproductive tract. J Reprod Immunol. 1999;45(2):127–158. doi: 10.1016/S0165-0378(99)00046-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PMNL Isolation: Blood (120 mL), collected in ACD solution A vacutainer tubes, was mixed well by inversion and placed on ice until PMN isolation (within ~30 min). Tubes were combined into three 50-mL conical tubes (Fisher Scientific, Pittsburgh, PA) and centrifuged at 918 × g for 30 min at 4 °C. The plasma, buffy coat, and approximately one-third of the red blood cells (RBC) were removed and discarded. Cells were lysed with 25 mL of deionized water at 4 °C, homogenized gently by inversion, and then 5 mL of 5 × PBS (pH 7.4) at 4 °C was added, in order to restore an iso-osmotic environment. The cell suspension was centrifuged at 330 × g for 10 min at 4 °C and the supernatants were decanted. Ten milliliters of 1 × PBS at 4 °C was added in each tube, homogenized until there was nothing attached to the bottom of the tube, and then the three tubes were combined in one. The cell suspension was centrifuged at 663 × g for 5 min at 4 °C and the supernatants were discarded. The remaining RBC were lysed with 8 mL of deionized water at 4 °C, homogenized gently by inversion and 2 mL of 5 × PBS at 4 °C was added. The samples were centrifuged at 663 × g for 5 min at 4 °C and the supernatant was discarded. Two subsequent washings using 10 mL of 1 × PBS at 4 °C were performed, centrifuged at 663 × g for 5 min at 4 °C and supernatant discarded. Prior to the last centrifugation, 100 μL of the cell suspension were aliquoted for further PMN concentration and cell viability analysis. (DOC 43 kb)
RNA extraction: Approximately 40 mg of frozen tissue was weighed and immediately placed in ice-cold 1 mL Qiazol reagent (Qiagen 75842; Qiagen Inc., Valencia, CA) for homogenization. After homogenization, the samples were centrifuged for 10 min at 12,000 × g at 4 °C to remove the insoluble material. The supernatant was transferred to a collection tube and incubated for 5 min on ice. Chloroform (200 µL) was added to each tube and the sample incubated at room temperature for 3 min. Subsequently, samples were centrifuged for 15 min at 12,000 × g at 4 °C, and the upper phase was transferred to a new collection tube without disturbing the mid and lower phases. A second wash was performed with 100% ethanol; 750 µL was added and transferred to a miRNeasy Mini Kit columns (Cat. No: 217004, Qiagen). Genomic DNA was removed on column from RNA samples with RNase-free DNase I, using the recommended protocol provided with the miRNeasy Mini Kit. RNA concentration was measured with a NanoDrop ND-1000 spectrophotometer (Thermo Fischer Scientific; Wilmington, DE), while the RNA quality was assessed using the Agilent 2100 Bioanalyzer system (Agilent Technologies, Santa Clara, CA). Samples of RNA used for analysis had an RNA integrity number ≥7.0. (DOC 44 kb)
Function of the genes measured in the PMNL. (DOC 67 kb)
Function of the genes measured in the endometrium. (DOC 61 kb)
Features of used primers for qPCR analysis. Hybridization position, sequence, and amplicon size of primers for Bos taurus used to analyze gene expression. (DOC 107 kb)
Sequencing results of PCR products from primers of genes used for this experiment. (DOC 62 kb)
qPCR performance among the genes measured in PMNL. (DOC 77 kb)
qPCR performance among the genes measured in the endometrium tissue. (DOC 71 kb)
Data Availability Statement
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.


