Abstract
Pituitary adenylate cyclase activating polypeptide (PACAP) signaling can increase guinea pig cardiac neuron excitability in part through extracellular signal-regulated kinase (ERK) activation. The present study examined the PACAP receptors and signaling cascades that stimulate guinea pig cardiac neuron ERK signaling using confocal microscopy to quantify PACAP-induced neuronal phosphorylated ERK (pERK) immunoreactivity. PACAP and maxadilan, but not vasoactive intestinal polypeptide (VIP), increased cardiac neuron pERK, implicating primary roles for PACAP selective PAC1 receptor (Adcyap1r1) signaling rather than VPAC receptors (Vipr1 and Vipr2) in the generation of cardiac neuron pERK. The adenylyl cyclase (AC) activator forskolin, but not the protein kinase C (PKC) activator phorbol myristate acetate (PMA) increased pERK, suggesting PAC1 receptor signal transduction via Gs/adenylyl cyclase (AC)/cAMP rather than Gq/phospholipase C (PLC) generated neuronal pERK. Activator and inhibitor studies suggested that the PACAP-mediated pERK activation was PKA-dependent rather than an exchange protein directly activated by a cAMP (EPAC), PKA-independent mechanism. The PACAP-induced pERK was inhibited by the clathrin inhibitor Pitstop2 to block receptor internalization and endosomal signaling. We propose that the PACAP-mediated MEK/ERK activation in cardiac neurons represents the summation of AC/cAMP/PKA signaling and PAC1 receptor internalization/activation of signaling endosomes.
Keywords: autonomic neuron, PACAP, PKA, PAC1 endocytosis, MAPK signaling
INTRODUCTION
The mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase 1/2 (ERK1/2) cascade is a primordial signaling network associated with nearly all facets of cellular development and function. Classically, following cell surface receptor activation, a cascading sequence of intracellular phosphorylation events is initiated via Ras, Raf and mitogen-activated ERK kinase (MEK), to result in the phosphorylation of ERK (pERK), a Ser/Thr kinase to more than 200 cytosolic and nuclear substrates resulting in the modulation of cellular transcription, translation and protein function in a temporal and spatial manner (Yoon and Seger, 2006; von Kriegsheim, Baiocchi, Birtwistle, et al., 2009). Neuronal ERK activation is a central means of transducing neurotransmitter and neurotrophic signals, and thereby participates in a wide range of activities including neural survival, proliferation, differentiation, protection and plasticity, hormonal regulation, autonomic function, nociception, learning and memory, and stress-related behavioral responses. Receptor tyrosine kinases, G-protein coupled receptors (GPCRs) and interleukin/cytokine gp130 receptors have been best studied with respect to pathway intersections to ERK activation, but even among these receptor classes, the mechanisms leading to ERK signaling can be quite varied (Goldsmith and Dhanasekaran, 2007; Wortzel and Seger, 2011). Several signaling pathways downstream of GPCR activation can intersect with the ERK pathway such as PLC/DAG/PKC, AC/cAMP/PKA or EPAC, and GPCR/arrestin-mediated endosomal signaling following receptor internalization (Goldsmith and Dhanasekaran, 2007) and notably, the preferential GPCR pathways appear cell-type specific.
PACAP binding at the selective G protein-coupled PAC1 receptor stimulates neuronal ERK phosphorylation and signaling (Barrie, Clohessy, Buensuceso, Rogers, and Allen, 1997; Villalba, Bockaert and Journot, 1997; Lazarovici, Jiang, and Fink, 1998; Bouschet, Perez, Fernandez, Bockaert, Eychene, and Journot, 2003; Obara, Horgan, and Stork, 2007; Monaghan, Mackenzie, Plevin and Lutz, 2008; May, Lutz, MacKenzie, Schutz, Dozark and Braas, 2010; Emery, Eiden, Mustafa, and Eiden, 2013). The PAC1 receptor can be dually coupled to Gq/11 and Gs to engage PLC and AC, respectively (Spengler, Waeber, Pantaloni, et al., 1993; Braas and May, 1999; Harmar, Fahrenkrug, Gozes, et al., 2012), and hence there are multiple cell type-specific options to transduce the ERK signaling cascade. PACAP/PAC1 receptor signaling has prominent and dynamic roles in autonomic function and we have shown previously that PACAP is co-localized with acetylcholine in virtually all preganglionic parasympathetic terminals innervating guinea pig postganglionic cardiac ganglia neurons (Braas, May, Harakall, Hardwick, and Parsons, 1998; Calupca, Vizzard, and Parsons, 2000). Both neurally-released and exogenously-applied PACAP can increase cardiac neuron excitability via PAC1 receptors (Braas, May, Harakall, Hardwick and Parsons, 1998; Tompkins, Ardell, Hoover, and Parsons, 2007). Importantly, the PACAP/PAC1 receptor-mediated increase in cardiac neuron excitability is suppressed following MEK1 inhibitor pretreatments, implicating activation of the MEK/ERK signaling cascade as one mechanism contributing to the modulation of excitability (Tompkins and Parsons, 2008). Despite the importance of the cardiac neuronal model in deciphering mechanisms controlling heart rate (i.e., bradycardia), and understanding PACAP/PAC1 receptor regulation of neuronal activity, the PAC1 receptor downstream mechanisms leading to neuronal ERK activation have not been clearly established. Various PACAP mechanisms have been described to drive ERK activation (Lazarovici, Jiang, and Fink, 1998; Bouschet, Perez, Fernandez, Bockaert, Eychene, and Journot, 2003; Shi, Rehmann, and Andres, 2006; Obara, Horgan, and Stork, 2007; Monaghan, Mackenzie, Plevin, and Lutz, 2008; May, Lutz, MacKenzie, Schutz, Dozark, and Braas, 2010; Emery and Eiden, 2012; Emery, Eiden, Mustafa, and Eiden, 2013). In a recent study using HEK cells stably expressing the human PACAP-selective PAC1 receptor (HEKPAC1 cells), we demonstrated that despite potent PACAP-mediated AC/cAMP generation, only PAC1 receptor-stimulated PLC/DAG/PKC activation and PAC1 receptor internalization/endosomal signaling activated the MEK/ERK cascade (May, Buttolph, Girard, Clason, and Parsons, 2014). Accordingly, from the many intracellular pathways, the current studies sought to identify the mechanisms by which PACAP signaling can recruit cardiac neuron MEK/ERK activation. Our results demonstrate that the PACAP-induced increase in cardiac neuron pERK immunoreactivity reflects solely PAC1 receptor signaling without contributions from the nonselective VPAC receptors, and that unlike mechanisms in other cell types, the resulting increase in cardiac ganglia pERK levels represents a summation of AC/cAMP/PKA and PACAP-induced PAC1 receptor internalization/endosomal signaling. From these observations, the mechanisms of PAC1 receptor-mediated ERK action appear cell specific.
METHODS
Animals
All experiments were performed using cardiac ganglia whole mount preparations from Hartley guinea pigs of mixed sex (250 – 350 g), following animal protocols approved by the University of Vermont Institutional Animal Care and Use Committee and methods described in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The guinea pigs were euthanized by isoflurane overdose and exsanguination; the hearts were quickly isolated and placed in cold Krebs solution (in mM: 121 NaCl, 5.9 KCl, 2.5 CaCl2, 1.2 MgCl2, 25 NaHCO3, 1.2 NaH2PO4, 8 glucose; pH 7.4 maintained by 95% O2 - 5% CO2 aeration) for cardiac ganglia dissection as whole mount explant preparations. All of the cardiac ganglia whole mount experiments were performed at 37°C.
Chemicals
PACAP27 (referred as PACAP throughout) was used exclusively in this study. The compounds obtained from commercial sources include: PACAP27 and vasoactive intestinal polypeptide (VIP) from American Peptide Co. (Sunnyvale CA); forskolin, BimI (bisindoylmaleimide I), PD 98059 (2′-amino-3′-methoxyflavone), PMA (phorbol myristate acetate) and EPAC activator (8-(4-chlorophenylthio)-2′-O-methyl-cAMP (8-pCPT-2′-O-cAMP)) from Calbiochem EMD Biosciences, Inc. (La Jolla CA); Pitstop 2 (N-[5-(4-bromobenzylidene)-4-oxo-4,5-dihydro-1,3-thiazol-2-yl]naphthalene-1-sulfonamide) from Abcam Biochemicals (Cambridge, UK); and KT 5720 from Tocris (Ellisville MO). Maxadilan was a kind gift from Dr. Ethan Lerner (Department of Dermatology, Massachusetts General Hospital, Boston MA). All drugs were applied directly to the bath solution from stocks prepared in DMSO (BimI, forskolin, Pitstop 2, PMA, EPAC activator and KT 5720) or water (PACAP, VIP, maxadilan).
Immunocytochemistry and confocal Imaging
Activation of neuronal MEK/ERK signaling by PACAP was quantified in cardiac ganglia immunostained with an antibody directed against pERK. Following different experimental treatments at 37°C, cardiac ganglia whole mounts were fixed by immersion in a 2% paraformaldehyde and 0.2% picric acid solution for 2 hours at 4°C, washed in blocking solution and treated with ice-cold methanol for 10 min before incubation overnight in 1:1,000 rabbit anti-phosphoERK1/2 (D13.14.4E, Cell Signaling Technology, Beverly, MA) for visualization with Cy3-conjugated donkey anti-rabbit IgG (1:500; Jackson ImmunoResearch, West Grove, PA).
For confocal imaging of pERK immunoreactivity, the guinea pig cardiac ganglia whole mounts were mounted on glass slides, cover slipped and imaged with a Nikon/Yokogawa CSU W1 Spinning Disk confocal using a Nikon Apo LWD 25X/1.10NA objective lens. Excitation was accomplished with a 100 mW 561 nm solid state laser, and emission collected from 590 nm-650 nm as 16-bit Nikon nd2 image files. Z-series in 0.4 μm-steps were taken on 5 random ganglia in each sample and regions of interest (ROIs) were generated for individual neurons at the axial midpoint adjacent to the nucleus and avoiding the cell membrane. Specifically, a single Z-slice was selected through the middle of the cell and a unique circular ROI was generated for each cell cytoplasm depending on cell size. Each ganglion generally contained 3–10 cells that met measurement criteria. All hardware settings were carefully maintained across samples and cross-checked by reviewing data header files. Data collection and analysis were all performed using Nikon Elements 4.30.10 (Build 1021). Data obtained from neurons in multiple cardiac ganglia from different whole mount preparations were averaged.
Statistics
Statistics were performed using GraphPad Prism statistical software (version 5.4; La Jolla, CA). Data are presented as mean ± SEM. Differences between means were either determined using an unpaired Students’ t-test or by one way ANOVA followed by Tukey post hoc analysis. Values were considered statistically significant at P < 0.05.
RESULTS
PACAP generation of pERK in cardiac neurons only requires PAC1 receptor activation
PACAP and VIP share receptor subtypes; the PAC1 receptor (Adcyap1r1) is selective for PACAP whereas PACAP and VIP can bind with near equal affinities at the nonselective VPAC1 and VPAC2 (Vipr1 and Vipr2, respectively) receptors (Harmar, Fahrenkrug, Gozes, et al., 2012). As PACAP and the PAC1 receptor selective agonist maxadilan, but not VIP, increase excitability of guinea pig cardiac neurons, the effects of PACAP on neuronal excitability are mediated solely through PAC1 receptor activation (Braas, May, Harakall, Hardwick, and Parsons, 1998; Hoover, Tompkins, and Parsons, 2009). In elucidating the mechanisms underlying the PACAP-stimulated responses, we have shown that neuronal pretreatments with the MEK inhibitor PD 98059 can suppress the PACAP-induced increase in cardiac neuron excitability (Tompkins and Parsons, 2008), consistent with PACAP activation of MEK/ERK signaling in the cardiac ganglia. Accordingly, we sought to demonstrate directly that PACAP can uniquely stimulate cardiac neuron ERK phosphorylation via PAC1 receptor activation in these preparations. We employed a quantitative immunocytochemical approach for these studies as the number of neurons in the ganglia explant preparations can vary widely (up to an order of magnitude), which precluded Western analyses.
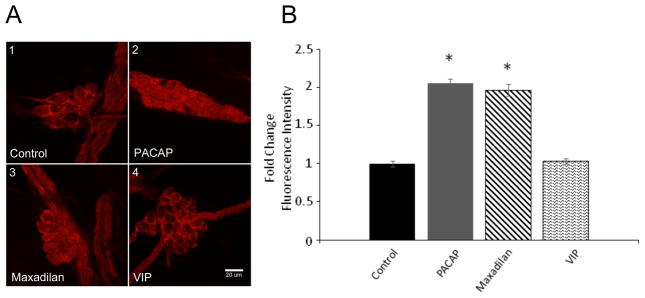
Under control basal conditions, limited endogenous neuronal pERK immunoreactivity was observed, whereas pERK immunoreactivity was very evident in the satellite cells encasing neurons within the ganglia and in non-neural cells in nerve bundles (Figure A1). Comparison of the confocal images presented in Figure 1A1 and 1A2 illustrates the increase in neuronal cytoplasmic pERK immunoreactivity following a 20-minute exposure to 25 nM PACAP. To determine whether the activation of MEK/ERK signaling by PACAP was mediated primarily through PAC1 receptor activation, the ability of the PAC1 receptor selective agonist, maxadilan (Moro and Lerner, 1997) and VIP, a VPAC1/VPAC2 agonist, to increase neuronal pERK immunoreactivity was compared to that noted for PACAP. As evident from Figure 1A3, exposure to 25 nM maxadilan for 20 minutes increased cytoplasmic pERK immunoreactivity above the control levels (Figure 1A1), whereas a 20-minute treatment with 25 nM VIP had no significant effect (Figure 1A4). Averaged results from analysis of multiple cells from different cardiac ganglia whole mount preparations exposed to PACAP, maxadilan or VIP are shown in Figure 1B. These results are consistent with PACAP activating the MEK/ERK cascade primarily through the PAC1 receptor to modulate guinea pig cardiac neuron excitability.
Figure 1.
PACAP activates MEK/ERK signaling through PAC1 receptors. A: Confocal images (~1 μm optical sections) of cardiac ganglia neurons immunostained with an antiserum against pERK. A1: A control cardiac ganglia preparation. A2: A cardiac ganglia preparation exposed to 25 nM PACAP for 20 minutes. A3: A cardiac ganglia preparation exposed to PAC1 receptor agonist maxadilan (25 nM) for 20 minutes. A4: A cardiac ganglia preparation exposed to 25 nM VIP for 20 minutes. Calibration bar in A4 equals 20 μm. Basal levels of pERK immunoreactivity were evident in axonal bundles and peri-neuronal cells. Only PAC1 receptor-mediated signaling increased cardiac ganglia neuron pERK immunoreactivity. B: Averaged fold-change in fluorescence intensity/area for the treatments shown in A. Results averaged from neurons in multiple cardiac ganglia from different whole mount preparations.
In addition to a PACAP-induced increase in cytoplasmic pERK immunoreactivity, nuclear pERK immunoreactivity also increased approximately 2-fold following the 20-minute exposure to 25 nM PACAP at 37°C. The present study focused on mechanisms responsible for the increase in cytosolic pERK immunoreactivity and no further analysis of nuclear pERK was undertaken.
Forskolin, but not PMA, stimulates pERK generation in guinea pig cardiac neurons
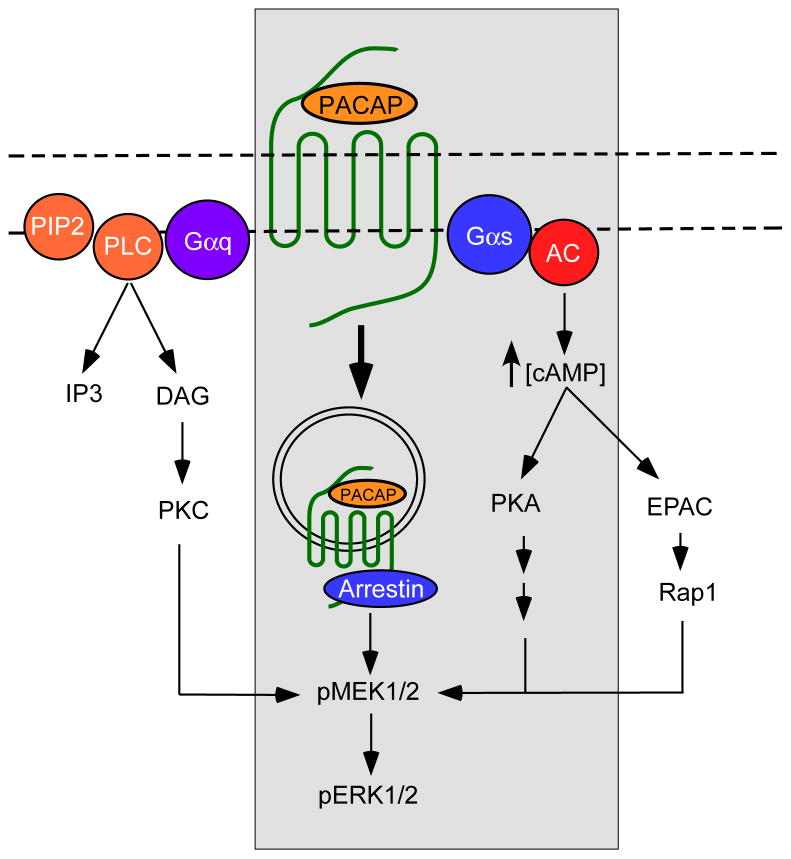
In our prior study of MEK/ERK activation in HEKPAC1 receptor expressing cells, PACAP activated adenylyl cyclase leading to a >100-fold increase in cAMP generation (May, Buttolph, Girard, Clason, and Parsons, 2014). However, treatment of the HEKPAC1 cells with the potent adenylyl cyclase (AC) activator forskolin or membrane permeable cAMP analogues failed to increase pERK; further, pretreatment of the cells with PKA inhibitors did not blunt the PACAP-induced generation of pERK. From these observations, activation of the AC/cAMP/PKA pathway did not contribute to the PACAP-induced generation of pERK in the HEKPAC1 cells (May, Buttolph, Girard, Clason, and Parsons, 2014). Notably, more detailed studies with PKC activators (PMA) and inhibitors (BimI), and with manipulations that blocked receptor-mediated endocytosis, all attenuated PACAP-stimulated ERK phosphorylation (May, Buttolph, Girard, Clason, and Parsons, 2014). Given the apparent diversity of PAC1 receptor mechanisms to ERK activation (see Figure 4), we first tested whether the AC/cAMP/PKA or EPAC, and PLC/DAG/PKC pathways contributed to the PACAP-induced pERK generation in the cardiac neurons.
Figure 4.
Schematic of PACAP/PAC1 receptor signaling to ERK activation. The PACAP/PAC1 receptor is dually coupled to Gs to activate adenylyl cyclase (AC) activity and Gq to stimulate phospholipase C (PLC) activity. From increased AC activity, the resulting increase in cAMP levels can engage PKA or EPAC (PKA-independent) pathways to ERK signaling; cardiac neurons employ the former PKA-dependent pathway. Despite potent activation of PLC signaling cascades, cardiac neuron PAC1 receptors do not appear to stimulate PKC mechanisms to ERK. However, following activation, PAC1 receptors can be associated with arrestin scaffolding molecules and internalized as signaling endosomes for long term MEK/ERK signaling. GPCR receptor signaling mechanisms to ERK is cell type specific; the pathways identified for the guinea pig cardiac neurons are boxed.
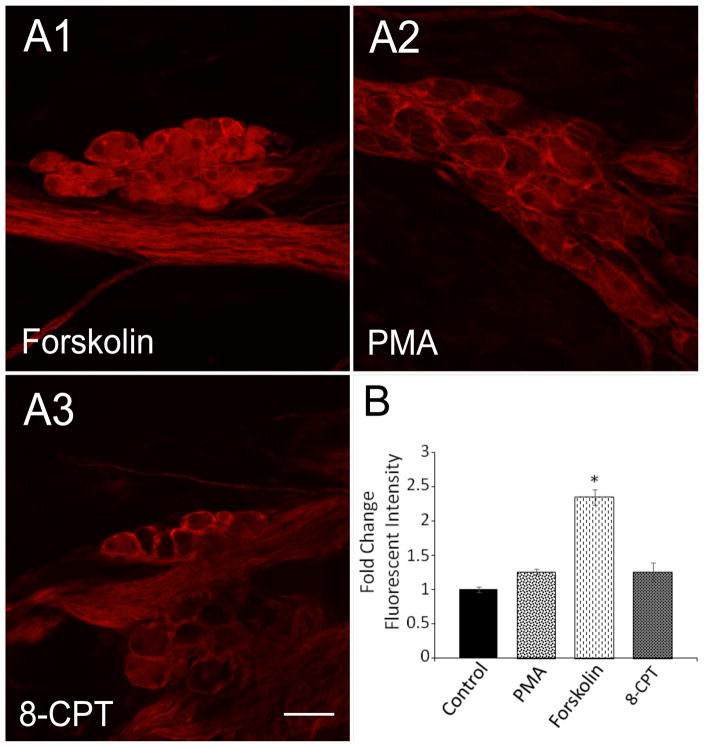
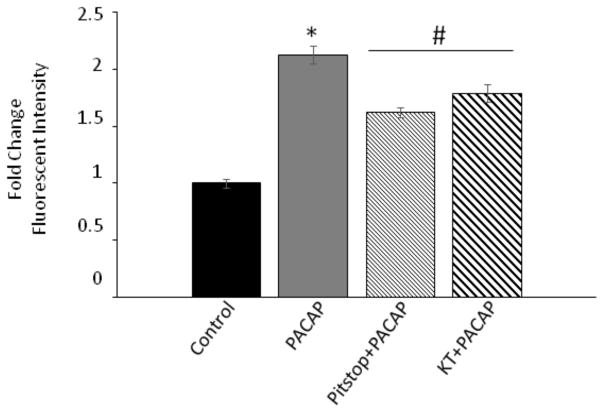
Unlike the HEKPAC1 receptor cells, adenylyl cyclase activation in cardiac ganglia explants with 5 μM forskolin for 20 minutes significantly increased neuronal pERK immunoreactivity levels nearly 2.5-fold (Figure 2), implicating AC and cAMP generation as a mechanism to the ERK pathway. In addition to activation of PKA, there are PKA-independent mechanisms including cAMP activation of EPAC that may lead to ERK stimulation. Hence to test the potential contribution cAMP/PKA roles in PACAP-mediated generation of pERK, the cardiac ganglia explants were pretreated with the PKA inhibitor KT 5720 (1 μM) before PACAP exposure and assessment of neuronal pERK immunoreactivity. Following KT5902 pretreatment, the averaged PACAP-induced increase in pERK immunoreactivity for neurons in 4 different cardiac ganglia whole mount preparations diminished significantly to approximately 50% of levels observed for cells exposed to PACAP alone (Figure 3). Hence even though inhibition of PKA reduced PACAP-induced pERK levels in the cardiac neurons, the PACAP effects were not completely blocked suggesting additional complementary PACAP mechanisms to ERK. To establish if a different arm of the cAMP pathway can also activate ERK signaling through an exchange protein activated by cAMP (EPAC); (Shi, Rehmann, and Andres, 2006), we tested whether the specific EPAC activator 8-(4-chlorophenylthio)-2′-O-methyl-cAMP (100 μM; 8-pCPT-2′-O-cAMP) can directly lead to cardiac neuron ERK phosphorylation. Following treatment with the EPAC activator for 20 minutes, cytosolic pERK levels in the cardiac neurons were not significantly different from those in control untreated neurons (Figure 2). This observation suggested that EPAC activation did not contribute to pERK generation in the cardiac neurons.
Figure 2.
cAMP-dependent, but EPAC-independent mechanisms increase cardiac neuron ERK phosphorylation. Cardiac ganglia whole mount preparations were treated with the adenylyl cyclase activator forskolin (5 μM) (A1), the protein kinase C activator PMA (500 nM) (A2) or the EPAC activator 8-(4-chlorophenylthio)-2′-O-methyl-cAMP (100 μM) (A3, 8-CPT) for 20 minutes at 37°C before fixation and immunocytochemical processing for pERK. Only forskolin treatments increased cardiac neuron pERK levels. B: Averaged fold change in fluorescence intensity/area for the different conditions shown in A. Data presented as averaged results from cells in multiple cardiac ganglia from different whole mount preparations.
Figure 3.
Inhibition of PKA or endocytosis mechanisms diminishes PACAP-induced pERK generation in cardiac neurons. Cardiac ganglia whole mount preparations were exposed to 25 nM PACAP alone or pretreated with the clathrin-mediated endocytosis inhibitor Pitstop2 (15 μM) or the PKA inhibitor KT5902 (1 μM) for 15 minutes before addition of 25 nM PACAP + inhibitor for 20 minutes at 37°C and immunocytochemical processing. Each inhibitor blocked PACAP-induced ERK phosphorylation approximately 50%. Data presented as averaged results from cells in multiple cardiac ganglia from different whole mount preparations under each condition (PACAP, 4 whole mount preparations, Pitstop2/PACAP, 3 whole mount preparations; KT5902/PACAP, 4 whole mount preparations).
PACAP activation of PKC did not appear to participate in the process as ganglia treatment with 500 nM PMA to activate directly PKC had no effect on pERK levels in the cardiac neurons (Figure 2). In sum, these results suggested that a PACAP-induced increase in guinea pig cardiac neuron MEK/ERK signaling was mediated in part by AC/cAMP via PKA and not by either EPAC or PKC signaling.
PACAP/PAC1 receptor-induced endosomal signaling contributes to cardiac neuron ERK activation
We showed previously that the PACAP-induced increase in cardiac neuron excitability and the PACAP-induced increase in HEKPAC1 cell pERK levels could be blunted following pretreatments with the small peptide clathrin inhibitor Pitstop2 (Merriam, Baran, Girard, Hardwick, May, and Parsons, 2013; May, Buttolph, Girard, Clason, and Parsons, 2014). These observations were consistent with the hypothesis that PAC1 receptor internalization and formation of a signaling endosome contributed to both actions of PACAP (Merriam, Baran, Girard, Hardwick, May, and Parsons, 2013; May, Buttolph, Girard, Clason, and Parsons, 2014). Consequently, we next tested whether treatment with 15 μM Pitstop2 could blunt the PACAP-induced increase in pERK immunoreactivity in the cardiac neurons. As summarized in Figure 3, pretreatment with Pitstop2 also significantly suppressed the PACAP-induced increase in cytosolic pERK immunoreactivity approximately 50%. Thus, in cardiac neurons, in addition to PACAP activation of the AC/cAMP/PKA pathway, PACAP stimulation of PAC1 receptor internalization and formation of a signaling endosome was an important component to the peptide-induced increase in cardiac neuron pERK immunoreactivity.
DISCUSSION
PACAP/PAC1 receptor mediated ERK signaling has been implicated in a variety of important neuronal responses including survival, proliferation and differentiation during development, neuroprotection following injury paradigms, nociceptive and behavioral responses, and channel regulation to gate neuronal excitability. The PACAP/PAC1 receptor activation can stimulate ERK for long durations, and as GPCRs can generate a myriad of signaling network schemes to engage the ERK in a cell specific manner, we sought to delineate some of the PAC1 receptor pathways that regulate ERK-stimulated neuronal excitability. The key observations in this study are (1) that the generation of MEK/ERK signaling by PACAP in guinea pig cardiac neurons uniquely involves PAC1 receptor activation and (2) that among signaling cascades downstream of the PAC1 receptor, activation of AC/cAMP/PKA and endosomal signaling following receptor internalization represent major mechanisms leading to PACAP-induced increase in cardiac neuron pERK immunoreactivity.
Although PACAP stimulates HEKPAC1 cell AC activity and can increase cAMP production nearly 100-fold, surprisingly the cAMP pathway did not contribute significantly to the elevation of pERK; an observation corroborated by the inability for forskolin to stimulate ERK in these cells (May, Buttolph, Girard, Clason, and Parsons, 2014). In contrast, PAC1 receptor Gq coupling to PLC/DAG/PKC in the HEKPAC1 receptor cell line was able to fully engage the ERK pathway as shown by direct PMA stimulation of PKC to activate ERK, and by the abilities for the PKC inhibitor BimI to block PACAP-mediated ERK phosphorylation (May, Buttolph, Girard, Clason, and Parsons, 2014). These PAC1 receptor plasma membrane signaling effectors were reversed in the cardiac ganglion neurons; whereas PLC/DAG/PKC had no apparent roles in ERK activation, AC/cAMP/PKA signaling did contribute to the PACAP-induced increase in cardiac neuron pERK immunoreactivity. An AC/cAMP/PKA-mediated mechanism to ERK activation has been described for a number of different neural cell types including PC12 pheochromocytoma cells (Bouschet, Perez, Fernandez, Bockaert, Eychene, and Journot, 2003) cerebellar granule cells (Botia, Basille, Allais, et al., 2007; Obara, Horgan, and Stork, 2007) sympathetic neurons (May, Lutz, MacKenzie, Schutz, Dozark and Braas, 2010) and hippocampal neurons (Gupte, Kadunganattil, Shepherd, et al. 2016). There are also cAMP-dependent but PKA-independent means to ERK activation described for PC12 cells (Lazarovici, Jiang, and Fink, 1998; Emery and Eiden, 2012; Emery, Eiden, Mustafa, and Eiden, 2013), SYHY neuroblastoma (Monaghan, Mackenzie, Plevin and Lutz, 2008), including mechanisms via EPAC, which has been described for PACAP signaling in P6 pheochromocytoma cells (Shi, Rehmann, and Andres, 2006). However, the EPAC activator 8-pCPT-2′-O-cAMP did not stimulate cardiac neuron ERK activation, an observation consistent with previous suggestions that EPAC expression is low in neural crest-derived cells and hence may not represent prevalent signaling mechanisms to ERK (Wang, Dillon, Pokala, et al., 2006; Liu, Takahashi, Li, et al., 2008). There may be other PKA-independent routes to ERK signaling including the recently described plasma membrane RapGEF2 (Emery, Eiden, Mustafa, and Eiden, 2013), but whether this pathway represents a separate and independent pathway or an integrated component of existing mechanisms is still relatively unclear and requires additional studies.
Cellular ERK signaling via PLC/DAG/PKC activation is perhaps best studied (Lazarovic, Jiang, and Fink, 1998; Bouschet, Perez, Fernandez, Bockaert, Eychene and Journot, 2003; Goldsmith and Dhanasekaran, 2007). In line with many other studies, PACAP stimulation of ERK in HEKPAC1 cell was mediated in part by PKC as the PKC activator PMA potently stimulated pERK levels and the PACAP-stimulated ERK responses could be blocked by PKC inhibitors (May, Buttolph, Girard, Clason, and Parsons, 2014). Yet despite abilities for the PAC1 receptor to couple Gq and activate the PLC pathway, the PLC/DAG/PKC pathways do not appear to have significant roles in cardiac neuron ERK activation. The PKC inhibitor BimI did not block PACAP-induced increases in cardiac neuron pERK immunoreactivity (data not shown), and direct PKC activation with PMA had no effects on cardiac ganglia pERK levels. These results are consistent with our previous PACAP data regulating cardiac neuron excitability. Even though PACAP-mediated cardiac neuron excitability is sensitive to ERK signaling, PACAP does not activate PLC signaling in the guinea pig cardiac neurons (Tompkins, Hardwick, Locknar, Merriam, and Parsons, 2006; Tompkins and Parsons 2008). Again, a similar result was observed in primary sympathetic neurons in which PKC inhibitor BimI had no effects on PACAP-mediated ERK activation (May, Lutz, MacKenzie, Schutz, Dozark, and Braas, 2010). Whether this reflects characteristics of neural crest derived cells is also unclear but again speaks to the cell specific mechanisms to ERK signaling.
GPCRs appear unique in that some receptors may continue to transduce signals upon internalization and trafficking in cellular endosomes (Murphy, Padilla, Hasdemir, Cottrell, and Bunnett, 2009; Calebiro, Nikolaev, Persani, and Lohse, 2010; Irannejad, Tomshine, Tomshine, et al., 2013; Irannejad and von Zastrow, 2014; Tsvetanova, Irannejad, and Zastrow, 2015). Class B GPCRs including PAC1 receptors have strong signature Ser residue clusters in the cytoplasmic carboxyl-terminal tail of the receptor for high affinity binding to beta-arrestins (Oakley, Laporte, Holt, Barak, and Caron, 1999; Oakley, Laporte, Holt, Caron, and Barak, 2000; Shenoy and Lefkowitz, 2003), which can act as scaffolding proteins for MEK/ERK assembly and signal generation. PAC1 receptors can associate with arrestin (Broca, Quoyer, Costes, et al., 2009) and our preliminary studies have suggested that internalized PAC1 receptors are associated with early endosomes and can be colocalized with beta-arrestin immunoreactivity (data not shown). The temporal dynamics of PAC1 receptor interactions and trafficking in endosomes have not been well studied, but may be critical as transient versus sustained cellular ERK activation has been associated with proliferation and differentiation, respectively (Marshall, 1995; Kao, Jaiswal, Kolch, and Landreth, 2001; Pellegrino and Stork, 2006). The abilities for PAC1 receptors to generate long duration ERK signals have implications in PACAP-mediated neurochemical, neuroarchitectural and synaptic plasticity which may participate in the maladaptive responses to chronic stress and other neurophysiological challenges leading to psychopathologies. Treatment of HEKPAC1 receptor cells with the clathrin inhibitor Pitstop2 suppressed PACAP-induced PAC1 internalization, attenuated pERK activation and blunted the PACAP-induced increase in cardiac neuron excitability (Merriam, Baran, Girard, Hardwick, May, and Parsons, 2013; May, Buttolph, Girard, Clason, and Parsons, 2014). Similarly, we show that Pitstop2 pretreatment significantly decreased the PACAP-induced increase in cardiac neuron pERK immunoreactivity. These results were also consistent with previous work in primary sympathetic neurons in which clathrin-mediated endocytosis blockade with a variety of inhibitors including chlorpromazine and monodansylcadaverine attenuated PACAP-mediated ERK phosphorylation, whereas inhibitors to caveolae-mediated endocytosis had no apparent effects (May, Lutz, MacKenzie, Schutz, Dozark, and Braas, 2010). Taken together, these observations indicate that PACAP/PAC1 internalization and formation of a signaling endosome may be a general mechanism contributing to the PACAP recruitment of MEK/ERK signaling in diverse cell types.
In conclusion, despite the many signaling cascades downstream of the PAC1 receptor, the present results support the view that both the AC/cAMP/PKA transduction cascade and PAC1 internalization/endosomal signaling contribute to the PACAP-induced activation of MEK/ERK signaling in cardiac neurons (Figure 4). Unique to this study is the direct observation that blunting agonist-induced internalization of the PAC1 receptor by pretreatment with the clathrin inhibitor Pitstop2 reduces MERK/ERK activation; a result that provides further support for the view that the internalization of GPCRs and formation of signaling endosomes can act as scaffolds for recruitment of intracellular transduction cascades.
Acknowledgments
This work was supported in part by NIH grant NIGMS P30 GM103498/NCRR P30 RR032135 (RLP).
Footnotes
Author contributions: RLP and VM designed the experiments; TAC and BMG collected and analysed the data; RLP and VM interpreted the data; RLP and VM wrote the paper with contributions from TAC and BMG. All authors approved the final version.
The authors declare that they have no conflict of interest.
References
- Barrie AP, Clohessy AM, Buensuceso CS, Rogers MV, Allen JM. Pituitary adenylyl cyclase-activating peptide stimulates extracellular signal-regulated kinase 1 or 2 (ERK1/2) activity in a Ras-independent, mitogen-activated protein Kinase/ERK kinase 1 or 2-dependent manner in PC12 cells. J Biol Chem. 1997;272:19666–71. doi: 10.1074/jbc.272.32.19666. [DOI] [PubMed] [Google Scholar]
- Botia B, Basille M, Allais A, Raoult E, Falluel-Morel A, Galas L, Jolivel V, Wurtz O, Komuro H, Fournier A, Vaudry H, Burel D, Gonzalez BJ, Vaudry D. Neurotrophic effects of PACAP in the cerebellar cortex. Peptides. 2007;28:746–52. doi: 10.1016/j.peptides.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Bouschet T, Perez V, Fernandez C, Bockaert J, Eychene A, Journot L. Stimulation of the ERK pathway by GTP-loaded Rap1 requires the concomitant activation of Ras, protein kinase C, and protein kinase A in neuronal cells. J Biol Chem. 2003;278:4778–85. doi: 10.1074/jbc.M204652200. [DOI] [PubMed] [Google Scholar]
- Braas KM, May V. Pituitary adenylate cyclase-activating polypeptides directly stimulate sympathetic neuron neuropeptide Y release through PAC(1) receptor isoform activation of specific intracellular signaling pathways. J Biol Chem. 1999;274:27702–10. doi: 10.1074/jbc.274.39.27702. [DOI] [PubMed] [Google Scholar]
- Braas KM, May V, Harakall SA, Hardwick JC, Parsons RL. Pituitary adenylate cyclase-activating polypeptide expression and modulation of neuronal excitability in guinea pig cardiac ganglia. J Neurosci. 1998;18:9766–9779. doi: 10.1523/JNEUROSCI.18-23-09766.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broca C, Quoyer J, Costes S, Linck N, Varrault A, Deffayet PM, Bockaert J, Dalle S, Bertrand G. Beta-Arrestin 1 is required for PAC1 receptor-mediated potentiation of long-lasting ERK1/2 activation by glucose in pancreatic beta-cells. J Biol Chem. 2009;284:4332–42. doi: 10.1074/jbc.M807595200. [DOI] [PubMed] [Google Scholar]
- Calebiro D, Nikolaev VO, Persani L, Lohse MJ. Signaling by internalized G-protein-coupled receptors. Trends Pharmacol Sci. 2010;3:221–8. doi: 10.1016/j.tips.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Calupca MA, Vizzard MA, Parsons RL. Origin of pituitary adenylate cyclase-activating polypeptide (PACAP)-immunoreactive fibers innervating guinea pig parasympathetic cardiac ganglia. J Comp Neurol. 2000;423:26–39. [PubMed] [Google Scholar]
- Emery AC, Eiden LE. Signaling through the neuropeptide GPCR PAC1 induces neuritogenesis via a single linear cAMP- and ERK-dependent pathway using a novel cAMP sensor. FASEB J. 2012;26:3199–3211. doi: 10.1096/fj.11-203042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery AC, Eiden MV, Mustafa T, Eiden LE. Rapgef2 connects GPCR-mediated cAMP signals to ERK activation in neuronal and endocrine cells. Sci Signal. 2013;6:ra51. doi: 10.1126/scisignal.2003993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith ZG, Dhanasekaran DN. G protein regulation of MAPK networks. Oncogene. 2007 May 14;26(22):3122–42. doi: 10.1038/sj.onc.1210407. [DOI] [PubMed] [Google Scholar]
- Gupte RP, Kadunganattil S, Shepherd AJ, Merrill R, Planer W, Bruchas MR, Strack S, Mohapatra DP. Convergent phosphomodulation of the major neuronal dendritic potassium channel Kv4.2 by pituitary adenylate cyclase-activating polypeptide. Neuropharmacology. 2016;101:291–308. doi: 10.1016/j.neuropharm.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmar AJ, Fahrenkrug J, Gozes I, Laburthe M, May V, Pisegna JR, Vaudry D, Vaudry H, Waschek JA, Said SI. Pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide: IUPHAR review 1. Br J Pharmacol. 2012;166:4–17. doi: 10.1111/j.1476-5381.2012.01871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover DB, Tompkins JD, Parsons RL. Differential activation of guinea pig intrinsic cardiac neurons by the PAC1 agonists maxadilan and pituitary adenylate cyclase-activating polypeptide 27 (PACAP27) J Pharmacol Exp Ther. 2009;331:197–203. doi: 10.1124/jpet.109.155747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irannejad R, Tomshine JC, Tomshine JR, Chevalier M, Mahoney JP, Steyaert J, Rasmussen SG, Sunahara RK, El-Samad H, Huang B, von Zastrow M. Conformational biosensors reveal GPCR signalling from endosomes. Nature. 2013;495:534–8. doi: 10.1038/nature12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irannejad R, von Zastrow M. GPCR signaling along the endocytic pathway. Curr Opin Cell Biol. 2014;27:109–16. doi: 10.1016/j.ceb.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao S, Jaiswal RK, Kolch W, Landreth GE. Identification of the mechanisms regulating the differential activation of the mapk cascade by epidermal growth factor and nerve growth factor in PC12 cells. J Biol Chem. 2001;276:18169–77. doi: 10.1074/jbc.M008870200. [DOI] [PubMed] [Google Scholar]
- Lazarovici P, Jiang H, Fink D., Jr The 38-amino-acid form of pituitary adenylate cyclase-activating polypeptide induces neurite outgrowth in PC12 cells that is dependent on protein kinase C and extracellular signal-regulated kinase but not on protein kinase A, nerve growth factor receptor tyrosine kinase, p21(ras) G protein, and pp60(c-src) cytoplasmic tyrosine kinase. Mol Pharmacol. 1998;54:547–58. doi: 10.1124/mol.54.3.547. [DOI] [PubMed] [Google Scholar]
- Liu C, Takahashi M, Li Y, Song S, Dillon TJ, Shinde U, Stork PJ. Ras is required for the cyclic AMP-dependent activation of Rap1 via Epac2. Mol Cell Biol. 2008;28:7109–25. doi: 10.1128/MCB.01060-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–85. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- May V, Buttolph TR, Girard BM, Clason TA, Parsons RL. PACAP-induced ERK activation in HEK cells expressing PAC1 receptors involves both receptor internalization and PKC signaling. Am J Physiol Cell Physiol. 2014;306:C1068–C1079. doi: 10.1152/ajpcell.00001.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May V, Lutz E, MacKenzie C, Schutz KC, Dozark K, Braas KM. Pituitary adenylate cyclase-activating polypeptide (PACAP)/PAC1HOP1 receptor activation coordinates multiple neurotrophic signaling pathways: Akt activation through phosphatidylinositol 3-kinase gamma and vesicle endocytosis for neuronal survival. J Biol Chem. 2010;285:9749–61. doi: 10.1074/jbc.M109.043117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merriam LA, Baran CN, Girard BM, Hardwick JC, May V, Parsons RL. Pituitary adenylate cyclase 1 receptor internalization and endosomal signaling mediate the pituitary adenylate cyclase activating polypeptide-induced increase in guinea pig cardiac neuron excitability. J Neurosci. 2013;33:4614–4622. doi: 10.1523/JNEUROSCI.4999-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan TK, Mackenzie CJ, Plevin R, Lutz EM. PACAP-38 induces neuronal differentiation of human SH-SY5Y neuroblastoma cells via cAMP–mediated activation of ERK and p38 MAP kinases. J Neurochem. 2008;104:74–88. doi: 10.1111/j.1471-4159.2007.05018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro O, Lerner EA. Maxidilan the vasodilator from sand flies, is a specific pituitary adenlyate cyclase activating peptide type I receptor agonist. J Biol Chem. 1997;272:966–970. doi: 10.1074/jbc.272.2.966. [DOI] [PubMed] [Google Scholar]
- Murphy JE, Padilla BE, Hasdemir B, Cottrell GS, Bunnett NW. Endosomes: a legitimate platform for the signaling train. Proc Natl Acad Sci U S A. 2009;106:17615–22. doi: 10.1073/pnas.0906541106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG. Association of beta-arrestin with G protein-coupled receptors during clathrin-mediated endocytosis dictates the profile of receptor resensitization. J Biol Chem. 1999;274:32248–57. doi: 10.1074/jbc.274.45.32248. [DOI] [PubMed] [Google Scholar]
- Oakley RH, Laporte SA, Holt JA, Caron MG, Barak LS. Differential affinities of visual arrestin, beta arrestin1, and beta arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J Biol Chem. 2000;275:17201–10. doi: 10.1074/jbc.M910348199. [DOI] [PubMed] [Google Scholar]
- Obara Y, Horgan AM, Stork PJ. The requirement of Ras and Rap1 for the activation of ERKs by cAMP, PACAP, and KCl in cerebellar granule cells. J Neurochem. 2007;101:470–82. doi: 10.1111/j.1471-4159.2006.04390.x. [DOI] [PubMed] [Google Scholar]
- Pellegrino MJ, Stork PJ. Sustained activation of extracellular signal-regulated kinase by nerve growth factor regulates c-fos protein stabilization and transactivation in PC12 cells. J Neurochem. 2006;99:1480–93. doi: 10.1111/j.1471-4159.2006.04250.x. [DOI] [PubMed] [Google Scholar]
- Shenoy SK, Lefkowitz RJ. Multifaceted roles of beta-arrestins in the regulation of seven-membrane-spanning receptor trafficking and signalling. Biochem J. 2003;375:503–15. doi: 10.1042/BJ20031076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi GX, Rehmann H, Andres DA. A novel cyclic AMP-dependent Epac-Rit signaling pathway contributes to PACAP38-mediated neuronal differentiation. Mol Cell Biol. 2006;26:9136–47. doi: 10.1128/MCB.00332-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spengler D, Waeber C, Pantaloni C, Holsboer F, Bockaert J, Seeburg PH, Journot L. Differential signal transduction by five splice variants of the PACAP receptor. Nature. 1993;365:170–5. doi: 10.1038/365170a0. [DOI] [PubMed] [Google Scholar]
- Tompkins JD, Ardell JL, Hoover DB, Parsons RL. Neurally released pituitary adenylate cyclase-activating polypeptide enhances guinea pig intrinsic cardiac neurone excitability. J Physiol. 2007;582:87–93. doi: 10.1113/jphysiol.2007.134965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins JD, Hardwick JC, Locknar SA, Merriam LA, Parsons RL. Ca2+ influx, but not Ca2+ release from internal stores, is required for the PACAP-induced increase in excitability in guinea pig intracardiac neurons. J Neurophysiol. 2006;95:2134–2142. doi: 10.1152/jn.01077.2005. [DOI] [PubMed] [Google Scholar]
- Tompkins JD, Parsons RL. Identification of intracellular signaling cascades mediating the PACAP-induced increase in guinea pig cardiac neuron excitability. J Mol Neurosci. 2008;36:292–298. doi: 10.1007/s12031-008-9086-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetanova NG, Irannejad R, Zastrow M. G protein-coupled receptor (GPCR) signaling via heterotrimeric G proteins from endosomes. J Biol Chem. 2015;290:6689–96. doi: 10.1074/jbc.R114.617951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba M, Bockaert J, Journot L. Pituitary adenylate cyclase-activating polypeptide (PACAP-38) protects cerebellar granule neurons from apoptosis by activating the mitogen-activated protein kinase (MAP kinase) pathway. J Neurosci. 1997;1:83–90. doi: 10.1523/JNEUROSCI.17-01-00083.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kriegsheim A, Baiocchi D, Birtwistle M, Sumpton D, Bienvenut W, Morrice N, Yamada K, Lamond A, Kalna G, Orton R, Gilbert D, Kolch W. Cell fate decisions are specified by the dynamic ERK interactome. Nat Cell Biol. 2009;11:1458–64. doi: 10.1038/ncb1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Dillon TJ, Pokala V, Mishra S, Labudda K, Hunter B, Stork PJ. Rap1-mediated activation of extracellular signal-regulated kinases by cyclic AMP is dependent on the mode of Rap1 activation. Mol Cell Biol. 2006;26:2130–45. doi: 10.1128/MCB.26.6.2130-2145.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wortzel I, Seger R. The ERK Cascade: Distinct Functions within Various Subcellular Organelles. Genes Cancer. 2011;2:195–209. doi: 10.1177/1947601911407328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors. 2006;24:21–44. doi: 10.1080/02699050500284218. [DOI] [PubMed] [Google Scholar]