Abstract
To further understand the risk of stomach cancer after fractionated high-dose radiotherapy, we pooled individual-level data from three recent stomach cancer case-control studies. These studies were nested in cohorts of five-year survivors of first primary Hodgkin lymphoma (HL), testicular cancer (TC) or cervical cancer (CX) from seven countries. Detailed data were abstracted from patient records and radiation doses were reconstructed to the site of the stomach cancer for cases and to the corresponding sites for matched controls. Among 327 cases and 678 controls, mean doses to the stomach were 15.3 Gy, 24.7 Gy and 1.9 Gy, respectively, for Hodgkin lymphoma, testicular cancer and cervical cancer survivors, with an overall mean dose of 10.3 Gy. Risk increased with increasing radiation dose to the stomach cancer site (P < 0.001) with no evidence of nonlinearity or of a downturn at the highest doses (≥35 Gy). The pooled excess odds ratio per Gy (EOR/Gy) was 0.091 [95% confidence interval (CI): 0.036–0.20] with estimates of 0.049 (95% CI: 0.007–0.16) for Hodgkin lymphoma, 0.27 (95% CI: 0.054–1.44) for testicular cancer and 0.096 (95% CI: −0.002–0.39) for cervical cancer (P homogeneity = 0.25). The EOR/Gy increased with time since exposure (P trend = 0.004), with an EOR/Gy of 0.38 (95% CI: 0.12–1.04) for stomach cancer occurring ≥20 years postirradiation corresponding to odds ratios of 4.8 and 10.5 at radiation doses to the stomach of 10 and 25 Gy, respectively. Of 111 stomach cancers occurring ≥20 years after radiotherapy, 63.8 (57%) could be attributed to radiotherapy. Our findings differ from those based on Japanese atomic-bomb survivors, where the overall EOR/Gy was higher and where there was no evidence of an increase with time since exposure. By pooling data from three studies, we demonstrated a clear increase in stomach cancer risk over a wide range of doses from fractionated radiotherapy with the highest risks occurring many years after exposure. These findings highlight the need to directly evaluate the health effects of high-dose fractionated radiotherapy rather than relying on the data of persons exposed at low and moderate acute doses.
INTRODUCTION
Risk estimates for radiation-related stomach cancer are based primarily on data from the Life Span Study (LSS) cohort of Japanese A-bomb survivors (1, 2). Because the doses of LSS survivors were received as a single instantaneous exposure and were generally under 3 Gy, LSS-based risk estimates may not be appropriate for estimating risks from the fractionated high doses received in therapeutic settings. In addition, baseline stomach cancer rates are much higher in Japan than in most Western countries, leading to uncertainty in how to use LSS data to estimate risks in other countries.
To provide direct data on the stomach cancer radiation-dose response in cancer survivors treated with radiotherapy, as well as additional data on radiation-related stomach cancer in Western populations, three case-control studies were launched in populations of five-year survivors of Hodgkin lymphoma (HL) (3), testicular cancer (TC) (4) and cervical cancer (CX) (5). To our knowledge, these are the first and only stomach cancer studies in cancer survivors with individual data on dose to the stomach tumor site. Hodgkin lymphoma, testicular cancer and cervical cancer were selected because cohort studies had revealed elevated stomach cancer risks and because the radiation fields used to treat them could be predicted to result in the wide range of doses needed for evaluation of the dose-response relationship with particularly high doses for patients treated with abdominal fields (often ≥25 Gy). All three studies, which used comparable epidemiologic and dosimetric methods, showed statistically significant increases in stomach cancer risk with increasing radiation dose to the stomach cancer site. None of the studies indicated departure from a linear radiation dose-response or dependency of this response on age at first cancer diagnosis, time since exposure or attained age, but statistical power for addressing these issues was limited in the individual studies.
Because of changes in radiotherapy techniques aimed at reducing doses to the stomach and other organs (6) and because of the general interest in risks at lower doses, it is important to quantify risk over the full range of radiation doses to the stomach included in these studies. Therefore, we present results of pooled analyses of these data, comprised of all the relevant quantitative data on the stomach cancer radiation dose response in cancer survivors. The overall objective of these analyses was to provide a summary of these data and to increase the contribution of these studies to understanding radiation-related stomach cancer. More specific objectives were to investigate statistical compatibility of the radiation dose response among the three studies and to combine all or selected portions of the data to obtain more precise estimates of risk and a more powerful assessment of variables that might modify the radiation dose response.
MATERIALS AND METHODS
Study methods have been described previously elsewhere (3–5). Briefly, population-based registries of five-year survivors of Hodgkin lymphoma, testicular cancer or cervical cancer in Denmark, Finland, Iowa (U.S.), Ontario (Canada) and Sweden, and Hodgkin lymphoma and testicular cancer survivors in Norway were used to identify cases and matched controls. Cases and controls from a previously published study in the Netherlands (7) were also included for Hodgkin lymphoma and testicular cancer. Controls were individually matched 2:1 by registry, sex, date of birth and first cancer diagnosis (each ±5 years), and survival without a subsequent primary cancer at least as long as the interval from first cancer diagnosis to stomach cancer diagnosis for the matched case. This study was approved by the institutional review board at each study center and exempted from review by the National Cancer Institute (NCI) because analyses used existing de-identified data.
Radiation Dose Reconstruction
The objective of the radiation dose reconstruction was to estimate the mean dose to the stomach tumor location specified as cardia, fundus, body, lesser curvature, greater curvature, antrum or pylorus, and to a comparable location for the matched controls, taking into account all radiotherapy fields. For brevity, “mean dose to the stomach cancer site” is sometimes referred to simply as “dose to the stomach” or “stomach dose”. Individual data were abstracted from patient records and included dates of administration, beam energy, dose delivered, and field location and configuration. Dose reconstruction methods made use of these variables and thus accounted for treatment differences by study and changes in treatments over calendar time. Primary analyses used doses calculated to each stomach region based on a typical J-shaped stomach (8, 9). Because of interindividual variability in stomach size, shape and location, doses were also calculated to two alternative stomach configurations, shown in Fig. 1 (10, 11). Most analyses were based on the sum of all stomach doses received at least five years before the stomach cancer diagnosis date or comparable date in controls. Although analyses addressing time since exposure and age at exposure reported in the individual studies (3–5) assumed that all exposure occurred at the time of diagnosis of the first cancer, the analyses reported here made use of dose in specific windows defined by these variables (for example, dose received 5–9, 10–14, 15–19, etc. years prior to stomach cancer diagnosis). The Supplementary Materials (http://dx.doi.org/10.1667/RR14453.1.S1) provide additional detail on radiation dose reconstruction, including information on the radiation fields used to treat each of the three first cancers (Supplementary Table S1).
FIG. 1.
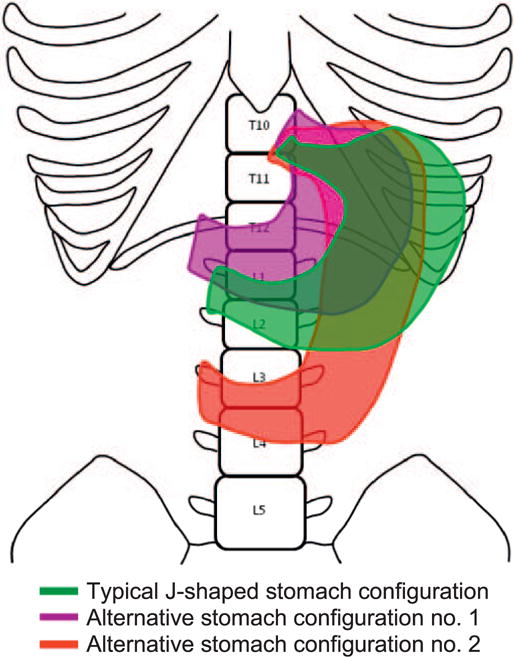
Typical and alternate stomach configurations used for radiation dose reconstruction. From Morton et al. (6). Used with the permission of the American Society of Clinical Oncology. All rights reserved.
Cervical cancer survivors were commonly treated with both external beam therapy and brachytherapy. Doses to the stomach from these two types of radiotherapy were estimated separately. For comparability with the Hodgkin lymphoma and testicular cancer studies (in which brachytherapy was not used), analyses were based on the external beam dose and did not include the dose contributed by brachytherapy. There was no evidence that the relatively small brachytherapy doses increased stomach cancer risks (5).
Statistical Methods
Conditional regression (12) was used to estimate odds ratios for stomach cancer risk by comparing radiation doses of cases to those of matched controls, using the Epicure software package (13). In most analyses, the odds ratio was assumed to be a linear function of dose with the linear coefficient referred to as the excess odds ratio per Gy (EOR/Gy). Parameter estimates were computed using maximum likelihood methods with likelihood ratio-based hypothesis tests and 95% confidence intervals (CI). Two-sided P < 0.05 was considered statistically significant. Because the Hodgkin lymphoma and testicular cancer studies were more comparable with respect to age at first cancer diagnosis and the magnitude of the doses (because of greater similarity in treatment fields), combined analyses were conducted both for all three studies and for Hodgkin lymphoma and testicular cancer only. We also evaluated trends in the EOR/Gy by time since exposure and age at exposure.
In previous analyses of data from the individual studies (3–5), effects of chemotherapy as well as radiotherapy were evaluated. Few testicular or cervical cancer patients received chemotherapy, and there was little evidence of chemotherapy-related stomach cancer risk in either study (4, 5). However, in the Hodgkin lymphoma study, a strong supramultiplicative interaction of radiation with high cumulative doses of oral procarbazine (≥5,600 mg/m2) was observed (3) with an estimated odds ratio (OR) of 78 (95% CI: 15–1,450) based on 25 cases and 2 controls who received both >25 Gy to the stomach tumor location and a high cumulative dose of procarbazine (≥5,600 mg/m2). Because the radiation dose response among patients receiving high cumulative doses of oral procarbazine is not likely to be typical of other populations for which risk estimates are needed, these patients (40 cases, 48 controls) were effectively excluded from the current analyses by including dummy variables as described below. Alternative analyses that simply excluded these 88 patients, and thus also excluded matched controls and some matched cases, yielded similar results but with slightly wider CIs for the Hodgkin lymphoma parameters. Further detail on chemotherapy (and radiotherapy) for Hodgkin lymphoma and testicular cancer patients is given by Hodson (14) and Hann et al. (15).
The simplest analyses were based on the model:
| (1) |
where z is radiation dose to the stomach in Gy and β is the EOR/Gy. This model, in which the EOR, βz, is a linear function of dose, has been used extensively in studies of persons exposed to radiation (1, 2). The variables x1 and x2 are respective indicator variables for Hodgkin lymphoma patients with procarbazine doses exceeding 5,600 mg/m2 and either radiation dose <25 Gy (x1) or radiation dose ≥25 Gy (x2), with the dose z set to zero for these patients. The variable x3 is an indicator variable for receipt of dacarbazine. The addition of other Hodgkin lymphoma chemotherapy variables, including dacarbazine dose, number of alkylating agent cycles, procarbazine dose (among those with cumulative doses less than 5,600 mg/m2), did not significantly improve the fit of the model (P > 0.45). The variables x4, x5 and x6 are indicator variables for missing radiation doses for Hodgkin lymphoma, testicular cancer and cervical cancer patients, respectively. In slightly more complex models, the EORs/Gy (β) were allowed to depend on the first cancer and/or categories of other variables such as time since exposure and stomach cancer site. Odds ratios by categories of dose were estimated by replacing βz with Σj βj vj, where the vj are indicator variables for categories of dose. To evaluate trends in the EOR/Gy, we used the following model:
| (2) |
where k indexes the three studies (first cancers) and the wj are modifying variables such as time since exposure, age at exposure and attained age. With this model, trends with the wj are adjusted for study. The variables x1–x6 are as defined above and additional xi variables are used to allow for trends in missing dose with the modifying variables. Attributable risk was calculated by summing the quantities (OR − 1)/ OR over cases.
These statistical methods are similar to those employed in the original published studies (3–5), as discussed in the Supplementary Materials (http://dx.doi.org/10.1667/RR14453.1.S1). The main radiation dose-response analyses from these studies are shown in Supplementary Table S2.
RESULTS
A total of 327 cases and 678 controls contributed to the pooled dose-response analyses (Table 1). The mean age at first cancer diagnosis was younger for Hodgkin lymphoma (36.2 years) and testicular cancer (39.4 years) than for cervical cancer (53.7 years) survivors. The mean time between first cancer and stomach cancer diagnosis was 17.8 years and 86.2% of all patients received external beam radiotherapy. Mean doses to the stomach tumor location (or comparable site in controls) were highest for testicular cancer (24.7 Gy), next highest for Hodgkin lymphoma (15.3 Gy) and lowest for cervical cancer (1.9 Gy). Both the Hodgkin lymphoma and testicular cancer studies included many survivors with doses to the stomach tumor site ≥25 Gy, reflecting common use of abdominal fields to treat these cancers in this time period (1953–1992). Many Hodgkin lymphoma survivors were treated only with supradiaphragmatic fields leading to more modest doses to the stomach (<25 Gy). Most cervical cancer survivors were treated only with pelvic fields and received doses to the stomach <5 Gy. Additional data on the distribution of cases and controls by dose, latency and age at exposure are shown in Tables 2–4.
TABLE 1.
Characteristics of Patients Who Developed a Second Stomach Cancer and Matched Controls by First Cancera
| Registry | First cancera; total no. of patients
(cases/controls) |
|||
|---|---|---|---|---|
| Hodgkin lymphoma | Testicular cancer | Cervical cancer | Combined | |
| Total no. of patients (cases/controls) | 183 (48/135) | 260 (86/174) | 562 (193/369) | 1,005 (327/678) |
| No. of cases/controls (%b) | ||||
| Denmark | 2/6 (4.4) | 18/26 (16.9) | 50/79 (22.9) | 70/111 (18.0) |
| Finland | 7/13 (10.9) | 6/12 (6.9) | 42/90 (23.5) | 55/115 (16.9) |
| Iowa | 2/9 (6.0) | 0/0 (0.0) | 1/2 (0.5) | 3/11 (1.4) |
| Ontario | 13/28 (22.4) | 4/10 (5.4) | 19/38 (10.1) | 36/76 (11.1) |
| Sweden | 11/28 (21.3) | 19/40 (22.7) | 81/160 (42.9) | 111/228 (33.7) |
| Norway | 8/13 (11.5) | 18/36 (20.8) | Not included | 26/49 (7.5) |
| Netherlands | 5/38 (23.5) | 21/50 (27.3) | Not included | 26/88 (11.3) |
| Age at first cancer diagnosis (years) | ||||
| Mean | 36.2 | 39.4 | 53.7 | 46.8 |
| Median | 30.0 | 38.6 | 54.2 | 46.5 |
| Range | 12.2–83.7 | 18.3–71.8 | 26.3–83.0 | 12.2–83.7 |
| Time since first cancer diagnosis (years) | ||||
| Mean | 16.9 | 17.9 | 19.0 | 17.8 |
| Median | 15.8 | 17.2 | 16.8 | 16.8 |
| Range | 5.2–35.8 | 7.3–38.8 | 5.0–41.8 | 5.0–41.8 |
| Year of first cancer diagnosis | ||||
| Mean | 1974.3 | 1973.7 | 1966.5 | 1969.8 |
| Median | 1973 | 1973 | 1966 | 1970 |
| Range | 1953–1992 | 1959–1987 | 1943–1995 | 1943–1995 |
| Age at stomach cancer diagnosis or comparable date for controls (years) | ||||
| Mean | 53.1 | 57.3 | 71.6 | 64.5 |
| Median | 50.4 | 57.6 | 72.3 | 65.9 |
| Range | 25.8–89.1 | 31.5–80.3 | 35.7–97.5 | 25.8–97.5 |
| No. of cases/controls treated with external beam therapy (%b) | 46/128 (95.1) | 82/145 (87.3) | 165/300 (82.7)d | 293/573 (86.2) |
| No. of cases/controls treated with chemotherapy (%b) | 17/56 (39.9) | 14/23 (14.2) | 1/7 (1.4) | 32/86 (11.7) |
| Radiation dose to the stomach (tumor location for cases, matched location for controls) (Gyc) | ||||
| Mean | 15.3 | 24.7 | 1.9 | 10.3 |
| Median | 2.6 | 28.0 | 1.3 | 1.9 |
| Rangee | 0.08–49.0 | 0.39–59.1 | 0.12–45.8 | 0.08–59.1 |
Excludes 16 cases and 2 controls for whom radiation dose could not be estimated and 40 Hodgkin lymphoma cases and 48 Hodgkin lymphoma controls who received ≥5,600 mg/m2 of procarbazine (1 case and 2 controls were in both excluded groups).
Percentages are based on combined cases and controls.
Excludes 2 Hodgkin lymphoma patients and 6 cervical cancer patients who received radiation only in the five-year period preceding the stomach cancer diagnosis or comparable date for controls. None of these patients received doses >2 Gy in this time period.
An additional 24 cervical cancer cases and 45 cervical cancer controls received brachytherapy but no external beam therapy.
Excludes doses of zero.
TABLE 2.
Risk of Stomach Cancer by Radiation Dose to the Specific Stomach Tumor Location for Each First Cancer and for Combined Data
| Stomach tumor dose (Gy) | Cases/controls | Mean dose (Gy) | OR (95% CI) | Cases/controls | Mean dose (Gy) | OR (95% CI) | Cases/controls | Mean dose (Gy) | OR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| First cancer: | Hodgkin lymphoma | Testicular cancer | Cervical cancer | ||||||
| 0–0.19a | 5a/15 | 0.07 | 1.0 | 4a/29 | 0.0 | 1.0 | 28/71 | 0.003 | 1.0 |
| 0.2–1.99 | 13/52 | 0.9 | 1.1 (0.27, 4.4) | 0/4 | 0.8 | 5.3b (0.93, 37) | 110/191 | 1.2 | 1.5 (0.90, 2.6) |
| 2–4.99 | 4/12 | 2.9 | 1.6 (0.20, 12) | 6/7 | 3.8 | 43/96 | 2.8 | 1.1 (0.55, 2.1) | |
| 5–24.99 | 3/15 | 15.0 | 1.3 (0.19, 8.0) | 20/43 | 16.3 | 4.0 (1.2, 19) | 9/9 | 7.3 | 3.8 (1.1, 16) |
| 25–34.99 | 5/10 | 31.4 | 2.1 (0.35, 14) | 23/45 | 29.9 | 9.0 (2.3, 49) | 0/1 | 30.4 | 3.9 (0.62, 31) |
| 35–39.99 | 10/16 | 37.8 | 5.1 (1.1, 28) | 14/19 | 36.8 | 14.4 (3.2, 87) | 0/0 | – | |
| 40–59.1 | 8/15 | 42.7 | 2.8 (0.56, 15) | 19/27 | 45.7 | 16.4 (3.6, 98) | 3/1 | 42.1 | |
| Missing dose | 1/7 | 0.56 (0.02, 5.5) | 6/6 | 8.9 (1.6, 60) | 8/9 | 2.7 (0.83, 8.8) | |||
| P trendc | 0.011 | <.001 | .061 | ||||||
| EOR/Gy (95% CI) | 0.049 (0.007, 0.16) | .27 (0.054, 1.44) | .096 (−0.002, 0.39) | ||||||
| Combined first cancers: | Hodgkin lymphoma, testicular cancer, cervical cancer | Hodgkin lymphoma, testicular cancer | |||||||
| 0–0.19 | 37/115 | 0.012 | 1.0 | 9a/44 | 0.028 | 1.0 | |||
| 0.2–1.99 | 123/247 | 1.1 | 1.6 (0.98–2.5) | 13/56 | 0.87 | 1.8 (0.58–5.7) | |||
| 2–4.99 | 53/115 | 2.9 | 1.3 (0.74–2.4) | 10/19 | 3.29 | 4.8 (1.2–21) | |||
| 5–24.99 | 32/67 | 14.4 | 2.4 (1.2–4.8) | 23/58 | 15.99 | 2.6 (0.98–7.2) | |||
| 25–34.99 | 28/56 | 30.2 | 3.6 (1.6–8.3) | 28/55 | 30.17 | 4.8 (1.7–14) | |||
| 35–39.99 | 24/35 | 37.3 | 6.6 (2.9–16.0) | 24/35 | 37.26 | 8.1 (2.8–25) | |||
| 40–59.1 | 30/43 | 44.6 | 6.1 (2.7–14.2) | 27/42 | 44.69 | 7.5 (2.6–23) | |||
| P trendc | <0.001 | <0.001 | |||||||
| EOR/Gy (95% CI) | 0.091 (0.036–0.20) | .090 (0.032–0.22) | |||||||
Note. CI = confidence interval; OR = odds ratio; EOR = excess odds ratio.
The referent group of <0.2 Gy was chosen to ensure that there were at least 3 informative cases in the referent group for each first cancer. For both Hodgkin lymphoma and testicular cancer, there were 3 informative cases in the referent group.
Odds ratio for the combined dose category, 0.2–4.99 Gy.
Based on analyses that considered dose as a continuous linear variable.
TABLE 4.
Radiation Dose Response for Two Latency Windows (<20 and ≥20 years) Based on Patients from Hodgkin Lymphoma, Testicular Cancer and Cervical Cancer Studies
| Stomach tumor dose (Gy) | Lateincy <20 years
|
Latency ≥20 years
|
All latencies |
|||||
|---|---|---|---|---|---|---|---|---|
| Cases/controlsb | Mean dose | OR (95% CI) | Cases/controlsb | Mean dose | OR (95% CI) | Cases/controls | OR (95% CI) | |
| 0–0.19a | 27/83 | 0.016 | 1.0 | 11/34 | 0.003 | 1.0 | 37/115 | 1.0 |
| >0.2–4.99 | 114/211 | 1.62 | 1.6 (0.96, 2.7) | 64/153 | 1.74 | 1.4 (0.66, 3.2) | 176/362 | 1.5 (0.95, 2.4) |
| 5–24.99 | 14/48 | 14.75 | 1.0 (0.4, 2.5) | 17/19 | 14.01 | 13.0 (3.8, 54) | 32/67 | 2.5 (1.2, 5.1) |
| 25–34.99 | 17/41 | 30.28 | 2.1 (0.82, 5.7) | 11/15 | 29.91 | 16.8 (4.1, 81) | 28/56 | 3.8 (1.7, 8.8) |
| >35 | 35/47 | 40.55 | 5.0 (2.2, 11.5) | 19/31 | 42.49 | 17.9 (4.9, 77) | 54/78 | 6.5 (3.1, 14.0) |
| P trendc, d | <0.001 | <0.001 | <0.001 | |||||
Note. CI = confidence interval; OR = odds ratio.
Cases and controls without any radiation dose in the specified windows were allocated according to the two latency groups by the length of the interval between first and second cancer diagnoses.
Numbers of cases and controls with nonzero radiation doses in the specified window. Patients could have radiation dose included in both the <20 and >20 year windows.
To evaluate trends with time since exposure, we used the year of the first annual radiation dose that exceeded 5 Gy or the date of the first dose, for patients who never received an annual dose of 5 Gy or more. See Supplementary Materials (http://dx.doi.org/10.1667/RR14453.1.S1) for additional details on the trend tests.
A four-degrees-of-freedom test for homogeneity of the categorical OR by latency yields P = 0.0024.
Significant dose-response relationships were demonstrated for both Hodgkin lymphoma (EOR/Gy = 0.049, 95% CI: 0.007–0.16) and testicular cancer (EOR/Gy = 0.27, 95% CI: 0.054–1.44) survivors, with a borderline significant dose response for cervical cancer survivors (EOR/Gy = 0.096, 95% CI: −0.002–0.39). The pooled EOR/Gy for all three studies was 0.091 (95% CI: 0.036–0.20, P homogeneity = 0.25), corresponding to an odds ratio of 1.91 at 10 Gy (1 + 0.091 × 10) and 3.28 at 25 Gy. For Hodgkin lymphoma and testicular cancer, the pooled EOR/Gy was nearly identical but with a slightly wider confidence interval (P homogeneity = 0.094). Even though the cervical cancer study had by far the largest number of cases, it did not greatly influence the overall dose-response relationship due to the much smaller stomach doses received from pelvic fields. The estimated odds ratios for specific dose categories were much higher for testicular cancer than for Hodgkin lymphoma patients, but had very wide confidence intervals due in part to the small number of testicular cancer cases receiving doses <5 Gy. Based on either of the combined analyses, there was no evidence of nonlinearity in the dose response, as determined by comparing with linear-quadratic, linear-exponential and log-linear functions (P > 0.4 in all cases). However, linear and log-linear models fitted the data equally well. Specifically, there was no evidence of downturn in the dose response at higher doses as evidenced by a positive estimated exponential coefficient in the linear-exponential model. The EOR/Gy restricted to patients receiving doses <5 Gy to the stomach cancer site was 0.089 (95% CI: −0.09, 0.42), similar to the estimate of 0.091 based on the full dose range. Figure 2 shows the categorical dose response and fitted EOR/Gy function.
FIG. 2.
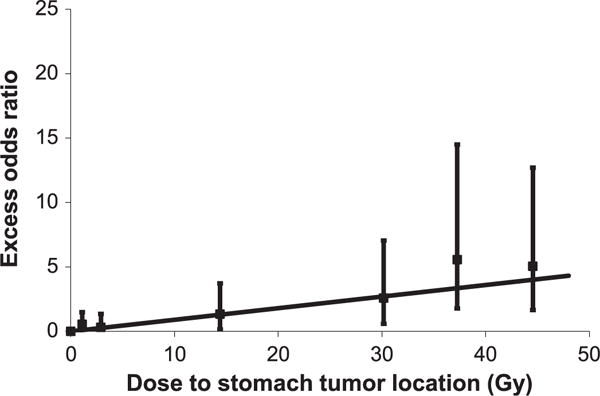
Radiation dose response for stomach cancer after Hodgkin lymphoma, testicular cancer or cervical cancer, showing the excess odds ratio estimates for dose categories (with 95% CI) and the fitted linear function.
The EOR/Gy was 0.14 for males (95% CI: 0.047, 0.37) and 0.048 for females (95% CI: 0.0024, 0.16) (P difference = 0.18). However, gender was largely confounded by study. Within the Hodgkin lymphoma study, the only study that included both sexes, the EOR/Gy was 0.078 for males (95% CI: 0.011–0.29) 0.032 for females (95% CI: −0.010, 0.14) (P difference = 0.32). There was no evidence of heterogeneity by study among males (P = 0.24) or females (P = 0.37) when the two groups were evaluated separately.
The EOR/Gy increased significantly with time since exposure (P trend = 0.0038), with significant trends persisting after taking into account age at exposure (Table 3). There was no indication that this trend varied among studies (P = 0.37), although the trend was significant only for testicular cancer (P = 0.039) compared to Hodgkin lymphoma (P = 0.069) and cervical cancer (P = 0.46) (see Supplementary Table S2; http://dx.doi.org/10.1667/RR14453.1.S1). The EOR/Gy for the 5–20-year latency period was 0.052 (95% CI: 0.014–0.13), whereas the EOR/ Gy for ≥20 years was 0.38 (0.12–1.04); this simple model described the data nearly as well as the six-category model shown in Table 3 (P > 0.5). The statistically significant trend with time since exposure and difference in results for the 5–20-year and ≥20-year latency periods persisted in analyses based on patients who were not treated with alkylating agent chemotherapy. The EOR/Gy estimate of 0.38 for the ≥ 20-year period corresponds to an odds ratio of 4.8 and 10.5 at 10 Gy and 25 Gy to the stomach, respectively. In categorical analyses of the ≥ 20-year latency period, the odds ratio exceeded 10 for all of the higher dose categories (≥5 Gy) (Table 4). We estimate that 50.7 of 184 (28%) stomach cancers occurring in the first 20 years postirradiation could be attributed to radiotherapy, whereas 63.8 of 111 (57%) stomach cancers occurring at later times could be attributed to radiotherapy. Findings regarding the shape of the overall dose response were not modified when allowing for a continuous trend with latency.
TABLE 3.
Excess Odds Ratio per Gy by Dose Defined by Latency or Age-at-Exposure Windows
| Combined first cancers: | Hodgkin lymphoma, testicular
cancer, cervical cancer |
Hodgkin lymphoma, testicular
cancer |
||
|---|---|---|---|---|
| Cases/controlsb | EOR/Gy (95% CI) | Cases/controlsb | EOR/Gy (95% CI) | |
| Latency windowa (years) | ||||
| 5–9.99 | 62/104 | 0.032 (−0.009, 0.21) | 18/33 | 0.048 (−0.007, 0.35) |
| 10–14.99 | 69/126 | 0.038 (−0.004, 0.16) | 37/77 | 0.028 (−0.008, 0.14) |
| 15–19.99 | 62/135 | 0.078 (0.016, 0.23) | 35/77 | 0.090 (0.014, 0.33) |
| 20–24.99 | 51/97 | 0.52 (0.10, 2.14) | 25/53 | 0.87 (0.13, 9.11) |
| 25–29.99 | 35/74 | 0.34 (0.064, 1.19) | 15/31 | 0.84 (0.11, 9.18) |
| 30–41.83 | 28/57 | 0.35 (0.002, 2.56) | 8/15 | |
| P trendc | 0.0038 | 0.0025 | ||
| P trend adjusted for age at exposurec | 0.018 | 0.015 | ||
| Latency window (years) | ||||
| <20 | 184/357 | 0.052 (0.014, 0.13) | 83/183 | 0.053 (0.012, 0.15) |
| >20 | 111/220 | 0.38 (0.12, 1.04) | 46/93 | 0.77 (0.16, 5.93) |
| P difference | 0.0030 | 0.0013 | ||
| Age at exposure window (years) | ||||
| 10–24.99 | 18/58 | 0.16 (0.027, 0.65) | 18/58 | 0.16 (0.025, 0.67) |
| 25–34.99 | 37/71 | 0.30 (0.091, 0.89) | 32/59 | 0.34 (0.094, 1.13) |
| 35–44.99 | 68/151 | 0.044 (0.000, 0.16) | 40/90 | 0.048 (0.000, 0.20) |
| 45–54.99 | 71/124 | 0.083 (0.004, 0.39) | 24/43 | 0.069 (−0.001, 0.42) |
| 55–64.99 | 61/116 | 0.019d (−0.017,0.17) | 9/24 | 0.017e (−0.017, 0.18) |
| 65–83.67 | 44/61 | 0.58d (0.023, 3.27) | 11/6 | |
| P trendc | 0.077 | 0.069 | ||
| P trend adjusted for latencyc | >0.5 | >0.5 | ||
Note. CI = confidence interval; EOR = excess odds ratio.
Interval between exposure and date of stomach cancer diagnosis or comparable date in controls.
Numbers of cases and controls with nonzero radiation doses in the specified window. Patients could have radiation dose included in more than one window. Thirty-four cases and 104 controls did not have any dose to include in any window, and of these, 28 cases and 78 controls were cervical cancer patients.
For the purpose of evaluating trends with age at exposure and time since exposure, we used the year of the first annual dose that exceeded 5 Gy or the date of the first dose, for patients who never received an annual dose of 5 Gy or more. See Supplementary Materials for additional details on the trend tests.
EOR/Gy for the combined group of ≥55-year window was 0.036 (95% CI: −0.011, 0.22).
EOR/Gy for the combined group of ≥55-year windows. EOR/Gy for the 55–64-year window was 0.013 (95% CI: −0.020, 0.24); EOR/Gy for >65 is infinite (95% CI: 8.5, ∞).
While a decline in risk with increasing age at exposure was suggested (P trend = 0.077), this was no longer evident after accounting for time since exposure (P > 0.5) (Table 3). If the date of first cancer diagnosis instead of the more accurate date of actual first exposure had been used to determine age at exposure and latency, all of the P values shown in Table 3 would have been larger, indicating that the use of the more accurate date increased statistical power for assessing these effects.
There was no indication of modification by attained age or year of first radiation treatment whether or not analyses accounted for time since exposure (data not shown). Analyses that included both age at exposure and attained age as modifiers of the dose response resulted in a model deviance that was only slightly less than that obtained in analyses in which only latency was included, reflecting that latency can be expressed as the difference between attained age and age at exposure; in fact, the estimated-age-at-exposure parameter was close to the negative of the estimated-attained-age parameter for both combined analyses and for the individual studies.
Previous analyses of the testicular cancer and cervical cancer data (4, 5) indicated heterogeneity by stomach cancer site with substantially lower EOR/Gy for tumors in the proximal stomach than for those in the body and distal sites. While our analyses confirmed these results, no evidence of such heterogeneity for Hodgkin lymphoma was detected (Supplementary Table S3; http://dx.doi.org/10.1667/RR14453.1.S1).
Table 5 shows results based on stomach doses that were estimated using alternative stomach configurations (Supplementary Materials; http://dx.doi.org/10.1667/RR14453.1.S1). The pooled EOR/Gy estimate was 0.096 (95% CI: 0.039–0.21) for alternative stomach configuration no. (1) and 0.074 (95% CI: 0.031–0.15) for alternative stomach configuration no. (2), compared to our primary pooled estimate of 0.091 (95% CI: 0.036–0.20) based on the J-shaped stomach. The slightly lower estimate for alternative stomach configuration no. (2), which is thought to be uncommon, results primarily from the cervical cancer data, where the EOR/Gy based on this stomach was only approximately half of that based on the J-shaped stomach. Alternative stomach configuration no. (2) is closer to the cervix than the J-shaped stomach, resulting in higher doses to the stomach and smaller risks per unit of exposure (EOR/Gy). Both the increase in risk with time since exposure and the heterogeneity in risks by stomach cancer site persisted in analyses that used doses based on alternative stomach configuration nos. (1) or (2) (data not shown).
TABLE 5.
Comparison of Excess Odds Ratio per Gy with Dosimetry Based on Three Different Stomach Configurationsa
| First cancers | ||||
|---|---|---|---|---|
| Stomach configuration used for dosimetry | Hodgkin lymphoma EOR/Gy (95% CI) | Testicular cancer EOR/Gy (95% CI) | Cervical cancer EOR/Gy (95% CI) | Combined Hodgkin lymphoma, testicular and cervical cancerb EOR/Gy (95% CI) |
| J-shaped | 0.049 (0.007, 0.16) | 0.27 (0.054, 1.44) | 0.096 (−0.002, 0.39) | 0.091 (0.036, 0.20) |
| Alternative configuration no. 1 | 0.045 (0.006, 0.15) | 0.26 (0.062, 1.29) | 0.12 (0.001, 0.54) | 0.096 (0.039, 0.21) |
| Alternative configuration no. 2 | 0.049 (0.007, 0.15) | 0.30 (0.064, 1.50) | 0.044 (−0.002, 0.16) | 0.074 (0.031, 0.15) |
Note. CI = confidence interval; EOR = excess odds ratio.
The J-shaped stomach configuration represents a typical shape for an adult of normal weight and no stomach pathology. Alternative stomach configuration no. (1) is located higher in the body than the J-shaped stomach and has been found more frequently in persons with massive body build and higher weight. Alternative stomach configuration no. (2) is located lower in the body and is longer than the J-shaped stomach and has been found more frequently in persons with thinner body build and lower weight (4, 5). (See Fig. 1.)
The combined estimates for Hodgkin lymphoma and testicular cancer were 0.090 (95% CI: 0.032, 0.22) for the J-shaped stomach, 0.092 (95% CI: 0.034, 0.22) for alternative configuration no. (1), and 0.094 (95% CI: 0.034, 0.23) for alternative configuration no. (2).
In analyses in which studies or registries were excluded one at a time, the EOR/Gy remained statistically significant regardless of which study or registry was excluded, ranging from 0.059 (95% CI: 0.014–0.16) when the testicular cancer study was excluded, to 0.16 (95% CI: 0.050–0.42) when the Hodgkin lymphoma study was excluded (Supplementary Table S4; http://dx.doi.org/10.1667/RR14453.1.S1). The positive trend with time since exposure also persisted, with P < 0.07 in each of these analyses.
To facilitate comparison of our results with those from the LSS cohort of Japanese atomic bomb survivors, we performed analyses on the publicly available cancer incidence data as described in the Supplementary Materials (http://dx.doi.org/10.1667/RR14453.1.S1) (16). To increase comparability of the data, LSS analyses were restricted to the first 40 years postirradiation and to LSS members exposed at age ≥15 years, and our data were restricted to follow-up of ≥12 years (237 cases, 498 controls) because LSS cancer incidence data were not available in the first 12 years. The overall LSS-based ERR/Gy was 0.39 (95% CI: 0.23–0.57), nearly four times the EOR/Gy of 0.11 (95% CI: 0.041–0.26) from our study (P difference = 0.0060). However, for exposures occurring ≥20 years prior to stomach cancer diagnosis, EOR/Gy estimates were comparable: 0.38 for the LSS and 0.41 for our study. When we fitted models that included both age at exposure and time since exposure, risk decreased with increasing age at exposure in both studies, but was statistically significant only in the LSS (P difference in age trends = 0.25). However, the time since exposure effects differed significantly (P = 0.022) with a nonsignificant decrease (P > 0.5) in the LSS and a significant increase in our study (P = 0.017).
DISCUSSION
By combining data from three stomach cancer case-control studies among cancer survivors with individual dosimetry, we obtained the most comprehensive evaluation of stomach cancer risk from therapeutic radiation to date. This included a single summary estimate, more precise quantification of the strong radiation dose response, described well by a linear function over a wide range of stomach doses, and a more powerful assessment of potential modifiers of this dose response than previously available. We demonstrated a significant increase in radiation risk with increasing time since exposure, a finding that was not apparent prior to these combined analyses and that has important implications for long-term cancer survivors.
To our knowledge, this is the first study of any second solid cancer to demonstrate a statistically significant increase in the linear radiation risk coefficient (EOR/Gy) with time since exposure, possibly because other studies in which the individual dosimetry was needed to evaluate the dose response have lacked statistical power and sufficient follow-up to detect such an effect (17). Because baseline stomach cancer risks increase as survivors age, absolute risks of radiation-related stomach cancer, which cannot be estimated from case-control data alone, are likely to show an even stronger increase with time since exposure than relative risks. However, it is unknown if the high risks observed 20–40 years after exposure persist throughout life.
After accounting for time since exposure, there was little indication that age at exposure or attained age modified risk. Several cohort studies have shown that overall risks relative to the general population decline with age at exposure (18–20). However, these studies did not account for radiation dose, and few studies adjusted age trends for latency or evaluated such trends for stomach cancer alone. Our study, like many studies of specific cancers in which radiation dose response was investigated (17), may have lacked adequate statistical power to demonstrate statistically significant effects of age at exposure. This is particularly true given the strong latency effect observed in this study. Due to the small radiation effect in the first 20 years of follow-up, the assessment of a latency-adjusted age effect depends primarily on long-term (≥20 year) survivors, thereby limiting statistical power.
We found no indication of nonlinearity in the dose-response relationship or of a decline in risk at higher radiation doses. However, even in this pooled study, power for detecting such effects was limited, due in part to the sparse data in the 5–25 Gy range (Table 2, Fig. 1).
Our overall EOR/Gy estimate was much lower than that from the LSS cohort, a finding that has been observed for several other cancer sites (21), likely resulting from dose fractionation and/or cell killing. The EOR/Gy estimate of 0.27 based on testicular cancer survivors alone was more comparable to LSS-based estimates, but is particularly uncertain because there were few testicular cancer cases with doses <2 Gy. The EOR/Gy estimate for ≥20 year survivors (0.58 per Gy) was also comparable to LSS-based estimates. Patients in our study were from Northern Europe and North America, where baseline stomach cancer rates are 5–10 times lower than in Japan (22). Thus, if absolute risks could be compared, the ratio of estimates from the LSS and our study would be even greater than the ratio of relative risks. Not only did the magnitude of the risk differ from that in the LSS, but the patterns of risk over time were also different, with an increase in risk with time since exposure in our study and no such effect in the LSS. While the reason for this difference is unclear, it is possibly related to the high fractionated doses, especially since the increase was not evident in cervical cancer survivors who received lower doses to the stomach from pelvic radiation. We note also that first cancer survivors may have higher susceptibility to cancer than LSS subjects and that LSS survivors were whole-body exposed in contrast to first cancer survivors.
Our estimate of the EOR/Gy is larger than the estimate of 0.042 (95% CI: −0.002, 0.12) (23) obtained from a study of patients treated for peptic ulcer with a mean radiation dose of approximately 15 Gy; this study suggested a decline in the EOR/Gy with age at exposure (P = 0.08), but found little evidence of modification by time since exposure (P = 0.27). Our EOR/Gy estimate for cervical cancer patients is smaller than the estimate of 0.54 (95% CI: 0.05 = 1.5) from Boice et al. (24). The difference comes about primarily because the Boice et al. estimate was based on mean dose to the entire stomach rather than dose to the stomach cancer site as in the current study. Since the mean dose to the entire stomach tends to be smaller, the resulting linear risk coefficient is larger. In addition, we fitted a linear coefficient that took account of all of the individual doses, whereas Boice et al. simply divided the excess relative risk (RR −1) by the overall average dose.
Tests for heterogeneity among the studies did not reach statistical significance, but the estimated EOR/Gy for testicular cancer was more than 5 times that for Hodgkin lymphoma (P difference = 0.094), although the uncertainty in the EOR/Gy estimate for testicular cancer was large with a ratio between the upper and lower confidence limits of 27. It is unlikely that the gender difference in the two studies contributed to this difference, since the LSS-based estimate for males was approximately half of that for females (16). A possible contributor to the difference in the Hodgkin lymphoma and testicular cancer estimates is the effective exclusion of Hodgkin lymphoma patients who received high cumulative doses of procarbazine (who had higher EOR/Gy) and the exclusion of a group of testicular cancer survivors from Norway due to a high proportion of missing data (who had lower EOR/Gy) (4). These data-driven exclusions were made to avoid bias. However, data were inadequate for precise characterization of the interaction in the Hodgkin lymphoma study or of the effect of missing data in the excluded Norway patients. Thus, the exclusions could have resulted in overcorrection of the biases they were intended to address. The variation in EOR/Gy estimates among the first cancers included in our study suggests that caution should be taken in generalizing our pooled estimates to all first cancer sites.
A limitation of our study was uncertainty in dose to the stomach cancer site due to variability in the size, shape and location of the stomach, but results were not modified greatly when doses based on alternative stomach configurations were analyzed. This uncertainty could also have contributed to the inconsistent variability of risk by stomach cancer site.
Although radiation treatments have changed over time, it is unlikely that these changes resulted in bias, since dosimetry practices accounted for the changes and there was no evidence that the calendar period of the first radiation treatment modified the radiation dose response. Another drawback was limited power for detecting risks at lower stomach doses, although the EOR/Gy limited to doses <5 Gy was similar to that for the full data set, suggesting that our estimate is reasonably appropriate across a wide dose range including the lower doses received in contemporary radiotherapy. We also could not effectively investigate modification by gender since gender is largely confounded by age and by the magnitude of the radiation dose to the stomach. Although we investigated and adjusted for the effects of chemotherapy, it is possible that chemotherapy influenced our results in ways that are not readily apparent.
With over 1,000 patients (327 cases/678 controls), this is one of the largest case-control studies with individual tumor doses of any single second cancer ever conducted. A total of 114 cases and 201 controls had radiation doses to the stomach tumor location of ≥5 Gy, providing valuable information on stomach cancer risks from fractionated high-dose radiotherapy. Other strengths of this study include the availability of individual estimates of dose to the stomach cancer site and the comparable methodologies used for all three studies. In addition, our analyses accounted for the actual time that radiation exposures occurred, which allowed a more powerful assessment of the effects of time since exposure and age at exposure than the more common approach of assuming that all exposures occurred close to the time of first cancer diagnosis.
Combining data on survivors of three first cancers resulted in a stronger basis for estimating stomach cancer risks from fractionated radiation exposure and a better understanding of how these risks are modified by other factors. Our study provides strong evidence that stomach cancer risk after radiotherapy increases with increasing stomach dose, with a smaller increase per unit of dose than that observed after the single lower dose exposure from the Japanese A-bomb, at least for the first several years after exposure. The strong increase in relative risk with increasing time since radiotherapy has important implications for the long-term clinical management of cancer survivors. The current findings also highlight the need for a comprehensive understanding of the long-term effects of treatment exposures, recognizing that the effects of high-dose fractionated radiotherapy may differ from those observed in persons exposed at low and moderate acute doses such as the Japanese atomic-bomb survivors.
Supplementary Material
Table S1. Numbers of cases and controls receiving radiotherapy five or more years prior to stomach cancer diagnosis (or comparable date in controls) and mean tumor doses by first cancer and type of radiotherapy fields.
Table S2. Risk of stomach cancer by radiation dose to the specific stomach tumor location as shown in the original studies.
Table S3. Excess odds ratio per Gy by windows of dose defined by latency or age at exposure for each first cancer.
Table S4. Excess odds ratio per Gy by stomach cancer site, first cancer and combined first cancers.
Table S5. Excess odds ratio per Gy and latency P value after excluding first cancers and registries one at a time.
Acknowledgments
This research was supported in part by the intramural research program of the NIH and the NCI, and with contracts from the NCI to: Cancer Care Ontario, Toronto, Canada (NO1-CP-31157); Danish Cancer Society, Copenhagen, Denmark (NO1-CP-31019); Finnish Cancer Registry, Helsinki, Finland (NO1-CP-31154); Information Management Services, Inc., Silver Spring, MD (N01-CP-31003); Karolinska Institute, Stockholm, Sweden (NO1-CP-31156); University of Iowa, Iowa City, IA (NO1-CP-31155); The University of Texas MD Anderson Cancer Center, Houston, TX (N02-CP-55503); and Westat Inc., Rockville, MD (N02-CP-31136). This report makes use of data obtained from the Radiation Effects Research Foundation (RERF) in Hiroshima, Japan. RERF is a private foundation funded equally by the Japanese Ministry of Health, Labour and Welfare and the U.S. Department of Energy through the U.S. National Academy of Sciences. The data include information obtained from the Hiroshima City, Hiroshima Prefecture, Nagasaki City, and Nagasaki Prefecture Tumor Registries and the Hiroshima and Nagasaki Tissue Registries. The conclusions in this report are those of the authors and do not necessarily reflect the scientific judgment of RERF or its funding agencies.
Footnotes
Editor’s note. The online version of this article (DOI: 10.1667/RR14453.1) contains supplementary information that is available to all authorized users.
References
- 1.National Research Council (NRC) and Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation. Health Risks from exposure to low levels of ionizing radiation: BEIR VII Phase 2. Washington DC: National Academy of Sciences; 2006. [Google Scholar]
- 2.Effects of ionizing radiation: UNSCEAR 2006 Report to the General Assembly with Scientific Annexes. I. New York: United Nations Scientific Committee on the Effects of Atomic Radiation; 2008. [Google Scholar]
- 3.Morton LM, Dores GM, Curtis RE, Lynch CF, Stovall M, Hall P, et al. Stomach cancer risk after treatment for Hodgkin lymphoma. J Clin Oncol. 2013;31:3369–77. doi: 10.1200/JCO.2013.50.6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hauptmann M, Fossa SD, Stovall M, von Leeuwen FE, Johannesen TB, Rajaraman P, et al. Increased stomach cancer risk following radiotherapy for testicular cancer. Br J Cancer. 2015;112:44–51. doi: 10.1038/bjc.2014.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kleinerman RA, Smith SA, Holowaty E, Hall P, Pukkala E, Vaalavirta L, et al. Radiation dose and subsequent risk for stomach cancer in long-term survivors of cervical cancer. Int J Radiat Oncol Biol Phys. 2013;86:922–9. doi: 10.1016/j.ijrobp.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Purdy JA. Dose to normal tissues outside the radiation therapy patent’s treated volume: a review of different radiation therapy techniques. Health Phys. 2008;95:666–76. doi: 10.1097/01.HP.0000326342.47348.06. [DOI] [PubMed] [Google Scholar]
- 7.van den Belt-Dusebout AW, Aleman BMP, Besseling G, de Bruin ML, Hauptmann M, van ’T Veer MB, et al. Roles of radiation dose and chemotherapy in the etiology of stomach cancer as a second malignancy. Int J Radiat Oncol Biol Phys. 2009;75:1420–9. doi: 10.1016/j.ijrobp.2009.01.073. [DOI] [PubMed] [Google Scholar]
- 8.Stovall M, Weathers R, Kasper C, Smith SA, Travis L, Ron E, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: use in epidemiological studies. Radiat Res. 2006;166:141–57. doi: 10.1667/RR3525.1. [DOI] [PubMed] [Google Scholar]
- 9.Leibel SA, Phillips TL. Textbook of radiation oncology. 2nd. Philadelphia: Elsevier Saunders; 2004. [Google Scholar]
- 10.Dowd SB, Wilson BG. Encyclopedia of radiographic positioning. Vol. 2. Philadelphia: Saunders; 1995. [Google Scholar]
- 11.Lamart S, Imran R, Simon SL, Doi K, Morton LM, Curtis RE, et al. Prediction of the location and size of the stomach using patient characteristics for retrospective radiation dose estimation following radiotherapy. Phys Med Biol. 2013;58:8739–53. doi: 10.1088/0031-9155/58/24/8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Breslow NE, Day NE. Statistical methods in cancer research Vol 1 The analysis of case-control studies Lyon. France: International Agency for Research on Cancer; 1980. [PubMed] [Google Scholar]
- 13.Preston DL, Lubin JH, Pierce DA, McConney MA. Epicure user’s guide. Seattle: Hirosoft International Corporation; 1993. [Google Scholar]
- 14.Hodson DC. Late effects in the era of modern therapy for Hodgkin lymphoma. Hematology, ASH Education Book. 2011;1:323–9. doi: 10.1182/asheducation-2011.1.323. [DOI] [PubMed] [Google Scholar]
- 15.Hanna N, Einhorn L. Testicular cancer: A reflection on 50 years of discovery. J Clin Oncol. 2014;32:3085–92. doi: 10.1200/JCO.2014.56.0896. [DOI] [PubMed] [Google Scholar]
- 16.Preston DL, Ron E, Tokuoka S, Funamoto S, Nichi N, Mabuchi K, et al. Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res. 2007;168:1–64. doi: 10.1667/RR0763.1. [DOI] [PubMed] [Google Scholar]
- 17.National Council on Radiation Protection and Measurements Scientific Committee 1–17. Second primary cancers and cardiovascular disease after radiotherapy. Bethesda: National Council on Radiation Protection and Measurements; 2012. (NCRP Report 170). Chapter 9. [Google Scholar]
- 18.Travis LB, Fossa SD, Schonfeld SJ, McMaster ML, Lynch CF, Storm H, et al. Second cancer among 40,576 testicular cancer patients: focus on long-term survivors. J Natl Cancer Inst. 2005;97:1354–65. doi: 10.1093/jnci/dji278. [DOI] [PubMed] [Google Scholar]
- 19.Hodgson DC, Gilbert ES, Dores GM, Schonfeld SJ, Lynch CF, Storm H, et al. Long-term solid cancer risk among 5-year survivors of Hodgkin’s lymphoma. J Clin Oncol. 2007;25:1489–97. doi: 10.1200/JCO.2006.09.0936. [DOI] [PubMed] [Google Scholar]
- 20.Chaturvedi AK, Engels EA, Gilbert ES, Chen BE, Storm H, Lynch CF, et al. Second cancers among 104,760 survivors of cervical cancer: Evaluation of long-term risk. J Natl Cancer Inst. 2007;99:1634–43. doi: 10.1093/jnci/djm201. [DOI] [PubMed] [Google Scholar]
- 21.Berrington de Gonzalez A, Gilbert E, Curtis R, Inskip P, Kleinerman R, Morton L, et al. Second cancers after radiotherapy: a systematic review of the epidemiological studies of the radiation dose-response relationship. Int J Radiat Oncol Biol Phys. 2013;86:224–33. doi: 10.1016/j.ijrobp.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cancer incidence in five continents Vol IX IARC Scientific Publication No 160. Lyon, France: International Agency for Research on Cancer; 2009. [Google Scholar]
- 23.Little MP, Stovall M, Smith SA, Kleinerman RA. A re-analysis of the curvature in the dose response for cancer and modifications by age at exposure following radiotherapy for benign disease. Int J Radiat Oncol Biol Phys. 2013;85:451–9. doi: 10.1016/j.ijrobp.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boice JD, Jr, Engholm G, Kleinerman RA, Blettner M, Stovall M, Lisco H, et al. Radiation dose and second cancer risk in patients treated for cancer of the cervix. Radiat Res. 1988;116:3–55. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Numbers of cases and controls receiving radiotherapy five or more years prior to stomach cancer diagnosis (or comparable date in controls) and mean tumor doses by first cancer and type of radiotherapy fields.
Table S2. Risk of stomach cancer by radiation dose to the specific stomach tumor location as shown in the original studies.
Table S3. Excess odds ratio per Gy by windows of dose defined by latency or age at exposure for each first cancer.
Table S4. Excess odds ratio per Gy by stomach cancer site, first cancer and combined first cancers.
Table S5. Excess odds ratio per Gy and latency P value after excluding first cancers and registries one at a time.


