Abstract
Background
In Bangladesh, nomadic duck flocks are groups of domestic ducks reared for egg production that are moved to access feeding sites beyond their owners’ village boundaries and are housed overnight in portable enclosures in scavenging areas. The objectives of this study were to measure the prevalence of influenza A virus RNA and H5‐specific antibodies in nomadic ducks and to characterize nomadic duck raising practices in northeastern Bangladesh.
Methods
We tested duck egg yolk specimens by competitive ELISA to detect antibodies against avian influenza A (H5) and environmental fecal samples by real‐time reverse‐transcription polymerase chain reaction (rRT‐PCR) to detect influenza A virus RNA and H5 subtype.
Results
The median age of the ducks was 24 months (range: 8‐36 months) and the median flock size was 300 ducks (range: 105‐1100). Of 1860 egg yolk samples, 556 (30%, 95% confidence interval (CI): 28‐32) were positive for antibodies against H5 and 58 flocks (94%) had at least one egg with H5‐specific antibodies. Of 496 fecal samples, 121 (24%, 95% CI: 22‐29) had detectable influenza A RNA. Thirty‐three flocks (53%) had at least one fecal sample positive for influenza A RNA.
Conclusions
Nomadic ducks in Bangladesh are commonly infected with avian influenza A (H5) virus and may serve as a bridging host for transmission of avian influenza A (H5) virus or other avian influenza A viruses subtypes between wild waterfowl, backyard poultry, and humans in Bangladesh.
Keywords: avian influenza A (H5) virus, egg yolk, H5‐antibodies, nomadic duck, wild waterfowl and Bangladesh
1. Introduction
Waterfowl are a natural reservoir for all subtypes of influenza A viruses.1, 2 Highly pathogenic avian influenza A (H5) viruses in domestic ducks may result in asymptomatic, subclinical, or clinical infections, and asymptomatic ducks often shed the viruses through feces and respiratory droplets.3, 4 In many Asian countries, farmers herd scavenging ducks from one feeding ground to another through the year and these practices can contribute to the spread of influenza A (H5) viruses.5, 6, 7 Allowing contact between domestic ducks, wild waterfowl 5, 6, 8 and other poultry and animal species, poses risks for spreading of influenza A (H5) viruses.5, 6, 9
Northeastern Bangladesh, with its high intensity domestic duck raising and an agroecological landscape with extensive interface between large water bodies and rice fields, acts as an important site for interaction between wild waterfowl and domestic ducks10 especially during the winter. Highly pathogenic avian influenza (HPAI) A(H5N1) virus clade 2.3.2.1 has been circulating among poultry in Bangladesh since 2011.11 However, HPAI A (H5N1) virus clade 2.3.2.1 was previously isolated from wild waterfowl in Bangladesh in 2010.12
Bangladesh had an estimated 41 million ducks in 2009.13 Ducks are mainly raised for egg production in Bangladesh14 where they provide an important source of protein, self‐employment, and livelihood for rural people.15, 16 Nomadic duck raising in Bangladesh occurs mainly in low‐lying areas around large water bodies17 and adjacent to harvested rice fields which provide feed and serve as sites of interaction between domestic ducks and wild waterfowl. Netrokona, Sunamganj, Noakhali, Habiganj, and Moulvibazar districts18 are the main nomadic duck raising areas in the country.19, 20 In these districts, nomadic ducks have opportunities for contact with wild waterfowl since they both scavenge in the same water bodies, whereas backyard ducks remain near their owners’ household premises.
Since March 2007, over 500 outbreaks of HPAI A(H5N1) virus have been reported in chickens in Bangladesh.18 Live bird market surveillance has identified HPAI A(H5N1) virus in apparently healthy ducks in Bangladesh since 2007,21 and in 2011, there was an outbreak with unusual duck mortality due to HPAI A(H5N1) 2.3.2.1a virus in northeastern Bangladesh.22
We conducted a study to measure the prevalence of H5‐specific antibodies and influenza A virus RNA in nomadic ducks and to characterize nomadic duck raising practices in northeastern Bangladesh. This study will help determine whether nomadic ducks are a substantial reservoir of avian influenza A (H5) viruses and describe the nomadic duck raising practices associated with AIV carriage that could be amenable to culturally appropriate, effective, and affordable intervention.
2. Methods
2.1. Study site and population
We selected Mohanganj subdistrict of Netrokona District in the northeastern part of Bangladesh because it has large bodies of water in low‐lying areas and domestic ducks raised in the nomadic system18 that interact with wild waterfowl during winter (November‐February).23 There were an estimated 2.5 million domestic ducks in the Mohanganj subdistrict in 2006.18 A large number are reared nomadically for egg production and the rest are backyard ducks (Department of Livestock Services). Ongoing live bird market surveillance frequently identifies H5N1 among domestic ducks in Mohanganj.21 Five months before our study began, a reported outbreak of HPAI A (H5N1) with high mortality occurred among poultry (ducks, geese, and chickens) in this study area.22
2.2. Study design
From December 2011 through February 2012, we conducted a cross‐sectional study of 62 nomadic duck flocks within Mohanganj to collect duck eggs and swab samples from fresh fecal droppings and interview flock owners. We chose egg yolk samples to detect antibodies against avian influenza A (H5) instead of serum samples because blood collection in egg‐laying ducks has practical difficulties: catching and collecting blood samples from laying ducks is stressful to the ducks which causes financial losses through reduced egg production.24 Others studies demonstrated that egg yolk is a good alternative source for the detection of antibodies of avian influenza viruses in laying hens and ducks.24, 25, 26 Another study found a high correlation between H5 antibodies in egg yolk and serum samples.27
We defined nomadic duck flocks as groups of domestic ducks reared for egg production that are moved to access feeding sites beyond their owners’ village boundaries and are housed overnight in portable enclosures in scavenging areas (Figure 1).
Figure 1.
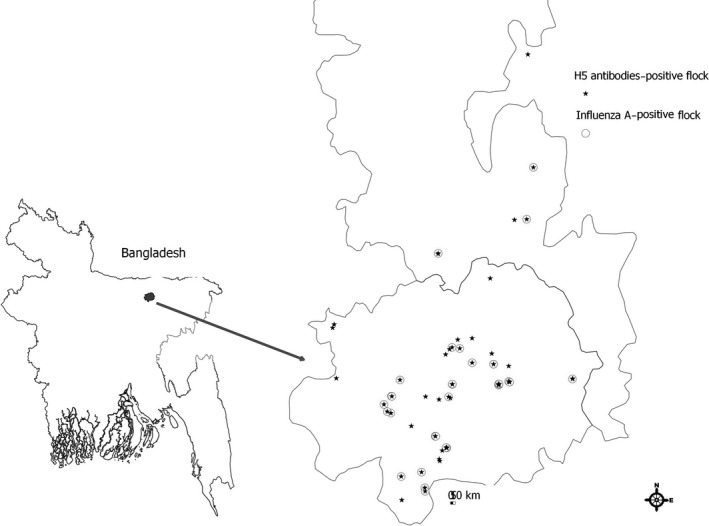
Location to detect avian influenza A RNA and with H5 antibody positive among nomadic duck flocks within northeastern Bangladesh, December 2011 to March 2012
2.3. Sample size
The (HPAI) virus (H5N1) is endemic in poultry in five countries: Bangladesh, China, Egypt, Indonesia, and Vietnam.28 Our sample size calculation was based on the prevalence of antibodies to H5 in duck flocks in Indonesia in 2007‐2008. The flock‐level prevalence of antibodies to H5 was 19.5%.6 To determine the number of nomadic duck flock, we assumed flock‐level prevalence of antibodies to H5 of 20%, a precision of 10%, and a 95% confidence interval (CI). Our estimated number of flocks needed for this study was 62.
To determine the number of egg samples necessary to detect an assumed H5 antibody prevalence of 2.6%6 with 1% precision and a 95% CI, we multiplied the calculated sample size by the assumed design effect of 229 to account for the cluster sampling strategy. Our calculated egg sample size was 1860. To determine the number of eggs sampled within a flock to detect H5 antibodies, we assumed 10% expected prevalence with 95% CI. Our estimated egg sample was 30 within a flock.
2.4. Sampling
We collected available information about nomadic duck flocks from the Mohanganj Upazila Government Veterinary Hospital and from the flock owners using the chain referral technique.30 We prepared a list of 90 nomadic duck flocks with at least 100 egg‐laying ducks in each flock and assigned a unique number to each flock owner. We generated a random number using Microsoft Excel to select 62 nomadic duck flocks from this list. Among them, 56 flock owners agreed to participate; the remaining six owners sold off their flocks before our study got underway. Therefore, we replaced these six with an additional six randomly selected flocks to achieve our target of 62 flocks. We followed a two‐stage cluster sampling strategy: First, we randomly selected nomadic duck flocks from Mohanganj, and then we selected convenience samples of eggs from within the flock. The field team collected 30 egg samples from each flock's night shelter during early morning (usually around 6 am) of a day.
2.5. Duck flock owner interviews
Using a structured questionnaire, we interviewed all 62 flock owners and collected information about flock movement history, trading practices of ducks and eggs, contact with wild waterfowl, vaccination history, and flock biosecurity practices in the past year.
2.6. Sample collection
Field workers visited the night shelter of each nomadic duck flock in the early morning (usually around 6 am) for sampling and data collection. After obtaining informed consent from flock owners, field workers purchased 30 eggs laid that morning. Field workers also collected eight swab samples of pooled fresh fecal droppings from the four corners and four central positions of the night shelters and placed them in a single tube containing 5 mL of viral transport medium (VTM). Egg samples and pooled fecal samples were kept in a cold box maintained between 2°C and 8°C for 5‐7 days and transported to the BSL‐2 animal specimen laboratory at icddr,b.
2.7. Preparation of egg yolk and pooled fecal samples
Eggs were individually cracked and the egg white separated from the yolk using a sterile egg yolk separator. The yolk sac was ruptured with a needle and 4 mL of yolk was collected with a syringe under sterile conditions. Then, the yolk was mixed with an equal volume of 0.01 mol/L phosphate‐buffered saline (PBS; pH 7.2) and homogenized. The mixture was left for 1 hour at room temperature followed by centrifugation at 1500 g for 30 minutes. The supernatant (1.5 mL) was collected in Eppendorf tubes and stored at −20°C until testing. Pooled fecal samples were aliquoted in a tubes containing 1.8 mL VTM.
2.8. Laboratory methods
2.8.1. H5 antibody detection by competitive enzyme‐linked immunosorbent assay (cELISA)
We tested egg yolk specimens to detect antibodies against avian influenza A (H5) using commercially available cELISA (AniGen H5 AIV Ab ELISA kit; BioNote, Gyeonggi‐do, South Korea). The kit uses a recombinant H5 hemagglutinin (HA) antigen that the manufacturer reports detects antibodies against avian influenza A (H5) in specimens with a higher sensitivity (100%) and specificity (99.9%) compared with hemagglutination inhibition (HI) assay (AniGen H5 AIV Ab ELISA kit; BioNote, Gyeonggi‐do, South Korea). The assay was performed according to the manufacturer's instructions (AniGen H5 AIV Ab ELISA kit; BioNote, Gyeonggi‐do, South Korea).The cELISA assay used in this study had 100% sensitivity and 96% specificity with egg yolk samples against H5N3 (A/wild bird feces/Korea/CSM2/2002 (H5N3) strain) compared with the hemagglutination inhibition assay.24 The cELISA and hemagglutination inhibition (HI) tests to detect avian influenza A virus antibodies in duck eggs had a good inter‐rater agreement (kappa) between tests (K>0.9).24 To classify the duck eggs as positive or negative, we used the manufacturer recommended cutoff value; percent inhibition (PI) values ≥75 were considered as positive and PI ≤75 as negative (AniGen H5 AIV Ab ELISA kit; BioNote, Gyeonggi‐do, South Korea).
2.8.2. Detection of influenza A RNA by real‐time reverse‐transcriptase polymerase chain reaction (rRT‐PCR)
From the fecal swabs, we extracted viral nucleic acid using InviMag virus DNA/RNA mini kit KF96 (Stratec Molecular, Germany) and an automated processing system (KingFisher Flex Magnetic Particle Processor, Thermo Fisher Scientific, Waltham, MA, USA) following the manufacturer's instructions. We performed one‐step rRT‐PCR to screen for influenza A virus by targeting the matrix (M) gene, and all influenza A‐positive samples were further subjected to rRT‐PCR for H5 subtyping using H5a‐ and H5b‐specific primers and probes as previously described.31 A sample was considered positive for detection of influenza A virus RNA if the cycle of threshold (C t) was lower than 40.32 We did not attempt to test for H9, H7, or other subtypes of influenza as our focus was on the H5 subtype which has occurred commonly in Bangladesh.
2.9. Data analysis
We calculated proportions and medians for reporting the variables related to duck flock‐level demographic characteristics and management practices. We estimated the proportion of fecal samples and flocks with influenza A virus RNA with a 95% confidence interval using a log linear model with flock‐level clustering effect adjustment through clustered sandwich estimate of standard error.33 We also estimated the proportion of eggs containing antibodies against avian influenza A (H5) virus after taking into account the sensitivity (100%) and specificity (91%) of the cELISA test.34
2.10. Ethical considerations
We obtained informed consent from the owners of the nomadic duck flocks that were surveyed and sampled. We paid approximately eight Bangladeshi Taka (BDT) for the duck egg depending on the market value. The study protocol was reviewed and approved by the Ethical Review Committee (ERC) and Animal Experimentation Ethical Committee (AEEC) of icddr,b Bangladesh. We also received CDC Institutional Review Board (IRB) approval.
3. Results
3.1. Demographic characteristics of nomadic duck flocks
The median age of the ducks was 24 months (range: 8‐36 months). The median flock size was 300 ducks (range: 105‐1100). The median number of eggs produced daily by each flock was 160 (range: 150‐1100). The majority (63%) of flocks consisted of two breeds (Khaki Campbell and a local indigenous breed).
3.2. Nomadic duck raising practices
3.2.1. Movement practices
Most flocks (98%) stayed within the scavenging area for a median time of 30 days (range: 15‐99). All flocks stayed in temporary confinement (made from bamboo skirt) during the night in the highlands near the scavenging area (Figure 3). All flock owners reported that scarcity of feed was the reason for moving flocks from one scavenging area to another. The median distance that flocks moved in a year was seven kilometers (range: 1‐150). Most owners moved flocks outside of their home village (87%) and most (79%) reported that they led the ducks on foot, while 16% transported ducks by boat and 5% by motor vehicle.
Figure 3.

A nomadic duck flock in the bodies of water and a night shelter
3.2.2. Marketing practices
Most of the owners (90%) sold their entire flock when their egg production decreased. All duck eggs were sold in the village market in the flock owners’ subdistricts (100% N=62). Most of the ducks (92%) were sold to vendors and the remaining ducks (8%) were sold directly to retail customers (Table 1).
Table 1.
Management practices of nomadic duck flocks reported by flock owners in northeastern Bangladesh, 2011‐2012
| Nomadic duck flock management practices | N=62n (%) |
|---|---|
| Movement practices | |
| Longest distance (in kilometers) of movement of duck flocks in the past year, median (range) | 7 (1‐150) |
| Places of movement of duck flocks in the past year | |
| Within own village | 8 (13) |
| Into another village | 36 (58) |
| Into another subdistrict | 10 (16) |
| Into another district | 8 (13) |
| Marketing practices | |
| Reasons for selling duck flocks | |
| Decreased egg production | 56 (90) |
| Scarcity of feed | 5 (8) |
| Disease outbreak(s) | 1 (2) |
| Methods of selling duck flocks | |
| Vendors came to herding places to purchase | 57 (92) |
| Brought to market | 5 (8) |
| Method of selling duck eggs | |
| Brought the eggs to village markets | 62 (100) |
| Biosecurity/biosafety practices | |
| Kept duck flocks away from chickens | 7 (11) |
| Used disinfectant in night shelters | 10 (16) |
| Took measures to prevent duck flocks from mixing with wild waterfowl in common feeding grounds | 5 (8) |
| Methods of disposal of dead ducks | |
| Throwing into a water body | 31 (50) |
| Burying | 29 (47) |
| Burning | 2 (3) |
| Reported hand washing techniques after collecting eggs | |
| With water alone | 48 (77) |
| With soap and water | 13 (21) |
| With ash | 1 (2) |
| Vaccinated of flock for either duck plague or cholera | 36 (58) |
3.2.3. Biosecurity practices
Most owners (71%) did not clean fecal droppings from the night shelter. A small number of owners (16%) used disinfectant in the night shelter. Half of the owners (50%) disposed of dead ducks by throwing them into adjacent water bodies. Almost all the owners (94%) reported that their duck flocks cofed with wild waterfowl. The majority of owners (58%) vaccinated their flocks against either duck plague or duck cholera. All owners reported that they did not vaccinate their duck against avian influenza A (H5N1). Most of the owners (77%) reported that they washed their hands with water from the nearby water bodies after collecting eggs. A few flock owners (11%) took measures to keep duck flocks away from chickens (Table 1). All duck flocks appeared healthy during sample collection.
3.3. Proportion of anti‐H5 antibodies in the eggs of nomadic duck flocks
Of the 1860 egg yolk samples collected, 556 (30%, 95%, CI: 28‐32) had H5 antibodies. Fifty‐eight flocks out of 62 (94%, 95% CI: 84‐98) had at least one egg with H5 antibodies. About half (47%) of the H5 antibody‐positive samples (261/556) had higher (≥90) percent inhibition values.
3.4. Proportion of influenza A virus RNA in the nomadic duck flocks
Of the 496 pooled fecal samples, 121 (24%, 95% CI: 22‐29) samples had detectable influenza A virus RNA by rRT‐PCR with a mean cycle threshold (C t) value of 36 (range: 24.9‐39.9), but none of the tested samples had detectable influenza type A (H5) RNA. Thirty‐three flocks (53%, 95% CI: 40‐66) had at least one pooled fecal sample that tested positive for influenza A virus RNA.
4. Discussion
The study provides molecular evidence of influenza A and antibody evidence of avian influenza A (H5) virus infections among nomadic ducks in northeastern Bangladesh. Nomadic duck raising activities provide important financial support to the duck owner's families, and low‐lying areas with large bodies of water are a favorable environment for nomadic duck raising. However, nomadic ducks are exposed to H5 influenza viruses and are substantial reservoirs of avian influenza A viruses in northeastern Bangladesh.
In this study, egg yolk samples had a much higher proportion (30%) of antibodies against avian influenza A (H5) virus in nomadic ducks than the reported proportion of antibodies detected from blood samples in studies from Indonesia (3%)6 and Vietnam (18%).8 The higher prevalence of antibodies may reflect higher exposure to H5N1 in this region of Bangladesh compared to the studied regions in Indonesia and Vietnam, although the age of the ducks may also have contributed to the high levels of seroprevalence. Most (90%) of the ducks studied in Bangladesh were adult (>12 months). One study found that adult ducks had higher seroprevalence of influenza A virus than subadults (<12 months) in Vietnam,8 presumably because of more opportunities for repeat exposure to influenza A viruses as a duck ages.35 Another possible explanation for these findings is that the ducks could have been exposed to avian influenza A (H5) virus which was circulating in this region a few months prior to our study.36
Nomadic ducks contact with wild waterfowl in the bodies of water during the winter months and contact with other poultry species and humans in the duck owner villages during the summer months. This may pose an increased risk of interspecies transmission of avian influenza A viruses in Bangladesh compared with Thailand, Indonesia, and Vietnam (Table S1).6, 7, 9 Infected wild waterfowl carry avian influenza A viruses and may spread them along their migratory route introducing these viruses into the poultry flocks.37 Our study shows that nomadic ducks were infected with influenza A viruses. Flock owners reported interaction between nomadic ducks and wild waterfowl during the winter period (November‐February) and nomadic ducks had close contact with backyard chickens and humans while staying in the owners’ home villages during the summer months (March‐June). Nomadic ducks in Bangladesh may serve as a bridging host for interspecies transmission of avian influenza A viruses from wild water fowl to backyard poultry or vice versa. Interspecies transmission is a public health concern because of the potential for viral adaption or reassortment between viruses affecting these varied hosts.38
Nomadic duck raising practices were characterized by movement outside of the owners’ home villages, transporting duck flocks on foot and marketing ducks and their eggs. This could contribute to regional spreading of avian influenza A viruses when nomadic ducks are actively shedding virus.5, 6, 7, 9 The practices and levels of infection reported in this study may help inform modeling efforts describing the potential bidirectional spread of avian influenza A viruses between wild waterfowl, nomadic ducks, and domestic poultry in Bangladesh.39
More than one quarter of nomadic duck flocks in our study shed influenza A viral RNA into the environment from their fresh fecal droppings, which is comparable to other studies.3, 4 Duck flocks that shed influenza A viruses while appearing healthy are also consistent with other studies.3, 6
We did not detect any H5 virus RNA in environmental fecal samples of nomadic duck flocks during our study period. Several factors may have contributed to this observation. The low nucleic acid content (mean C t value was 36) among influenza A‐positive samples provided low sensitivity to detect H5. There may have been no active H5 circulation at the time of our study. Many of the nomadic ducks in our study may have been immune to avian influenza A (H5) infection due to previous exposure as indicated by high (30%) antibody positivity against avian influenza A (H5) virus.
Few flock owners reported cleaning fecal material or disinfecting their duck night shelters (Figures 2 and 3). Duck night shelters do not have a floor so duck feces remain on the ground after the shelter is relocated. However, fecal contamination from night shelters may contribute to influenza A virus maintenance in the environment as well as infection of other ducks within the flock. A study in Cambodia suggests that influenza A virus‐contaminated environmental materials may act as potential sources for human and/or animal infection.40 Moreover, the shelters have sides, which made from bamboo skirts could act as vehicles for AIV transmission to another location, because they frequently soiled with duck feces and not properly cleaned.
Figure 2.

A nomadic duck night shelter in study area
Half of the duck flock owners reported that they disposed of dead ducks by throwing them into adjacent water bodies, which is similar to other studies conducted in Bangladesh.41 If ducks are infected, this practice may also spread influenza A viruses in the environment.
Limited biosecurity and hygiene practices may also contribute to the risk of interspecies transmission of avian influenza A viruses including gene exchange of different subtypes of influenza A viruses between nomadic ducks, wild waterfowl, chickens, and possibly humans.
The study has some limitations. First, we examined duck flocks in only one subdistrict so the findings are not statistically representative of all nomadic duck raising areas in Bangladesh. However, this is a major duck raising area and represents a substantial risk for avian influenza virus transmission. Second, we conducted the study during a short period in the winter and exposure to, and shedding of, influenza A viruses may be subject to seasonal variations.21, 28 Nevertheless, the study was conducted during peak season for avian influenza A (H5) virus circulation in Bangladesh.42 Third, instead of structured observation, we depended on reports from duck raisers to describe duck raising practices over the past year which may have been affected by social desirability bias 43 and so the reports of high risk behavior should be seen as minimal estimates. Fourth, we utilized cELISA kit to detect antibodies against avian influenza A (H5), which does not distinguish highly pathogenic from low pathogenic H5 strains. However, widespread outbreaks of highly pathogenic H5N1 in Bangladesh22, 44, 45 since 2007, including HPAI H5N1 outbreak among waterfowl that reported in the study area before 5 months of this study,22 suggest that widespread infection with low pathogenic H5 infections is an unlikely explanation for these results.
Nomadic duck raising is the primary livelihood for the low‐income nomadic duck owners in our study. Investments in improved hygiene and biosecurity measure risk being unaffordable. To develop an affordable and effective intervention, it is important to understand the duck flock owners’ perspectives to identify which practices to target and how to change these practices.46 Interventions to change behavior are more likely to be successful when aligned with the financial incentives of the target population.47, 48 Specifically, biosecurity interventions that cost effectively improve duck survival and egg production are probably more likely to be adopted. We recommend further research to develop and evaluate interventions that simultaneously improve duck raisers profitability and biosecurity.
Supporting information
Acknowledgements
The research protocol was funded by the Centers for Diseases Control and Prevention (CDC), USA, through their cooperative agreement no. U01CI000628‐05. icddr,b acknowledges with gratitude the commitment of CDC to its research efforts. icddr,b is also grateful to the Governments of Bangladesh, Canada, Sweden, and the UK for providing core/unrestricted support. We thank the nomadic duck flock owners and herders who participated in the study for their cooperation and time and the field workers who collected samples and conducted field interviews. We gratefully acknowledge the Department of Livestock Services (DLS) of Bangladesh, veterinary surgeon of Mohanganj Upazila Veterinary Government Hospital and the veterinarian Dr. Mozzafar Goni Osmani from DLS for their cooperation in this study. We thank Dr. Sue Trock of CDC for suggesting egg yolk testing instead of serology, to avoid invasive sampling. We are grateful to Dorothy Southern, Diana DiazGranados, Carrie Read, Meghan Scott, and Astrid Dier for providing support with the scientific writing and clarity of this manuscript.
Sarkar S, Khan SU, Mikolon A, Rahman MZ, Abedin J, Zeidner N, Sturm‐Ramirez K, and Luby SP. An epidemiological study of avian influenza A (H5) virus in nomadic ducks and their raising practices in northeastern Bangladesh, 2011‐2012. Influenza Other Respi Viruses. 2017;11,275–282. https://doi.org/10.1111/irv.12438
References
- 1. Hinshaw VS, Wood J, Webster R, Deibel R, Turner B. Circulation of influenza viruses and paramyxoviruses in waterfowl originating from two different areas of North America. Bull World Health Organ. 1985;63:711. [PMC free article] [PubMed] [Google Scholar]
- 2. Shortridge K. Avian influenza A viruses of southern China and Hong Kong: ecological aspects and implications for man. Bull World Health Organ. 1982;60:129. [PMC free article] [PubMed] [Google Scholar]
- 3. Hulse‐Post DJ, Sturm‐Ramirez KM, Humberd J, et al. Role of domestic ducks in the propagation and biological evolution of highly pathogenic H5N1 influenza viruses in Asia. Proc Natl Acad Sci USA. 2005;102:10682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sturm‐Ramirez KM, Hulse‐Post DJ, Govorkova EA, et al. Are ducks contributing to the endemicity of highly pathogenic H5N1 influenza virus in Asia? J Virol. 2005;79:11269–11279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Henning J, Henning KA, Long NT, Ha NT, Meers J. Characteristics of two duck farming systems in the Mekong Delta of Viet Nam: stationary flocks and moving flocks, and their potential relevance to the spread of highly pathogenic avian influenza. Trop Anim Health Prod. 2013;45:837–848. [DOI] [PubMed] [Google Scholar]
- 6. Henning J, Wibawa H, Morton J, Usman TB, Junaidi A, Meers J. Scavenging ducks and transmission of highly pathogenic avian influenza, Java, Indonesia. Emerg Infect Dis. 2010;16:1244–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Minh PQ, Stevenson MA, Schauer B, Morris RS, Quy TD. A description of the management of itinerant grazing ducks in the Mekong River Delta of Vietnam. Prev Vet Med. 2010;94:101–107. [DOI] [PubMed] [Google Scholar]
- 8. Henning J, Henning KA, Morton JM, et al. Highly pathogenic avian influenza (H5N1) in ducks and in‐contact chickens in backyard and smallholder commercial duck farms in Viet Nam. Prev Vet Med. 2011;101:229–240. [DOI] [PubMed] [Google Scholar]
- 9. Gilbert M, Chaitaweesub P, Parakamawongsa T, et al. Free‐grazing ducks and highly pathogenic avian influenza, Thailand. Emerg Infect Dis. 2006;12:227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cappelle J, Zhao D, Gilbert M, et al. Risks of avian influenza transmission in areas of intensive free‐ranging duck production with wild waterfowl. EcoHealth. 2014;11:109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Islam M, Haque M, Giasuddin M, et al. New introduction of clade 2.3. 2.1 avian influenza virus (H5N1) into Bangladesh. Transbound Emerg Dis. 2012;59:460–463. [DOI] [PubMed] [Google Scholar]
- 12. Parvin R, Kamal AH, Haque ME, et al. Genetic characterization of highly pathogenic H5N1 avian influenza virus from live migratory birds in Bangladesh. Virus Genes. 2014;49:438–448. [DOI] [PubMed] [Google Scholar]
- 13. Ministry of Finance . Bangladesh Economic Review‐2009. Available at: http://www.mof.gov.bd/en/index.php?option=com_content&view=article&id=158&Itemid=1. Accessed on January 10, 2016.
- 14. Das S, Chowdhury S, Khatun M, Nishibori M, Isobe N, Yoshimura Y. Poultry production profile and expected future projection in Bangladesh. Worlds Poult Sci J. 2008;64:99–118. [Google Scholar]
- 15. Sultana R, Nahar N, Rimi NA, et al. Backyard poultry raising in Bangladesh: a valued resource for the villagers and a setting for zoonotic transmission of avian influenza A qualitative study. Rural Remote Health. 2012;12:1927. [PubMed] [Google Scholar]
- 16. The World Bank . Implementation completion and results report (IDA‐43400 TF‐90662) on a credit in the amount of SDR 10.5 million (US$16.0 million equivalent) to the People's Republic of Bangladesh for an avian influenza preparedness and response project under the global program for avian influenza and human pandemic preparedness and response. Washington, DC: The World Bank; 2013. http://www-wds.worldbank.org/external/default/WDSContentServer/WDSP/IB/2013/07/04/000442464_20130704100805/Rendered/PDF/ICR21770ICR0Av0Box0377341B00PUBLIC0.pdf. Accessed 23 October 2014. [Google Scholar]
- 17. Directorate General of Health Services, Bangladesh . 2nd National Avian and Pandemic Influenza Preparedness and Response Plan, Bangladesh 2009–2011, 2009. http://www.who.int/influenza/preparedness/plans/influenza_preparedness_plan_bangladesh/en/index.html. Accessed July 19, 2012.
- 18. Department of Livestock Services, Bangladesh, Daily bird flu Situation, Dhaka, Ministry of Fisheries and Livestock, Government of the People’s Republic of Bangladesh, 2011. Available: http://www.mofl.gov.bd/. Accessed August 1, 2013.
- 19. Fattah KA. Poultry as a tool on poverty eradication and promotion of equality In: Dolberg F, Petersen PH, eds. Proceedings of the Danish Agricultural and Rural Development Advisers’ Forum Workshop on Poultry as a Tool in Poverty Eradication and Promotion of Gender Equality; 1999 Mar 22–26; Tune Landboskole, Denmark. Copenhagen (Denmark): DSR Forlag; 1999:16–28. [Google Scholar]
- 20. Development Wheel. Netrokona Duck Sub‐sector, Inception Report, December 2007. http://dewbd.org/dew/images/stories/dew_report/INCEPTION%20REPORT%20Duckl.pdf. Accessed 1 November 2014.
- 21. Khan MSU, Gurley ES, Rahman M, et al. Live bird market surveillance for avian influenza in Bangladesh, 2007–2009. International Conference on Emerging Infectious Diseases, ICEID 2012. 2010; Abstract book.
- 22. Haider N, Sturm‐Ramirez K, Khan S, et al. Unusually high mortality in waterfowl caused by highly pathogenic avian influenza A (H5N1) in Bangladesh. Transbound Emerg Dis. 2015. doi:10.1111/tbed.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Directorate General of Health Services, Bangladesh. Second National Avian and Pandemic Influenza Preparedness and Response Plan, Bangladesh, 2009‐2011, 2009. http://www.who.int/influenza/preparedness/plans/influenza_preparedness_plan_bangladesh/en/index.html. Accessed July 19, 2012.
- 24. Jeong O‐M, Kim M‐C, Kang H‐M, et al. Validation of egg yolk antibody based C‐ELISA for avian influenza surveillance in breeder duck. Vet Microbiol. 2010;144:287–292. [DOI] [PubMed] [Google Scholar]
- 25. Beck JR, Swayne DE, Davison S, Casavant S, Gutierrez C. Validation of egg yolk antibody testing as a method to determine influenza status in white leghorn hens. Avian Dis. 2003;47(3 Suppl):1196–1199. [DOI] [PubMed] [Google Scholar]
- 26. Trampel DW, Zhou E‐M, Yoon K‐J, Koehler KJ. Detection of antibodies in serum and egg yolk following infection of chickens with an H6N2 avian influenza virus. J Vet Diagn Invest. 2006;18:437–442. [DOI] [PubMed] [Google Scholar]
- 27. Hotta K, Takakuwa H, Yabuta T, et al. Antibody survey on avian influenza viruses using egg yolks of ducks in Hanoi between 2010 and 2012. Vet Microbiol. 2013;116:179–183. [DOI] [PubMed] [Google Scholar]
- 28. Durand LO, Glew P, Gross D, et al. Timing of influenza A (H5N1) in poultry and humans and seasonal influenza activity worldwide, 2004–2013. Emerg Infect Dis. 2015;21:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kish L. Survey Sampling. New York, NY: John Wiley & Sons; 1965. [Google Scholar]
- 30. Heckathorn DD. Comment: snowball versus respondent‐driven sampling. Sociol Methodol. 2011;41:355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kis Z, Jones J, Creanga A, et al. Real‐time RT‐PCR assay to differentiate clades of H5N1 avian influenza viruses circulating in Vietnam. J Virol Methods. 2013;193:452–458. [DOI] [PubMed] [Google Scholar]
- 32. Wille M, van Run P, Waldenström J, Kuiken T. Infected or not: are PCR‐positive oropharyngeal swabs indicative of low pathogenic influenza A virus infection in the respiratory tract of Mallard Anas platyrhynchos? Vet Res. 2014;45:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McCullagh P, Nelder JA. Generalized Linear Models, 2nd edn London: Chapman & Hall/CRC; 1989. [Google Scholar]
- 34. Reiczigel J, Földi J, Ózsvári L. Exact confidence limits for prevalence of a disease with an imperfect diagnostic test. Epidemiol Infect. 2010;138:1674–1678. [DOI] [PubMed] [Google Scholar]
- 35. Karki S, Lupiani B, Budke C, Manandhar S, Ivanek R. Cross‐sectional Serosurvey of Avian Influenza Antibodies Presence in Domestic Ducks of Kathmandu. Nepal: Zoonoses and Public Health; 2013. [DOI] [PubMed] [Google Scholar]
- 36. Haider N, Sturm‐Ramirez K, Khan SU, et al. Unusually high mortality in waterfowl caused by highly pathogenic avian influenza A(H5N1) in Bangladesh. Transbound Emerg Dis. 2015. doi: 10.1111/tbed.12354. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fan Z, Ci Y, Liu L, et al. Phylogenetic and pathogenic analyses of three H5N1 avian influenza viruses (Clade 2.3. 2.1) isolated from wild birds in Northeast China. Infect Genet Evol. 2014;29:138–145. [DOI] [PubMed] [Google Scholar]
- 38. Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992;56:152–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Real L, Biek R. Infectious disease modeling and the dynamics of transmission In: Wildlife and Emerging Zoonotic Diseases: The Biology, Circumstances and Consequences of Cross‐Species Transmission. Berlin: Springer; 2007:33–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Horm SV, Gutierrez RA, Sorn S, Buchy P. Environment: a potential source of animal and human infection with influenza A (H5N1) virus. Influenza Other Respir Viruses. 2012;6:442–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sultana R, Rimi NA, Azad S, et al. Bangladeshi backyard poultry raisers perceptions and practices related to zoonotic transmission of avian influenza. J Infect Dev Ctries. 2011;6:156–165. [DOI] [PubMed] [Google Scholar]
- 42. Chowdhury S. Avian influenza virus surveillance at live bird markets in Bangladesh, 2007–2012. ICDDR,B Health Sci Bull. 2013;11:8–16. [Google Scholar]
- 43. Fadnes LT, Taube A, Tylleskär T. How to identify information bias due to self‐reporting in epidemiological research. Int J Epidemiol. 2009;7:3. [Google Scholar]
- 44. Marinova‐Petkova A, Feeroz MM, Alam SR, et al. Multiple introductions of highly pathogenic avian influenza H5N1 viruses into Bangladesh. Emerg Microbes Infect. 2014;3:e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gerloff NA, Khan SU, Balish A, et al. Multiple reassortment events among highly pathogenic avian influenza A (H5N1) viruses detected in Bangladesh. Virology. 2014;450:297–307. [DOI] [PubMed] [Google Scholar]
- 46. Aboud FE, Singla DR. Challenges to changing health behaviours in developing countries: a critical overview. Soc Sci Med. 2012;75:589–594. [DOI] [PubMed] [Google Scholar]
- 47. Morris SS, Flores R, Olinto P, Medina JM. Monetary incentives in primary health care and effects on use and coverage of preventive health care interventions in rural Honduras: cluster randomised trial. Lancet. 2004;364:2030–2037. [DOI] [PubMed] [Google Scholar]
- 48. Gonzalez AE, Garcıa H, Gilman RH, Tsang VC, in Peru CWG . Control of Taeniasolium. Acta Trop. 2003;87:103–109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


