Abstract
Background:
Proximal Femoral shaft fractures are commonly associated with marked blood loss which can lead to postoperative acute anemia and some other complications.
Tranexamic acid (TA) is an antifibrinolytic medication that reduces intra-and postoperative blood loss and transfusion requirements during some elective surgeries.
The aim of this study is to evaluate the effect of intravenous Tranexamic acid (TA) on intraoperative blood loss and a subsequent need for transfusion in patients who were undergoing surgery for femoral shaft fractures in trauma setting.
Methods:
Thirty-eight ASA grade I-II patients undergoing proximal femoral shaft fracture surgery with intra medullary nailing were included in this double blind randomized controlled clinical trial. They were allocated into two groups. Group I, the intervention group with eighteen patients received 15 mg/kg (TA) via intravenous infusion before surgical incision. Patients in the placebo group received an identical volume of normal saline.
Hemoglobin level was measured four hours before and after the surgeries. Postoperative blood loss and hemoglobin change as well as transfusion rates and volumes were compared between the two groups.
Results:
Mean Percentage fall in hemoglobin after surgery were 1.75±0.84 and 2.04±1.9 in the study and placebo groups, respectively (P=0.570). Hemoglobin loss was higher in the placebo group. Transfusion rates was lower in TA group (5.6%) compared to the placebo group (30%) (P=0.06). No significant difference in The Allowable Blood Loss during the surgery was found between the two groups (P=0.894).
Conclusion:
Preoperative treatment with TA reduces postoperative blood loss and the need for blood transfusion during traumatic femoral fracture operation.
Keywords: Blood loss, Blood transfusion, Femoral fracture, Tranexamic acid
Introduction
Femoral fractures occur frequently following trauma especially in the elderly (1-3). Although bleeding in femoral or hip fracture is usually controllable, there may be significant blood loss, which can lead to severe anemia and hence, need for transfusion and prolonged hospital stay (1, 4, 5). Furthermore, transfusion can cause complications, including anaphylactic and allergic reaction to blood products and infections and even death (1, 6). Blood transfusion can cause complications including allergic and anaphylactic reactions, infections and even death (1-7).
Several approaches have been used to reduce intraoperative blood loss, including: controlled hypotensive anesthesia, local application of cold saline, and pharmacologic treatment like aprotinin and thromboplastic factors, each of which has their own consequences(8-10). Administration of antifibinolytic agents such as tranexamic acid is the alternate approach for hemostasis. Tranexamic acid is a synthetic lysine product which binds to the plasminogen molecules and prevents fibrin clots to dissolve (1, 4, 6, 7, 11). More than 95% of the TA is exerted unchanged through the urine. Although TA is generally well tolerated, however, it can cause uncommon dose dependent side effects including nausea, vomiting, diarrhea, headache, blurred vision, and vertigo. Hypotension can occur suddenly after rapid infusion and in rare cases, thromboembolism may be seen (1-15). TA is an easy accessible and cost effective treatment that has been used in gynecological and obstetric, urologic, orthopedic, spinal and thoracic surgeries and has reduced the amount of blood loss and subsequent need for blood transfusion; However, there are limited data on its use in femoral fracture surgeries (9, 16, 17).The current study was designed to assess the efficacy of TA in decreasing the Hb loss and need for transfusion in patients undergoing femoral shaft fracture surgery.
Materials and Methods
Thirty eight patients aged between 20-50 year old (ASA grade I-II) referring to Poursina Hospital, Rasht Iran, undergoing surgery for femoral fracture with intramedullary nailing were included in this double blind randomized controlled trial study (IRCTregistered code: 201104256280 NI). The ethic committee at our hospital and all patients signed a written informed consent approved the study. Coronary artery disease, history of arterial fibrillation, thrombophilia, chronic renal failure, hemoglobin<10 g/dl, thromboembolic episodes (DVT or pulmonary embolus), taking anticoagulant medication or oral contraceptive pills (OCP) and allergy to TA, presence of subarachnoid hemorrhage (SAH), pregnancy and breast feeding were defined as the exclusion criteria. All patients were examined the day before surgery; their heights and weights were recorded, and the necessary test for transfusion and blood reservation were done. Rapid infusion of TA can lead to gastrointestinal disorder such as nausea, vomiting and hypotension; Therefore, we infused TA slowly in our cases (4).
Patients Randomization
Patients were allocated into two groups based on randomized block method. Group I patients received 15 mg/kg intravenous tranexamic acid (Caspian, Iran) injections dissolved in 100 ml normal saline and 20 min before skin incision; while Group II patients received identical volumes of normal saline (14). Routine monitoring including on- invasive blood pressure (NIBP), electrocardiogram (ECG) and pulse oximetry were performed every five minutes. All patients received midazolam (0.03 mg/kg) and fentanyl (2 mg/kg) as premedication. The surgery was performed under general anesthesia. The induction of anesthesia was accomplished with propofol (2 mg/kg) followed by cisatracurium (0.2 mg/kg) and then tracheal intubation was performed. Anesthesia was maintained with administration of 1-1.2% isoflurane and 10 mg atracurium every 30minutes.
Replacement fluid therapy with crystalloid solution was done according to the calculated maintenance therapy, fluid deficit and third space loss in both groups. Packed red blood cells were transfused based on Allowable Blood loss (ABL) and recorded in milliliters in cases of need for blood transfusion. Neuromuscular reversal agents were administered at the end of the surgery after recording the vital signs. Postoperative hemoglobin concentration was measured four hours after readmission at the orthopedic ward. The amount of transfused Red Blood Cell during the hospital stay was recorded in milliliters.
The total amount of blood transfusion during operation and four hours after the surgery was measured and considered in the formula. The total hemoglobin concentration, before and four hours after the surgery, was calculated based on Nadler et al formula; then, data was compared between the groups.
PBV=Patient Total Blood Volume=(K1×H(m))+(K2×W(kg)) +K3…(Equation 1)
Where:
For men: K1=0.3669, K2=0.03219, K3=0.6041
For women: K1=03361, K2=0.03308, K3=0.1833
Blood Loss=Change in Blood Volume+Transfused Volume …(Equation 2)
Change in Blood Volume=(Hctpreop-Hctpostop)/HctMean …(Equation 3)
Statistical Analysis
Data analysis was performed using SPSS, version 16.0. The independent t-test, MANOVA, Mann-whitney U test, and chi-square test were used for the statistical analyses. Also, P< 0.05 was considered as significant.
Results
Forty patients were enrolled in the study. Two patients refused to participate in the study. The demographic data were similar in both groups; there were no significant differences in the mean age (P=0.652), weight (P=0.463), body mass index (BMI)(P=0.540), gender (P=0.931), the American society of anesthesiologists (ASA) class (P=0.654), pre- and post-op hemoglobin level (P=0.467 and P=0.864 respectively), mean arterial pressure before the surgery (P=0.476), heart rate before anesthesia (P=0.746), duration of anesthesia and surgery (P=0.551 and P=0.199 respectively), and allowable blood loss during the surgery between the two groups (P= 0.894) [Tables 1, 2]. No significant difference was found between the groups in the drain blood volume four hours after entrance to the ward (P=0.295) [Figures 1, 2].
Table 1.
Demographic Characteristics
| Groups | P value | |||
|---|---|---|---|---|
| Placebo | TA | |||
| Age (year) | 66.15±8.51 | 65.11±4.89 | 0.652 | |
| Gender | Male | 17(%85.0) | 14(77.8%) | 0.931 |
| Female | 3(%15.0) | 4(22%) | ||
| BMI* | 25.47±2.53 | 25.35±5.75 | 0.540 | |
| ASA Class ** | class 1 | 0(%0.0) | 0(%0.0) | 0.654 |
| class 2 | 15(%75.0) | 15(83.3%) | ||
| class 3 | 5(%25.0) | 3(16.6%) | ||
| Time of surgery (min) | 115.00±66.47 | 93.89±16.94 | 0.199 | |
| Duration of anesthesia (min) | 144.50±69.17 | 133.89±29.68 | 0.551 | |
BMI, body mass index;
ASA, the American society of anesthesiologists
Table 2.
Allowable Blood Loss, Amount of Bleeding and Transfusion and Surgeon Satisfaction
| The variables | Groups | ||||
|---|---|---|---|---|---|
| Placebo Group | TXA Group | Total | P value | ||
| ABL intraoperative (ml) | 850.55±460.75** | 830.22±476.22 | 840.92 | 0.894 | |
| Blood in Vacuum 4h after transferring to ward (ml) | 242.72±211.02 | 179.55±143.80 | 209.47 | 0.295 | |
| Transfusion received units P/C* | No need transfusion | 14(%70.0) | 17(%94.4) | 0.061 | 0.061 |
| Need to transfusion | 6(%30.0) | 1(%5.6) | 0.173 | ||
| Crystalloid Infusion (L) Induction to ward | 2.42±0.90 | 2.53±0.55 | 2.47 | 0.643 | |
| Surgeon Satisfaction | good | 16±80.0 | 17±94.4 | 0.194 | 0.194 |
| moderate | 4(%20.0) | 1(%5.6) | 5 | ||
Packed Red Blood Cell,
Mean±Sd
Figure 1.
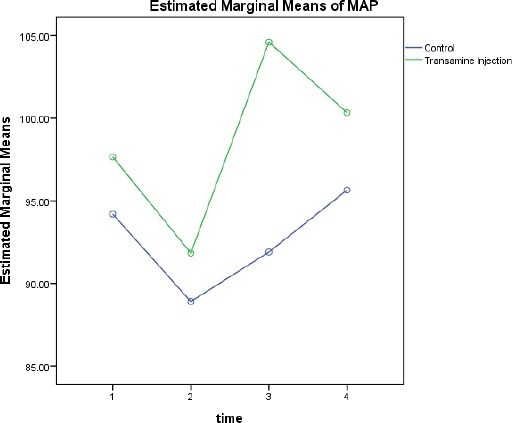
Comparing the hemodynamic changes.
Figure 2.
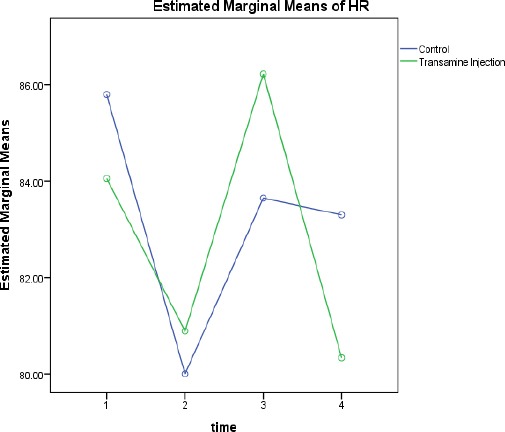
Comparing the changes in HR.
Although the mean fall in hemoglobin concentration in placebo group was higher than the test group, however, the difference was not statistically significant (P= 0.570) [Figure 3; Table 3]. The difference in transfusion rate between the placebo group (30%) and study group (5.6%) was not statistically significant (P=0.06). Although the surgeon satisfaction level was higher in the study group (94.4%) compared to the placebo group (80%), the difference was not statistically significant. It was noteworthy that no dissatisfaction (lower than medium level) was reported in both two groups [Table 2].
Figure 3.
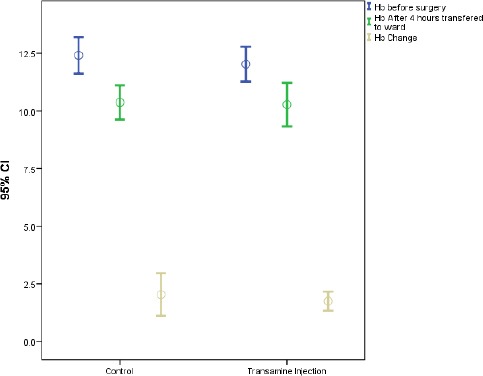
comparing the changes in HB level.
Table 3.
Hemoglobin and Hemodynamic Changes
| Groups Statistics | |||||
|---|---|---|---|---|---|
| Groups | N | Mean | Std. Deviation | P value | |
| Hb(mg) (Initial Level) | Placebo Group | 20 | 12.62 | 1.94 | 0.744 |
| TXA Group | 18 | 12.42 | 1.76 | ||
| Hb(mg) (Before Surgery) | Placebo Group | 20 | 12.41 | 1.69 | 0.467 |
| TXA*** Group | 18 | 12.02 | 1.52 | ||
| Hb(mg) 4h In ward | Placebo Group | 20 | 10.37 | 1.58 | 0.864 |
| TXA Group | 18 | 10.27 | 1.90 | ||
| Hemoglobin Changes During The Surgery | Placebo Group | 20 | 2.04 | 1.97 | 0.570 |
| TXA Group | 18 | 1.75 | 0.74 | ||
| MAP* before induction | Placebo Group | 20 | 94.20 | 14.08 | 0.476 |
| TXA Group | 18 | 97.67 | 15.61 | ||
| MAP (0.5 h) after surgery | Placebo Group | 20 | 88.90 | 17.11 | 0.592 |
| TXA Group | 18 | 91.83 | 16.24 | ||
| MAP before transferring to recovery | Placebo Group | 20 | 91.90 | 16.26 | 0.026 |
| TXA Group | 18 | 104.61 | 17.54 | ||
| MAP 4 h in ward | Placebo Group | 20 | 95.65 | 13.96 | 0.318 |
| TXA Group | 18 | 100.33 | 14.54 | ||
| HR** before induction | Placebo Group | 20 | 85.80 | 18.18 | 0.746 |
| TXA Group | 18 | 84.06 | 14.19 | ||
| HR 0.5 h after surgery | Placebo Group | 20 | 80.00 | 17.97 | 0.870 |
| TXA Group | 18 | 80.89 | 15.00 | ||
| HR before transferring to recovery | Placebo Group | 20 | 83.65 | 20.81 | 0.679 |
| TXA Group | 18 | 86.22 | 16.71 | ||
| HR 4 hr In ward | Placebo Group | 20 | 83.30 | 14.62 | 0.514 |
| TXA Group | 18 | 80.33 | 12.91 | ||
MAP=Mean Arterial Pressure,
HR= heart Rate,
Tranexamic Acid
Independent T-test showed a significant difference in mean arterial pressure (MAP) between the groups (P=0.026). In addition, heart rate changes before and four hours after the surgery, were similar in both groups with no significant difference (P= 0.617) [Table 3].
Discussion
Numerous studies have reported favorable safety and efficacy of TA in orthopedic surgeries like total hip, total knee replacement, and fractures (2). There is no universal standard of its administration and its use has not yet become the standard of practice. Especially, limited research has shown that TA is effective in reducing perioperative blood loss in femoral fractures compared to placebo.
This study showed that although the effect of preoperative intravenous administration of TA on hemoglobin fall, rate of blood loss, and the need for transfusions in patient undergoing proximal femoral shaft fractures surgery is statistically nonsignificant, a 25% lower transfusion requirement was seen in the study group compared to the placebo. Tranexamic acid accumulates in the extracellular space, inhibiting the tissue fibrinolysis and accordingly stabilizes the clot, but, has no effect on coagulation parameters (13). (Tyler C. Wind et al) evaluated the efficacy and safety of topical TA compared to its intravenous administration in patient undergoing total knee arthroplasty (TKA). They found that TA reduces the transfusion requirement in patients undergoing TKA (12). Robin G. mac Gilli Vary et al. showed that TA reduces bleeding in patients undergoing bilateral knee arthroplasty and reported that the mean amount of bleeding in the placebo group and groups of 15 and 10 mg/kg TA were 918, 462 and 678 mL, respectively. The amount of transfusion was similar in both study groups and significantly lower than that of the placebo group which is in line with the results of the current study (18). Sadeghi et al. showed that intravenous TA reduces blood loss and decreases the transfusion rate and volumes and the length of hospitalization in patients undergoing TKA (9). In a different study by Rannikko A. et al. oral TA 2 gram was given trice a day in the operating day and the day after surgery to the patients undergoing transurethral resection of the prostate (TURP) for benign prostatic hyperplasia (BPH). They concluded that oral TA significantly reduces intraoperative blood loss while it has no effect on the amount of postoperative bleeding (13).
The rout of TA administration and the type of surgery may be the reasons for their different results (13). Tranexamic acid has also been shown to reduce bleeding in patients undergoing posterior lumbar spine surgery (11).
A lower transfusion rate (34%) was reported in TA-treated patients undergoing retropubic prostatectomy compared to the non-treated controls (55%) in a study performed in Milan, Italy. In addition, the -relative risk of transfusion in the study group was reported to be 0.62 (0.45-0.85). Also, no significant difference was found in the incidence of thromboembolic event between the two groups (19). Rate of vascular injury was 16% in the TA administered group and 6% in the placebo group P=0.1 (20). A study on patients undergoing hip fracture surgery showed a significant difference (P=0.06) in transfusion rate in TA treated group (42%) with the placebo group (60%). It should be noticed that four studies are conducted on the strategies to attenuate PRBc transfusion ratio, and there is no universally accepted method yet.
Numerous research studies have showed that intraoperative treatment with proper dose of TA in order to reducing blood loss is a simple, safe, and more economic method compared to aprotinin and recombinant factor VII. In this current study, the efficacy of tranexamic acid administration in patients undergoing intramedullary nailing fixation for formal surgery was evaluated. However, According to the small sample size (38 patients), the results did not have enough strength to prove the usefulness of Tranexamic Acid in traumatic femoral fracture operation. Also, as the inclusion criteria were very wide, TA might be useful for other orthopedic surgeries (10). In agreement with other studies conducted in this filed, we did not have any sever complications due to use of intravenous TA (12, 14, 15). Also, due to the insufficient follow up period, long term effects of TA on the incidence of thromboembolic events could not be evaluated in our study. On the other hand, a larger sample size with a longer follow up time are necessary to precisely determine the effectiveness and complications of TA (1). Use of Topical tranexamic acid is a new challenge in controlling intra operative blood loss among the major orthopedic surgery and there is strong recommendation in orthopedic surgery to compare these two from of TA topical and intravenous with each other (21, 22).
The present study demonstrated that, intravenous TA before skin incision in patient undergoing proximal femoral shaft fractures surgery, may reduce intraoperative blood loss and hemoglobin fall but without significant reduction in postoperative anemia. However, further investigations are necessary.
Acknowledgments
The authors would like to thank Anesthesiology Research Center, Guilan University of Medical Science for their help and cooperations.
This study was financially supported by Vice- Chancellorship of research and technology of Guilan University of Medical Science.
There was no conflict of interest.
References
- 1.Henry DA, Carless PA, Moxey AJ, O’Connell D, Stokes BJ, Fergusson DA, et al. Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2011;16(3):CD001886. doi: 10.1002/14651858.CD001886.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Kim C, Park SS, Davey JR. Tranexamic acid for the prevention and management of orthopedic surgical hemorrhage: current evidence. J Blood Med. 2015;6(25):239–44. doi: 10.2147/JBM.S61915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keyhani S, Esmailiejah AA, Abbasian MR, Safdari F. Which route of tranexamic acid administration is more effective to reduce blood loss following total knee arthroplasty? Arch Bone Jt Surg. 2016;4(1):65–9. [PMC free article] [PubMed] [Google Scholar]
- 4.Soleimanha M, Haghighi M, Mirbolook A, Sedighinejad A, Mardani-Kivi M, Naderi-Nabi B, et al. A survey on transfusion status in orthopedic surgery at a trauma center. Arch Bone Jt Surg. 2016;4(1):70–4. [PMC free article] [PubMed] [Google Scholar]
- 5.Lin SY, Chen CH, Fu YC, Huang PJ, Chang JK, Huang HT. The efficacy of combined use of intraarticular and intravenous tranexamic acid on reducing blood loss and transfusion rate in total knee arthroplasty. J Arthroplasty. 2015;30(5):776–80. doi: 10.1016/j.arth.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Vijay BS, Bedi V, Mitra S, Das B. Role of tranexamic acid in reducing postoperative blood loss and transfusion requirement in patients undergoing hip and femoral surgeries. Saudi J Anaesth. 2013;7(1):29–32. doi: 10.4103/1658-354X.109803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong J, El Beheiry H, Rampersaud YR, Lewis S, Ahn H, De Silva Y, et al. Tranexamic Acid reduces perioperative blood loss in adult patients having spinal fusion surgery. Anesth Analg. 2008;107(5):1479–86. doi: 10.1213/ane.0b013e3181831e44. [DOI] [PubMed] [Google Scholar]
- 8.Keating EM, Meding JB. Perioperative blood management practices in elective orthopaedic surgery. J Am Acad Orthop Surg. 2002;10(6):393–400. doi: 10.5435/00124635-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Noordin S, Waters TS, Garbuz DS, Duncan CP, Masri BA. Tranexamic acid reduces allogenic transfusion in revision hip arthroplasty. Clin Orthop Relat Res. 2011;469(2):541–6. doi: 10.1007/s11999-010-1441-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soleimanha M, Sedighinejad A, Haghighi M, Nabi BN, Mirbolook AR, Mardani-Kivi M. Hemodynamic and arterial blood gas parameters during cemented hip hemiarthroplasty in elderly patients. Arch Bone Jt Surg. 2014;2(3):163–7. [PMC free article] [PubMed] [Google Scholar]
- 11.Foss NB, Kehlet H. Hidden blood loss after surgery for hip fracture. J Bone Joint Surg Br. 2006;88(8):1053–9. doi: 10.1302/0301-620X.88B8.17534. [DOI] [PubMed] [Google Scholar]
- 12.Wind TC, Barfield WR, Moskal JT. The effect of tranexamic acid on blood loss and transfusion rate in primary total knee arthroplasty. J Arthroplasty. 2013;28(7):1080–3. doi: 10.1016/j.arth.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 13.Rannikko A, Petas A, Taari K. Tranexamic acid in control of primary hemorrhage during transurethral prostatectomy. Urology. 2004;64(5):955–8. doi: 10.1016/j.urology.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Shakur H, Roberts I, Bautista R, Caballero J, Coats T, et al. CRASH-2 trial collaborators. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376(9734):23–32. doi: 10.1016/S0140-6736(10)60835-5. [DOI] [PubMed] [Google Scholar]
- 15.Turpie AG, Eriksson BI, Bauer KA, Lassen MR. New pentasaccharides for the prophylaxis of venous thromboembolism: clinical studies. Chest. 2003;124(6 Suppl):371S–8. [PubMed] [Google Scholar]
- 16.Sethna NF, Zurakowski D, Brustowicz RM, Bacsik J, Sullivan LJ, Shapiro F. Tranexamic acid reduces intraoperative blood loss in pediatric patients undergoing scoliosis surgery. Anesthesiology. 2005;102(4):727–32. doi: 10.1097/00000542-200504000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Dunn CJ, Goa KL. Tranexamic acid: a review of its use in surgery and other indications. Drugs. 1999;57(6):1005–32. doi: 10.2165/00003495-199957060-00017. [DOI] [PubMed] [Google Scholar]
- 18.MacGillivray RG, Tarabichi SB, Hawari MF, Raoof NT. Tranexamic acid to reduce blood loss after bilateral total knee arthroplasty: a prospective, randomized double blind study. J Arthroplasty. 2011;26(1):24–8. doi: 10.1016/j.arth.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 19.Crescenti A, Borghi G, Bignami E, Bertarelli G, Landoni G, Casiraghi GM, et al. Intraoperative use of tranexamic acid to reduce transfusion rate in patients undergoing radical retropubic prostatectomy: double blind, randomised, placebo controlled trial. BMJ. 2011;343:5701. doi: 10.1136/bmj.d5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zufferey PJ, Miquet M, Quenet S, Martin P, Adam P, Albaladejo P, et al. Tranexamic acid in hip fracture surgery: a randomized controlled trial. Br J Anaesth. 2010;104(1):23–30. doi: 10.1093/bja/aep314. [DOI] [PubMed] [Google Scholar]
- 21.Rasouli MR, Parvizi J. Tranexamic Acid in total joint arthroplasty: efficacy and safety. Arch Bone Jt Surg. 2015;3(1):1–2. [PMC free article] [PubMed] [Google Scholar]
- 22.Patel JN, Spanyer JM, Smith LS, Huang J, Yakkanti MR, Malkani AL. Comparison of intravenous versus topical tranexamic acid in total knee arthroplasty: a prospective randomized study. J Arthroplasty. 2014;29(8):1528–31. doi: 10.1016/j.arth.2014.03.011. [DOI] [PubMed] [Google Scholar]


