Abstract
The hippocampal formation undergoes significant morphological and functional changes after prolonged caloric and dietary restriction (DR). In this study we tested whether prolonged DR results in deleterious alterations in hippocampal neurogenesis, density of granule cell neurons and mossy fibers, all of which support plasticity in the dentate gyrus. Young adult animals either experienced free access to food (control condition), or every-other-day feeding regimen (DR condition) for 3 months. The number of Ki-67 cells and 28-day old 5-bromo-2’-deoxyuridine (BrdU) cells were quantified in the dorsal and ventral dentate gyrus to determine the effect of DR on cellular proliferation and survival of neural progenitor cells in the anatomically defined regions of the dentate gyrus. The density of granule cell neurons and synaptoporin were also quantified to determine the effect of DR on granule cell neurons and mossy fiber projections in the dentate gyrus. Our results show that DR increases cellular proliferation and concurrently reduces survival of newly born neurons in the ventral dentate gyrus without effecting the number of cells in the dorsal dentate gyrus. DR reduced density of granule cell neurons in the dorsal dentate gyrus. These alterations in the number of granule cell neurons did not affect mossy fiber density in DR animals, which was visualized as no differences in synaptoporin expression. Our findings demonstrate that granule cell neurons in the dentate gyrus are vulnerable to chronic DR and that the reorganization of granule cells in the dentate gyrus subregions is not producing concomitant alterations in dentate gyrus neuronal circuitry with this type of dietary restriction.
Keywords: Dentate gyrus, BrdU, Ki-67, Synaptoporin, Neurogenesis, Food restriction
1. Introduction
Nutrient composition, frequency, and quantity of diet can influence the brain in a multitude of ways (Prehn et al., 2016). For example, dietary restriction (DR) studies conducted on pregnant dams have shown that perinatal DR produces deleterious and often permanent effects on the physiology and morphology of the developing hippocampus, and function dependent on the hippocampus during periods of neurogenesis and rapid cell growth (Morgane et al., 2002). DR studies conducted in young adult and adult rodents (2 to 6 months of age) also show that neurogenesis in the postnatal and adult hippocampus continues to be vulnerable to nutritional insults, with DR animals showing greater number of newly born neurons in the dentate gyrus (Lee et al., 2000; Kim et al., 2015). Furthermore, the detrimental effects of DR on hippocampal plasticity and hippocampal dependent behaviors in adult rodents are recognized to a lesser extent than those observed during development, suggesting that compensatory reorganization in the structural plasticity of hippocampal neurons is capable of minimizing the functional impairments that were expected to occur following DR conditions during adulthood (Andrade et al., 2002; Rezende et al., 2015). Contradictory to the effects of DR on hippocampal plasticity and function during development and adulthood, DR has been unanimously indicated to be beneficial during aging and in aged subjects (10 to 22 months of age; (Ingram et al., 1987; Barger et al., 2003; Park et al., 2013)). Therefore, it appears that effects of DR on hippocampal structure and function are influenced by the age of the subject, where it is less beneficial during postnatal development and young adulthood and enhances several aspects of hippocampal structure and function in aged subjects.
Particularly interesting is the findings on hippocampal plasticity after DR in adult subjects, where DR does not alter proliferation of neural progenitor cells, however, enhances survival of newly born granule cell neurons, and these alterations do not affect the total number of granule cell neurons (Lee et al., 2000; Andrade et al., 2002; Kim et al., 2015). Notably, while DR reduces the number and structural arborization of granule cell neurons, it does not alter mossy fiber sprouting in the dentate gyrus (Lukoyanov & Andrade, 2000; Andrade et al., 2002; Rezende et al., 2015), suggesting that subtle adaptations in dentate gyrus granule cell neuron structure do not produce overt effects on dentate gyrus synaptic plasticity. Similar findings are noted in hippocampal function after DR in adult subjects, where DR does not alter learning and spatial memory dependent on the hippocampus (Andrade et al., 2002), but does reduce the ability to cope with stress provoked by aversive stimuli (Campbell & Richardson, 1988; Heiderstadt et al., 2000; Lukoyanov & Andrade, 2000), alters internal homeostasis by enhancing alliesthesia (Roberts et al., 1983; Carr & Wolinsky, 1993; Abrahamsen et al., 1997), and enhances rewarding properties of illicit drugs (Carroll & Meisch, 1981; Cabeza de Vaca & Carr, 1998). Given that hippocampal function and neurogenesis vary in a subregion-specific fashion (for reviews see (Bannerman et al., 2004; O’Leary & Cryan, 2014)), where dorsal hippocampus regulates spatial processing and has higher levels of neurogenesis, whereas ventral hippocampus regulates anxiety-related behaviors (such as coping to stress, drug seeking), the behavioral alterations produced by DR suggest that DR animals display a number of impairments that can be ascribed to maladaptive plasticity in the ventral hippocampus (Moser et al., 1995; Kjelstrup et al., 2002; Bannerman et al., 2004; Pothuizen et al., 2004; Pentkowski et al., 2006). To the best of our knowledge, the dentate gyrus subregion specific sequelae of chronic DR in young adult animals have not yet been quantitatively characterized. Therefore, we hypothesized that chronic DR might differently affect proliferation and survival of neural progenitors in dorsal versus ventral dentate gyrus. We also hypothesized that DR-induced changes in neurogenesis would be associated with alterations in granule cell neuron density and mossy fiber density in the dentate gyrus.
2. Results
2.1 Every-other-day feeding regimen reduces body weight in young adult rats
Young adult rats were maintained on either control (ad lib) or a DR feeding regimen (where they were fed every other day). Repeated measures two-way ANOVA demonstrated a significant DR regimen × weeks interaction (F (12, 72) = 28.60, p <0.001), significant main effect of DR regimen (F (1, 6) = 44.53, p <0.001) and weeks maintained on DR regimen (F (12, 72) = 508.3, p<0.001; Figure 1). Post-hoc analysis revealed significant reduction in body weight as early as 2 weeks into the DR regimen (p <0.01). The body weight of rats in either experimental group progressively increased over the entire period of observation, as indicated by a significant effect of weeks on the mean body weight of controls (F (12, 36) = 312.2, p = 0.0001) and DR animals (F (12, 36) = 199.8, p = 0.0001). At the end of the study, DR rats weighed 22% less than the control rats.
Figure 1.
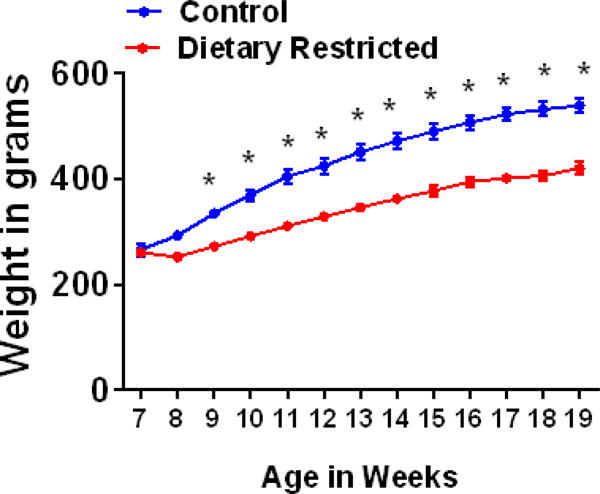
Changes in body weight over weeks of DR in male Wistar rats. Body weight is indicated in grams. BrdU was injected when animals were 15 weeks of age. *p<0.05 vs controls. Data is represented as mean ± SEM.
2.2 DR enhances proliferation of neural progenitors and reduces survival of neural progenitors in the ventral dentate gyrus
The number of Ki-67 immunoreactive cells were measured to quantify the cell proliferation in control and DR animals. Cells were quantified in the dorsal and ventral dentate gyrus. Two-way ANOVA demonstrated a significant DR = dentate gyrus subregion interaction (F (1, 12) = 8.957, p = 0.01) and a significant effect of subregion (F (1, 12) = 15.15, p = 0.002), but did not demonstrate significant effect of DR. Post-hoc analysis showed that control animals had a higher number of Ki-67 cells in the dorsal dentate gyrus compared with ventral sections (p <0.05, Figure 2b), and DR abolished this subregion difference by enhancing the number of Ki-67 cells in the ventral DG (p <0.05).
Figure 2.
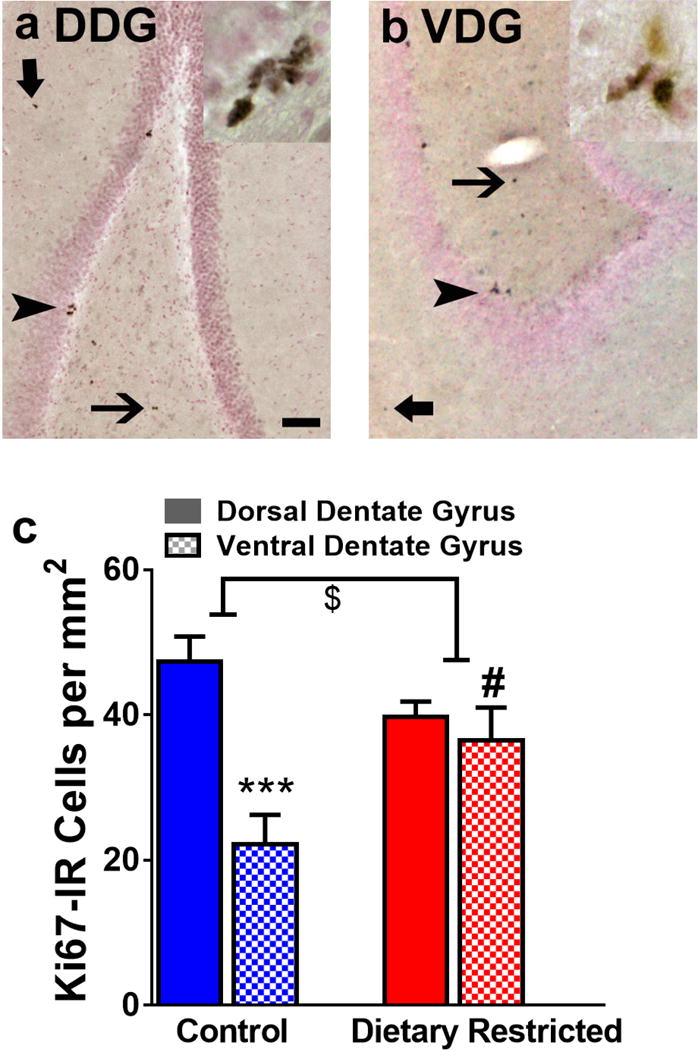
Photomicrographs of Ki-67 immunoreactive cells in dorsal (a, DDG) and ventral (b, VDG) dentate gyrus. Arrowhead in a-b points to a cluster of Ki-67 cells in the subgranular zone. Thick arrow in a–b points to a cell in the molecular layer and thin arrow in a–b points to a cell in the hilus of the dentate gyrus. Inset in a–b show Ki-67 cells at 400x magnification. Scale bar in (a) is 50 μm applies a–b, main panel and 10 μm in the inset. Quantitative analysis of Ki-67 cells in both subregions in the subgranular zone (c). $ indicated significant interaction, ***p<0.001 vs. dorsal region, #p<0.05 vs. control animals. Data is represented as mean ± SEM.
The number of 28-day-old BrdU immunoreactive cells were measured to quantify the cell survival in control and DR animals. Cells were quantified in the dorsal and ventral dentate gyrus. While two-way ANOVA did not show an interaction or effect of subregion, there was a strong trend towards effect of DR (F (1, 12) = 4.272, p = 0.06). Unpaired t test was performed between groups and the number of BrdU cells in the controls in the ventral dentate gyrus was higher than the number of BrdU cells in the DR animals (p = 0.02; Figure 3b).
Figure 3.
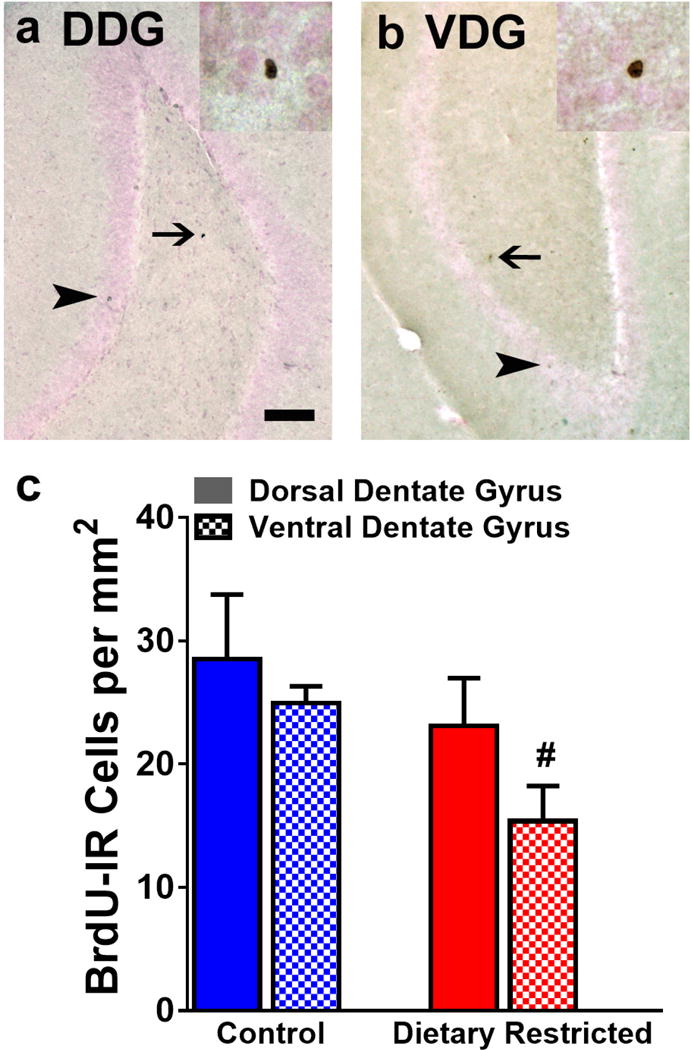
Photomicrographs of BrdU immunoreactive cells in dorsal (a, DDG) and ventral (b, VDG) dentate gyrus. Arrowhead in a-b points to a mature BrdU cell in the granule cell layer. Thin arrow in a-b points to a cell in the hilus of the dentate gyrus. Inset in a–b show BrdU cells at 400x magnification. Scale bar in (a) is 50 μm applies a–b, main panel and 10 μm in the inset. Quantitative analysis of BrdU cells in both subregions (c). #p<0.05 vs. control animals. Data is represented as mean ± SEM.
2.3 DR reduces the number of granule cell neurons in the dorsal dentate gyrus and does not alter the number of granule cell neurons in the ventral dentate gyrus
The number of granule cell neurons were quantified using stereological methods in control and DR dorsal and ventral dentate gyrus sections. Two-way ANOVA did not detect treatment × subregion interaction (F (1, 12) = 1.74, p = 0.2), main effect of subregion (F (1, 12) = 2.455, p = 0.14) or treatment (F (1, 12) = 0.03, p = 0.8). A priori comparisons were performed separately, and control animals show higher number of granule cell neurons compared with DR animals in the dorsal dentate gyrus (p = 0.05 by Unpaired t test; Figure 4). No effect was seen in the ventral dentate gyrus.
Figure 4.
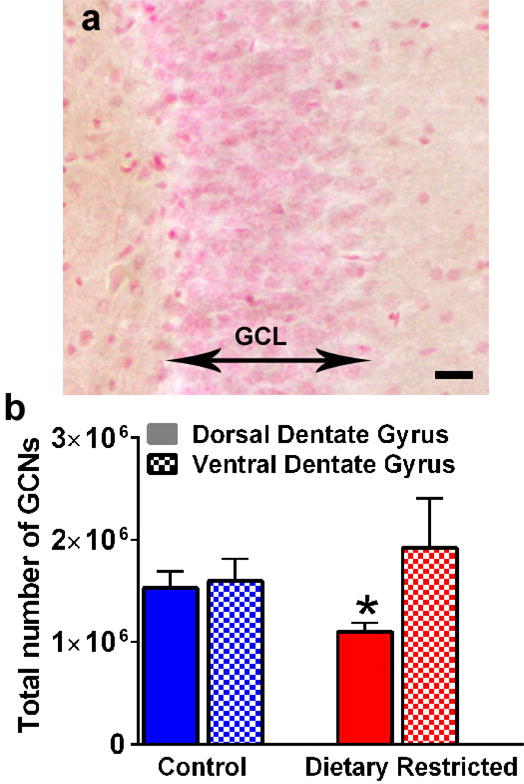
Photomicrograph of granule cell neurons in the dorsal dentate gyrus used for stereological analysis (a). Two sided arrow indicates the granule cell layer. Granule cells are pink in color visualized with Vector FastRed staining. Scale bar in (a) is 25 μm. Quantitative analysis of granule cells in both subregions by optical fractionator method (b). *p≤0.05 vs. control animals. Data is represented as mean ± SEM.
2.4 DR does not affect the density of mossy fiber projections in the dorsal and ventral dentate gyrus
The density of mossy fiber subfields was analyzed in dorsal and ventral hippocampal sections from control and DR animals. Two-way ANOVA did not detect treatment = subregion interaction (F (1, 12) = 0.4052, p = 0.5), main effect of subregion (F (1, 12) = 3.007, p = 0.1) or treatment (F (1, 12) = 1.455, p = 0.2). No differences between the two groups were found regarding the density of staining in the hilus, the infrapyramidal layer and the suprapyramidal layer (Figure 5).
Figure 5.
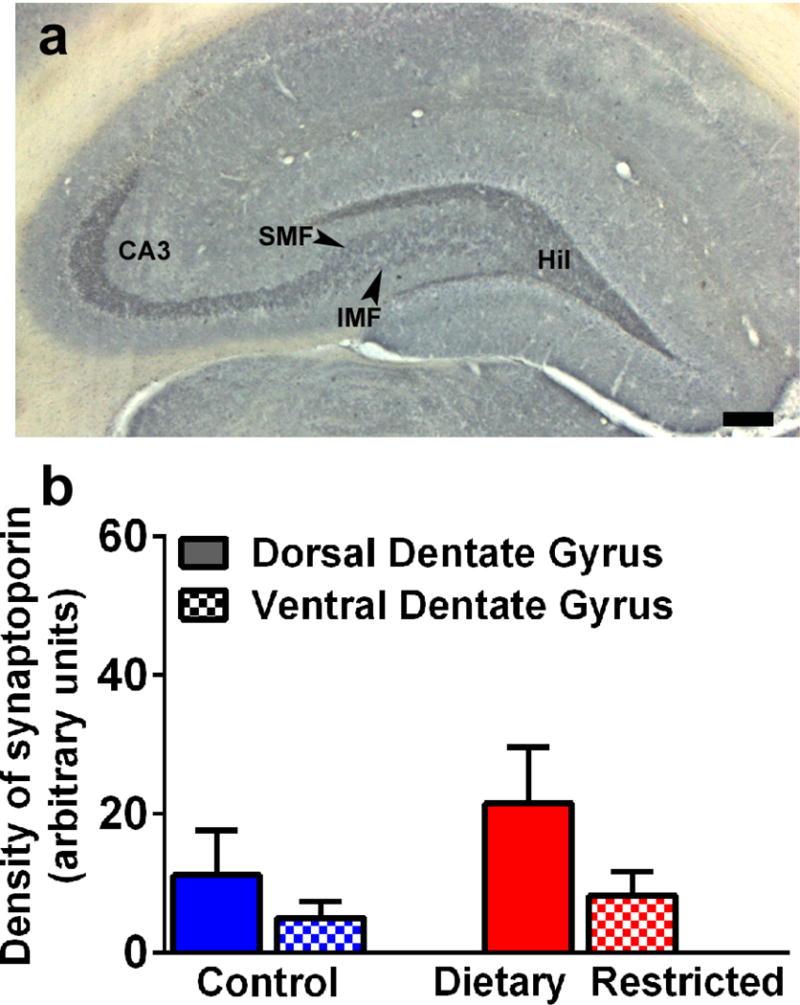
Photomicrograph showing mossy fiber projections via staining for synaptoporin in the dorsal dentate gyrus (a). Staining for synaptoporin was evident in the hilus (Hil), CA3 region, with infrapyramidal mossy fibers (IMF) and suprapyramidal mossy fibers (SMF) indicated in the hilus region. Scale bar in (a) is 100 μm. Densitometric analysis of mossy fiber projections in the dorsal and ventral dentate gyrus (b). Data is represented as mean ± SEM.
3. Discussion
The present results show that the DR paradigm employed was effective at reducing body weight since a significant difference in weight between control (ad lib fed) and DR groups was observed during the early weeks on the diet and was maintained until the end of the experiment. The DR rats showed 22% lower body weight than the control rats, indicating that the feeding paradigm used in this study produced chronic undernutrition (Goodrick et al., 1983). Although our data confirm the weight loss effects associated with DR, the neuroanatomical and morphometric findings do not necessarily indicate protection against hippocampal neuroplasticity (Lee et al., 2000; Lukoyanov & Andrade, 2000; Andrade et al., 2002; Qiu et al., 2012; Kim et al., 2015; Rezende et al., 2015).
In the adult hippocampus, functional granule cell neurons are generated in the dentate gyrus throughout life by a multistep process called neurogenesis (Garcia et al., 2004; Spalding et al., 2013). The process of neurogenesis involves stem-like precursor cells that proliferate into preneuronal progenitors, which in turn differentiate into immature neurons and eventually mature into granule cell neurons (Abrous et al., 2005). Proliferating progenitor cells in the subgranular zone (SGZ) of the dentate gyrus are heterogeneous, and Ki-67 labels cell types of varied proliferative activity, namely, types 2a/2b/3 (Kronenberg et al., 2003). Furthermore, a cell that is actively dividing during synthesis (S) phase, undergoing mitosis during gap2 (G2) phase and trapped in the gap1 (G1) phase of the cell cycle would still express Ki-67 (Scholzen & Gerdes, 2000), suggesting that alterations in Ki-67 labeling would reveal changes in progenitors in multiple phases of the cell cycle. Therefore, we used Ki-67 to determine whether DR alters several subtypes of SGZ progenitors. Unlike Ki-67 that is endogenously expressed by proliferating cells, BrdU is used for experimentally labeling proliferating cells (Dayer et al., 2003; Mandyam et al., 2007; Taupin, 2007). Injections of BrdU are also indicated to have cytotoxic and teratologic effects (Kolb et al., 1999; Sekerkova et al., 2004; Ogawa et al., 2005; Kuwagata et al., 2007; Duque & Rakic, 2011; Rowell & Ragsdale, 2012), primarily because BrdU is a marker of DNA synthesis and not of cell division per se (Breunig et al., 2007). However, in adult rodent models, BrdU cytotoxicity is typically evident at greater than 2 times the currently used dose ((Cameron & McKay, 2001; Eadie et al., 2005); for review, (Taupin, 2007)), suggesting that the BrdU dose used in the current study could be suitable to evaluate survival of progenitors in adult rats. In the dentate gyrus, newborn cells that survive ~28 days are predominantly neuronal (~70% become mature neurons, (Palmer et al., 2000)) and are stably incorporated into the granule cell layer (Kempermann et al., 2003). Therefore, we used BrdU to determine whether DR alters survival of SGZ progenitors.
Granule cell neurons generated during adulthood assist with neuronal turnover (Ming & Song, 2011). Computational and behavioral models combined with electrophysiological findings indicate that the dentate gyrus (with active neuronal turnover and communications with CA3 neurons via mossy fiber projections) participates in an array of behaviors to assist with hippocampal dependent spatial memory (Sahay et al., 2011; Niibori et al., 2012; Park et al., 2015). Functional dissociation also exists along the dorsal-ventral gradient in the rat hippocampus. For example, the ventral hippocampus when compared with the dorsal hippocampus has greater output connections with the areas of the brain implicated in stress responses (Henke, 1990; Pitkanen et al., 2000; Ishikawa & Nakamura, 2006), suggesting that neuroadaptations in the ventral hippocampus may be strongly associated with impaired emotional responsiveness. Indeed, DR is known to produce hyperlocomotor activity in open field testing, suggesting that DR animals exhibit higher emotional responsiveness to a novel environment (Andrade et al., 2002). DR is known to enhance hippocampal neurogenesis (dorsal and ventral combined) by promoting the survival of neural progenitor cells without affecting active cell proliferation (Lee et al., 2000; Kim et al., 2015). Although the mechanisms underlying enhanced survival of newly born progenitors are unknown, it has been speculated that the metabolic stress induced by DR could regulate the later stages of neuronal development of these neural progenitor cells (Kim et al., 2015). Contrary to the previous findings in the field, our results via Ki-67 labeling show that DR did not alter the developmental stages of newly born cells in the dorsal dentate gyrus. Furthermore, DR enhanced proliferation of newly born cells and reduced survival of newly born cells in the ventral dentate gyrus, suggesting that the newly born cells were unstable and unable to survive into immature neurons in the ventral dentate gyrus. It is possible that DR-induced maladaptive changes in protein biosynthesis, neurotransmitter and neuropeptide release in the hippocampus may have contributed to the subregion specific changes in cell survival of newly born progenitors (Wiggins et al., 1984; Shoham et al., 2000; Rotta et al., 2003). For example, there are differences in the density of dopamine receptors, noradrenergic inputs, glutamatergic receptor-dependent plasticity along the dorso-ventral axis of the hippocampus (Kempadoo et al., 2016; Kouvaros & Papatheodoropoulos, 2016; Weitemier & McHugh, 2016; Zhang et al., 2016), all of which regulate neurogenesis in the dentate gyrus (Cameron et al., 1995; Mu et al., 2011). Nevertheless, the distinct alterations in cell survival in the ventral dentate gyrus could support the behavioral deficits in emotional responsiveness with this type of DR paradigm (Morse et al., 1995; Altemus et al., 1996; Weed et al., 1997; Heiderstadt et al., 2000). Taken together, our findings demonstrate that ventral dentate gyrus of adult rats may be more sensitive to the effects of DR.
DR also reduced the number of granule cell neurons in the dorsal dentate gyrus without effecting the cells in the ventral dentate gyrus. These findings suggest that DR produces detrimental effects on newly born and preexisting granule neurons. One possible explanation for these effects could be due to the fact that DR is a stressor and produces elevated plasma corticosterone levels (Carr, 1996; Heiderstadt et al., 2000). For example, the hippocampus is sensitive to changes in corticosterone levels in response to stress, and this has been associated with reduced neurogenesis, reduced hippocampal neuron number, dendritic atrophy of hippocampal neurons, and reduced hippocampal volume (Sapolsky et al., 1985; Woolley et al., 1990; Gould et al., 1991; Coburn-Litvak et al., 2004). These studies suggest that reduced survival of newly born neurons and overall reduction in the number of granule cell neurons in DR animals could be due to altered hypothalamic-pituitary-adrenal responses to stress endured by these animals.
Notably, the alterations in newly born granule cell neurons and preexisting granule cell neurons did not alter the density of mossy fiber projections in dorsal and ventral dentate gyrus. Therefore, it is possible that alterations in the number of granule cell neurons are not sufficient to trigger changes in mossy fiber projections. Furthermore, our results indicate that reduced neurogenesis and granule cell neuron numbers in DR animals are not directly predictive of alterations in mossy fiber density in these animals. These compensatory changes could assist with intact spatial memory behaviors in DR animals despite the changes in the morphology and cellular changes in granule cell neurons (Andrade et al., 2002; Rezende et al., 2015; Babits et al., 2016). In conclusion, these data lend support to the idea that the neuronal circuitry of the hippocampal formation in adult animals was not severely affected by DR, supporting the functional efficacy of the compensatory dendritic and synaptic changes in the dentate granule cell neurons.
4. Methods
4.1 Animals and Feeding Paradigm
A total of eight male Wistar rats (aged eight weeks at the beginning of dietary changes) were used for this study with four animals assigned to each feeding protocol. Animals were maintained in pair housing under temperature, humidity, and light controlled (reverse 12h light–12h dark) conditions. Animals in the ad lib feeding paradigm (controls) were allowed free and continual access to food in their home cage for the duration of the experimental period. In contrast, animals in the DR feeding paradigm were allowed free access in their home cage to the same food as those in the ad lib paradigm, but only every other day (24h food access, 24h no food access) for the duration of the experimental period. We chose this established DR paradigm because rats maintained on this schedule consume approximately 30% less food over time compared to animals fed ad lib (Goodrick et al., 1983). Furthermore, this type of DR paradigm is a well-known metabolic stressor, which is known to enhance adult hippocampal neurogenesis, enhance neurotrophic factors associated with neurogenesis and reduce mossy fiber projections in the dentate gyrus (Lee et al., 2000; Lee et al., 2002a; Lee et al., 2002b; Kim et al., 2015; Rezende et al., 2015). Water was available at all times to all animal regardless of experimental feeding paradigm. Animals were weighed daily, and eight weeks after DR or control conditions all animals were administered a single intraperitoneal injection of 5-bromo-2’-deoxyuridine (BrdU, 150 mg/kg body weight). Feeding paradigms continued for a total of 12 weeks, at which point the animals were anesthetized with chloral hydrate, transcardially perfused as previously described (Sobieraj et al., 2014) and briefly detailed below. All animal treatments were approved and overseen by the IACUC at The Scripps Research Institute.
4.2 Tissue collection, processing and histological analysis
Following perfusion with saline followed by 4% paraformaldehyde, brain tissue from each animal was removed and post fixed in 4% paraformaldehyde and cryoprotected in 30% sucrose at 4°C until sectioning. Tissue was cut into 40 μm serial coronal sections on a sliding microtome and stored in PBS. Hippocampal tissue (dorsal dentate gyrus: representing −2.56, −3.14, −4.16 and −4.8mm from bregma; ventral dentate gyrus: representing −5.20, −5.6, −6.04 and −6.30 mm from bregma; (Klomp et al., 2014; Vetreno & Crews, 2015) 8 sections per rat) was mounted and stained for Ki-67 (1:700, Rabbit polyclonal, Thermo Scientific) and BrdU (1:500, Sheep polyclonal, Abcam) followed by biotin-tagged secondary antibodies and visualized with DAB. For Ki-67 and BrdU analyses, all immunoreactive cells in the subgranular zone (Ki-67) and granule cell layer (BrdU) were counted per animal in dorsal and ventral granule cell layer. In addition to cell counting, area measures of the granule cell layers were also determined for each section for each animal using StereoInvestigator software (MicroBrightField), and the raw cell counts per section per animal were divided by the area of the granule cell layer and are indicated as cells per mm2 of the granule cell layer per subregion per animal.
For morphometric analysis of the density of mossy fiber projections, dorsal hippocampal sections (representing −2.56 and −4.8mm from bregma, 4 sections per rat) were separately stained for synaptoporin (1:50, Rabbit polyclonal, SynapticSystems) followed by biotin-tagged secondary antibodies and visualized with DAB. We chose to use the presynaptic vesicle protein for detecting the density of mossy fiber projections due to the expression of the protein in measurable amounts in the mossy fiber tracts (Romer et al., 2011). The images were captured with Zeiss AxioImagerA2 and the infrapyramidal and suprapyramidal mossy fiber tracts were combined for density measures of mossy fiber projections. Colored, white-balanced images were captured with StereoInvestigator software (MicroBrightField); synaptoporin in the DG, and the CA3 was evaluated by quantifying DAB stain (% area stained) using ImageJ software (NIH). Briefly, the infrapyramidal and suprapyramidal mossy fiber tracts were contoured using the polygonal selection feature. A circular area above the CA3 was used to quantify non-specific/background staining. The image was then converted to red-green-blue stacks. The green stack was used for quantification of DAB using the threshold function; the maximum and minimum threshold for all the images was set to 130 and 90, respectively. Area stained (% area) was measured for the mossy fiber tract contour and the background; specific staining was calculated by subtracting the background. Area stained was compared between groups using Unpaired t test.
For measurement of granule cell number, sections were selected via systematic random sampling, stained with Vector FastRed and were used for cell quantification via optical fractionator method using the StereoInvestigator software (MicroBrightField). The average density of granule cells was found by examining 6 sections from each rat from dorsal granule cell layer and 6 sections from each rat from the ventral granule cell layer. Live video images were used to draw contours delineating the granule cell layer. All contours were drawn at low magnification (Zeiss AxioImagerA2 at 100× final magnification), and the contours were realigned at high magnification (400× final magnification). Following determination of mounted section thickness (cut section thickness 40 μm; measured mounted section thickness 28 μm), z plane values and selection of contours, an optical fractionator analysis was used to determine bilateral estimates of granule cell neuron number per granule cell layer of each dentate. A counting frame of appropriate dimensions, denoting forbidden and nonforbidden boundaries, was superimposed on the video monitor, and the optical fractionator analysis was performed at 400×. Cells were identified as granule cell neurons based on standard morphology, and only neurons with a focused nucleus within the nonforbidden regions of the counting frame were counted. Over 400 cells were counted at a 10 × 10 × 2 μm counting grid, and a 2 μm top and bottom guard zone. The total number of granule cells was calculated by an unbiased stereological estimation, where the average density of the granule cells (cells/μm3) was multiplied by total volume of the granule cell layer of the hippocampal dentate gyrus (West et al., 1991; Mandyam et al., 2008).
4.3 Statistical analysis
All cellular quantifications were done by an observer blinded to the study and animal groups. Animal weight was assessed as a repeated measures two-way ANOVA (feeding paradigm × time). Cell counts for each marker (expressed as positive cells per mm2) and density of granule cells and synaptoporin were analyzed by two-way ANOVA or by Students-t-test. All graphs and statistical analysis were generated using Graph Pad version 6 for PC and p<0.05 was considered statistically significant.
Highlights.
DR differentially modulates proliferation and survival in the DG
DR differentially effects density of GCNs along the dorso-ventral axis
DR does not alter density of mossy fiber projections in the DG
Acknowledgments
The study was supported by funds from the National Institute on Alcoholism and Alcohol Abuse and National Institute on Drug Abuse (AA020098, AA06420 and DA034140 to CDM; T32AA00747 and F32AA023690 to MCS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to report.
References
- Abrahamsen GC, Kandawire MJ, Carr KD. Aminoglutethimide, a corticosteroid synthesis inhibitor, facilitates brain stimulation reward in food-restricted rats: an investigation of underlying mechanisms. Psychopharmacology (Berl) 1997;133:405–412. doi: 10.1007/s002130050421. [DOI] [PubMed] [Google Scholar]
- Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol Rev. 2005;85:523–569. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- Altemus M, Glowa JR, Galliven E, Leong YM, Murphy DL. Effects of serotonergic agents on food-restriction-induced hyperactivity. Pharmacol Biochem Behav. 1996;53:123–131. doi: 10.1016/0091-3057(95)02003-9. [DOI] [PubMed] [Google Scholar]
- Andrade JP, Lukoyanov NV, Paula-Barbosa MM. Chronic food restriction is associated with subtle dendritic alterations in granule cells of the rat hippocampal formation. Hippocampus. 2002;12:149–164. doi: 10.1002/hipo.1102. [DOI] [PubMed] [Google Scholar]
- Babits R, Szoke B, Sotonyi P, Racz B. Food restriction modifies ultrastructure of hippocampal synapses. Hippocampus. 2016;26:437–444. doi: 10.1002/hipo.22533. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, Zhang WN, Pothuizen HH, Feldon J. Regional dissociations within the hippocampus--memory and anxiety. Neurosci Biobehav Rev. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Barger JL, Walford RL, Weindruch R. The retardation of aging by caloric restriction: its significance in the transgenic era. Exp Gerontol. 2003;38:1343–1351. doi: 10.1016/j.exger.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Breunig JJ, Arellano JI, Macklis JD, Rakic P. Everything that glitters isn’t gold: a critical review of postnatal neural precursor analyses. Cell Stem Cell. 2007;1:612–627. doi: 10.1016/j.stem.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Cabeza de Vaca S, Carr KD. Food restriction enhances the central rewarding effect of abused drugs. J Neurosci. 1998;18:7502–7510. doi: 10.1523/JNEUROSCI.18-18-07502.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, McEwen BS, Gould E. Regulation of adult neurogenesis by excitatory input and NMDA receptor activation in the dentate gyrus. J Neurosci. 1995;15:4687–4692. doi: 10.1523/JNEUROSCI.15-06-04687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. Journal of Comparative Neurology. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Campbell BA, Richardson R. Effects of chronic dietary restriction on sensory-motor function and susceptibility to stressor stimuli in the laboratory rat. Exp Gerontol. 1988;23:417–427. doi: 10.1016/0531-5565(88)90047-2. [DOI] [PubMed] [Google Scholar]
- Carr KD. Feeding, drug abuse, and the sensitization of reward by metabolic need. Neurochem Res. 1996;21:1455–1467. doi: 10.1007/BF02532386. [DOI] [PubMed] [Google Scholar]
- Carr KD, Wolinsky TD. Chronic food restriction and weight loss produce opioid facilitation of perifornical hypothalamic self-stimulation. Brain Res. 1993;607:141–148. doi: 10.1016/0006-8993(93)91499-i. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Meisch RA. Determinants of increased drug self-administration due to food deprivation. Psychopharmacology (Berl) 1981;74:197–200. doi: 10.1007/BF00427092. [DOI] [PubMed] [Google Scholar]
- Coburn-Litvak PS, Tata DA, Gorby HE, McCloskey DP, Richardson G, Anderson BJ. Chronic corticosterone affects brain weight, and mitochondrial, but not glial volume fraction in hippocampal area CA3. Neuroscience. 2004;124:429–438. doi: 10.1016/j.neuroscience.2003.11.031. [DOI] [PubMed] [Google Scholar]
- Dayer AG, Ford AA, Cleaver KM, Yassaee M, Cameron HA. Short-term and long-term survival of new neurons in the rat dentate gyrus. J Comp Neurol. 2003;460:563–572. doi: 10.1002/cne.10675. [DOI] [PubMed] [Google Scholar]
- Duque A, Rakic P. Different effects of bromodeoxyuridine and [3H]thymidine incorporation into DNA on cell proliferation, position, and fate. J Neurosci. 2011;31:15205–15217. doi: 10.1523/JNEUROSCI.3092-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eadie BD, Redila VA, Christie BR. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J Comp Neurol. 2005;486:39–47. doi: 10.1002/cne.20493. [DOI] [PubMed] [Google Scholar]
- Garcia AD, Doan NB, Imura T, Bush TG, Sofroniew MV. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci. 2004;7:1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider NL. Differential effects of intermittent feeding and voluntary exercise on body weight and lifespan in adult rats. Journal of gerontology. 1983;38:36–45. doi: 10.1093/geronj/38.1.36. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Cameron HA, Daniels DC, McEwen BS. Adrenal steroids regulate postnatal development of the rat dentate gyrus: II. Effects of glucocorticoids and mineralocorticoids on cell birth. J Comp Neurol. 1991;313:486–493. doi: 10.1002/cne.903130309. [DOI] [PubMed] [Google Scholar]
- Heiderstadt KM, McLaughlin RM, Wright DC, Walker SE, Gomez-Sanchez CE. The effect of chronic food and water restriction on open-field behaviour and serum corticosterone levels in rats. Laboratory animals. 2000;34:20–28. doi: 10.1258/002367700780578028. [DOI] [PubMed] [Google Scholar]
- Henke PG. Hippocampal pathway to the amygdala and stress ulcer development. Brain Res Bull. 1990;25:691–695. doi: 10.1016/0361-9230(90)90044-z. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Weindruch R, Spangler EL, Freeman JR, Walford RL. Dietary restriction benefits learning and motor performance of aged mice. Journal of gerontology. 1987;42:78–81. doi: 10.1093/geronj/42.1.78. [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Nakamura S. Ventral hippocampal neurons project axons simultaneously to the medial prefrontal cortex and amygdala in the rat. J Neurophysiol. 2006;96:2134–2138. doi: 10.1152/jn.00069.2006. [DOI] [PubMed] [Google Scholar]
- Kempadoo KA, Mosharov EV, Choi SJ, Sulzer D, Kandel ER. Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory. Proc Natl Acad Sci U S A. 2016;113:14835–14840. doi: 10.1073/pnas.1616515114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- Kim Y, Kim S, Kim C, Sato T, Kojima M, Park S. Ghrelin is required for dietary restriction-induced enhancement of hippocampal neurogenesis: lessons from ghrelin knockout mice. Endocrine journal. 2015;62:269–275. doi: 10.1507/endocrj.EJ14-0436. [DOI] [PubMed] [Google Scholar]
- Kjelstrup KG, Tuvnes FA, Steffenach HA, Murison R, Moser EI, Moser MB. Reduced fear expression after lesions of the ventral hippocampus. Proc Natl Acad Sci U S A. 2002;99:10825–10830. doi: 10.1073/pnas.152112399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klomp A, Vaclavu L, Meerhoff GF, Reneman L, Lucassen PJ. Effects of chronic fluoxetine treatment on neurogenesis and tryptophan hydroxylase expression in adolescent and adult rats. PLoS One. 2014;9:e97603. doi: 10.1371/journal.pone.0097603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Pedersen B, Ballermann M, Gibb R, Whishaw IQ. Embryonic and postnatal injections of bromodeoxyuridine produce age-dependent morphological and behavioral abnormalities. J Neurosci. 1999;19:2337–2346. doi: 10.1523/JNEUROSCI.19-06-02337.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouvaros S, Papatheodoropoulos C. Theta burst stimulation-induced LTP: Differences and similarities between the dorsal and ventral CA1 hippocampal synapses. Hippocampus. 2016;26:1542–1559. doi: 10.1002/hipo.22655. [DOI] [PubMed] [Google Scholar]
- Kronenberg G, Reuter K, Steiner B, Brandt MD, Jessberger S, Yamaguchi M, Kempermann G. Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J Comp Neurol. 2003;467:455–463. doi: 10.1002/cne.10945. [DOI] [PubMed] [Google Scholar]
- Kuwagata M, Ogawa T, Nagata T, Shioda S. The evaluation of early embryonic neurogenesis after exposure to the genotoxic agent 5-bromo-2′-deoxyuridine in mice. Neurotoxicology. 2007;28:780–789. doi: 10.1016/j.neuro.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Lee J, Duan W, Long JM, Ingram DK, Mattson MP. Dietary restriction increases the number of newly generated neural cells, and induces BDNF expression, in the dentate gyrus of rats. J Mol Neurosci. 2000;15:99–108. doi: 10.1385/JMN:15:2:99. [DOI] [PubMed] [Google Scholar]
- Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002a;82:1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- Lee J, Seroogy KB, Mattson MP. Dietary restriction enhances neurotrophin expression and neurogenesis in the hippocampus of adult mice. J Neurochem. 2002b;80:539–547. doi: 10.1046/j.0022-3042.2001.00747.x. [DOI] [PubMed] [Google Scholar]
- Lukoyanov NV, Andrade JP. Behavioral effects of protein deprivation and rehabilitation in adult rats: relevance to morphological alterations in the hippocampal formation. Behav Brain Res. 2000;112:85–97. doi: 10.1016/s0166-4328(00)00164-9. [DOI] [PubMed] [Google Scholar]
- Mandyam CD, Harburg GC, Eisch AJ. Determination of key aspects of precursor cell proliferation, cell cycle length and kinetics in the adult mouse subgranular zone. Neuroscience. 2007;146:108–122. doi: 10.1016/j.neuroscience.2006.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandyam CD, Wee S, Crawford EF, Eisch AJ, Richardson HN, Koob GF. Varied access to intravenous methamphetamine self-administration differentially alters adult hippocampal neurogenesis. Biol Psychiatry. 2008;64:958–965. doi: 10.1016/j.biopsych.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgane PJ, Mokler DJ, Galler JR. Effects of prenatal protein malnutrition on the hippocampal formation. Neurosci Biobehav Rev. 2002;26:471–483. doi: 10.1016/s0149-7634(02)00012-x. [DOI] [PubMed] [Google Scholar]
- Morse AD, Russell JC, Hunt TW, Wood GO, Epling WF, Pierce WD. Diurnal variation of intensive running in food-deprived rats. Can J Physiol Pharmacol. 1995;73:1519–1523. doi: 10.1139/y95-210. [DOI] [PubMed] [Google Scholar]
- Moser MB, Moser EI, Forrest E, Andersen P, Morris RG. Spatial learning with a minislab in the dorsal hippocampus. Proc Natl Acad Sci U S A. 1995;92:9697–9701. doi: 10.1073/pnas.92.21.9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Y, Zhao C, Gage FH. Dopaminergic modulation of cortical inputs during maturation of adult-born dentate granule cells. J Neurosci. 2011;31:4113–4123. doi: 10.1523/JNEUROSCI.4913-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niibori Y, Yu TS, Epp JR, Akers KG, Josselyn SA, Frankland PW. Suppression of adult neurogenesis impairs population coding of similar contexts in hippocampal CA3 region. Nat Commun. 2012;3:1253. doi: 10.1038/ncomms2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary OF, Cryan JF. A ventral view on antidepressant action: roles for adult hippocampal neurogenesis along the dorsoventral axis. Trends Pharmacol Sci. 2014;35:675–687. doi: 10.1016/j.tips.2014.09.011. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Kuwagata M, Muneoka KT, Shioda S. Neuropathological examination of fetal rat brain in the 5-bromo-2′-deoxyuridine-induced neurodevelopmental disorder model. Congenital anomalies. 2005;45:14–20. doi: 10.1111/j.1741-4520.2005.00055.x. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Park EH, Burghardt NS, Dvorak D, Hen R, Fenton AA. Experience-Dependent Regulation of Dentate Gyrus Excitability by Adult-Born Granule Cells. J Neurosci. 2015;35:11656–11666. doi: 10.1523/JNEUROSCI.0885-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Glass Z, Sayed K, Michurina TV, Lazutkin A, Mineyeva O, Velmeshev D, Ward WF, Richardson A, Enikolopov G. Calorie restriction alleviates the age-related decrease in neural progenitor cell division in the aging brain. Eur J Neurosci. 2013;37:1987–1993. doi: 10.1111/ejn.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentkowski NS, Blanchard DC, Lever C, Litvin Y, Blanchard RJ. Effects of lesions to the dorsal and ventral hippocampus on defensive behaviors in rats. Eur J Neurosci. 2006;23:2185–2196. doi: 10.1111/j.1460-9568.2006.04754.x. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann N Y Acad Sci. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Pothuizen HH, Zhang WN, Jongen-Relo AL, Feldon J, Yee BK. Dissociation of function between the dorsal and the ventral hippocampus in spatial learning abilities of the rat: a within-subject, within-task comparison of reference and working spatial memory. Eur J Neurosci. 2004;19:705–712. doi: 10.1111/j.0953-816x.2004.03170.x. [DOI] [PubMed] [Google Scholar]
- Prehn K, Jumpertz von Schwartzenberg R, Mai K, Zeitz U, Witte AV, Hampel D, Szela AM, Fabian S, Grittner U, Spranger J, Floel A. Caloric Restriction in Older Adults-Differential Effects of Weight Loss and Reduced Weight on Brain Structure and Function. Cereb Cortex. 2016 doi: 10.1093/cercor/bhw008. [DOI] [PubMed] [Google Scholar]
- Qiu G, Spangler EL, Wan R, Miller M, Mattson MP, So KF, de Cabo R, Zou S, Ingram DK. Neuroprotection provided by dietary restriction in rats is further enhanced by reducing glucocortocoids. Neurobiol Aging. 2012;33:2398–2410. doi: 10.1016/j.neurobiolaging.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezende GH, Guidine PA, Medeiros Dde C, Moraes-Santos T, Mello LE, Moraes MF. Protein-caloric dietary restriction inhibits mossy fiber sprouting in the pilocarpine model of TLE without significantly altering seizure phenotype. Epilepsy Res. 2015;117:85–89. doi: 10.1016/j.eplepsyres.2015.08.011. [DOI] [PubMed] [Google Scholar]
- Roberts HJ, Wearden JH, Smart JL. Undernutrition of weanling and adult rats: effects on operant responding. Behav Brain Res. 1983;10:287–296. doi: 10.1016/0166-4328(83)90035-9. [DOI] [PubMed] [Google Scholar]
- Romer B, Krebs J, Overall RW, Fabel K, Babu H, Overstreet-Wadiche L, Brandt MD, Williams RW, Jessberger S, Kempermann G. Adult hippocampal neurogenesis and plasticity in the infrapyramidal bundle of the mossy fiber projection: I. Co-regulation by activity. Front Neurosci. 2011;5:107. doi: 10.3389/fnins.2011.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotta LN, Schmidt AP, Mello e Souza T, Nogueira CW, Souza KB, Izquierdo IA, Perry ML, Souza DO. Effects of undernutrition on glutamatergic parameters in rat brain. Neurochem Res. 2003;28:1181–1186. doi: 10.1023/a:1024272227219. [DOI] [PubMed] [Google Scholar]
- Rowell JJ, Ragsdale CW. BrdU birth dating can produce errors in cell fate specification in chick brain development. J Histochem Cytochem. 2012;60:801–810. doi: 10.1369/0022155412458588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, ’Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. Prolonged glucocorticoid exposure reduces hippocampal neuron number: implications for aging. J Neurosci. 1985;5:1222–1227. doi: 10.1523/JNEUROSCI.05-05-01222.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Sekerkova G, Ilijic E, Mugnaini E. Bromodeoxyuridine administered during neurogenesis of the projection neurons causes cerebellar defects in rat. J Comp Neurol. 2004;470:221–239. doi: 10.1002/cne.11016. [DOI] [PubMed] [Google Scholar]
- Shoham S, Marcus EL, Avraham Y, Berry EM. Diet Restriction Increases Enkephalin- and Dynorphin-like Immunoreactivity in Rat Brain and Attenuates Long-term Retention of Passive Avoidance. Nutritional neuroscience. 2000;3:41–55. doi: 10.1080/1028415X.2000.11747302. [DOI] [PubMed] [Google Scholar]
- Sobieraj JC, Kim A, Fannon MJ, Mandyam CD. Chronic wheel running-induced reduction of extinction and reinstatement of methamphetamine seeking in methamphetamine dependent rats is associated with reduced number of periaqueductal gray dopamine neurons. Brain Struct Funct. 2014 doi: 10.1007/s00429-014-0905-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, Bostrom E, Westerlund I, Vial C, Buchholz BA, Possnert G, Mash DC, Druid H, Frisen J. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153:1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taupin P. BrdU immunohistochemistry for studying adult neurogenesis: paradigms, pitfalls, limitations, and validation. Brain Res Rev. 2007;53:198–214. doi: 10.1016/j.brainresrev.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Vetreno RP, Crews FT. Binge ethanol exposure during adolescence leads to a persistent loss of neurogenesis in the dorsal and ventral hippocampus that is associated with impaired adult cognitive functioning. Front Neurosci. 2015;9:35. doi: 10.3389/fnins.2015.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed JL, Lane MA, Roth GS, Speer DL, Ingram DK. Activity measures in rhesus monkeys on long-term calorie restriction. Physiol Behav. 1997;62:97–103. doi: 10.1016/s0031-9384(97)00147-9. [DOI] [PubMed] [Google Scholar]
- Weitemier AZ, McHugh TJ. Noradrenergic modulation of evoked dopamine release and pH shift in the mouse dorsal hippocampus and ventral striatum. Brain Res. 2016 doi: 10.1016/j.brainres.2016.12.002. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Wiggins RC, Fuller G, Enna SJ. Undernutrition and the development of brain neurotransmitter systems. Life Sci. 1984;35:2085–2094. doi: 10.1016/0024-3205(84)90507-1. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, McEwen BS. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990;531:225–231. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]
- Zhang T, Hong J, Di T, Chen L. MPTP Impairs Dopamine D1 Receptor-Mediated Survival of Newborn Neurons in Ventral Hippocampus to Cause Depressive-Like Behaviors in Adult Mice. Frontiers in molecular neuroscience. 2016;9:101. doi: 10.3389/fnmol.2016.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]


