Significance
This study demonstrated that intranasal (IN) administration of A1-exosomes alleviates multiple adverse changes that typically emerge after status epilepticus (SE), a medical crisis that presents a high propensity to evolve into chronic hippocampus dysfunction. Specifically, A1-exosome treatment after SE led to reduced neuron loss and inflammation, maintenance of normal neurogenesis, and preservation of cognitive and memory function. The results have significance for clinical application of A1-exosomes for curbing the evolution of SE-induced injury into chronic hippocampus dysfunction. The results also imply that IN administration of A1-exosomes is therapeutic for other neurological conditions that present with significant neuroinflammation.
Keywords: status epilepticus, memory dysfunction, neuroinflammation, exosomes, adult neurogenesis
Abstract
Status epilepticus (SE), a medical emergency that is typically terminated through antiepileptic drug treatment, leads to hippocampus dysfunction typified by neurodegeneration, inflammation, altered neurogenesis, as well as cognitive and memory deficits. Here, we examined the effects of intranasal (IN) administration of extracellular vesicles (EVs) secreted from human bone marrow-derived mesenchymal stem cells (MSCs) on SE-induced adverse changes. The EVs used in this study are referred to as A1-exosomes because of their robust antiinflammatory properties. We subjected young mice to pilocarpine-induced SE for 2 h and then administered A1-exosomes or vehicle IN twice over 24 h. The A1-exosomes reached the hippocampus within 6 h of administration, and animals receiving them exhibited diminished loss of glutamatergic and GABAergic neurons and greatly reduced inflammation in the hippocampus. Moreover, the neuroprotective and antiinflammatory effects of A1-exosomes were coupled with long-term preservation of normal hippocampal neurogenesis and cognitive and memory function, in contrast to waned and abnormal neurogenesis, persistent inflammation, and functional deficits in animals receiving vehicle. These results provide evidence that IN administration of A1-exosomes is efficient for minimizing the adverse effects of SE in the hippocampus and preventing SE-induced cognitive and memory impairments.
Status epilepticus (SE) is a grave medical crisis that requires swift remedy through all age groups (1, 2). It can produce substantial neurodegeneration, blood–brain barrier disruption, and inflammation in the hippocampus if not extinguished quickly by antiepileptic drug (AED) treatment (3–5). An episode of extended SE is sufficient to cause chronic hippocampus dysfunction, exemplified by persistent inflammation with activation of microglia and monocyte infiltration, loss of sizable fractions of several subclasses of inhibitory interneurons, aberrant and waned neurogenesis, hippocampus-dependent cognitive and memory impairments, and chronic epilepsy (5–12). Numerous situations such as head trauma, stroke, Alzheimer’s disease, brain tumor, and encephalitis can engender SE. Although administration of AEDs leads to termination of SE in most instances, it does not thwart the evolution of SE into chronic epilepsy (13–16). A multitude of changes ensue in the hippocampus after an episode of SE, which evolve over a period of months, years, or even decades, and result in chronic epilepsy when they have reached certain thresholds (11, 17, 18). Hence, there is an urgent need to find an adjuvant therapy with AEDs that not only provides neuroprotection and suppression of inflammation in the early phase after SE but also maintains normal neurogenesis, preserves cognitive and memory function, and thwarts epilepsy development in the chronic phase after SE. The i.v. administration of bone marrow-derived mononuclear cells (MNCs) or mesenchymal stem cells (MSCs) has shown potential for modulating SE-induced adverse effects in the hippocampus and reducing the severity of chronic epilepsy (19). However, clinical therapies with MNCs or MSCs face considerable challenges in regard to the variable biological activity of different preparations of the cells and the logistics of delivering the cells to the bedside or emergency room.
A feasible alternative would be the dispensation of prebanked extracellular vesicles (EVs) generated from MSCs, as human MSC (hMSC)-derived EVs seemed to have most of the antiinflammatory and neuroprotective activity of MSCs (20). Moreover, several studies suggest that therapeutic benefits of MSC administration are largely explained by paracrine effects mediated by soluble factors or EVs secreted by MSCs (21–24). Furthermore, EVs can cross the blood–brain barrier and deliver various therapeutic factors to the brain (25, 26). EVs containing a multitude of mRNAs, miRNAs, and proteins (26, 27) can be harvested, characterized, and banked from MSCs obtained from several sources such as bone marrow, lipoaspirate of liposuction procedures, umbilical cord, and human-induced pluripotent stem cells (28–30). The use of EVs also avoids several potential safety hazards such as the risk of tumors. Besides, EVs have several distinct advantages over cells for use in clinical therapies. Their compositions can be defined and standardized because they are stable and not responsive to external stimuli. EVs can be made readily available for use in patients, as they are far more stable to freezing and thawing. Also, if the small size of EVs makes it possible to administer them via an IN route, they may be broadly applicable as a noninvasive therapy.
Therefore, we rigorously ascertained the efficacy of IN administration of EVs derived from human bone marrow-derived MSCs by using a pilocarpine model of SE in mice. The EVs used in this study have been well characterized and are referred to as A1-exosomes because of their robust antiinflammatory properties (20). First, we measured the ability of IN administered A1-exosomes to enter the hippocampus, suppress inflammation, and protect glutamatergic and GABAergic neurons in the early phase after SE. Next, we measured the proficiency of IN-administered A1-exosomes for maintaining normal neurogenesis and cognitive and memory function with persistent suppression of inflammation in the chronic phase after SE.
Results
Preparation, Selection and Characterization of A1-Exosomes from Human Bone Marrow-Derived MSCs.
The generation, isolation, and capture of EVs of uniform size (80–100 nm in diameter) from human bone marrow-derived MSCs were performed as detailed in our recent report (20). The EVs generated through this procedure were positive for classical EV markers such as CD63 and CD81 but negative for CD9 and 13 other epitopes found on the surface of MSCs. Each batch of EVs was also tested for antiinflammatory activity in the spleen by using a model of systemic inflammation induced by administration of LPS. Only EVs that exhibited antiinflammatory activity in the spleen were labeled as A1-exosomes and used in the SE model. Further details are provided in SI Materials and Methods.
IN-Dispensed A1-Exosomes Incorporated into Cortical and Hippocampal Neurons.
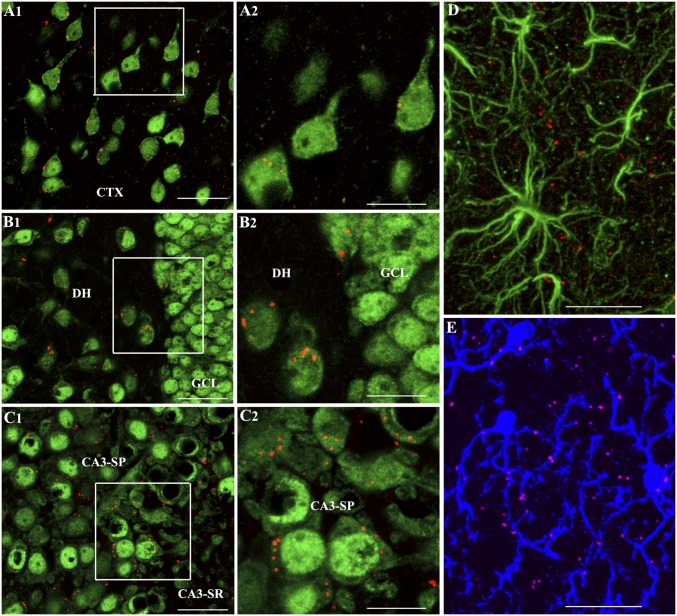
We investigated whether IN administration of A1-exosomes after SE would result in targeting of these exosomes into the hippocampus, the region exhibiting intense hyperactivity of neurons, increased oxidative stress, and inflammation with infiltration of peripheral monocytes during and/or after SE (5, 12). We administered PKH26-labeled A1-exosomes via IN route (15 μg, ∼7.5 × 109) immediately after the termination of 2 h of SE by an injection of diazepam. Six hours later, animals were perfused (n = 4), and serial sections through the entire brain were processed for immunofluorescence by using markers of neurons (neuronal nuclear antigen [NeuN]), astrocytes (GFAP), and microglia (IBA-1) and Z-sectioning in a confocal microscope. We found red-colored PKH26+ particles (i.e., A1-exosomes) throughout the olfactory bulb, frontoparietal cortex, basal forebrain, striatum, and dorsal hippocampus. At dorsal hippocampal levels, most exosomes were in small clusters and were seen within the cytoplasm of neurons or attached to the cell membrane of neurons (Fig. 1 A1–C2). In the hippocampus, exosomes were clearly seen within dentate hilar neurons (Fig. 1 B1 and B2) and the CA3 pyramidal neurons (Fig. 1 C1 and C2). Occasionally, exosomes were also found in the cytoplasm of dentate granule cells (Fig. 1 B1 and B2) and the CA1 pyramidal neurons. We also examined their presence within GFAP+ astrocytes (Fig. 1D) and IBA-1+ microglial cells (Fig. 1E) in the hippocampus. None were seen in the cell body of astrocytes but were found inside the cell body of some microglia. However, exosomes were frequently seen in close proximity to astrocyte and microglial processes. In rostral regions of the cerebral cortex, accumulation of exosomes could be seen in virtually all neurons and a vast majority of microglia (Figs. S1 and S2). Exosomes were also seen in close proximity to processes of astrocytes and microglia. Interestingly, whereas neurons displayed isolated or smaller clusters of exosomes, a greater fraction of microglia displayed larger clusters of exosomes within their cytoplasm (Fig. S2). Thus, within 6 h of IN administration, A1-exosomes incorporated robustly into neurons and microglia in rostral regions of the cerebral cortex, and predominantly into neurons in the cortex and the hippocampus at dorsal hippocampal levels.
Fig. 1.
A1-exosomes invade the frontoparietal cerebral cortex and the dorsal hippocampus within 6 h after IN administration. (A1–C2) The presence of PKH26+ exosomes (red dots) within the cytoplasm or in close contact with the cell membrane of NeuN+ neurons in the cerebral cortex (A1), the DH and GCL (B1), and CA3 pyramidal neurons (C1) of the hippocampus at 6 h after their IN administration. (A2, B2, and C2) Magnified views of boxed regions in A1, B1, and C1. (D) Lack of exosomes within the soma of GFAP+ astrocytes and the presence of some exosomes adjacent to astrocyte processes. (E) Presence of exosomes within the soma or processes of some IBA-1+ microglia. CA3-SP, CA3 stratum pyramidale; CA3-SR, CA3 stratum radiatum; CTX, cortex. (Scale bars: A1, B1, and C1, 50 µm; A2, B2, and C2, 25 µm; D and E, 25 µm.)
Fig. S1.
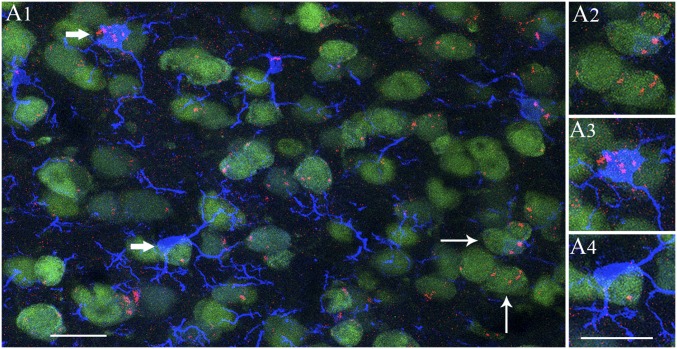
A1-exosomes displayed comparable affinity toward neurons and microglia. (A1) Distribution of A1-exosomes within NeuN-expressing neurons and IBA-1–positive microglia in the anterior-most part of the motor cortex at 6 h after IN administration. Note that A1-exosomes are seen in the cytoplasm of majority of neurons in this region even though the density of exosomes varied between neurons. (A2) Magnified view of neurons from A1 (thin arrows) displaying clumps of exosomes. A1-exosomes also incorporated into the cytoplasm of all microglia in this region (A1). (A3 and A4) Magnified views of microglia from A1 (thick arrows). One of these microglia displays clusters of exosomes in the soma (A3) whereas the other shows scattered exosomes in the soma and processes (A3). (Scale bars: 20 µm.)
Fig. S2.
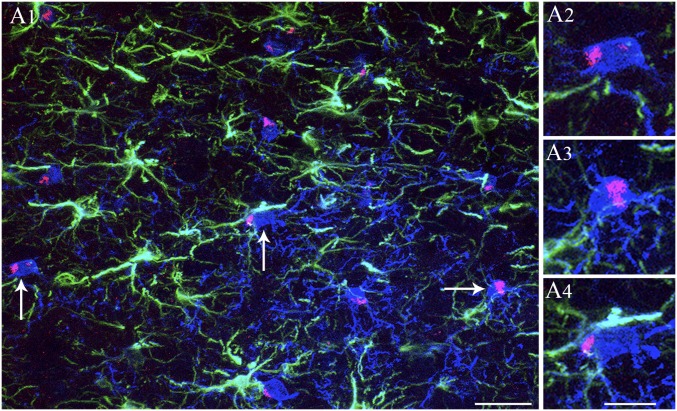
A1-exosomes showed greater affinity for microglia in comparison with astrocytes. (A1) GFAP-positive astrocytes (green), IBA-1–positive microglia (blue), and A1-exosomes (red) in the frontal association cortex at 6 h after IN administration. Note that clusters of A1-exosomes are seen in the cytoplasm of virtually all microglia in this region. (A2–A4) Magnified views of microglia from A1 (arrows) displaying larger clumps of exosomes. Interestingly, exosomes are not found in the soma of astrocytes, but scattered exosomes are seen in close proximity to processes of astrocytes (A1). (Scale bars: A1, 25 µm; A2–A4, 10 µm.)
IN Delivery of A1-Exosomes After SE Prevented the Rise of Multiple Proinflammatory Cytokines and Increased the Concentration of Some Antiinflammatory Cytokines and Growth Factors in the Hippocampus.
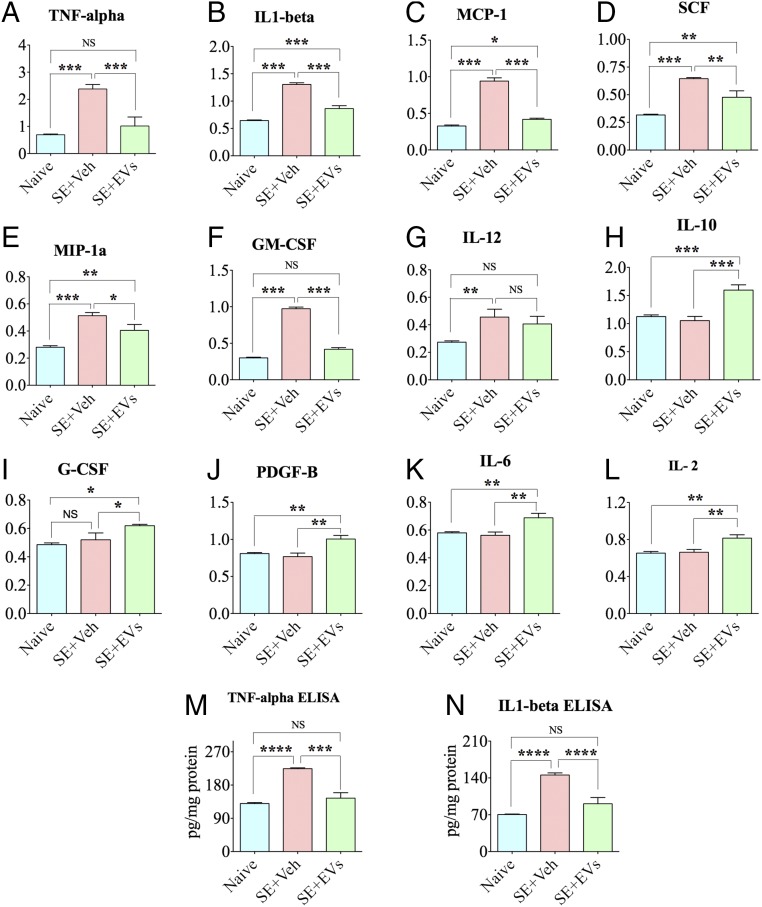
We measured 24 cytokines in hippocampal lysates obtained from animals belonging to different groups (n = 6 per group) at 24 h post-SE by using 96-well array plates that were precoated with specific cytokine capture antibodies. Sixteen proinflammatory cytokines exhibited up-regulation in animals receiving vehicle after SE (SE-VEH group) in comparison with naive control animals (Table S1). Among these, the concentrations of seven proinflammatory cytokines was significantly reduced in animals receiving A1-exosomes after SE (SE-EVs group; Fig. 2 A–G) in comparison with animals in the SE-VEH group. These include TNF-α, IL-1β, monocyte chemoattractant protein-1 (MCP-1), stem cell factor (SCF), macrophage inflammatory protein-1α (MIP-1α), GM-CSF, and IL-12. Animals in the SE-EVs group also displayed enhanced concentrations of antiinflammatory cytokine IL-10 (Fig. 2H), granulocyte colony-stimulating factor (G-CSF; Fig. 2I), platelet-derived growth factor-β (PDGF-β, Fig. 2J), IL-6 (Fig. 2K), and IL-2 (Fig. 2L). As TNF-α and IL-1β are among the major proinflammatory cytokines that are implicated in brain diseases exhibiting inflammation and/or cognitive and memory dysfunction and have proconvulsive properties (31), we further confirmed their concentration through independent quantitative ELISAs. The results clearly showed their up-regulation at 24 h post-SE in animals in the SE-VEH group and normalized levels in the SE-EVs group (Fig. 2 M and N). Thus, IN administration of A1-exosomes commencing 2 h post-SE was adequate for greatly easing the inflammatory storm triggered by SE.
Table S1.
List of 16 proinflammatory cytokines that were up-regulated in the SE+VEH group
| Cytokine | Naive | SE+VEH | P value |
| TNF-α | 0.6922 ± 0.028 | 2.378 ± 0.167 | < 0.0001 |
| IGF1 | 0.3175 ± 0.022 | 0.5125 ± 0.028 | < 0.001 |
| IL-1β | 0.6437 ± 0.011 | 1.304 ± 0.031 | < 0.0001 |
| GM-CSF | 0.3002 ± 0.009 | 0.9728 ± 0.023 | < 0.0001 |
| MCP-1 | 0.3275 ± 0.013 | 0.94 ± 0.045 | < 0.0001 |
| MIP-1a | 0.2805 ± 0.011 | 0.5127 ± 0.024 | < 0.001 |
| SCF | 0.317 ± 0.007 | 0.6450 ± 0.01 | < 0.001 |
| IL-1α | 0.4125 ± 0.01 | 0.5217 ± 0.025 | < 0.01 |
| VEGF | 0.3402 ± 0.016 | 0.4362 ± 0.034 | < 0.05 |
| FGF b | 0.4565 ± 0.014 | 0.5468 ± 0.028 | < 0.05 |
| IL-4 | 0.4897 ± 0.016 | 0.6382 ± 0.021 | < 0.001 |
| IFN -γ | 0.3942 ± 0.013 | 0.6030 ± 0.025 | < 0.001 |
| Resistin | 0.3493 ± 0.012 | 0.4842 ± 0.053 | < 0.05 |
| Leptin | 0.6070 ± 0.008 | 0.6867 ± 0.026 | < 0.05 |
| Rantes | 0.3878 ± 0.011 | 0.4832 ± 0.022 | < 0.01 |
| IL-12 | 0.2735 ± 0.01 | 0.4558 ± 0.06 | < 0.01 |
Fig. 2.
IN administration of A1-exosomes 2 h after SE eases inflammation in the hippocampus when examined 24 h post-SE. Bar charts compare the relative concentrations of multiple cytokines between naive control animals, SE+VEH animals, and SE+EVs animals. Assays were by multiplexed ELISAs. Animals in the SE+VEH group display increased concentration of proinflammatory cytokines TNF-α, IL-1β, MCP-1, SCF, MIP-1α, GM-CSF, and IL-12 (A–G), whereas animals in the SE+EVs group exhibit significantly reduced concentrations of these cytokines. This group also showed increased concentration of antiinflammatory cytokines and growth factors such as IL-10, G-CSF, PDGF-β, IL-6, and IL-2 (H–L). Bar charts in M and N compare levels of TNF-α and IL-1β in the hippocampus measured through independent ELISAs. In comparison with naive controls, the concentrations of these proinflammatory cytokines are increased in the SE+VEH group but normalized in the SE+EVs group (*P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001).
IN Delivery of A1-Exosomes After SE Greatly Reduced the Activation of Microglia in the Hippocampus.
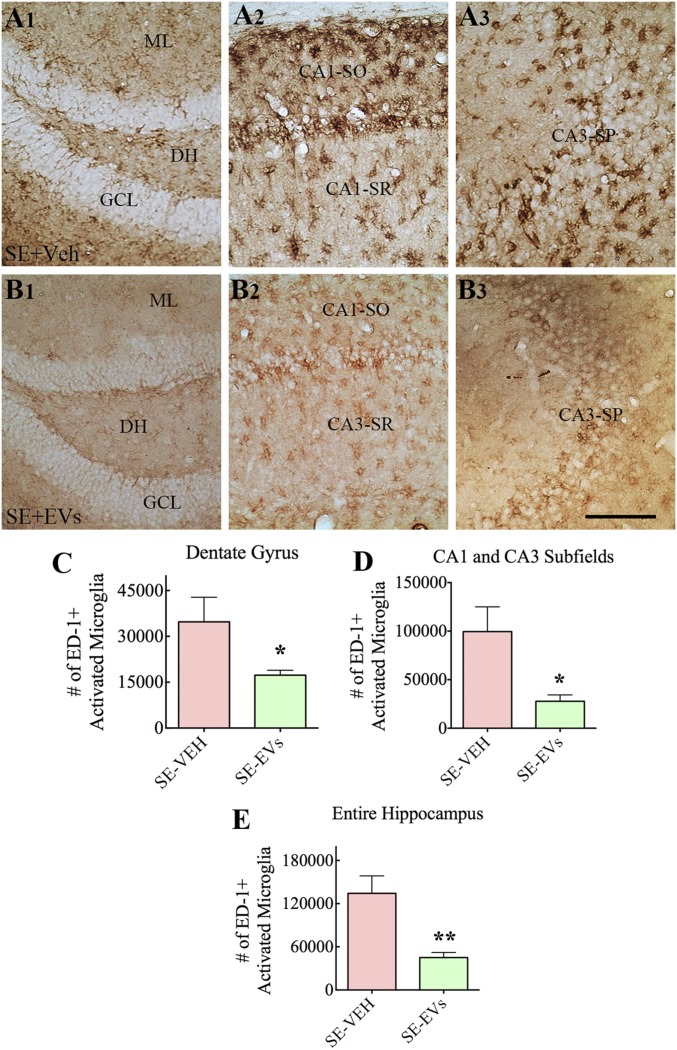
We measured the extent of inflammation in the hippocampus at 4 d post-SE in animals receiving vehicle or A1-exosomes through immunohistochemical staining of serial sections for ED-1 (CD68, a marker of activated microglia or macrophages in the brain) and stereological quantification of ED-1+ cells in the dentate gyrus (DG) and CA1 and CA3 subfields of the hippocampus (n = 5–6 per group; Fig. 3 A1–E). Animals in the SE-VEH group exhibited increased density of ED-1+ microglia, with several morphological changes, particularly in the CA1 and CA3 subfields. A fraction of microglia exhibited hypertrophy of soma with multiple short processes whereas some others exhibited round or oval-shaped soma with no or minimal processes, both of which are characteristics of activated microglia (Fig. 3 A2 and A3). In contrast, animals in the SE-EVs group exhibited not only reduced density of ED-1+ microglia but also a greatly diminished intensity of ED-1 staining (Fig. 3 B1–B3). Stereological quantification confirmed reduced numbers of ED-1+ microglia in the DG (Fig. 3C), CA1 and CA3 subfields (Fig. 3D), and in the entire hippocampus (Fig. 3E). The reductions were 50% for the DG, 72% for the CA1 and CA3 subfields, and 66% for the entire hippocampus (P < 0.05–0.01; Fig. 3 C–E).
Fig. 3.
IN administration of A1-exosomes 2 h after SE greatly reduces the density of ED-1+ (CD68+) activated microglia in the hippocampus when examined 4 d post-SE. (A1–B3) Distribution of ED-1+ activated microglia in the DG (A1 and B1), the CA1 subfield (A2 and B2), and the CA3 subfield (A3 and B3) of an animal in the SE-VEH group (A1–A3) and an animal in the SE-EVs group (B1–B3). ML, molecular layer; SO, stratum oriens; SP, stratum pyramidale; SR, stratum radiatum. Bar charts in C–E compare the numbers of ED-1+ microglia in the DG (C), CA1 and CA3 subfields (D), and the entire hippocampus (E). Note that animals receiving A1-exosomes (i.e., SE-EVs group) display reduced numbers of ED-1+ activated microglia compared with animals in the SE-VEH group (*P < 0.05 and **P < 0.01). (Scale bar: 100 µm.)
IN Delivery of A1-Exosomes After SE Reduced the Overall Loss of Neurons in the Dentate Hilus and the CA1 Cell Layer of the Hippocampus.
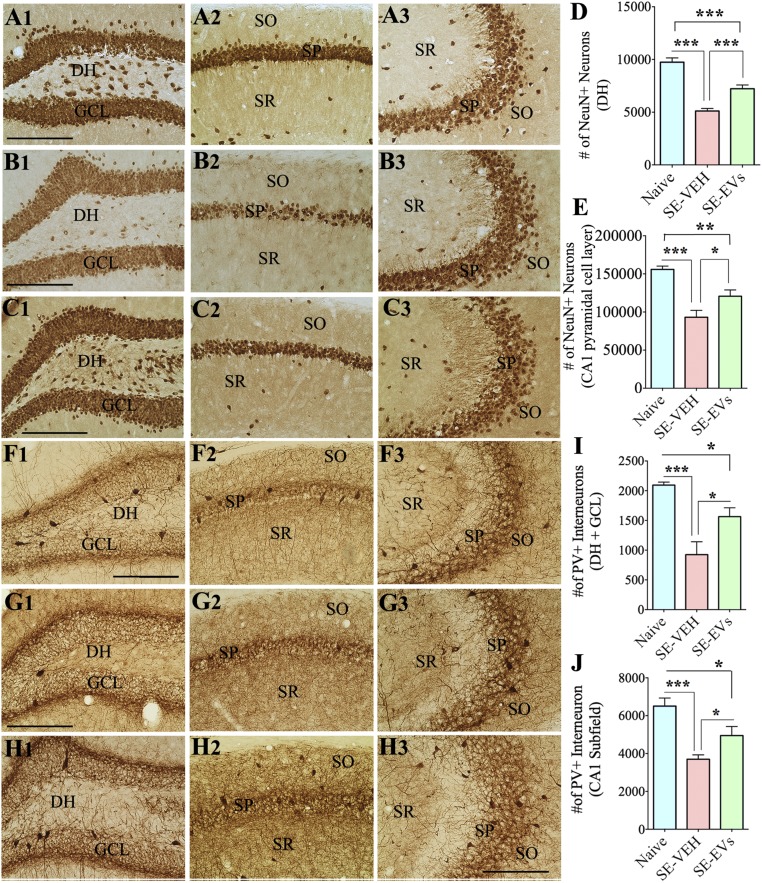
SE typically causes degeneration of neurons in certain regions/layers of the hippocampus. To ascertain the extent of SE-induced neurodegeneration in the hippocampus of animals receiving vehicle or A1-exosomes after SE, we performed NeuN immunostaining of serial sections through the entire hippocampus at 4 d post-SE (Fig. 4 A1–C3). In comparison with naive control animals, the SE-VEH and SE-EVs groups showed reduced densities of neurons in the dentate hilus (DH) and the CA1 pyramidal cell layer but no discernable changes in the granule cell layer (GCL) and the CA3 pyramidal cell layer (n = 5–6 per group; Fig. 4 A1–C3). Stereological quantification revealed that the overall neuron loss in the DH and CA1 cell layer ranged from 40% to 47% in the SE-VEH group (P < 0.001) and from 25% to 26% in the SE-EVs group (P < 0.01–0.001). Because of neuroprotection mediated by A1-exosomes, animals in the SE-EVs group displayed a 30–41% greater number of neurons than animals in the SE-VEH group (P < 0.01–0.001; Fig. 4 D and E). Thus, IN administration of A1-exosomes after SE reduced the loss of neurons in regions of the hippocampus that are highly susceptible to SE-induced neurodegeneration.
Fig. 4.
IN administration of A1-exosomes 2 h after SE reduces the loss of NeuN+ neurons and PV+ interneurons in the DG and the CA1 subfield when examined 4 d post-SE. (A1–C3) Distribution of NeuN+ neurons in the DG (A1, B1, and C1), the CA1 subfield (A2, B2, and C2), and the CA3 subfield (A3, B3, and C3) of a naive control mouse (A1–A3), a mouse in the SE-VEH group (B1–B3), and a mouse in the SE-EVs group (C1–C3). Bar charts in D and E compare the numbers of NeuN+ neurons in the DH (D) and the CA1 pyramidal cell layer (E) of the hippocampus. Although both SE groups display reduced numbers of NeuN+ neurons in comparison with the naive control group, the SE-EVs group exhibits greater numbers of surviving neurons than the SE-VEH group, implying neuroprotection after IN administration of A1-exosomes. (F1–H3) Distribution of PV+ interneurons in the DG (F1, G1, and H1), the CA1 subfield (F2, G2, and H2), and the CA3 subfield (F3, G3, and H3) of a naive control mouse (F1–F3), a mouse from the SE-VEH group (G1–G3), and a mouse from the SE-EVs group (H1–H3). Bar charts in I and J compare the numbers of PV+ interneurons in the DH+GCL (I) and the CA1 subfield (J) of the hippocampus. Although both SE groups display reduced numbers of PV+ interneurons in the DH+GCL and CA1 subfield in comparison with the naive control group, the SE-EVs group exhibits greater numbers of PV+ interneurons than the SE-VEH group, implying protection of these interneurons after IN administration of A1-exosomes. SO, stratum oriens, SP, stratum pyramidale; SR, stratum radiatum (*P < 0.05; **P < 0.01; ***P < 0.001). (Scale bar: 200 µm.)
IN Delivery of A1-Exosomes After SE Restrained the Loss of Several Subclasses of Inhibitory Interneurons in the Hippocampus.
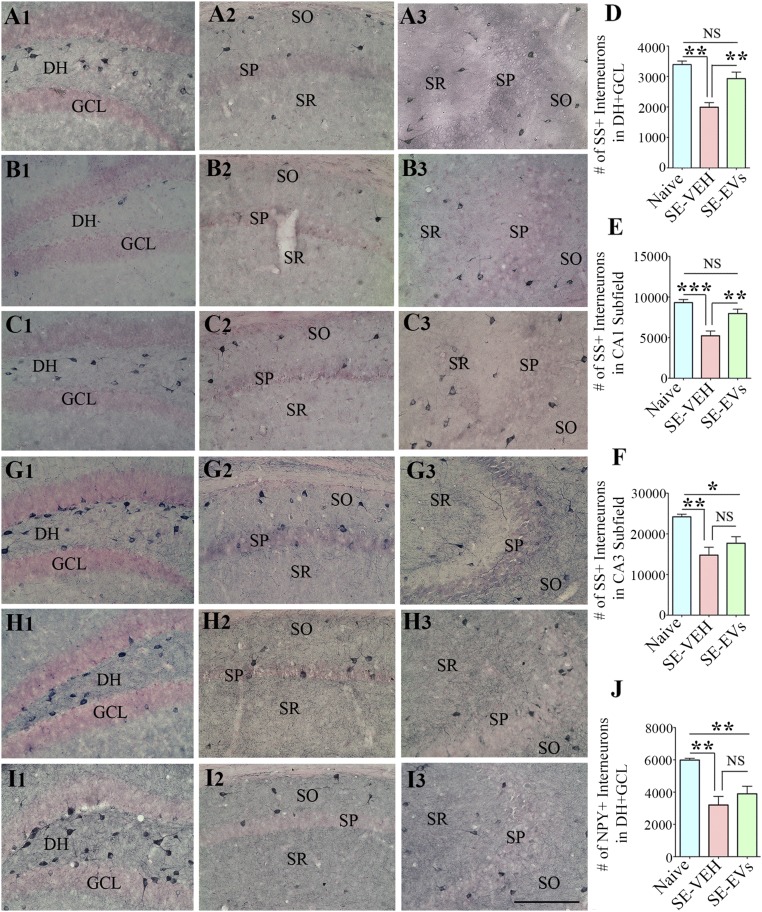
Several subclasses of inhibitory GABAergic interneurons in the hippocampus are highly susceptible to SE. To measure the extent of SE-induced loss of inhibitory interneurons in animals receiving vehicle or A1-exosomes after SE, we performed immunostaining of serial sections through the entire hippocampus for the calcium binding protein parvalbumin (PV), and neuropeptides somatostatin (SS) and neuropeptide Y (NPY) at 4 d post-SE (n = 5–6 per group). The interneurons positive for PV displayed reduced density in the DH-GCL region and the CA1 subfield after SE (Fig. 4 F1–H3). Stereological measurement demonstrated that the overall PV+ interneuron loss in the DH-GCL and the CA1 subfield varied from 43% to 56% in the SE-VEH group (P < 0.001) and from 24% to 25% in the SE-EVs group (P < 0.01–0.001). Because of the protection mediated by A1-exosomes, the SE-EVs group displayed 34–69% greater numbers of PV+ interneurons than the SE-VEH group (P < 0.05; Fig. 4 I and J). Interneurons expressing SS exhibited reduced densities in the DH+GCL, CA1, and CA3 regions after SE (Fig. 5 A1–C3). Stereological cell counting showed that the overall SS+ interneuron loss in these regions ranged from 39% to 44% in the SE-VEH group (P < 0.01–0.001). In contrast, the SE-EVs group displayed no significant loss in the DH+GCL region and the CA1 subfield (P > 0.05) but a 27% loss in the CA3 subfield (P < 0.05). In comparison with the SE-VEH group, the SE-EVs group displayed 47–52% greater numbers of SS+ interneurons in the DH+GCL and CA1 regions (P < 0.01; Fig. 5 D and E) and a 20% higher number in the CA3 subfield (P > 0.05; Fig. 5F). The interneurons positive for NPY displayed reduced density only in the DH-GCL region after SE (Fig. 5 G1–I3). Stereological quantification revealed that the NPY+ interneuron loss in the DH+GCL region is 46% in the SE-VEH group and 35% in the SE-EVs group (P < 0.01; Fig. 5J). In comparison with the SE-VEH group, the SE-EVs group displayed 22% higher numbers of NPY+ interneurons (P > 0.05; Fig. 5J). Thus, IN administration of A1-exosomes after SE diminished the loss of several subclasses of GABAergic interneurons in the hippocampus.
Fig. 5.
IN administration of A1-exosomes 2 h after SE reduces the loss of SS+ and NPY+ interneurons in the hippocampus when examined 4 d post-SE. (A1–C3) Distribution of SS+ interneurons in the DG (A1, B1, and C1), the CA1 subfield (A2, B2, and C2), and the CA3 subfield (A3, B3, and C3) of a naive control mouse (A1–A3), a mouse in the SE-VEH group (B1–B3), and a mouse in the SE-EVs group (C1–C3). Bar charts in D–F compare the numbers of SS+ interneurons in the DH+GCL (D) and the CA1 and CA3 subfields (E and F) of the hippocampus. All regions display a significant loss of SS+ interneurons in the SE-VEH group, but only the CA3 subfield shows some loss in the SE-EVs group. Overall, the SE-EVs group exhibits greater numbers of SS+ interneurons than the SE-VEH group in all regions, implying a considerable protection after IN administration of A1-exosomes. (G1–I3) Distribution of NPY+ interneurons in the DG (G1, H1, and I1), the CA1 subfield (G2, H2, and I2), and the CA3 subfield (G3, H3, and I3) of a naive control mouse (G1–G3), a mouse from the SE-VEH group (H1–H3), and a mouse from the SE-EVs group (I1–I3). Bar chart in J compares the numbers of NPY+ interneurons in the DH+GCL (I) of the hippocampus. Although both SE groups display reduced numbers of NPY+ interneurons in the DH+GCL in comparison with the naive control group, the SE-EVs group exhibits relatively greater numbers of PV+ interneurons than the SE-VEH group, implying some protection of these interneurons after IN administration of A1-exosomes. SO, stratum oriens, SP, stratum pyramidale; SR, stratum radiatum (*P < 0.05, **P < 0.01, and ***P < 0.001). (Scale bar: 200 µm.)
IN Delivery of A1-Exosomes After SE Averted Cognitive and Memory Impairments in the Chronic Phase.
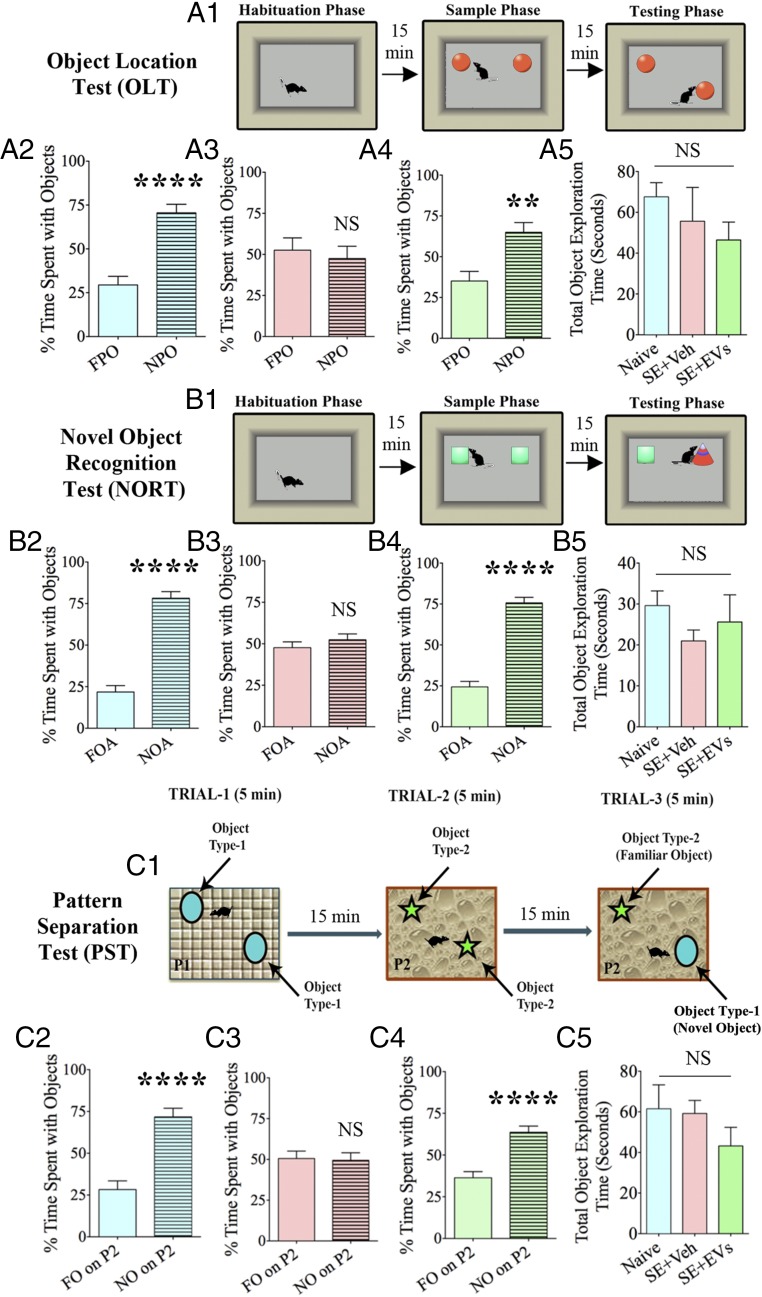
Cognitive and memory impairments typically ensue after SE. We examined animals 5–6 wk after SE with vehicle or A1-exosome treatment via three distinct behavioral tests. These include an object location test (OLT), a novel object recognition test (NORT), and a pattern separation test (PST; n = 8–10 per group). We first measured the cognitive ability of animals through an OLT. The choice to explore an object displaced to a novel location in this test reflects the ability of the animal to discern minor changes in its immediate environment (Fig. 6A1). Maintenance of this function depends on the integrity of the hippocampus circuitry (32). Animals in the SE-VEH group were impaired, as they did not show affinity for the object moved to a novel place (Fig. 6A3). Rather, they spent nearly equal amounts of time with the object in the familiar place and the object in the novel place (P > 0.05). In contrast, animals belonging to the SE-EVs group showed a greater affinity for exploring the novel place object (NPO) over the familiar place object (FPO; P < 0.01; Fig. 6A4), which matched the normal behavior typically observed in naive control animals (P < 0.0001; Fig. 6A2). As animals belonging to different groups explored objects for comparable durations (P > 0.05; Fig. 6A5) in the testing phase, the results were not influenced by variable object exploration times between groups. Thus, SE causes hippocampus-dependent cognitive dysfunction, but early intervention with A1-exosomes prevents this impairment.
Fig. 6.
IN administration of A1-exosomes after SE prevents cognitive, memory, and pattern-separation impairments. (A1, B1, and C1) Depiction of various phases involved in an OLT (A1), a NORT (B1), and a PST (C1). Bar charts in A2–A4, B2–B4, and C2–C4 compare percentages of time spent with different objects. Naive control animals showed a greater affinity for (i) the NPO over the FPO in an OLT (A2), (ii) the NO area (NOA) over the FO area (FOA) in a NORT (B2), and (iii) NO on P2 over the FO on P2 in a PST (C2), implying normal cognitive, memory, and pattern-separation function. However, animals in the SE+VEH group were impaired in all three tests (A3, B3, and C3). This was evinced by their behavior of spending nearly equal amounts of the object exploration time with the FPO and the NPO in OLT (A3), FOA and NOA in NORT (B3), and FO on P2 and NO on P2 in the PST (C3). In contrast, animals in the SE-EVs group showed a greater affinity for exploring the NPO in OLT (A4), NOA in NORT (B4), and NO on P2 in PST (C4), suggesting a similar cognitive, memory, and pattern-separation function as naive control animals. Bar charts in A5, B5, and C5 show that animals in different groups explored objects for comparable durations (**P < 0.01 and ****P < 0.0001).
We next examined recognition memory function by using a NORT. Recognition memory function depends on the integrity of the perirhinal cortex and the hippocampus. We examined animals with a 15-min delay between the “object exploration phase” comprising the exploration of two identical objects for 5 min in an arena and the “testing phase” involving the exploration of objects in the same arena but with replacement of one of the objects with a new object (Fig. 6B1) (32). Animals belonging to the SE-VEH group showed an inability of novel object (NO) discrimination, as they spent similar percentages of time exploring the familiar object (FO) and the NO (P > 0.05; Fig. 6B3). However, animals in the SE-EVs group spent greater percentages of their object exploration time with the NO (P < 0.0001; Fig. 6B4), akin to that observed in naive control animals (P < 0.0001; Fig. 6B2). Again, animals in all groups explored objects for comparable durations (P > 0.05; Fig. 6B5) in the testing phase. These results demonstrated that A1-exosome treatment after SE prevents recognition memory impairment.
Following the aforementioned cognitive and memory tests, we further investigated the ability of animals for pattern separation by using an PST, a relatively complex test for discriminating analogous experiences through storage of similar representations in a nonoverlapping manner (33, 34). In this test, each animal successively explored two different sets of identical objects (object types 1 and 2) placed on distinct types of floor patterns (patterns 1 and 2 [P1 and P2]) for 5 min each in the two acquisition trials separated by 15 min (Fig. 6C1). Fifteen minutes later, in the testing phase (trial 3), each animal explored an object from trial 2 (which is now an FO) and an object from trial 1 (which is now an NO) placed on the floor pattern used in trial 2 (i.e., P2). Excellent pattern separation ability in naive animals was revealed by a greater exploration of the object from trial 1 (i.e., NO on P2) than the object from trial 2 (i.e., FO on P2; P < 0.0001; Fig. 6C2). In contrast, animals belonging to the SE-VEH group showed no preference for the NO on P2, as they spent nearly similar amounts of time with the NO and the FO on P2 (P > 0.05; Fig. 6C3), implying an impaired ability for pattern separation. However, animals in the SE-EVs group spent greater percentages of their object exploration time with the NO (P < 0.0001; Fig. 6C4), like the behavior seen in naive control animals (P < 0.0001; Fig. 6C2). These findings were not influenced by variable object exploration times between groups, as animals belonging to different groups explored objects for comparable durations (P > 0.05; Fig. 6C5). Thus, A1-exosome treatment rescues animals from developing SE-induced pattern separation dysfunction.
IN Delivery of A1-Exosomes After SE Promoted Normal Hippocampal Neurogenesis in the Chronic Phase.
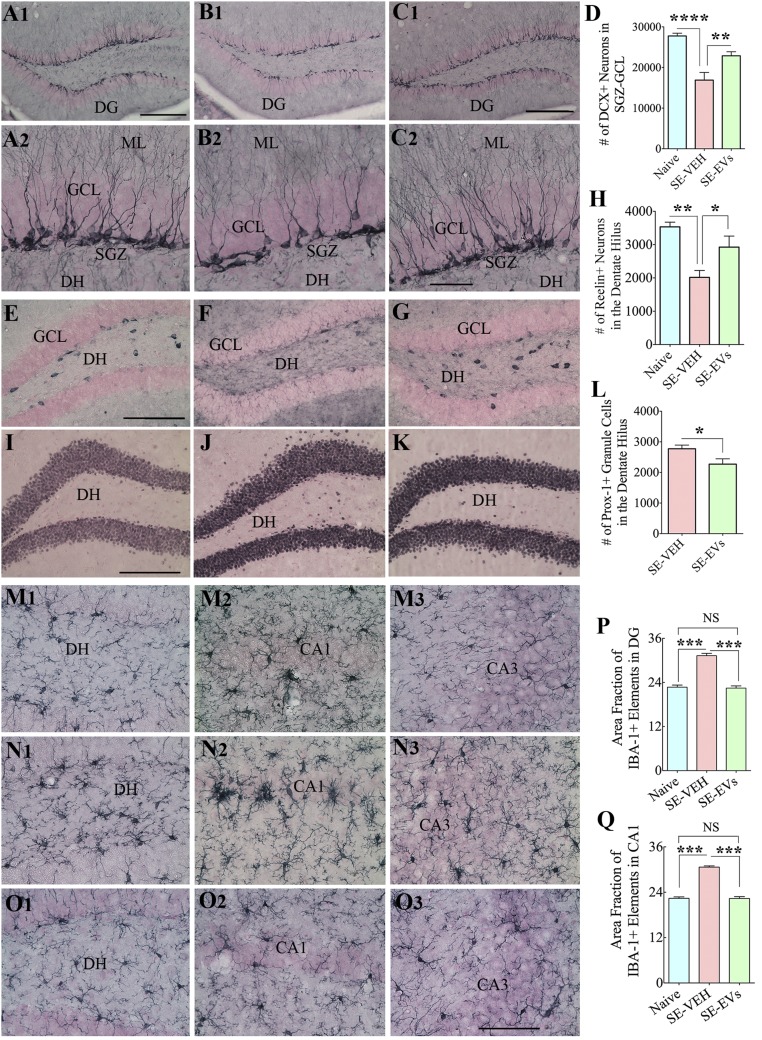
Hippocampal neurogenesis exhibits a biphasic response to SE, with increased and abnormal neurogenesis in the early phase and persistently declined neurogenesis in the chronic phase (6, 7). We investigated the effects of IN administration of A1-exosomes after SE on long-term neurogenesis in the hippocampus (i.e., 6 wk after SE; n = 6 per group). In comparison with naive controls (Fig. 7 A1 and A2), animals in SE-VEH group exhibited decreased neurogenesis (P < 0.0001; Fig. 7 B1 and B2), whereas animals in the SE-EVs group (Fig. 7 C1 and C2) displayed a pattern and extent of neurogenesis that is equivalent to age-matched naive control animals (P > 0.05) and a greater extent of neurogenesis than animals in the SE-VEH group (P < 0.01; Fig. 7D). Furthermore, SE-VEH animals showed significant loss of dentate hilar neurons positive for reelin, a protein important for directing the migration of newly born neurons in the subgranular zone (SGZ) to the GCL (P < 0.01; Fig. 7 E–H). Interestingly, reelin+ positive neuron numbers in the SE-EVs group were comparable to those in naive control animals (P > 0.05; Fig. 7 E–H) and greater than in the SE-VEH group (P < 0.05). To determine the extent of abnormal migration of newly born granule cells into the DH, we quantified the numbers of neurons positive for prox-1 (a marker of dentate granule cells) in the DH (Fig. 7 I–L). This revealed reduced abnormal migration of newly born granule cells into the DH in animals belonging to the SE-EVs group compared with the SE-VEH group (P < 0.05; Fig. 7L). Thus, A1-exosome treatment after SE facilitated maintenance of normal pattern and extent of neurogenesis with preservation of reelin+ neurons and minimal aberrant migration of newly born granule cells.
Fig. 7.
IN administration of A1-exosomes 2 h after SE restrains multiple adverse changes that are typically seen in the chronic phase after SE. In comparison with naive control animals (A1, A2, E, I, and M1–M3), animals in the SE-VEH group showed waning of hippocampal neurogenesis [doublecortin (DCX) immunostaining; B1 and B2], loss of reelin+ interneurons in the DG (F), aberrant migration of newly born prox-1+ granule cells into the DH (J), and persistent hippocampal inflammation (with increased density and hypertrophy of IBA-1+ microglia; N1–N3). In animals in the SE-EVs group, the extent of neurogenesis (C1 and C2), the survival of reelin+ interneurons (G), and the morphology and density of IBA-1+ microglia (O1–O3) were comparable to those observed in naive control animals (A1, A2, E, I, and M1–M3). In addition, aberrant migration of newly born cells into the DH was reduced (K) in these animals. ML, molecular layer. Bar charts compare numbers of DCX+ newly born neurons in the SGZ-GCL (D), reelin+ interneurons in the DH (H), numbers of prox-1+ newly born granule cells in the DH (L), and IBA-1+ microglia in the DG (P) and the CA1 subfield (Q) between groups. Note that the extent of neurogenesis (D) and numbers of reelin+ interneurons (H) and IBA-1+ structures (P and Q) in SE-EVs group animals were comparable to those seen in naive control animals. In addition, SE-EVs animals showed reduced numbers of prox-1+ cells in the DH (L), implying a reduced abnormal migration of newly born granule cells with A1-exosome treatment after SE (*P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001). (Scale bars: A1, B1, and C1, 200 µm; A2, B2, and C2, 50 µm; E–G and I–K, 200 µm; M1–O3, 100 µm.)
IN Delivery of A1-Exosomes After SE Led to Reduced Hippocampal Inflammation in the Chronic Phase.
To examine whether A1-exosome–mediated suppression of hippocampal inflammation observed in the early phase after SE persists in the chronic phase, we examined microglia in the hippocampus through IBA-1 immunostaining 6 wk after SE (Fig. 7 M1–Q). Animals in the SE-VEH group exhibited enhanced density of microglia with hypertrophied soma and thick, short processes (Fig. 7 N1–N3). Such microglia were prominently seen in the DG and the CA1 subfield. In contrast, animals in the SE-EVs group showed highly ramified microglia (Fig. 7 O1–O3), akin to those seen in age-matched naive control animals (Fig. 7 M1–M3). Measurement of the area occupied by IBA-1–reactive elements revealed increased microglial activity in DG and CA1 regions of animals belonging to the SE-VEH group compared with the naive control group and the SE-EVs group (P < 0.001; n = 4 per group; Fig. 7 P–Q). Thus, A1-exosome treatment early after SE restrained hippocampal inflammation for prolonged periods.
SI Materials and Methods
hMSCs from Bone Marrow.
hMSCs were obtained from the National Institutes of Health-sponsored Center for the Preparation and Distribution of Adult Stem Cells (medicine.tamhsc.edu/irm/msc-distribution.html). The cells were from bone marrow aspirates of normal, healthy donors (donor 2015) with informed consent under Scott & White and Texas A&M Institutional Review Board-approved procedures. A frozen vial of approximately 1 million passage-1 hMSCs was thawed at 37 °C and plated in complete culture medium (CCM) consisting of α-minimum essential medium (α-MEM; Gibco), 17% FBS (prescreened for rapid growth of MSCs; Atlanta Biologicals), 100 U/mL penicillin (Gibco), 100 µg/mL streptomycin (Gibco), and 2 mM l-glutamine (Gibco) on a 152-cm2 culture dish (Corning). After 15–24 h, the medium was removed, the cell layer was washed with PBS solution, and the adherent viable cells were harvested by using 0.25% trypsin and 1 mM EDTA (Gibco) for 3–4 min at 37 °C. The cells were reseeded at 500 cells per square centimeter in CCM and incubated for 5–7 d (with medium change on day 3) until 70–80% confluence (i.e., 6,000–10,000 cells per square centimeter). The medium was removed, the cell layer washed with PBS solution, and the cells were lifted with trypsin/EDTA and frozen at a concentration of approximately 1 million cells per milliliter in α-MEM containing 30% FBS and 5% dimethyl sulfoxide (Sigma). For experiments described here, the cells were expanded under the same conditions, and passage-4 cells were used.
Culture Conditions for Producing EVs.
A frozen vial of passage-4 hMSCs was thawed at 37 °C and plated directly at approximately 500 cells per square centimeter in 150 × 20-mm-diameter tissue culture plates (no. 430599; Corning) in CCM. The CCM medium was replaced after 2–3 d. After the cells reached approximately 70% confluence in 4–6 d, the medium was replaced with a medium initially optimized by supplements (58) to a commercial medium CHO cells (CD-CHO medium; cat. no. 10743–002; Invitrogen). The medium was recovered after 6 h and was discarded. The medium was replaced, and the medium recovered between 6 and 48 h was stored at −80 °C or used directly to isolate EVs.
Isolation of EVs by Chromatography.
For isolation of EVs, the medium harvested from 40–45 plates (approximately 1.2 L) was used directly or after thawing (20). The medium was centrifuged at 2,565 × g for 15 min to remove cellular debris and the supernatant was applied directly at room temperature to a column containing the anion exchange resin (100-mL bed volume; Express Q; cat. no. 4079302; Whatman) that had been equilibrated with 50 mM NaCl in 50 mM Tris buffer (pH 8.0). The medium was applied at a flow rate of 4 mL/min at room temperature. The column resin was washed with 10 volumes of the equilibration buffer and then eluted with 25 volumes of 500 mM NaCl in 50 mM Tris buffer (pH 8.0). Fractions of 20–30 mL were collected and stored at 4 °C or −20 °C. The protein content of the EVs was assayed by the Bradford method (Bio-Rad) and the size and number by nanoparticle tracking analysis (Nanosight LM10; Malvern).
Assays of Antiinflammatory Activity of EVs.
Male C57BL/6 mice (Jackson Laboratories) 6–8 wk of age were injected through a tail vein with 150 µL of PBS solution, 50 µg LPS from Escherichia coli 055:B5 (L2880; Sigma) in PBS solution, 50 µg LPS plus 30 µg dexamethasone (D4902; Sigma) in PBS solution, or 50 µg LPS plus EVs (30 µg protein and 15 billion vesicles) in PBS solution. After 3 h, the mice were killed and the spleens assayed by RT-PCR with commercial kits for IL-6, IFN-γ, and IL-1β using β-actin as an internal standard. EVs that did not produce a significant decrease (P < 0.05) in all three of the proinflammatory factors were rejected for further use. Batches of EVs that decreased the levels of all three proinflammatory cytokines were chosen and referred to as A1-exosomes.
Labeling of A1-Exosomes.
A1-exosomes were labeled with the red fluorescent membrane dye PKH26 (MINI26; Sigma). This was done by transferring A1-exosomes from PBS solution to diluent C solution (Sigma) by centrifugation at 100,000 × g for 70 min. PKH26, diluted to 4 mM, and the A1-exosomes (200 μg/mL) were filtered separately through small 0.2-μm syringe filters before mixing at 1:1 for 5 min, followed by the addition of 5% BSA and washing by centrifugation. The pellet of A1-exosomes was suspended in 0.5 mL PBS solution. To avoid dye-stained aggregates, the A1-exosomes were filtered through a 0.2-μm syringe filter immediately before use.
Animals for Induction of SE.
Male C57BL/6J mice were purchased from the Jackson Laboratory. They were 6–8 wk of age at the time of commencement of experiments. Animals were housed in an environmentally controlled room with a 12:12-h light:dark cycle and were given food and water ad libitum. All animals were treated in accordance with a protocol approved by the institutional animal care and use committee of Texas A&M Health Science Center College of Medicine.
Induction of SE.
Animals first received an s.c. injection of scopolamine methyl nitrate (1 mg/kg; S2250; Sigma-Aldrich) as a measure to reduce the peripheral cholinergic effects of pilocarpine. Following a waiting period of 30 min, animals received an i.p. injection of pilocarpine hydrochloride (P6503; Sigma-Aldrich) at a dose of 290–350 mg/kg (59–61), which induced SE. Animals were closely monitored for the severity and duration of the behavioral seizures. To limit the duration of SE and mortality to comparable periods, seizures were terminated in all animals with a diazepam injection (10 mg/kg, s.c.) at 2 h after the onset of SE. The severity of convulsive responses was monitored and classified according to the modified Racine scale (62). Mice that showed consistent stage 4 (i.e., bilateral forelimb myoclonus and rearing) or stage 5 (i.e., bilateral fore- and hindlimb myoclonus and transient falling) seizures were chosen for further experimentation. Animals that did not show consistent acute seizure activity (i.e., nonresponders exhibiting no seizures or isolated milder seizures) were excluded from the study. Furthermore, animals that demonstrated extensive and severe tonic-clonic seizures (i.e., overresponders) were euthanized to avoid severe pain and distress.
IN Administration of A1-Exosomes or Vehicle.
A1-exosomes were prepared using sterile PBS solution at a concentration of 200 µg/mL and stored at −80 °C. Mice that displayed SE after a pilocarpine injection were randomly assigned to the SE+VEH group or SE+EVs group. Following termination of 2 h of SE via a diazepam injection, each nostril was treated with 5 µL of hyaluronidase (100 U; H3506; Sigma-Aldrich) in sterile PBS solution to enhance the permeability of the nasal mucous membrane. Thirty minutes later, each mouse was held ventral side up with the head facing downward. Each nostril was then carefully administered PBS solution or A1-exosomes in ∼5-µL spurts separated by 5 min via a 10-μL micropipette. Each mouse received a total volume of 75 µL on the day of SE. Eighteen hours later, another 75 µL of PBS solution or A1-exosomes was administered in a similar manner. Overall, each mouse received a total of 150 µL of PBS solution or A1-exosomes (30 µg, approximately 15 × 109) within 18 h after 2 h of SE. However, mice in A1-exosome tracking studies received administration of 75 µL A1-exosomes only on SE day.
Tracking of IN-Administered A1-Exosomes.
For tracking IN-administered exosomes, PKH26-labeled exosomes (15 μg, approximately 7.5 billion per mouse) were administered only on SE day by using methods described here earlier (n = 4). Each mouse was transcardially perfused with saline solution and paraformaldehyde (pH 7.4) at 6 h following A1-exosome administration. The brains were removed, postfixed in 4% paraformaldehyde overnight, and cryoprotected with different grades of sucrose solution. Coronal sections 30 µm thick were cut through the entire brain using a cryostat, and the sections were collected serially in 24-well plates containing phosphate buffer. Representative sets of sections (every 10th) through different levels of the cortex and the hippocampus were chosen for tracking the IN-administered A1-exosomes through dual immunofluorescence and confocal microscopy. Briefly, different sets of sections were labeled with primary antibodies for NeuN (a pan-neuronal marker; ABN78; Millipore), GFAP (a marker of astrocytes; MAB360; Millipore), or IBA-1 (a marker of microglia; ab5076; Abcam). The secondary antibodies comprised Cy2-conjugated donkey anti-goat IgG (no. 715–225-150; Jackson Immuno Research), Cy2-conjugated donkey anti-rabbit IgG (no. 711–545-152; Jackson Immuno Research), or A488 anti-mouse IgG (A-21202; Thermo Fisher Scientific). Sections were mounted by using an antifade reagent (S7114; Sigma). Optical Z-sections 1 μm thick were sampled from different regions of the cortex and various subfields of the hippocampus by using a confocal microscope [FV10i (Olympus) or Ti-Eclipse (Nikon)], and the images were analyzed by using an Olympus FV-10i image browser.
Preparation of Hippocampal Extract and Evaluation of Cytokines.
Twenty-four hours after the first administration of PBS solution or A1-exosomes (i.e., 75 µL administration performed ∼2 h after SE induction), mice were anesthetized with isoflurane, and the brains were rapidly dissected from the skull and stored at −80 °C (n = 6 per group). For comparison, brains were similarly collected from naive control animals (n = 6). For the evaluation of cytokine levels in the hippocampus, each brain was thawed and the hippocampus was rapidly dissected under a stereomicroscope and sonicated on ice in lysis buffer containing protease inhibitor mixture (P2714; Sigma) and centrifuged at 9,391 × g for 5 min at 4 °C. The supernatant was collected, the total protein concentration was measured, and the lysate was diluted for the required concentration. Each 96-well cytokine array plate (EA-4005; Signosis) used in this study displayed four segments (24 wells per segment) adequate for measuring 24 different cytokines from four samples. The wells were precoated with specific cytokine capture antibodies. The assay was performed per the manufacturer’s guidelines, with each well receiving 10 µg of lysate (in 100 µL volume). In this assay, the concentration of each of 24 cytokines in hippocampal lysates is directly proportional to the intensity of color. For TNF-α (EA-2203; Signosis) and IL1-β (EA-2508; Signosis) enzyme-linked quantitative immunoassays, 100 µL of serially diluted standard and 100 µL of hippocampal lysate were used. The assay was performed per the manufacturer’s guidelines. The levels of TNF-α and IL-1β were quantified by using the standard graph and expressed as picograms per milligram of protein.
Tissue Processing and Immunohistochemistry.
Four days after SE, subgroups of animals belonging to SE+VEH and SE+EVs groups (n = 6 per group) were perfused with 4% paraformaldehyde as described here previously. A subgroup of age-matched naive control animals (n = 5) were also perfused similarly. Several sets of serial sections (every 15th or 20th) through the entire hippocampus were selected and processed for immunohistochemistry. The procedures used for immunostaining are described in our previous reports (5). Briefly, the sections were etched with PBS solution containing 20% methanol and 3% hydrogen peroxide for 20 min, rinsed thrice in PBS solution, and treated for 30 min in PBS solution containing 0.1% Triton-X 100 and an appropriate serum (10%) selected on the basis of the species in which the chosen secondary antibody was raised. The primary antibodies comprised anti-CD68 (ED-1, an activated microglia marker; MACA341R; Bio-Rad Laboratories), anti-NeuN (a pan-neuronal marker; ABN78; Millipore), anti-PV (a calcium-binding protein found in a subclass of GABAergic interneurons; P3088; Sigma-Aldrich), anti-SS (a neuropeptide found in a subclass of GABAergic interneurons; T-4546; Peninsula Laboratories), or anti-NPY (another neuropeptide found in a subclass of GABAergic interneurons; T-4070; Peninsula Laboratories). After an overnight incubation with the respective primary antibody solution, sections were washed thrice in PBS solution and incubated in an appropriate secondary antibody solution for 1 h. Biotinylated anti-rabbit (H+L; BA-1000; Vector Lab) and biotinylated anti-mouse [heavy- and light-chains (H+L); BA-2000; Vector Lab] were used for the study. The sections were washed thrice in PBS solution and treated with avidin-biotin complex reagent (PK-6100; Vector Lab) for 1 h. Peroxidase reaction was developed by using diaminobenzidine (SK-4100; Vector Lab) or vector SG (SK-4700; Vector Lab) as chromogens, and the sections were mounted on gelatin-coated slides, dehydrated, cleared, and coverslipped with Permount.
Measurement of the Number of Various Immunostained Cells.
The optical fractionator method in the Stereo Investigator system (Microbrightfield) interfaced with a Nikon E600 microscope through a color digital video camera (Optronics) was used for all cell counts performed at 4 d post-SE. This comprised quantification of numbers of (i) activated microglia positive for ED-1 in the DG and CA1 and CA3 subfields; (ii) neurons positive for NeuN in the DH and the CA1 pyramidal cell layer; and (iii) interneurons positive for PV, SS, and NPY in the DH-GCL and CA1 and CA3 subfields. Detailed methodology used in these counts is described in our previous reports (5, 63).
Behavioral Tests.
We examined animals with three object-based tests at 5–6 wk after SE in the SE+VEH and SE+EVs groups (n = 8–10 per group). Age-matched naive control animals (n = 10) were also included for comparison. The tests comprised an OLT, a NORT, and a PST. All tests were performed by using an open-field apparatus (measuring 45 × 45 cm).
OLT.
Each mouse was observed in an open field with three successive trials separated by 15-min intervals. A detailed description of this test is available in our previous report (32). In brief, the mouse was placed in an open field for 5 min in the first trial for acclimatization to the testing apparatus (i.e., habituation phase), whereas, in trial 2, the mouse was allowed to explore two identical objects placed in distant areas of the open field (i.e., sample phase). In trial 3 (i.e., testing phase), one of the objects was moved to a new area (i.e., NPO) while the other object remained in the previous place (i.e., FPO). Trials 2 and 3 were video-recorded by using a Noldus-Ethovision video-tracking system to measure the amount of time spent with each of the two objects. Exploration of the object was defined as the length of time a mouse’s nose was 1 cm away from the marked object area. The results, such as the percentage of object exploration time spent in exploring the NPO and FPO, as well as the total object exploration time in trial 3, were computed. The percentage of time spent with the NPO and FPO was calculated by dividing the time spent with the particular object by the total object exploration time and multiplying by 100.
NORT.
Each mouse was examined in an open field with three consecutive trials separated by 15-min intervals. A detailed description of this test is available in our previous report (32). The first two trials comprised an acclimatization period of 5 min to an open field (i.e., trial 1 or habituation phase) and exploration of two similar objects placed in distant areas within the open field for 5 min (i.e., sampling phase). In trial 3 (i.e., testing phase), the mouse was allowed to explore a different pair of objects comprising one of the objects used in trial 2 and a NO for 5 min. Trials 2 and 3 were video-recorded by using a Noldus-Ethovision video-tracking system. The amounts of time spent with the FOA and the NOA and the total object exploration time in trial 3 were computed. Exploration of the FOA or NOA was defined as the length of time a mouse’s nose was 1 cm away from the respective area. The percentages of time spent with the NOA or FOA were calculated by dividing the time spent with the particular object by the total object exploration time and multiplying by 100.
PST.
This test comprised three successive trials separated by 15-min intervals following an acclimatization period of 5 min in an open-field apparatus. The first trial comprised exploration of a pair of identical objects (i.e., type 1 objects) placed in distant areas on a floor pattern (i.e., P1) for 5 min. The second trial involved exploration of a second pair of identical objects (i.e., type 2 objects) placed in distant areas on a different floor pattern (i.e., P2) for 5 min. In trial 3, one of the objects from trial 2 was replaced with an object from trial 1, which became an NO on pattern 2, whereas the object retained from trial 2 became an FO on P2. The mouse was allowed to explore objects for 5 min. Trials 2 and 3 were video-recorded bu using a Noldus-Ethovision video-tracking system. Exploration of objects was defined as the length of time a mouse’s nose was 1 cm away from the object area. The results such as the time spent in exploring the NO on P2 and the FO on P2 and the total object exploration time were computed from trial 3. Furthermore, the NO and FO discrimination index was calculated by dividing the time spent with a particular object on P2 by the total object exploration time and multiplying by 100.
Analyses of Hippocampal Neurogenesis and Inflammation in the Chronic Phase After SE.
Animals were perfused 6 wk post-SE by using 4% paraformaldehyde. Tissue processing, section cutting, and storage of sections were done as described earlier. Serial sections (every 10th) through the entire hippocampus were selected from animals belonging to naive control, SE+VEH, and SE+EVs groups (n = 6 per group) and processed for immunohistochemistry. The detailed procedures used for immunostaining are available in our previous report (5). The primary antibodies comprised anti-DCX [a marker of newly born neurons (63); sc-8066; Santa Cruz Biotechnology; ab5076; Abcam], anti-reelin (marker of a subclass of interneurons that secrete reelin in the hippocampus; MAB5364; Millipore), Prox-1 (a marker of dentate granule cells; AB5475; Millipore), and anti–IBA-1(a pan-microglial marker; ab5076; Abcam). The secondary antibodies comprised biotinylated anti-goat (H+L; BA-9500; Vector Lab), biotinylated anti-mouse (H+L; BA-2001; Vector Lab), or biotinylated anti-rabbit (H+L; BA-1000; Vector Lab). Following incubation in secondary antibody solutions, sections were incubated with avidin–biotin complex reagent (PK-6100; Vector Lab). The peroxidase reaction was developed by using diaminobenzidine (SK-4100; Vector Lab) or vector SG (SK-4700; Vector Lab) as chromogens, and the sections were mounted on gelatin-coated slides, dehydrated, cleared, and coverslipped with Permount.
Quantification of Numbers of DCX+, prox-1+, and reelin+ Neurons.
An optical fractionator method available in the Stereo Investigator system (Microbrightfield) was used for counting (i) newly born neurons positive for DCX in the SGZ-GCL of the hippocampus, (ii) prox-1+ granule cells in the DH, and (iii) reelin+ interneurons in the DH. Quantification was done from five or six animals per group. A detailed methodology used in these counts is described in our previous report (5, 63).
Measurement of the Area Fraction of IBA-1+ Immunoreactive Elements.
The area occupied by IBA-1+ immunoreactive elements (soma and processes of microglia) in the DG and CA1 and CA3 subfields were quantified by using ImageJ software as described in our previous report (5). In brief, images from different regions of the hippocampus were digitized by using a 20× objective lens in a Nikon E600 microscope equipped with a digital video camera connected to a computer. Each image saved in grayscale as a bitmap file was opened in ImageJ software, and a binary image was created by selecting a threshold value that retained all IBA-1+ structures but no background. The area occupied by the IBA-1+ structures (i.e., the area fraction) in the binary image was then measured by selecting the Analyze command in the program. Area fraction of IBA-1+ immunoreactive elements was calculated separately for every hippocampal region in each animal by using data from all chosen serial sections before the mean and SEM were determined for the total number of animals included per group (n = 4 per group).
Statistical Analysis.
Statistical analyses were performed by using Prism software. One-way ANOVA with Newman–Keuls multiple comparison post hoc tests was used when three groups were compared. Comparison within groups in the behavioral tests or comparison between the two groups (e.g., prox-1 counts) used unpaired, two-tailed Student’s t test. Numerical data were presented as mean ± SEM, and a P value less than 0.05 was considered as statistically significant.
Discussion
The present results demonstrate that IN delivery of A1-exosomes 2 h after SE onset is efficacious for reducing multiple SE-induced adverse effects in the hippocampus, a region of the brain vital for learning and memory. The beneficial effects of A1-exosome treatment in the early phase after SE comprised (i) suppression of SE-induced surge in multiple proinflammatory cytokines, (ii) enhanced expression of a few antiinflammatory cytokines and trophic factors, (iii) diminished activation of microglia, (iv) reduced loss of dentate hilar neurons (presumably comprising excitatory and inhibitory hilar neurons), (v) robust protection of glutamatergic CA1 pyramidal neurons, and (vi) reduced loss of several subclasses of GABAergic inhibitory interneurons, including those expressing the calcium binding protein PV and neuropeptides SS and NPY. The early favorable effects after SE led to maintenance of a normal pattern and extent of neurogenesis with minimal loss of reelin+ interneurons, reduced aberrant migration of newly born granule cells into the DH, and diminished inflammation in the chronic phase after SE. Moreover, the extent of neuroprotective and antiinflammatory effects mediated by A1-exosome treatment was sufficient for preserving normal cognitive and memory function. Importantly, these changes were preceded by incorporation of IN-administered A1-exosomes into a large number of neurons in the frontoparietal cortex and the dorsal hippocampus, and a vast majority of microglia in rostral regions of the cortex. In addition, exosomes were frequently seen adjacent to processes of astrocytes and microglia throughout the frontoparietal cortex and the hippocampus. A proinflammatory cytokine storm and loss of glutamatergic and GABAergic neurons in the early phase after SE and an abnormal and reduced neurogenesis with persistent inflammation in the chronic phase after SE are considered as epileptogenic and cognitive and memory-impairing alterations. Hence, the favorable outcomes mediated through IN administration of A1-exosomes have considerable significance for developing a therapy that eases the advancement of primary hippocampal injury into a chronic epileptic state.
Protracted and orchestrated hyperactivity of multiple populations of neurons typically lead to continuous seizures or SE, which cause excitotoxic neurodegeneration and neuroinflammation associated with an increase in the concentration of multiple proinflammatory cytokines and activation of microglia (5, 35, 36). In this study, IN treatment with A1-exosomes 2 h after SE resulted in their targeting into the hippocampus within 6 h of administration, which seemed to provide considerable protection to glutamatergic neurons and GABAergic interneurons in the hippocampus. The potential of A1-exosomes as a neurotherapeutic product was first discovered in a model of traumatic brain injury (TBI) when a single i.v. administration of A1-exosomes after TBI considerably suppressed inflammation and improved spatial learning and pattern separation ability (20). Nonetheless, the efficacy of these exosomes when dispensed through an IN route or in an excitotoxic injury model was unknown. In this regard, the present study provides compelling evidence that IN administration of A1-exosomes is neuroprotective when application is commenced 2 h after SE. Measurement of proinflammatory cytokines 24 h post-SE and activation of microglia 4 d post-SE revealed that IN treatment of A1-exosomes considerably eases neuroinflammation resulting from SE. This was exemplified by the reduced concentration of multiple proinflammatory cytokines, enhanced expression of a few antiinflammatory cytokines and trophic factors, and reduced occurrence of activated microglia. The proinflammatory cytokines that showed increased expression with SE and VEH treatment but reduced expression with SE and A1-exosome treatment comprised (i) TNF-α, a prominent cytokine secreted by activated macrophages involved in inflammation and several brain diseases; (ii) IL1-β, another prominent cytokine secreted by activated macrophages and involved in inflammation; (iii) MCP-1, a chemokine that regulates the migration and infiltration of monocytes and macrophages; (iv) SCF, a cytokine involved in hematopoiesis; (v) MIP-1α, a protein involved in leukocyte recruitment; and (vi) GM-CSF, a protein that stimulates stem cells to produce granulocytes. Among these, TNF-α and IL-1β are also considered as proconvulsant cytokines because of their propensity for contributing to epileptogenesis and chronic epilepsy development after an initial injury (31).
The antiinflammatory cytokines or factors that showed enhanced expression with A1-exosome treatment include (i) IL-10, a well-known antiinflammatory cytokine; (ii) G-CSF, a factor that stimulates bone marrow to produce new blood cells; (iii) PDGF-β, a factor that promotes angiogenesis; (iv) IL-6, a cytokine that acts as a proinflammatory and antiinflammatory protein; and (v) IL-2, a cytokine involved in immune tolerance and immunity. Furthermore, A1-exosome treatment reduced the overall number of ED1+ activated microglia in the hippocampus by 66% when examined 4 d post-SE. Additionally, examination of IBA-1+ microglia at 6 wk post-SE revealed persistent inflammation in animals belonging to the SE-VEH group but not in the SE-EVs group. Greatly reduced inflammation observed after SE with A1-exosome treatment has implications because persistent inflammation is one of the key players in the evolution of neurodegenerative diseases, including temporal-lobe epilepsy (37). Indeed, enhanced concentrations of proinflammatory cytokines are constantly observed in the epileptic hippocampus (38), and are derived from activated microglia/macrophages and reactive astrocytes (38, 39). In this regard, diminished inflammation with normalization of TNF-α and IL-1β concentrations and reduced numbers of ED1+ activated microglia in the SE-EVs group imply that A1-exosome treatment is effective for curbing the SE-induced inflammation cascade. Considering the robust incorporation of exosomes into microglia in rostral regions of the cortex and association of exosomes with processes of microglia and astrocytes in the hippocampus, it is plausible that antiinflammatory effects of IN administered A1-exosomes were mediated by modulation of microglia and astrocytes directly or indirectly through the release of antiinflammatory proteins.
The antiinflammatory effects of A1-exosome treatment were also accompanied by robust neuroprotection, epitomized by diminished loss of neurons in the DH and the CA1 pyramidal cell layer, two regions in the hippocampus that typically display increased vulnerability to SE-induced neuronal death (5, 40, 41). Moreover, IN administration of A1-exosomes after SE promoted preservation of subclasses of GABAergic interneurons, particularly those expressing PV and SS. This was evident in the finding of 34–69% greater numbers of these interneurons in the DG and CA1 subfield of animals in the SE-EVs group compared with animals in the SE-VEH group. Interneuron preservation has great importance because severe loss of PV+ and SS+ interneurons leads to chronic epilepsy typified by spontaneous recurrent seizures and cognitive and mood impairments (41–45). Neuroprotective effects were likely a consequence of robust antiinflammatory effects of exosomes. Nonetheless, some direct neuroprotective and/or neuron-reparative effects through the release of neurotrophic factors cannot be ruled out as exosomes incorporated into many neurons in the hippocampus and the frontoparietal cortex.
Another important feature of A1-exosome treatment after SE was its proficiency in maintaining a normal pattern and extent of neurogenesis for an extended period after SE and in reducing the size of aberrant neurogenesis. Animals in the SE-VEH group exhibited clearly reduced neurogenesis in the SGZ-GCL, whereas animals in the SE-EVs group presented neurogenesis that was comparable to age-matched naive control animals. Deviant neurogenesis after SE is exemplified by an anomalous migration of newly born dentate granule cells into the DH (46, 47). In the present study, such aberrant migration was ostensible in the SE-VEH group but significantly lowered in the SE-EVs group. Dampening of SE-induced aberrant neurogenesis through A1-exosome treatment has implications because deviant neurogenesis has been shown to facilitate the expansion of epileptogenic circuitry between dentate granule cells relocated to the DH and CA3 pyramidal neurons. It is currently unknown whether these anomalies by themselves are adequate to induce spontaneous seizures (48). Nonetheless, their involvement in manifestations of spontaneous seizures after SE has been well documented (49–54). We measured reelin+ interneurons in the DH to identify the mechanism behind A1-exosome–mediated inhibition of aberrant neurogenesis, as migration of newly born dentate granule cells from the SGZ to GCL is steered by reelin protein secreted by reelin+ interneurons in the DH, and considerable loss of these neurons fosters aberrant displacement of newly born neurons into the DH (55). Abnormally migrated prox-1+ new granule cells were accompanied with a sizable loss of reelin+ interneurons in the DH of animals in the SE-VEH group. In contrast, a diminished extent of aberrant neurogenesis was apparent with the salvation of reelin+ interneurons in the DH of animals in the SE-EVs group. Thus, A1-exosome treatment after SE eased aberrant neurogenesis via protection of reelin+ interneurons, which is likely a result of repression of inflammation facilitated by A1-exosomes. Decreased inflammation and normal levels of neurogenesis likely also contributed to the preservation of cognitive and memory function in the SE-EVs group, as inflammation and decreased neurogenesis can impair cognitive and memory function (56, 57). A series of object-based tests in the present study demonstrated the impaired ability of animals in the SE-VEH group for cognition and memory. These include inability to discern minor changes in their environment in an OLT, identifying an NO in an NORT, and pattern separation in a PST. However, animals in the SE-EVs group exhibited similar ability as age-matched naive control animals in these tests, underscoring that A1-exosome treatment shortly after SE is efficacious for preventing cognitive and memory impairments.
In summary, the results of the present study establish that IN A1-exosome treatment after the termination of 2 h of SE can considerably repress neurodegeneration, neuroinflammation, aberrant neurogenesis, and cognitive and memory impairments. The results suggest that A1-exosomes may be used clinically as an adjunct to AED therapy after SE. Although an ideal combo of AEDs would terminate an SE crisis, IN administration of A1-exosomes shortly after SE termination would help in reducing the SE-induced neurodegeneration, neuroinflammation, and cognitive and memory impairments, which in turn may considerably reduce the predilection of SE to evolve into a chronic epileptic state. In such situations, IN dispensation of prebanked A1-exosomes generated from allogenic MSCs from multiple sources may be used (28–30). There are several advantages of the use of EVs as opposed to cells. These include their ability to cross the blood–brain barrier and deliver various therapeutic factors to the brain (25, 26); amenability for engineering to package specific mRNAs, miRNAs, and proteins (26, 27); and minimal risk for developing tumors or causing thrombosis. Future studies will be focused on determining whether the extent of neuroprotective, antiinflammatory, neurogenic, cognitive, and memory-protective effects mediated by A1-exosomes is adequate to prevent the evolution of SE-induced initial precipitating injury into a chronic epileptic state.
Materials and Methods
SI Materials and Methods includes descriptions of all materials and methods used in the present study. This includes culture conditions, chromatographic isolation of EVs, timeline of various experiments, induction of SE, IN administration of A1-exosomes, tracking of PKH-26+ labeled exosomes in the brain, behavioral testing procedures, immunohistochemistry for neuroinflammation and neuroprotection analyses, stereological cell-counting methods, and measurement of microglia with ImageJ software. Human bone marrow-derived MSCs were from the Center for MSC Distribution (medicine.tamhsc.edu/irm/msc-distribution.html), and the Texas A&M Animal Care and Use Committee approved all animal protocols. Human bone marrow-derived MSCs were obtained from normal, healthy donors after informed consent in accordance with procedures approved by the Scott & White and Texas A&M Institutional Review Boards.
Acknowledgments
This study was supported by Emerging Technology Funds from the State of Texas (A.K.S.); Department of Veterans Affairs Merit Award I01 BX002351 (to A.K.S.), National Institutes of Health Grant P40OD011050 (to D.J.P.), Biomedical Laboratory Research and Development (BLR&D) Research Career Scientist Award 1IK6BX003612 from the Department of Veterans Affairs (to A.K.S.), and Government of China Grant NSFC-No.31301216; 2014KJXX-29 (to Q.L.). Q.L. was a visiting research scholar from the Department of Neurosurgery, Xi’an Central Hospital, School of Medicine, Xi’an Jiao Tong University, Xi’an, P.R. China. The contents of this article suggest the views of authors and do not represent the views of the US Department of Veterans Affairs or the United States Government.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1703920114/-/DCSupplemental.
References
- 1.Seinfeld S, Goodkin HP, Shinnar S. Status epilepticus. Cold Spring Harb Perspect Med. 2016;6:a022830. doi: 10.1101/cshperspect.a022830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khoujah D, Abraham MK. Status epilepticus: What’s new? Emerg Med Clin North Am. 2016;34:759–776. doi: 10.1016/j.emc.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 3.Vezzani A, Dingledine R, Rossetti AO. Immunity and inflammation in status epilepticus and its sequelae: Possibilities for therapeutic application. Expert Rev Neurother. 2015;15:1081–1092. doi: 10.1586/14737175.2015.1079130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Löscher W. Single versus combinatorial therapies in status epilepticus: Novel data from preclinical models. Epilepsy Behav. 2015;49:20–25. doi: 10.1016/j.yebeh.2015.02.027. [DOI] [PubMed] [Google Scholar]
- 5.Mishra V, et al. Resveratrol treatment after status epilepticus restrains neurodegeneration and abnormal neurogenesis with suppression of oxidative stress and inflammation. Sci Rep. 2015;5:17807. doi: 10.1038/srep17807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shetty AK, Hattiangady B. Concise review: Prospects of stem cell therapy for temporal lobe epilepsy. Stem Cells. 2007;25:2396–2407. doi: 10.1634/stemcells.2007-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hattiangady B, Shetty AK. Decreased neuronal differentiation of newly generated cells underlies reduced hippocampal neurogenesis in chronic temporal lobe epilepsy. Hippocampus. 2010;20:97–112. doi: 10.1002/hipo.20594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ben-Ari Y. Blocking seizures with the diuretic bumetanide: Promises and pitfalls. Epilepsia. 2012;53:394–396. doi: 10.1111/j.1528-1167.2011.03378.x. [DOI] [PubMed] [Google Scholar]
- 9.Loscher W. Strategies for antiepileptogenesis: Antiepileptic drugs versus novel approaches evaluated in post-status epilepticus models of temporal lobe epilepsy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s Basic Mechanisms of the Epilepsies. 4th Ed. National Center for Biotechnology Information; Bethesda, MD: 2012. pp. 1055–1065. [PubMed] [Google Scholar]
- 10.Sankar R, Mazarati A. Neurobiology of depression as a comorbidity of epilepsy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s Basic Mechanisms of the Epilepsies. 4th Ed. National Center for Biotechnology Information; Bethesda, MD: 2012. pp. 945–956. [PubMed] [Google Scholar]
- 11.Shetty AK. Hippocampal injury-induced cognitive and mood dysfunction, altered neurogenesis, and epilepsy: Can early neural stem cell grafting intervention provide protection? Epilepsy Behav. 2014;38:117–124. doi: 10.1016/j.yebeh.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varvel NH, et al. Infiltrating monocytes promote brain inflammation and exacerbate neuronal damage after status epilepticus. Proc Natl Acad Sci USA. 2016;113:E5665–E5674. doi: 10.1073/pnas.1604263113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sahin M, Menache CC, Holmes GL, Riviello JJ. Outcome of severe refractory status epilepticus in children. Epilepsia. 2001;42:1461–1467. doi: 10.1046/j.1528-1157.2001.21301.x. [DOI] [PubMed] [Google Scholar]
- 14.Temkin NR. Antiepileptogenesis and seizure prevention trials with antiepileptic drugs: Meta-analysis of controlled trials. Epilepsia. 2001;42:515–524. doi: 10.1046/j.1528-1157.2001.28900.x. [DOI] [PubMed] [Google Scholar]
- 15.Dichter MA. Emerging concepts in the pathogenesis of epilepsy and epileptogenesis. Arch Neurol. 2009;66:443–447. doi: 10.1001/archneurol.2009.10. [DOI] [PubMed] [Google Scholar]
- 16.Trinka E, Kälviäinen R. 25 years of advances in the definition, classification and treatment of status epilepticus. Seizure. 2017;44:65–73. doi: 10.1016/j.seizure.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Sloviter RS, Bumanglag AV. Defining “epileptogenesis” and identifying “antiepileptogenic targets” in animal models of acquired temporal lobe epilepsy is not as simple as it might seem. Neuropharmacology. 2013;69:3–15. doi: 10.1016/j.neuropharm.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patterson V, Pant P, Gautam N, Bhandari A. A Bayesian tool for epilepsy diagnosis in the resource-poor world: development and early validation. Seizure. 2014;23:567–569. doi: 10.1016/j.seizure.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Agadi S, Shetty AK. Concise review: Prospects of bone marrow mononuclear cells and mesenchymal stem cells for treating status epilepticus and chronic epilepsy. Stem Cells. 2015;33:2093–2103. doi: 10.1002/stem.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim DK, et al. Chromatographically isolated CD63+CD81+ extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after TBI. Proc Natl Acad Sci USA. 2016a;113:170–175. doi: 10.1073/pnas.1522297113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salem HK, Thiemermann C. Mesenchymal stromal cells: Current understanding and clinical status. Stem Cells. 2010;28:585–596. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phinney DG, et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun. 2015;6:8472. doi: 10.1038/ncomms9472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heldring N, Mäger I, Wood MJ, Le Blanc K, Andaloussi SE. Therapeutic potential of multipotent mesenchymal stromal cells and their extracellular vesicles. Hum Gene Ther. 2015;26:506–517. doi: 10.1089/hum.2015.072. [DOI] [PubMed] [Google Scholar]
- 24.Anderson JD, et al. Comprehensive proteomic analysis of mesenchymal stem cell exosomes reveals modulation of angiogenesis via nuclear factor-kappaB signaling. Stem Cells. 2016;34:601–613. doi: 10.1002/stem.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cossetti C, et al. Extracellular vesicles from neural stem cells transfer IFN-γ via Ifngr1 to activate Stat1 signaling in target cells. Mol Cell. 2014;56:193–204. doi: 10.1016/j.molcel.2014.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Batrakova EV, Kim MS. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Control Release. 2015;219:396–405. doi: 10.1016/j.jconrel.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.EL Andaloussi S, Mäger I, Breakefield XO, Wood MJ. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 28.Kimbrel EA, et al. Mesenchymal stem cell population derived from human pluripotent stem cells displays potent immunomodulatory and therapeutic properties. Stem Cells Dev. 2014;23:1611–1624. doi: 10.1089/scd.2013.0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watson N, et al. Discarded Wharton jelly of the human umbilical cord: A viable source for mesenchymal stromal cells. Cytotherapy. 2015;17:18–24. doi: 10.1016/j.jcyt.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim MH, Ong WK, Sugii S. The current landscape of adipose-derived stem cells in clinical applications. Expert Rev Mol Med. 2014;16:e8. doi: 10.1017/erm.2014.8. [DOI] [PubMed] [Google Scholar]
- 31.Li G, et al. Cytokines and epilepsy. Seizure. 2011;20:249–256. doi: 10.1016/j.seizure.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 32.Hattiangady B, et al. Object location and object recognition memory impairments, motivation deficits and depression in a model of Gulf War illness. Front Behav Neurosci. 2014;8:78. doi: 10.3389/fnbeh.2014.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yassa MA, Stark CE. Pattern separation in the hippocampus. Trends Neurosci. 2011;34:515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- 35.Wasterlain CG, Naylor DE, Liu H, Niquet J, Baldwin R. Trafficking of NMDA receptors during status epilepticus: Therapeutic implications. Epilepsia. 2013;54:78–80. doi: 10.1111/epi.12285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Janigro D, Iffland PH, 2nd, Marchi N, Granata T. A role for inflammation in status epilepticus is revealed by a review of current therapeutic approaches. Epilepsia. 2013;54:30–32. doi: 10.1111/epi.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amor MM, Eltawansy SA, Osofsky J, Holland N. Recurrent sinus pauses: An atypical presentation of temporal lobe epilepsy. Case Rep Crit Care. 2014;2014:918247. doi: 10.1155/2014/918247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vezzani A. Fetal brain inflammation may prime hyperexcitability and behavioral dysfunction later in life. Ann Neurol. 2013;74:1–3. doi: 10.1002/ana.23930. [DOI] [PubMed] [Google Scholar]
- 39.Prinz M, Priller J. Microglia and brain macrophages in the molecular age: From origin to neuropsychiatric disease. Nat Rev Neurosci. 2014;15:300–312. doi: 10.1038/nrn3722. [DOI] [PubMed] [Google Scholar]
- 40.Rao MS, Hattiangady B, Reddy DS, Shetty AK. Hippocampal neurodegeneration, spontaneous seizures, and mossy fiber sprouting in the F344 rat model of temporal lobe epilepsy. J Neurosci Res. 2006;83:1088–1105. doi: 10.1002/jnr.20802. [DOI] [PubMed] [Google Scholar]
- 41.Hattiangady B, Kuruba R, Shetty AK. Acute seizures in old age leads to a greater loss of CA1 pyramidal neurons, an increased propensity for developing chronic TLE and a severe cognitive dysfunction. Aging Dis. 2011;2:1–17. [PMC free article] [PubMed] [Google Scholar]
- 42.Vezzani A, Sperk G, Colmers WF. Neuropeptide Y: Emerging evidence for a functional role in seizure modulation. Trends Neurosci. 1999;22:25–30. doi: 10.1016/s0166-2236(98)01284-3. [DOI] [PubMed] [Google Scholar]
- 43.Schwaller B, et al. Parvalbumin deficiency affects network properties resulting in increased susceptibility to epileptic seizures. Mol Cell Neurosci. 2004;25:650–663. doi: 10.1016/j.mcn.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 44.Houser CR. Do structural changes in GABA neurons give rise to the epileptic state? Adv Exp Med Biol. 2014;813:151–160. doi: 10.1007/978-94-017-8914-1_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murray AJ, et al. Parvalbumin-positive CA1 interneurons are required for spatial working but not for reference memory. Nat Neurosci. 2011;14:297–299. doi: 10.1038/nn.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parent JM, et al. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hattiangady B, Rao MS, Shetty AK. Chronic temporal lobe epilepsy is associated with severely declined dentate neurogenesis in the adult hippocampus. Neurobiol Dis. 2004;17:473–490. doi: 10.1016/j.nbd.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 48.Cho KO, et al. Aberrant hippocampal neurogenesis contributes to epilepsy and associated cognitive decline. Nat Commun. 2015;6:6606. doi: 10.1038/ncomms7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuruba R, Hattiangady B, Shetty AK. Hippocampal neurogenesis and neural stem cells in temporal lobe epilepsy. Epilepsy Behav. 2009;14:65–73. doi: 10.1016/j.yebeh.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scharfman HE, Goodman JH, Sollas AL. Granule-like neurons at the hilar/CA3 border after status epilepticus and their synchrony with area CA3 pyramidal cells: Functional implications of seizure-induced neurogenesis. J Neurosci. 2000;20:6144–6158. doi: 10.1523/JNEUROSCI.20-16-06144.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scharfman HE, Sollas AL, Smith KL, Jackson MB, Goodman JH. Structural and functional asymmetry in the normal and epileptic rat dentate gyrus. J Comp Neurol. 2002;454:424–439. doi: 10.1002/cne.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scharfman HE, Sollas AL, Berger RE, Goodman JH. Electrophysiological evidence of monosynaptic excitatory transmission between granule cells after seizure-induced mossy fiber sprouting. J Neurophysiol. 2003;90:2536–2547. doi: 10.1152/jn.00251.2003. [DOI] [PubMed] [Google Scholar]
- 53.McCloskey DP, Hintz TM, Pierce JP, Scharfman HE. Stereological methods reveal the robust size and stability of ectopic hilar granule cells after pilocarpine-induced status epilepticus in the adult rat. Eur J Neurosci. 2006;24:2203–2210. doi: 10.1111/j.1460-9568.2006.05101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hester MS, Danzer SC. Hippocampal granule cell pathology in epilepsy - A possible structural basis for comorbidities of epilepsy? Epilepsy Behav. 2014;38:105–116. doi: 10.1016/j.yebeh.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gong C, Wang TW, Huang HS, Parent JM. Reelin regulates neuronal progenitor migration in intact and epileptic hippocampus. J Neurosci. 2007;27:1803–1811. doi: 10.1523/JNEUROSCI.3111-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ryan SM, Nolan YM. Neuroinflammation negatively affects adult hippocampal neurogenesis and cognition: can exercise compensate? Neurosci Biobehav Rev. 2016;61:121–131. doi: 10.1016/j.neubiorev.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 57.Mendelsohn AR, Larrick JW. Pharmaceutical rejuvenation of age-associated decline in spatial memory. Rejuvenation Res. 2016;19:521–524. doi: 10.1089/rej.2016.1903. [DOI] [PubMed] [Google Scholar]
- 58.Kim DK, et al. Scalable production of a multifunctional protein (TSG-6) that aggregates with itself and the CHO cells that synthesize it. PLoS One. 2016a;11:e0147553. doi: 10.1371/journal.pone.0147553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cunningham M, et al. hPSC-derived maturing GABAergic interneurons ameliorate seizures and abnormal behavior in epileptic mice. Cell Stem Cell. 2014;15:559–573. doi: 10.1016/j.stem.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen X, et al. A reduced susceptibility to chemoconvulsant stimulation in adenylyl cyclase 8 knockout mice. Epilepsy Res. 2016;119:24–29. doi: 10.1016/j.eplepsyres.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shibley H, Smith BN. Pilocarpine-induced status epilepticus results in mossy fiber sprouting and spontaneous seizures in C57BL/6 and CD-1 mice. Epilepsy Res. 2002;49:109–120. doi: 10.1016/s0920-1211(02)00012-8. [DOI] [PubMed] [Google Scholar]
- 62.Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 63.Rao MS, Shetty AK. Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur J Neurosci. 2004;19:234–246. doi: 10.1111/j.0953-816x.2003.03123.x. [DOI] [PubMed] [Google Scholar]