Significance
Why do so many species occur in mountains? A popular but little-tested hypothesis is that tectonic uplift creates environmental conditions (new habitats, dispersal barriers, etc.) that increase the rate at which resident species divide and evolve to form new ones. In China’s Hengduan Mountains region, a biodiversity hotspot uplifted over the last 8 million years, this rate does in fact show a significant increase during that time, relative to the rate for adjacent older mountains, and to the rate of species immigration. The Hengduan Mountains flora is thus made up disproportionately of species that evolved within the region during its uplift, supporting the original hypothesis and helping to explain the prevalence of mountains as global biodiversity hotspots.
Keywords: biogeography, vascular plants, molecular clocks, dispersal, speciation
Abstract
A common hypothesis for the rich biodiversity found in mountains is uplift-driven diversification—that orogeny creates conditions favoring rapid in situ speciation of resident lineages. We tested this hypothesis in the context of the Qinghai–Tibetan Plateau (QTP) and adjoining mountain ranges, using the phylogenetic and geographic histories of multiple groups of plants to infer the tempo (rate) and mode (colonization versus in situ diversification) of biotic assembly through time and across regions. We focused on the Hengduan Mountains region, which in comparison with the QTP and Himalayas was uplifted more recently (since the late Miocene) and is smaller in area and richer in species. Time-calibrated phylogenetic analyses show that about 8 million y ago the rate of in situ diversification increased in the Hengduan Mountains, significantly exceeding that in the geologically older QTP and Himalayas. By contrast, in the QTP and Himalayas during the same period the rate of in situ diversification remained relatively flat, with colonization dominating lineage accumulation. The Hengduan Mountains flora was thus assembled disproportionately by recent in situ diversification, temporally congruent with independent estimates of orogeny. This study shows quantitative evidence for uplift-driven diversification in this region, and more generally, tests the hypothesis by comparing the rate and mode of biotic assembly jointly across time and space. It thus complements the more prevalent method of examining endemic radiations individually and could be used as a template to augment such studies in other biodiversity hotspots.
Central to understanding global patterns of biodiversity are considerations of biotic assembly: For a given region, when and how did resident species accumulate? Of primary interest is tempo (the rate of accumulation) and mode (the process, whether by colonization via dispersal or in situ lineage diversification). We wish to know how and why these vary in time and space.
For mountains, well-known for harboring a disproportionate fraction of terrestrial species, a common hypothesis is that of uplift-driven diversification—that orogeny creates conditions favoring in situ speciation of resident lineages (1–6). Among global biodiversity hotspots, the mountain ranges surrounding the Qinghai–Tibetan Plateau (QTP) are unusual and enigmatic: They harbor one of the world’s richest temperate floras, and (unlike other hotspots) they are neither tropical nor Mediterranean in climate. Moreover, despite increasing interest from biogeographers, their biotic assembly remains poorly understood (3, 4, 7). The mountains form three distinct hotspots of biodiversity that respectively lie to the west, south, and east of the QTP’s central high desert: the Central Asian mountains (Altai and Tianshan ranges), the Himalayas, and the Hengduan Mountains region (4) (Fig. 1). Of these, the richest in plant diversity is the Hengduan Mountains, with a vascular flora of about 12,000 species in an area of about 500,000 km2 (8–10). In this study we seek to better understand the origins of this remarkable flora through the lens of historical biogeography. In particular, was in situ diversification accelerated by uplift of the Hengduan Mountains?
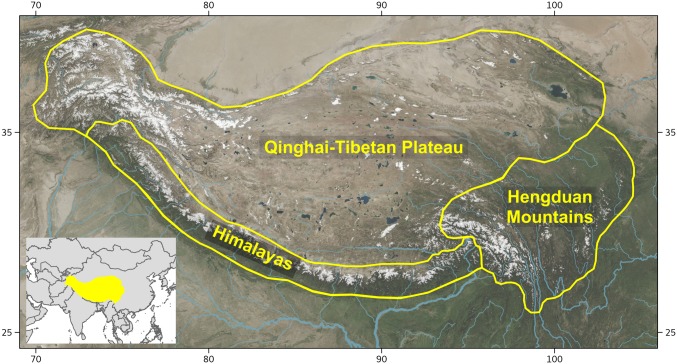
Fig. 1.
Map of the Hengduan Mountains region in relation to the QTP and Himalayas.
The geological history of the QTP and its surrounding ranges is complex, and many details remain controversial, with different lines of evidence often yielding conflicting inferences. However, some points of consensus have emerged from recent syntheses (4, 11–13). One is that the central plateau was uplifted first, forming a “proto-QTP” as early as 40 Mya, with subsequent outward extensions by the early Miocene (11, 14). By the late Miocene, 8 to 10 Mya, all of the mountains surrounding the QTP to the south, west, and north had reached their current elevations (12, 15–17). By contrast, uplift of the Hengduan Mountains region, at the southeastern margin (Fig. 1), is generally believed to have been rapid and recent, occurring mainly between the late Miocene and late Pliocene (18–23).
The Hengduan Mountains are thus younger than the rest of the QTP and relatively small in area, but conspicuously rich in species. If floristic assembly tracks orogeny, this suggests an elevated tempo since the late Miocene, compared with adjacent regions, but what of mode? Either colonization, in situ diversification, or both processes must have been accelerated. In biogeographic studies of QTP-associated clades, the hypothesis of uplift-driven in situ diversification has been clearly favored over colonization, being commonly invoked to explain phylogenetic and phylogeographic divergences (e.g., refs. 24–27). However, conspicuously lacking from these studies are quantitative analyses that explicitly measure rates of diversification and colonization and compare them across regions and time (3, 4). Consequently, the idea that uplift-driven diversification has contributed disproportionately to floristic assembly in the Hengduan Mountains has yet to be rigorously tested.
In this study we used the evolutionary histories of multiple plant groups to study the floristic assembly of the Hengduan Mountains region, focusing on comparisons to adjacent regions, especially the Himalayas and other geologically older parts of the QTP (Fig. 1). We inferred regional rates of diversification and colonization through time from fossil-calibrated molecular chronograms and reconstructions of ancestral range and rates of lineage diversification, using data from 19 clades of vascular plants chosen for their potential to inform the biogeographic history of the Hengduan Mountains. Our primary aim was to discover the differences in tempo and mode of biotic assembly that help account for the remarkable diversity of the Hengduan flora.
Results
Contrasting Histories of Floristic Assembly.
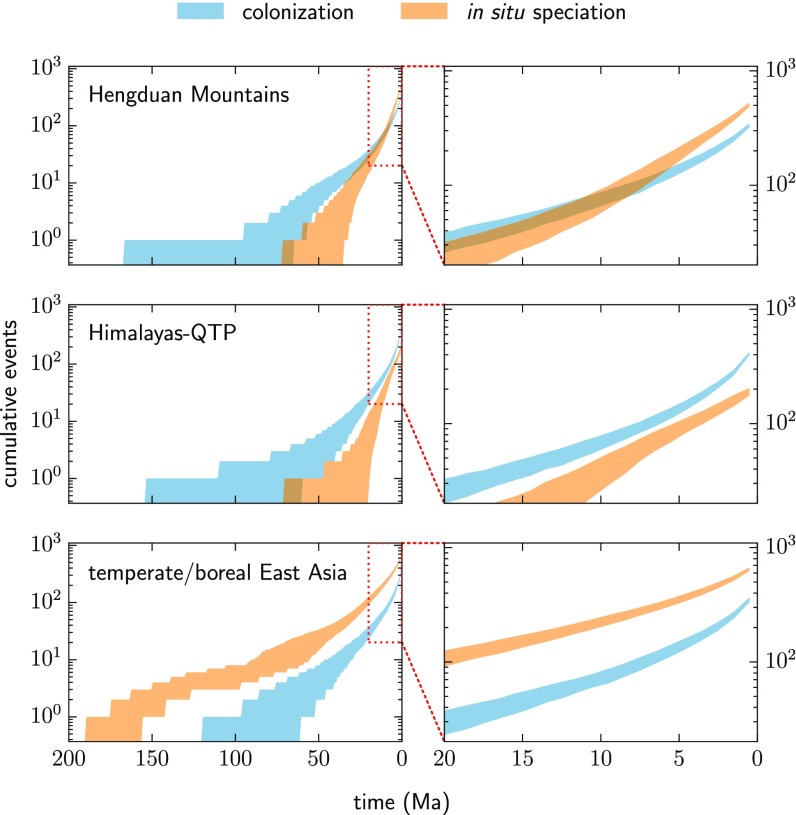
Reconstructed biogeographic histories of the selected clades reveal distinct patterns of floristic assembly across regions when plotted as the cumulative number of colonization and in situ speciation events through time (Fig. 2). We focus here on contrasting the Hengduan Mountains region with a combined Himalayas–QTP region to the west and temperate/boreal East Asia to the east (Delimitation of Biogeographic Regions). Given geological and paleontological evidence that the Hengduan Mountains are younger than the Himalayas–QTP, we expected to find that its flora was assembled more recently. Instead, we found surprisingly deep phylogenetic histories of Hengduan occupancy, with initial assembly of its flora predating that of the Himalayas–QTP. In more than 95% of the pseudoreplicated joint histories the earliest colonization event occurs by 64 Ma for the Hengduan Mountains and by 59 Ma for the Himalayas–QTP; in situ speciation begins later, starting by 34 Ma and 19 Ma, respectively. By contrast, in temperate/boreal East Asia, which was commonly reconstructed as the root ancestral area for clades, in situ speciation initially occurs by 155 Ma and colonization initially occurs by 60 Ma.
Fig. 2.
Assembly of regional floras by colonization and in situ speciation events in 19 plant clades, inferred from ancestral-range reconstructions on time-calibrated molecular phylogenies. Shaded regions indicate the 5 to 95% quantile intervals for the cumulative number of events through time from 500 pseudoreplicated joint biogeographic histories designed to account for phylogenetic uncertainty (discussed in the text). Panels on the right focus on the last 20 Ma, in which differences in regional assembly are most apparent. In the Hengduan Mountains region, cumulative in situ speciation overtakes colonization about 8 Ma, whereas for the Himalayas–QTP colonization remains the dominant process. In situ speciation thus seems to have played a disproportionately large role in assembling the Hengduan Mountains flora since the late Miocene compared with the Himalayas–QTP, consistent with the theory of uplift-driven diversification in the Hengduan Mountains region.
For all regions, species accumulation by each process is approximately exponential (log-linear) following initiation until about the last 8 Ma. During this earlier “constant” phase, assembly in both the Hengduan Mountains and Himalayas–QTP is dominated by colonization, whereas in temperate/boreal East Asia the dominant process is in situ speciation. Following this phase, the assembly dynamics of the Hengduan Mountains and Himalayas diverge considerably, with relatively little change in temperate/boreal East Asia. In the Hengduan Mountains, the cumulative number of in situ speciation events overtakes that of colonization around 8 Ma, and by the present the median number is 508 versus 335. In the Himalayas–QTP in situ speciation never overtakes colonization, and by the present the median number of events is 192 versus 411. The relative contribution of in situ speciation to floristic assembly is thus for the Hengduan Mountains, and for the Himalayas–QTP. In other words, in situ speciation has contributed almost twice as much to the assembly of the Hengduan Mountains flora as it has to the Himalayas–QTP flora, especially since the late Miocene.
Regional Rates of in Situ Speciation and Dispersal Through Time.
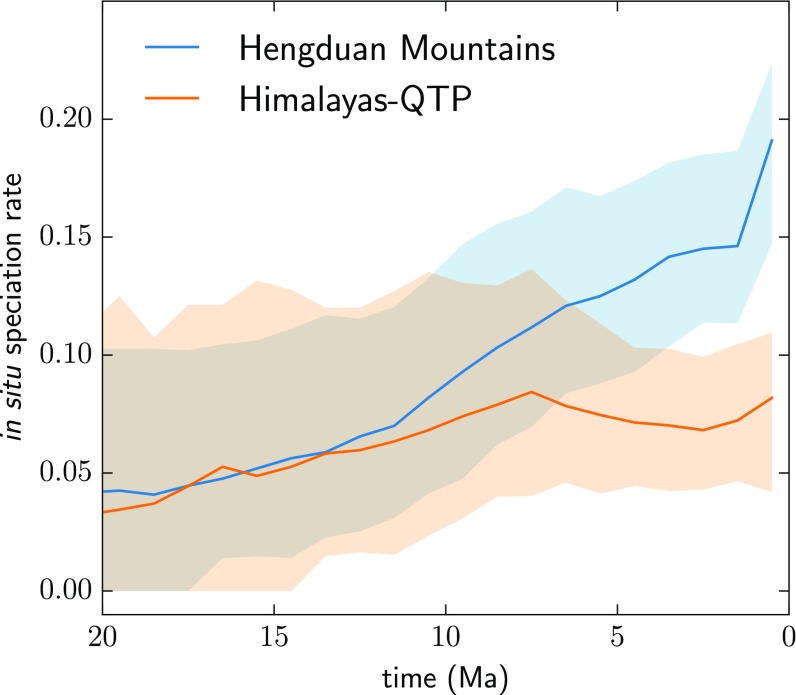
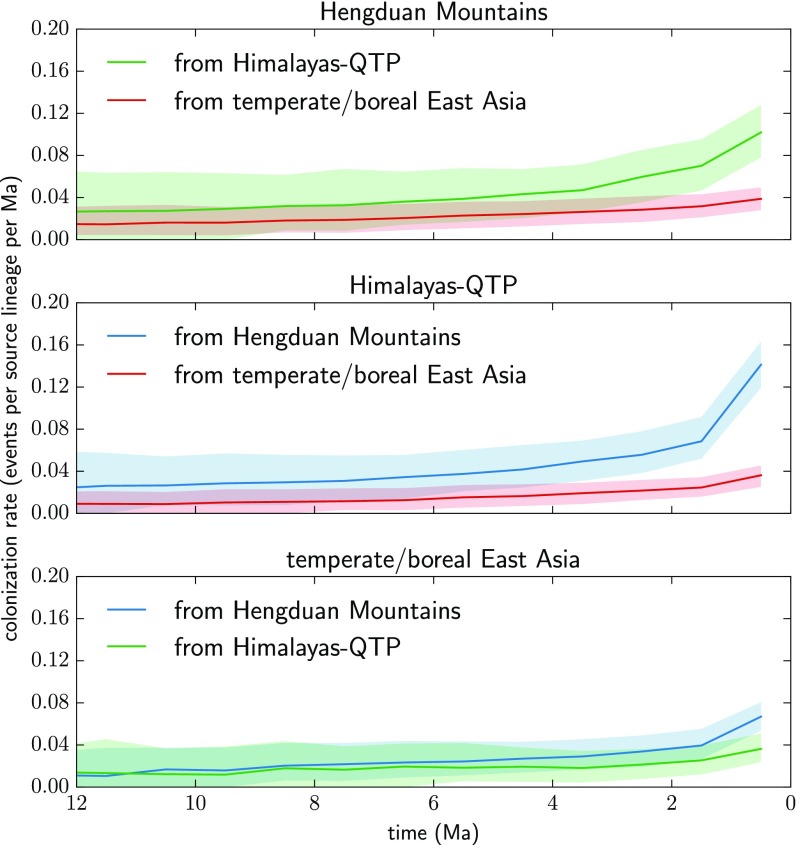
The rate of in situ speciation for the Hengduan Mountains region, in events per resident lineage per million years, increased almost twofold over the past 10 Ma, whereas for the Himalayas–QTP, it remained more or less constant (Fig. 3). Dispersal rates between the Hengduan Mountains and Himalayas–QTP increase gradually over the last 4 to 5 Ma, and sharply in the last 1 Ma. By contrast, dispersal from each region to temperate/boreal East Asia increases only slightly in the same time period (Fig. 4).
Fig. 3.
Rolling estimates of in situ speciation rates through time for the Hengduan Mountains and Himalayas–QTP regions from inferred biogeographic histories of 19 plant clades. Lines indicate medians and shaded areas indicate 5 to 95% quantile intervals from 500 pseudoreplicated joint histories designed to account for phylogenetic uncertainty (discussed in the text). Regional rates begin to diverge about 8 Ma, with the Hengduan Mountains showing a striking increase in in situ speciation relative to the Himalayas–QTP.
Fig. 4.
Rolling estimates of colonization rates through time for the Hengduan Mountains, Himalayas–QTP, and temperate/boreal East Asia regions from inferred biogeographic histories of 19 plant clades. Lines indicate medians and shaded areas indicate 5 to 95% quantile intervals from 500 pseudoreplicated joint histories designed to account for phylogenetic uncertainty (discussed in the text). Dispersal between the Hengduan Mountains and Himalayas–QTP increases in the last 2 Ma relative to dispersal between either region and temperate/boreal East Asia.
Shifts in Diversification Rate.
Seven out of the 19 clades showed strong evidence (Bayes factor >15) for one or more shifts in diversification regime (Table 1). However, inspection of branch-specific evidence (the cumulative probability of occurring in the credible set of shift configurations, and the marginal odds ratio in favor of a shift) in light of ancestral-range reconstructions does not reveal any clear geographic patterns in the phylogenetic locations of regime shifts (SI Appendix, Fig. S2). That is, across clades, macroevolutionary jumps in diversification rate are not obviously associated with the colonization or occupancy of any particular region.
Table 1.
Bayes factor support for shifts in diversification in sampled clades, relative to the null hypothesis of no shifts
| No. of regime shifts | |||||||||||
| Clade | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
| Acer | 31.76 | 71.62 | 26.07 | 5.59 | 1.05 | 0.12 | |||||
| Allium | 14.24 | 182.48 | 1,048.16 | 3,836.48 | 6,668.16 | 7,038.72 | 5,401.60 | 3,015.68 | 1,382.40 | 409.60 | 163.84 |
| Clematidinae + Anemoninae | 6.74 | 6.82 | 4.10 | 1.72 | 0.44 | 0.16 | 0.05 | ||||
| Cyananthus | 0.96 | 0.52 | 0.18 | 0.06 | 0.02 | 0.00 | |||||
| Delphineae | 377.43 | 5,871.71 | 6,889.71 | 4,316.57 | 1,872.00 | 566.86 | 137.14 | 36.57 | |||
| Isodon | 8.18 | 8.60 | 5.23 | 2.23 | 0.94 | 0.19 | 0.06 | ||||
| Ligularia–Cremanthodium– | |||||||||||
| Parasenecio complex | 1.64 | 1.41 | 0.80 | 0.32 | 0.12 | 0.04 | 0.01 | ||||
| Lilium + Nomocharis | 0.17 | 0.041 | 0.01 | ||||||||
| Meconopsis | 0.77 | 0.32 | 0.10 | 0.02 | 0.01 | ||||||
| Microsoroideae | 3.83 | 4.37 | 2.79 | 1.19 | 0.38 | 0.11 | 0.07 | ||||
| Pinaceae | 40.78 | 29.36 | 10.15 | 2.17 | 0.34 | 0.05 | |||||
| Polygoneae | 550.12 | 1,841.18 | 7,241.41 | 8,086.59 | 5,225.41 | 2,304.00 | 783.06 | 165.65 | |||
| Primulaceae | 2.13 | 2.18 | 1.54 | 0.77 | 0.36 | 0.09 | 0.06 | 0.02 | |||
| Rhodiola | 1.94 | 1.92 | 1.33 | 0.71 | 0.26 | 0.08 | 0.02 | ||||
| Rhododendron | 17.76 | 87.97 | 247.00 | 289.51 | 215.75 | 111.64 | 40.47 | 9.76 | 2.30 | ||
| Rosa | 1.31 | 0.90 | 0.42 | 0.18 | 0.03 | 0.01 | |||||
| Saussurea | 7.30 | 20.21 | 21.68 | 13.56 | 6.26 | 2.01 | 0.35 | ||||
| Saxifragaceae + Grossulariaceae | 1.91 | 1.52 | 0.72 | 0.26 | 0.04 | 0.00 | |||||
| Thalictrum | 1.57 | 1.25 | 0.67 | 0.28 | 0.10 | 0.02 | 0.01 | ||||
Strong support (value > 15) is indicated in bold.
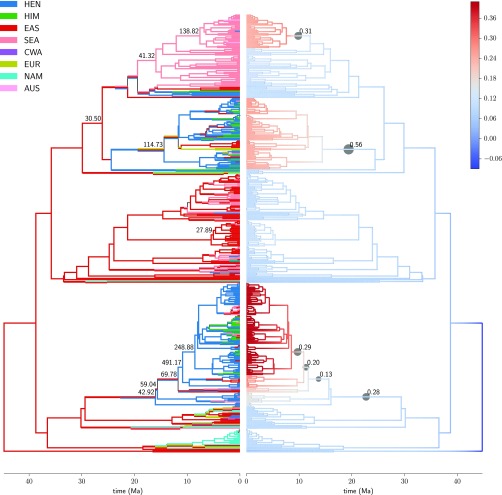
A notable exception to this general pattern is Rhododendron, in which results from BAMM (Bayesian Analysis of Macroevolutionary Mixtures) and Lagrange suggest that in a window of about 9 to 15 Ma net diversification increased independently in two clades that each originated in the Hengduan region and are currently dominated by Hengduan species (Fig. 5). In Saussurea, an increase in net diversification is inferred about 1.7 Ma along the stem of a branch containing most of the clade’s Hengduan species (SI Appendix, Fig. S2O). Similarly, in Delphineae, two separate increases are inferred in clades that are ancestrally Hengduan and together hold most of the Hengduan species; however, the times of these rate shifts (about 28 Ma and 37 Ma, respectively) predate the uplift of the Hengduan Mountains (SI Appendix, Fig. S2D). Finally, in Isodon and the Ligularia–Cremanthodium–Parasenecio complex, branch-specific measures show one increase in net diversification for each clade in the context of Hengduan ancestry about 7 and 5 Ma, respectively, but in both cases models with regime shifts are not supported by Bayes factors, and the Hengduan Mountains region is reconstructed as ancestral across most of the phylogeny, rendering the geographic context of the shift less informative.
Fig. 5.
Reconstructions of ancestral geographic range (Left) and net diversification rate (Right) on the maximum clade credibility tree, with branch lengths set to posterior means, for Rhododendron. Ancestral ranges are maximum-likelihood estimates at the start and end of each branch. Net diversification values are branch-segment means of the posterior distribution estimated by BAMM. Filled circles on the right indicate branches that appear in the 95% credible set of distinct shift configurations, with the size and label of a circle indicating the cumulative probability of the branch over all configurations in the credible set. On the left, the marginal odds ratio for a shift in diversification regime along a branch is drawn for branches where the ratio exceeds 20. Geographic regions are coded as follows: AUS, Australasia; CWA, central/western Asia; EAS, temperate-boreal East Asia; EUR, Europe; HEN, Hengduan Mountains; HIM, Himalayas–QTP; NAM, North America; and SEA, Southeast Asia. Hengduan species cluster primarily in two clades, both of which show evidence of ancestral shifts to higher diversification rate in the mid-to-late Miocene.
Discussion
Our analysis quantifies the relative contributions of in situ lineage diversification and colonization to the assembly of one of the world’s richest temperate floras, that of the Hengduan Mountains. We expected regional differences to reflect contrasting times of orogeny, in particular the prediction of the uplift-driven diversification hypothesis that in situ speciation increases with mountain-building activity. In this context, the late Miocene emerges as an important reference point, because previous studies of geology and paleontology indicate that the Hengduan Mountains achieved their current height only after this time, whereas the Himalayas and central QTP did so before (4, 13, 15, 17, 21). Here, our phylogenetic inferences, which make no prior assumptions about the timing of geological events, show that after about 8 Ma the rate of in situ diversification increased in the Hengduan Mountains (Fig. 3), yielding a remarkable inflection point at which cumulative speciation overtakes colonization (Fig. 2). This indicates that the Hengduan Mountains flora has been assembled disproportionately by recent in situ diversification that coincides temporally with independent estimates of orogeny.
Rapid Himalayan orogeny during the early to middle Miocene (15, 21, 28) would predict a similar pulse of uplift-driven diversification, but none is evident whether the Himalayas are treated as part of the QTP (Fig. 3) or separately (SI Appendix, Fig. S4). It is possible that the Himalayas were uplifted more gradually; alternatively, the signal of such a pulse could be masked by the greater uncertainty associated with estimating clade ages and ancestral ranges in deeper time, and/or subsequent extinction and turnover in the affected lineages.
In a recent review, Renner (13) argued that numerous phylogenetic studies have misinterpreted the Earth sciences literature and incorrectly attributed relatively young (Miocene and later) estimates of lineage divergence to QTP uplift, and recommended that “Biogeographical discussions should also keep in mind expected differences in the biota of the TP and those in the Himalayas, the Kunlun range to the N, the Pamir and Karakoram to the W, and Hengduan Mountains to the E” (ref. 13, p. 7). Here, our focus on distinguishing the younger and more species-rich Hengduan Mountains region from the Himalayas–QTP is in the spirit of that advice, and our results suggest that at least some of the referenced cases of diversification could be more accurately interpreted as coincident with orogeny of the Hengduan region, not the older QTP. Indeed, several of the studies cited in Renner (13) are the original sources of molecular data for the clades analyzed here and include many Hengduan species. It thus seems important to emphasize that, despite precedent in the literature for considering the Hengduan Mountains to be simply part of a greater biogeographic region that includes the Himalayas and QTP (e.g., refs. 26, 27, 29, and 30), the Hengduan region actually has a very distinct history of assembly that reflects its younger age.
Why did in situ diversification in the Hengduan Mountains increase since the late Miocene, and, more specifically, to what extent was it driven by orogeny? Further studies are needed. One might look for contrasts between the Hengduan Mountains and adjacent regions in the evolution of ecological traits and environmental tolerances (e.g., ref. 31) across multiple clades. These may reveal, for example, the extent to which speciation within a region is associated with adaptive divergence and niche-filling, as opposed to nonadaptive processes such as genetic isolation and drift arising from dispersal limitation and topographic effects (vicariance, emergence of sky islands, etc.). These analyses would require denser and more fine-grained taxonomic and geographic sampling than was possible here, in addition to the requisite trait data. However, we suspect that evidence for common processes will prove elusive, because the proximate causes of diversification are likely to be idiosyncratic and involve a variety of contingencies and associated variables (e.g., refs. 32 and 33). For many clades, factors unrelated to uplift per se, such as biotic interactions, are almost certain to have played important roles (3, 13, 34). For example, in Pedicularis (Orobanchaceae) diversification of the many Hengduan species may have been facilitated by recurrent divergence in floral traits associated with pollinator sharing and reproductive interference (e.g., ref. 35), in combination with other mechanisms promoting population isolation, including uplift and associated aspects of landscape and resource heterogeneity.
A coarser-grained view is that diversification in the Hengduan region reflects a macroevolutionary response to the rapid expansion of moist temperate montane and alpine habitats in the late Miocene. At this level the effects of orogeny and climate change are not clearly separable, because they concurrently and jointly set a stage of ecological/evolutionary opportunity. During the early to middle Miocene, the southern QTP had already achieved its current elevation (15) and was covered by coniferous and deciduous-leaved forests (36, 37). As Hengduan uplift proceeded, falling temperatures would have driven the treeline lower, increasing the extent of shrublands, meadows, and scree slopes—habitats where most of the species in our dataset occur and would have diversified. Similar changes would also have occurred in the Himalayas and QTP, but in comparison with those areas moisture in the Hengduan region would have been abundant, because by then the summer monsoon was established, and the central QTP and Central Asian interior had aridified (13). This predicts that carrying capacities—and by extension, diversification potential—of the Hengduan region’s newly formed habitats were enhanced by greater summer rainfall.
Although not a driver of diversification per se, it is important to consider the effects of glaciation and climatic oscillations during the Pleistocene, and the possibility that our results can be explained, at least in part, by regional differences in extinction caused by these factors. In the Hengduan region, the north–south orientation of major valleys suggests that extinction was relatively low, because species would have been able to persist by migrating relatively unhindered toward southern ice-free areas during glacial cycles; as a result, the region’s history of in situ diversification would be better preserved in time-calibrated phylogenies, simply because fewer branches would have been “pruned” by extinction. By contrast, in the Himalayas–QTP, the east–west orientation of valleys would have inhibited southern displacement, leading to greater extinction. In this case, the pruning effect of extinction would yield lower estimates of in situ diversification during the Pleistocene.
This pattern is probably not due solely to differences in glacial extent. Numerous studies of species distributions (e.g., refs. 38 and 39) and phylogeography (e.g., refs. 40–43) support the idea that refugia occurred along the southeastern margin of the QTP as well as in areas further west and north, even at high elevation (e.g., refs. 44–46). This aligns with geological evidence that ice-free areas were present across the QTP and adjacent mountains throughout the Quaternary (47). It may be that greater Pleistocene extinction in the Himalayas–QTP compared with the Hengduan region was driven as much (or more) by aridification as glaciation, because the former experienced reduced summer monsoon strength during interglacials (48).
These effects may be reflected in temporal patterns of dispersal between the Hengduan Mountains and Himalayas–QTP. The older age of the latter predicts that its flora should have been an early source of lineage dispersal to the younger Hengduan Mountains. However, we do not find any marked asymmetry in dispersal between these regions through the end of the Miocene; instead, rates in both directions are relatively low and increase only gradually until about 2 to 3 Ma, when both increase more sharply, but more so from the Hengduan Mountains to the Himalayas–QTP (Fig. 4). That is, in the Quaternary, the Hengduan Mountains seem to have acted as a biogeographic source, from which lineages buffered from extinction colonized the relatively depauperate regions to the west and north.
Other than plants, the group that has received the most biogeographic scrutiny in relation to the QTP and adjacent regions is birds, which present similar patterns (49), notably that in the species-rich Hengduan Mountains region and adjacent forests of SE Asia, in situ diversification during and subsequent to the Miocene led to repeated colonization of the Himalayas and QTP (e.g., refs. 31, 50, and 51). In the Himalayas, the lack of in situ diversification of songbirds has been attributed to the filling of niches by preadapted colonizing lineages (52).
Among studies inferring biodiversity hotspot assembly from multiple clades, quantitative analyses of in situ diversification and/or colonization are still rare, and ours estimates rates of both processes through time and across regions. This comparative framework has allowed us to characterize, at a very basic level, changes in the tempo (rate) and mode (process) of biotic assembly in relation to independently inferred timelines of regional orogeny and climate change. By contrast, studies of other hotspots have tended to focus on single regions or biomes, and subsets of these dimensions (time, space, tempo, and mode). For example, the high species diversity in the California Floristic Province is associated with lower rates of extinction more than elevated speciation or immigration, using models in which rates of lineage birth, death, and movement are dependent on geographic occupancy but not time (53). Assembly of the Cerrado biome in Brazil has been characterized by recent (late Miocene onward) in situ adaptation of lineages to fire resistance (54), with some clades, especially in the campos rupestres highlands, showing evidence of endemic radiation [e.g., Mimosa (54, 55), Calliandra (56), and Chamaecrista (57)]. In the Páramo biome of the Andes, analyses of net diversification (but not immigration) yielded the highest average rate compared with eight other hotspots and appear driven more by Pleistocene climate oscillations than orogeny (58). Clearly, for the Hengduan Mountains, much remains to be studied about how patterns of assembly through time break down at a finer scale of particular biomes and habitats within the region.
The Andes, perhaps the world’s richest mountain hotspot (2), are a compelling subject for comparison with the present results. The tropical montane/alpine habitats of the Andes are geographically isolated, whereas the temperate Hengduan region is embedded in a network of high mountains surrounding the QTP and proximate to cool high-latitude habitats. It thus stands to reason that dispersal figures prominently in Hengduan biotic assembly, compared with the Andes, in which endemic radiations are the more dominant pattern (e.g., refs. 5, 7, 34, 59, and 60).
Prevalence of dispersal is likely the main reason that we find little evidence of macroevolutionary jumps in diversification rate associated with Hengduan colonization in individual clades. In our phylogenies, clades tend not to be entirely endemic to the Hengduan Mountains or Himalayas–QTP; dispersal is common and reflects the fact that these regions are not defined by differences in biome but share broad physiographic similarities that presumably have facilitated biogeographic exchange. That is, dispersal between these regions should not necessarily require extensive ecophysiological adaptations that may be difficult to evolve (61), countering expectations of infrequent colonization events followed by endemic radiations (cf. ref. 60). For this reason our data are perhaps not well-suited for BAMM, which models diversification shifts as relatively rare events. Moreover, the link between dispersal events and shifts in diversification regime is further obscured by the inherent uncertainty associated with inferring their phylogenetic locations. The most appropriate interpretation may simply be that the signal of higher diversification in the Hengduan region is more or less diffuse across clades and emerges only when their biogeographic histories are considered jointly. This helps explain why previous analyses of single clades have not yielded the pattern inferred here.
The validity of our results hinges on the degree to which the data are representative of the focal regions (Hengduan Mountains and Himalayas–QTP). The clades we selected span a wide taxonomic range, and the species exhibit a diversity of growth forms, life histories, and habitat preferences. Taxon sampling, although incomplete, is not generally biased with respect to the focal regions (SI Appendix, Table S1) and therefore should not unduly affect inferences of the relative rates of assembly processes across regions. We also expect the phylogenetic distribution of unsampled species within each clade to be effectively random, which means that reconstructed phylogenies would tend to be missing more nodes—and thus inferred biogeographic events—closer to the present. In other words, the extent to which we have underestimated the frequencies of those events is likely greater in the Pliocene and Quaternary than in the Miocene and earlier.
Another potential source of error is in the demarcation of discrete areas as required for biogeographic analysis. Although the QTP, Himalayas, and Hengduan Mountains may have broadly distinct orogenic histories, this does not translate into clearly defined borders, and to the extent such borders existed they surely would have shifted through time, leading to ambiguity in the present. Our definitions of these areas (Fig. 1) are thus necessarily somewhat subjective and represent our best attempts to capture the essential elements of this complex system. That being said, our results are robust to the treatment of the Himalayas and QTP as a single biogeographic area; an analyis of recoded species range data treating them as separate areas yielded the same signature of uplift-driven Hengduan diversification (SI Appendix, Additional Analyses of Biotic Assembly and Figs. S3–S5).
Materials and Methods
Clade Selection and Phylogeny Reconstruction.
Our criteria were that each clade (i) included a substantial number of species that occur in the Hengduan Mountains, as well as their closest known relatives in other biogeographic regions, (ii) had sufficient molecular data available to infer a phylogeny that was broadly representative of the clade’s taxonomic diversity and geographic range, and (iii) had fossil data suitable for molecular clock calibration or secondary calibrations inferred from fossil dated phylogenies. We found 19 clades—17 of angiosperms and one each of gymnosperms (Pinaceae) and ferns (Microsorioideae)—that met these criteria. The combined taxon sample included 4,668 ingroup species, of which 930 occur in the Hengduan Mountains region, 703 in the Himalayas–QTP, and 1,045 in the remainder of temperate/boreal East Asia (Delimitation of Biogeographic Regions). About 370 species are shared between the Hengduan Mountains and Himalayas–QTP regions. Across clades, the proportion of species sampled ranged from 20 to 97% globally and 26 to 94% for the Hengduan Mountains region (SI Appendix, Table S1).
For each clade, we assembled molecular sequence alignments (Dataset S1) and fossil calibration data and used relaxed molecular clock models implemented in BEAST (Bayesian Evolutionary Analysis Sampling Trees) (62, 63) to generate a Bayesian posterior sample of time-calibrated phylogenies and the associated maximum-clade-credibility tree (SI Appendix).
Inference of Range Evolution and Lineage Diversification.
Delimitation of biogeographic regions.
We delimited 11 biogeographic regions (SI Appendix, Fig. S1) and scored the range of each sampled species as its presence or absence in these regions based on floras, online databases, and published datasets (SI Appendix and Dataset S2). Delimitations reflected our need to accommodate the global distributions of the selected clades and the granularity of available species range data, and most importantly to focus on distinguishing the Hengduan Mountains region from adjacent, geologically older parts of the QTP, especially the Himalayas (Fig. 1).
The Hengduan Mountains region, following Boufford (8), is bounded to the west by the Yarlung Tsangpo River in eastern Xizang (Tibet), to the northwest by the high plateau in Qinghai, to the north by the Tao River in southern Gansu, to the east by the Sichuan Basin, and to the south by subtropical forests and the Yunnan–Guizhou Plateau (Fig. 1). We delimited the complementary “Himalayas–QTP” region as including the plateau itself, the Himalayas to the south, the Kunlun Mountains to the north, and the Qilian Mountains to the northeast. The distinct geological histories of the Himalayas and QTP argue against their treatment as a single unit for biogeographic analysis; however, the granularity of available data on species ranges made it difficult to differentiate between them. We attempted to do so, with no effect on outcomes (SI Appendix, Additional Analyses of Biotic Assembly and Figs. S3–S5), but we are most confident with analyses based on the combined delimitation, which effectively serves the purpose of contrasting with the Hengduan region.
The other regions we delimited are temperate/boreal East Asia (the eastern boreal part of Russia and temperate regions of East Asia, including Japan and Taiwan), central/western Asia (including the Xinjiang Uyghur Autonomous Region to the north of the Kunlun Range), southeast Asia (including tropical regions of China, Malesia, and Papuasia), Australasia (Australia, New Zealand, and the southwestern Pacific), India (south of the Himalyas, including Sri Lanka), Africa, Europe, North America, and South America (south of the Panama Canal).
Ancestral range reconstruction.
We estimated ancestral geographic ranges for all ingroups using a dispersal-extinction-cladogenesis model in Lagrange (64, 65) in which dispersal was allowed based on spatial adjacency (SI Appendix, Fig. S1) and the maximum range size was set to 3. We explicitly avoided placing any temporal constraints on dispersal, so that biogeographic inferences were independent of prior beliefs about the ages of the Hengduan Mountains or Himalayas–QTP regions. For each ingroup we inferred a distribution of biogeographic histories to account for phylogenetic uncertainty. A single history was generated in three steps. First, a chronogram was randomly sampled from the clade’s Bayesian posterior distribution. Second, the maximum-likelihood set of ancestral ranges (geographic speciation scenarios) at internal nodes was estimated using Lagrange. Third, parsimonious sequences of dispersal and local extinction events were randomly interpolated along branches having different ancestral and descendant ranges. This procedure yielded a complete chronology of biogeographic events (i.e., where and when ancestral lineages moved and underwent speciation and local extinction). To account for phylogenetic uncertainty, one history was generated for each of 500 chronograms sampled from the posterior for each clade.
Regional assembly processes through time.
We focused our analysis on the dynamics of the Hengduan Mountains region in comparison with the Himalayas–QTP and temperate East Asia. Our objective was to estimate, for each region, rates of in situ diversification and colonization and their cumulative contributions to biotic assembly through time, while accounting for phylogenetic uncertainy. Taking the biogeographic histories of clades from the ancestral-range analysis, we generated 500 pseudoreplicated joint histories—sets of one history drawn randomly from each clade’s pool without replacement. From each joint history we extracted the chronology of in situ speciation, colonization, and local extinction events affecting the Hengduan Mountains, Himalayas–QTP, and temperate/boreal East Asia, and binned them into 1-My periods. This allowed region-specific estimates of assembly processes through time. We calculated rolling estimates of per capita in situ speciation rates as , where is the number of in situ speciation events inferred in a region in a 1-My period and is the number of inferred lineages in the region in the previous period (the cumulative sum of in situ speciation and colonization minus local extinction). We also calculated rolling per capita rates of colonization between regions as , where is the number of inferred colonization events of area from area . For all estimates, confidence intervals (5 to 95% quantiles) were calculated from the pseudoreplicated joint histories. Our results thus do not condition on any particular tree topologies or branch lengths and reflect the levels of clade support, and confidence in divergence times, provided by the sequence data.
Geography-independent rates of diversification.
To complement the regional-process analysis and to better understand heterogeneity in rates of lineage diversification independently of geography, we generated Bayesian inferences of diversification rate for each clade’s maximum-clade-credibility tree, with posterior mean branch lengths, using BAMM version 2.5 and the BAMMtools R package (66). BAMM uses Markov chain Monte Carlo procedures to jointly estimate the number, parameters, and locations of distinct macroevolutionary regimes (rates of lineage birth and death, possibly time-dependent) on a phylogenetic tree. It can account statistically for incomplete taxon sampling, so we assigned sampling fractions at the finest level of taxonomic resolution possible (SI Appendix). Priors for speciation and extinction were set empirically using the setBAMMpriors function. For all clades smaller than 500 species we set the geometric prior on the expected number of regime shifts to 1, as recommended in the BAMM documentation; for larger clades we also tested a prior of 10 expected shifts. We ran each BAMM Markov chain Monte Carlo analysis for 10 million generations with a sampling frequency of 1/1,000 and assessed convergence by visually inspecting plots of the likelihood trace and calculating the effective sample size after discarding the first 10% of the run as burn-in. For each clade, we calculated Bayes factors for the distinct numbers of regime shifts sampled, marginal odds ratios (relative evidence in favor of a shift) for individual branches, and the 95% credible set of distinct shift configurations.
Supplementary Material
Acknowledgments
We thank D. Boufford and three anonymous reviewers for valuable comments on previous drafts. This work was supported by a Boyd Postdoctoral Fellowship at the Field Museum, Swiss Advanced Postdoc.Mobility Fellowship P300P3_158528, the Pioneer Hundred Talents Program of the Chinese Academy of Sciences (Y.X.), and NSF Grant DEB-1119098 (to R.H.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 4275.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1616063114/-/DCSupplemental.
References
- 1.Hoorn C, Mosbrugger V, Mulch A, Antonelli A. Biodiversity from mountain building. Nat Geosci. 2013;6:154–154. [Google Scholar]
- 2.Hughes CE. The tropical Andean plant diversity powerhouse. New Phytol. 2016;210:1152–1154. doi: 10.1111/nph.13958. [DOI] [PubMed] [Google Scholar]
- 3.Wen J, Zhang JQ, Nie ZL, Zhong Y, Sun H. Evolutionary diversifications of plants on the Qinghai-Tibetan Plateau. Front Genet. 2014;5:1–16. doi: 10.3389/fgene.2014.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Favre A, et al. The role of the uplift of the Qinghai-Tibetan Plateau for the evolution of Tibetan biotas. Biol Rev. 2015;90:236–253. doi: 10.1111/brv.12107. [DOI] [PubMed] [Google Scholar]
- 5.Lagomarsino LP, Condamine FL, Antonelli A, Mulch A, Davis CC. The abiotic and biotic drivers of rapid diversification in Andean bellflowers (Campanulaceae) New Phytol. 2016;210:1430–1442. doi: 10.1111/nph.13920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwery O, et al. As old as the mountains: The radiations of the Ericaceae. New Phytol. 2015;207:355–367. doi: 10.1111/nph.13234. [DOI] [PubMed] [Google Scholar]
- 7.Hughes CE, Atchison GW. The ubiquity of alpine plant radiations: From the Andes to the Hengduan Mountains. New Phytol. 2015;207:275–282. doi: 10.1111/nph.13230. [DOI] [PubMed] [Google Scholar]
- 8.Boufford D. Biodiversity hotspot: China’s Hengduan Mountains. Arnoldia. 2014;72:24–35. [Google Scholar]
- 9.Li XW, Li J. A preliminary floristic study on the seed plants from the region of Hengduan Mountain. Yunnan-zhiwu-yanjiu. 1993;15:217–231. [Google Scholar]
- 10.Wu ZY. Hengduan mountain flora and her significance. J Jpn Bot. 1988;63:297–311. [Google Scholar]
- 11.Wang C, et al. Outward-growth of the Tibetan Plateau during the Cenozoic: A review. Tectonophysics. 2014;621:1–43. [Google Scholar]
- 12.Deng T, Ding L. Paleoaltimetry reconstructions of the Tibetan Plateau: Progress and contradictions. Natl Sci Rev. 2015;2:417–437. [Google Scholar]
- 13.Renner SS. Available data point to a 4-km-high Tibetan Plateau by 40 Ma but 100 molecular-clock papers have linked supposed recent uplift to young node ages. J Biogeogr. 2016;43:1479–1487. [Google Scholar]
- 14.Rowley DB, Currie BS. Palaeo-altimetry of the late Eocene to Miocene Lunpola basin, central Tibet. Nature. 2006;439:677–681. doi: 10.1038/nature04506. [DOI] [PubMed] [Google Scholar]
- 15.Spicer RA, et al. Constant elevation of southern Tibet over the past 15 million years. Nature. 2003;421:622–624. doi: 10.1038/nature01356. [DOI] [PubMed] [Google Scholar]
- 16.Fang X, et al. Magnetostratigraphy of the late Cenozoic Laojunmiao anticline in the northern Qilian Mountains and its implications for the northern Tibetan Plateau uplift. Sci China Ser D. 2005;48:1040–1051. [Google Scholar]
- 17.Wang Y, et al. Cenozoic uplift of the Tibetan Plateau: Evidence from the tectonic–sedimentary evolution of the western Qaidam Basin. Geosci Front. 2012;3:175–187. [Google Scholar]
- 18.Kirby E, et al. Late Cenozoic evolution of the eastern margin of the Tibetan Plateau: Inferences from 40ar/39ar and (U-Th)/He thermochronology. Tectonics. 2002;21:1–20. [Google Scholar]
- 19.Clark MK, et al. Late Cenozoic uplift of southeastern Tibet. Geology. 2005;33:525–528. [Google Scholar]
- 20.Wang E, et al. Two-phase growth of high topography in eastern Tibet during the Cenozoic. Nat Geosci. 2012;5:640–645. [Google Scholar]
- 21.Wang P, et al. Tectonic control of Yarlung Tsangpo Gorge revealed by a buried canyon in Southern Tibet. Science. 2014;346:978–981. doi: 10.1126/science.1259041. [DOI] [PubMed] [Google Scholar]
- 22.Meng K, Wang E, Wang G. Uplift of the Emei Shan, western Sichuan Basin: Implication for eastward propagation of the Tibetan Plateau in Early Miocene. J Asian Earth Sci. 2016;115:29–39. [Google Scholar]
- 23.Sun BN, et al. Reconstructing Neogene vegetation and climates to infer tectonic uplift in western Yunnan, China. Palaeogeogr, Palaeoclimatol, Palaeoecol. 2011;304:328–336. [Google Scholar]
- 24.Liu JQ, Wang YJ, Wang AL, Hideaki O, Abbott RJ. Radiation and diversification within the Ligularia–Cremanthodium–Parasenecio complex (Asteraceae) triggered by uplift of the Qinghai-Tibetan Plateau. Mol Phylogenet Evol. 2006;38:31–49. doi: 10.1016/j.ympev.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Susanna A, von Raab-Straube E, Milne R, Liu J. Island-like radiation of Saussurea (Asteraceae: Cardueae) triggered by uplifts of the Qinghai–Tibetan Plateau. Biol J Linn Soc. 2009;97:893–903. [Google Scholar]
- 26.Zhang JQ, Meng SY, Allen GA, Wen J, Rao GY. Rapid radiation and dispersal out of the Qinghai-Tibetan Plateau of an alpine plant lineage Rhodiola (Crassulaceae) Mol Phylogenet Evol. 2014;77:147–158. doi: 10.1016/j.ympev.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 27.Gao YD, Harris AJ, Zhou SD, He XJ. Evolutionary events in Lilium (including Nomocharis, Liliaceae) are temporally correlated with orogenies of the Q–T plateau and the Hengduan Mountains. Mol Phylogenet Evol. 2013;68:443–460. doi: 10.1016/j.ympev.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 28.Searle MP. Geological evolution of the Karakoram Ranges. Ital J Geosci. 2011;130:147–159. [Google Scholar]
- 29.Nie ZL, et al. Molecular phylogeny of Anaphalis (Asteraceae, Gnaphalieae) with biogeographic implications in the Northern Hemisphere. J Plant Res. 2013;126:17–32. doi: 10.1007/s10265-012-0506-6. [DOI] [PubMed] [Google Scholar]
- 30.Matuszak S, Muellner-Riehl AN, Sun H, Favre A. Dispersal routes between biodiversity hotspots in Asia: The case of the mountain genus Tripterospermum (Gentianinae, Gentianaceae) and its close relatives. J Biogeogr. 2016;43:580–590. [Google Scholar]
- 31.Liu Y, et al. Sino-Himalayan mountains act as cradles of diversity and immigration centres in the diversification of parrotbills (Paradoxornithidae) J Biogeogr. 2016;43:1488–1501. [Google Scholar]
- 32.Donoghue MJ, Sanderson MJ. Confluence, synnovation, and depauperons in plant diversification. New Phytol. 2015;207:260–274. doi: 10.1111/nph.13367. [DOI] [PubMed] [Google Scholar]
- 33.Bouchenak-Khelladi Y, Onstein RE, Xing Y, Schwery O, Linder HP. On the complexity of triggering evolutionary radiations. New Phytol. 2015;207:313–326. doi: 10.1111/nph.13331. [DOI] [PubMed] [Google Scholar]
- 34.Luebert F, Weigend M. Phylogenetic insights into Andean plant diversification. Evol Popul Genet. 2014;2:27. [Google Scholar]
- 35.Eaton DAR, Fenster CB, Hereford J, Huang SQ, Ree RH. Floral diversity and community structure in Pedicularis (Orobanchaceae) Ecology. 2012;93:S182–S194. [Google Scholar]
- 36.Sun J, et al. Palynological evidence for the latest Oligocene-early Miocene paleoelevation estimate in the Lunpola Basin, central Tibet. Palaeogeogr, Palaeoclimatol, Palaeoecol. 2014;399:21–30. [Google Scholar]
- 37.Li H, Guo S. The Miocene flora Namling of Xizang. Gu sheng wu xue bao. 1976;15:7–17. [Google Scholar]
- 38.Srinivasan U, Tamma K, Ramakrishnan U. Past climate and species ecology drive nested species richness patterns along an east-west axis in the Himalaya: Nestedness in Himalayan fauna. Global Ecol Biogeogr. 2014;23:52–60. [Google Scholar]
- 39.López-Pujol J, Zhang FM, Sun HQ, Ying TS, Ge S. Centres of plant endemism in China: Places for survival or for speciation? J Biogeogr. 2011;38:1267–1280. [Google Scholar]
- 40.Cun YZ, Wang XQ. Plant recolonization in the Himalaya from the southeastern Qinghai-Tibetan Plateau: Geographical isolation contributed to high population differentiation. Mol Phylogenet Evol. 2010;56:972–982. doi: 10.1016/j.ympev.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 41.Wang B, Mao JF, Gao J, Zhao W, Wang XR. Colonization of the Tibetan Plateau by the homoploid hybrid pine Pinus densata. Mol Evol. 2011;20:3796–3811. doi: 10.1111/j.1365-294X.2011.05157.x. [DOI] [PubMed] [Google Scholar]
- 42.Lei F, Qu Y, Song G. Species diversification and phylogeographical patterns of birds in response to the uplift of the Qinghai-Tibet Plateau and Quaternary glaciations. Curr Zool. 2014;60:149–161. [Google Scholar]
- 43.Meng L, et al. Refugial isolation and range expansions drive the genetic structure of Oxyria sinensis (Polygonaceae) in the Himalaya-Hengduan Mountains. Sci Rep. 2015;5:10396. doi: 10.1038/srep10396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang L, et al. History and evolution of alpine plants endemic to the Qinghai-Tibetan Plateau: Aconitum gymnandrum (Ranunculaceae) Mol Evol. 2009;18:709–721. doi: 10.1111/j.1365-294X.2008.04055.x. [DOI] [PubMed] [Google Scholar]
- 45.Sun YS, Ikeda H, Wang YJ, Liu JQ. Phylogeography of Potentilla fruticosa (Rosaceae) in the Qinghai-Tibetan Plateau revisited: A reappraisal and new insights. Plant Ecol Divers. 2010;3:249–257. [Google Scholar]
- 46.Opgenoorth L, et al. Tree endurance on the Tibetan Plateau marks the world’s highest known tree line of the Last Glacial Maximum. New Phytol. 2010;185:332–342. doi: 10.1111/j.1469-8137.2009.03007.x. [DOI] [PubMed] [Google Scholar]
- 47.Owen LA, Dortch JM. Nature and timing of Quaternary glaciation in the Himalayan–Tibetan orogen. Quaternary Sci Rev. 2014;88:14–54. [Google Scholar]
- 48.Owen LA, Caffee MW, Finkel RC, Seong YB. Quaternary glaciation of the Himalayan–Tibetan orogen. J Quaternary Sci. 2008;23:513–531. [Google Scholar]
- 49.Päckert M, Martens J, Sun YH, Tietze DT. Evolutionary history of passerine birds (Aves: Passeriformes) from the Qinghai–Tibetan plateau: From a pre-Quarternary perspective to an integrative biodiversity assessment. J Ornithol. 2015;156:355–365. [Google Scholar]
- 50.Tietze DT, Päckert M, Martens J, Lehmann H, Sun YH. Complete phylogeny and historical biogeography of true rosefinches (Aves: Carpodacus) Zool J Linn Soc. 2013;169:215–234. [Google Scholar]
- 51.Johansson US, et al. Build-up of the Himalayan avifauna through immigration: A biogeographical analysis of the Phylloscopus and Seicercus warblers. Evolution. 2007;61:324–333. doi: 10.1111/j.1558-5646.2007.00024.x. [DOI] [PubMed] [Google Scholar]
- 52.Price TD, et al. Niche filling slows the diversification of Himalayan songbirds. Nature. 2014;509:222–225. doi: 10.1038/nature13272. [DOI] [PubMed] [Google Scholar]
- 53.Lancaster LT, Kay KM. Origin and diversification of the California flora: Re-examining classic hypotheses with molecular phylogenies. Evolution. 2013;67:1041–1054. doi: 10.1111/evo.12016. [DOI] [PubMed] [Google Scholar]
- 54.Simon MF, et al. Recent assembly of the Cerrado, a neotropical plant diversity hotspot, by in situ evolution of adaptations to fire. Proc Natl Acad Sci USA. 2009;106:20359–20364. doi: 10.1073/pnas.0903410106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koenen EJM, et al. Exploring the tempo of species diversification in legumes. S Afr J Bot. 2013;89:19–30. [Google Scholar]
- 56.de Souza ER, et al. Phylogeny of Calliandra (Leguminosae: Mimosoideae) based on nuclear and plastid molecular markers. Taxon. 2013;62:1200–1219. [Google Scholar]
- 57.Rando JG, et al. Phylogeny of Chamaecrista ser. Coriaceae (Leguminosae) unveils a lineage recently diversified in Brazilian campo rupestre vegetation. Int J Plant Sci. 2015;177:3–17. [Google Scholar]
- 58.Madriñán S, Cortés AJ, Richardson JE. Páramo is the world’s fastest evolving and coolest biodiversity hotspot. Front Genet. 2013;4:192. doi: 10.3389/fgene.2013.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pennington RT, et al. Contrasting plant diversification histories within the Andean biodiversity hotspot. Proc Natl Acad Sci USA. 2010;107:13783–13787. doi: 10.1073/pnas.1001317107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hughes C, Eastwood R. Island radiation on a continental scale: Exceptional rates of plant diversification after uplift of the Andes. Proc Natl Acad Sci USA. 2006;103:10334–10339. doi: 10.1073/pnas.0601928103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Donoghue MJ, Edwards EJ. Biome shifts and niche evolution in plants. Annu Rev Ecol Evol Systemat. 2014;45:547–572. [Google Scholar]
- 62.Drummond AJ, Suchard MA, Xie D, Rambaut A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Molecular Biology And Evolution. 2012;29:1969–1973. doi: 10.1093/molbev/mss075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bouckaert R, et al. BEAST 2: A software platform for Bayesian evolutionary analysis. PLoS Comput Biol. 2014;10:e1003537. doi: 10.1371/journal.pcbi.1003537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ree RH, Moore BR, Webb CO, Donoghue MJ. A likelihood framework for inferring the evolution of geographic range on phylogenetic trees. Evolution. 2005;59:2299–2311. [PubMed] [Google Scholar]
- 65.Ree RH, Smith SA. Maximum likelihood inference of geographic range evolution by dispersal, local extinction, and cladogenesis. Syst Biol. 2008;57:4–14. doi: 10.1080/10635150701883881. [DOI] [PubMed] [Google Scholar]
- 66.Rabosky DL. Automatic detection of key innovations, rate shifts, and diversity-dependence on phylogenetic trees. PloS One. 2014;9:e89543. doi: 10.1371/journal.pone.0089543. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.