Recently, Eklund et al. (1) analyzed clustering methods in standard fMRI packages: AFNI (which we maintain), FSL, and SPM. They claim that (i) false-positive rates (FPRs) in traditional approaches are greatly inflated, questioning the validity of “countless published fMRI studies”; (ii) nonparametric methods produce valid, but slightly conservative, FPRs; (iii) a common flawed assumption is that the spatial autocorrelation function (ACF) of fMRI noise is Gaussian-shaped; and (iv) a 15-y-old bug in AFNI’s 3dClustSim significantly contributed to producing “particularly high” FPRs compared with other software. We repeated simulations from ref. 1 [Beijing_Zang data (2), cf. ref. 3) and comment on each point briefly.
AFNI and 3dClustSim
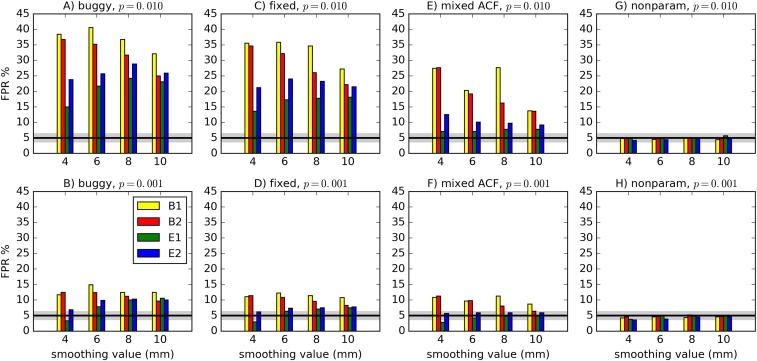
Fig. 1 A–D compares results of the “buggy” and “fixed” 3dClustSim. For each simulation, the typical difference was small: at per-voxel and for . The bug had only a minor impact.
Fig. 1.
FPRs for various software scenarios, with 1,000 two-sample one-sided -tests (as in ref. 1; cf. ref. 3 for more details) using 20 subjects’ data in each sample. For “buggy” (A and B) and “fixed” (C and D), cluster-size thresholds were selected using the Gaussian shape model with the FWHM being the median of the 40 individual subjects’ values: “buggy” via 3dClustSim before the bug fix, “fixed” via 3dClustSim after the bug fix. For “mixed ACF” (E and F), the cluster-size threshold was selected using a non-Gaussian ACF model allowing for heavy tails (3). For “nonparam” (G and H), 3dttest++ was used to perform spatial model-free, nonparametric permutation testing (3); paired, two-sided, and tests with covariates gave similar results. Two different per-voxel P-value thresholds are shown. The black line shows the nominal 5% FPR (out of 1,000 trials), and the gray band shows its binomial 95% confidence interval, 3.65–6.35%. As in ref. 1, different smoothing values were tested (4–10 mm), and four test designs were used: B1 = 10-s block; B2 = 30-s block; E1 = regular event-related; E2 = randomized event-related.
Figures 1 and 2 of ref. 1 actually show similar FPRs for AFNI, FSL-OLS, and SPM: Most tests were in a range of FPR at and FPR at (nor did their famous 70% FPR come from AFNI). The data given in the Results section of ref. 1 simply do not support the statement in the Discussion section that AFNI had “particularly high” FPRs.
Smoothness
To test the effect of assuming a Gaussian ACF in fMRI noise, an empirical “mixed ACF” allowing for longer tails was computed from residuals (3). All FPRs (Fig. 1 E and F) decreased. Block designs remained , likely reflecting dependence of the noise’s spatial smoothness on temporal frequency. Heavy tails in spatial smoothness indeed have significant consequences for clustering.
Nonparametric Approach
A spatial model-free, nonparametric randomization approach was added to AFNI’s group-level GLM program, 3dttest++ (3). All FPRs (Fig. 1 G and H) were within the nominal confidence interval. Although this approach shows promise (as in ref. 1), it may not be feasible to generalize nonparametric permutations to complicated covariate structures and models (e.g., complex ANOVA, analysis of covariance, or linear mixed effects) (4, 5).
Inflated FPRs
Several cases showed significant FPR inflation across existing fMRI software within the testing framework of ref. 1. However, deviations from nominal FPR were not uniformly large and depended strongly on several factors. Fig. 1 and figure 1 of ref. 1 show quite good cluster results for stricter per-voxel P values (which ref. 6 found to be predominantly used in fMRI analyses) and for event-related stimuli (emphasizing the importance of good experimental design): FPR inflation was often (Beijing) or (Cambridge), affecting only clusters with marginally significant volume.
We strongly disagree with Eklund et al.’s (1) summary statement: “Alarmingly, the parametric methods can give a very high degree of false positives (up to , compared with the nominal ).” For comparison, their own nonparametric method’s results actually showed up to 40% FPR. When characterizing results, medians or percentile ranges are generally more informative summary statistics than maxima. Looking backward, the typical ranges show much smaller FPR inflation than what had been highlighted, and looking forward they provide useful suggestions for experimental design and analyses (lower voxelwise , event-related paradigms, etc.). By concentrating on the highest observed FPRs, the conclusions of Eklund et al. (1) are unnecessarily alarmist.
Acknowledgments
This work was supported by the National Institute of Mental Health and National Institute of Neurological Disorders and Stroke Intramural Research Programs ZICMH002888 of the NIH, US Department of Health and Human Services. This work used the computational resources of the NIH High-Performance Computing Biowulf cluster (https://hpc.nih.gov/).
Footnotes
The authors declare no conflict of interest.
References
- 1.Eklund A, Nichols TE, Knutsson H. 2016 Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci USA 113:7900–7905. Erratum in Proc Natl Acad Sci USA 113:E4929. [Google Scholar]
- 2.Biswal BB, et al. Toward discovery science of human brain function. Proc Natl Acad Sci USA. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cox RW, Reynolds RC, Taylor PA. AFNI and clustering: False positive rates redux. bioRxiv. 2016;065862 doi: 10.1101/065862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen G, Adleman NE, Saad ZS, Leibenluft E, Cox RW. Applications of multivariate modeling to neuroimaging group analysis: A comprehensive alternative to univariate general linear model. Neuroimage. 2014;99:571–588. doi: 10.1016/j.neuroimage.2014.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen G, Saad ZS, Britton JC, Pine DS, Cox RW. Linear mixed-effects modeling approach to FMRI group analysis. Neuroimage. 2013;73:176–190. doi: 10.1016/j.neuroimage.2013.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carp J. The secret lives of experiments: Methods reporting in the fMRI literature. Neuroimage. 2012;63:289–300. doi: 10.1016/j.neuroimage.2012.07.004. [DOI] [PubMed] [Google Scholar]