Significance
Neutrophil infiltration into infected tissues is a fundamental process of the innate immune response. Here we found that lipopolysaccharide was a potent stop signal for chemotactic neutrophil migration. Knockout of the ATP receptor (P2X1) in neutrophil-like differentiated HL-60 cells recovered neutrophil chemotaxis. Further observations showed that LPS-induced ATP release through connexin-43 hemichannels was responsible for the activation of the P2X1 and the subsequent calcium influx. Increased intracellular calcium stopped neutrophil chemotaxis by activating MLC through the MLCK-dependent pathway. These data identify a previously unknown function of LPS-induced autocrine ATP signaling in inhibiting neutrophil chemotaxis by enhancing MLC phosphorylation, which provides important evidence that stoppage of neutrophil chemotaxis at infectious foci plays a key role for the defense against invading pathogens.
Keywords: endotoxin, neutrophil, chemotaxis, ATP, myosin light chain
Abstract
Although the neutrophil recruitment cascade during inflammation has been well described, the molecular players that halt neutrophil chemotaxis remain unclear. In this study, we found that lipopolysaccharide (LPS) was a potent stop signal for chemotactic neutrophil migration. Treatment with an antagonist of the ATP receptor (P2X1) in primary human neutrophils or knockout of the P2X1 receptor in neutrophil-like differentiated HL-60 (dHL-60) cells recovered neutrophil chemotaxis. Further observations showed that LPS-induced ATP release through connexin 43 (Cx43) hemichannels was responsible for the activation of the P2X1 receptor and the subsequent calcium influx. Increased intracellular calcium stopped neutrophil chemotaxis by activating myosin light chain (MLC) through the myosin light chain kinase (MLCK)-dependent pathway. Taken together, these data identify a previously unknown function of LPS-induced autocrine ATP signaling in inhibiting neutrophil chemotaxis by enhancing MLC phosphorylation, which provides important evidence that stoppage of neutrophil chemotaxis at infectious foci plays a key role in the defense against invading pathogens.
Neutrophils are the most abundant leukocytes in humans and the first blood cells to arrive at infectious sites as part of the innate cellular immune response. Following transmigration out of the blood vessel, neutrophils migrate through interstitial tissue toward the foci of damage. Then, neutrophil recruitment mobilizes near the pathogenic sites to eliminate pathogens and necrotic tissue. Although the neutrophil recruitment cascade during inflammation has been well described, the molecules that stop neutrophil chemotaxis remain unclear.
Studies in recent decades have confirmed that extracellular ATP, as a purinergic signaling molecule, participates in the pathogenesis of various inflammatory diseases such as transplantation rejection, autoimmune disease, and bacterial infection (1). As a result of ectoapyrases and ectoadenosine triphosphatases (ecto-ATPases), which hydrolyze ATP into adenosine diphosphate (ADP), adenosine monophosphate (AMP), and adenosine (ADO), the concentration of extracellular ATP is maintained at ∼10 nmol/L (2). However, the intracellular ATP concentration in mammalian cells is 5–8 mmol/L. Owing to the 106-fold ATP gradient difference, cell necrosis or activation at inflammatory sites causes a dramatic ATP release (2). By activating the P2X (P2X1–7 subtypes) and P2Y (P2Y1, -2, -4, -6, and -11–14 subtypes) receptor families, extracellular ATP contributes to the regulation of a variety of inflammatory cell responses (3). Given the high concentration of ATP in inflammatory tissues, the effects of extracellular ATP/P2 receptors on neutrophil migration have attracted much attention. ATP released from the leading edge of the neutrophil surface amplifies chemotactic signals and directs cell orientation by feedback through the P2Y2 receptor (4). Knockout of the P2Y2 receptor decreases liver infiltration by neutrophils and subsequent liver damage in mice with acute liver injury (5). The P2X1 receptor is also critical for neutrophil sensing of extracellular ATP, but its role in neutrophil migration during inflammation has not been thoroughly characterized. Lecut et al. reported that the P2X1 receptor protects against lipopolysaccharide (LPS)-induced mice endotoxemia by dampening neutrophil infiltration and activation (6), whereas Maître et al. observed that the P2X1 receptor is required for neutrophil extravasation during LPS-induced mouse endotoxemia (7).
In this study, we set out to elucidate the mechanism underlying the effect of the P2X1 receptor on neutrophil chemotaxis during endotoxin inflammation. Under-agarose chemotaxis analyses show that bacterial LPS generates a strong neutrophil chemotaxis stop signal by activating the P2X1 receptor. Opening of connexin 43 (Cx43) hemichannels on the neutrophil membrane releases extracellular ATP, which activates the P2X1 receptor. Further observations show that, as an ATP-gated cation channel, P2X1 receptor activation leads to a calcium influx and subsequent initiation of the calcium/myosin light chain kinase (MLCK)/myosin light chain (MLC) signaling pathway, which may be the cause of the neutrophil chemotaxis stop signal. Our findings indicate that after sensing bacterial LPS, ATP release and autocrine feedback through the P2X1 receptor provide a signal for neutrophils effectively arriving at the inflammatory site to exert their bactericidal functions.
Results
Bacterial LPS Triggers the Stop Signal of Human Neutrophil Chemotaxis.
Once the neutrophils are in close proximity to the bacterial pathogens, the neutrophils sense a chemotaxis stop signal and eliminate bacteria by phagocytosis, degranulation, and neutrophil extracellular traps. In the microenvironment of a Gram-negative bacterial infection, LPS is released from dead bacteria and acts as an important danger signal to immune cells via Toll-like receptor 4 (TLR4). To evaluate the effects of bacterial LPS on neutrophil chemotaxis, under agarose chemotaxis assays were performed. N-formyl-Met-Leu-Phe (fMLP) and C5a are bacteria-associated end-target chemoattractants, whereas interleukin-8 (IL-8), platelet-activating factor (PAF), and leukotriene B4 (LTB4) are endogenous intermediary chemoattractants (8). We observed that bacterial LPS was a potent termination signal for neutrophil chemotaxis toward both types of chemoattractants (SI Appendix, Fig. S1 A–C and Movies S1 and S2). Considering the distinct molecules involved as different chemoattractants, we used both fMLP and IL-8 as chemoattractants in further investigations. Internalization of membrane chemoattractant receptors was the most studied neutrophil stop cue (9). However, flow cytometry (FCM) analyses displayed that LPS administration increased the membrane fMLP receptor (FPR1) but decreased the membrane IL-8 receptors (CXCR1 and CXCR2) (SI Appendix, Fig. S1 D and E). Therefore, internalization of the chemoattractant receptors seems to be uninvolved in the LPS-induced stop signal. Cell viability is also not involved in the LPS-induced stop signal. The early apoptosis rate (annexin V+/7-AAD−) of LPS-treated neutrophils was lower than control neutrophils, which indicated that the lifespan of LPS-administered neutrophils was prolonged (SI Appendix, Fig. S1 F and G). Nonviable neutrophils (annexin V+/7-AAD+) were not significantly changed by LPS (SI Appendix, Fig. S1 F and G). In addition, we measured the effects of other inflammatory stimuli on neutrophil chemotaxis using the under-agarose chemotaxis assay (SI Appendix, Fig. S1 H and I). IFN-γ and lipoteichoic acid (LTA) exerted no effects on neutrophil chemotaxis. Interleukin-1β (IL-1β) slightly inhibited neutrophil chemotaxis even at a concentration much higher than endogenous inflammatory conditions, which suggested the inhibitory mechanisms of IL-1β were distinct from that of LPS. Tumor necrosis factor-α (TNF-α) was previously reported to stop neutrophil migration, and our result was consistent with that finding (10).
P2X1 Receptor Is Required for the LPS-Induced Neutrophil Stop Signal.
Activation of ATP receptors has been proven to play a crucial role in regulating neutrophil chemotaxis. Junger and colleagues demonstrated that human neutrophils released ATP from the leading edge of the cell surface to amplify chemotactic signals by feedback through P2Y2 receptor (4). They found that extracellular nonhydrolyzable ATP analog adenosine 5′-O-(3-thiotriphosphate) (ATP-γ-S) induced chemokinesis (random cell migration) but not chemotaxis and uniformly added ATP-γ-S impaired chemotaxis to a point source of fMLP, with only 31% of cells migrating along the correct path, which implied that extracellular ATP might also be a negative regulator of neutrophil chemotaxis (4). The molecular mechanisms underlying inhibitory effects of extracellular ATP on neutrophil migration were unknown. Consistent with their reports, we found that neither hydrolyzable ATP nor the nonhydrolyzable ATP-γ-S induced neutrophil chemotaxis using the under-agarose chemotaxis model (SI Appendix, Fig. S1J), and ATP-γ-S inhibited neutrophil chemotaxis (SI Appendix, Fig. S1K) when uniformly added to neutrophils. Moreover, ATP-γ-S promoted the inhibitory effects of LPS on neutrophil chemotaxis (Fig. 1A). But using pan-P2X purinergic antagonists (Evans Blue and iso-PPADS) to block signal transduction of extracellular ATP, we found that neutrophil chemotaxis was significantly recovered either in the presence of LPS alone or in combination with ATP-γ-S (SI Appendix, Fig. S2A). These data suggested that P2X receptor activation was essential for the LPS-induced stop signal. Interestingly, hydrolyzable ATP did not achieve similar results as ATP-γ-S did because neutrophils possess strong ecto-ATPases, and the hydrolysis products AMP and ADO were capable of restoring the LPS-induced neutrophil chemotaxis stop signal (SI Appendix, Fig. S2 B and C).
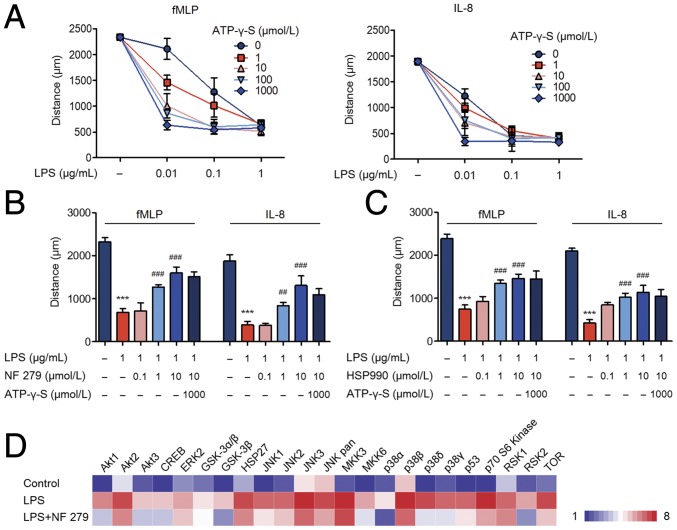
Fig. 1.
The P2X1 receptor is required for the LPS-induced neutrophil stop signal. (A) LPS combined with gradient concentrations of ATP-γ-S was administered to neutrophils. The chemotaxis distances were assayed. (B and C) Neutrophils were preincubated with the P2X1 receptor antagonist (NF 279) or HSP90 inhibitor (HSP990). Subsequently, LPS and ATP-γ-S were introduced, and neutrophil chemotaxis was detected. (D) Neutrophils pretreated with the P2X1 receptor antagonist. Activation of the MAPK and AKT pathways was determined by the phospho-MAPK proteome array. Data are representative of six (A–C) or three (D) independent experiments. Mean and SD are presented. ***P < 0.001, compared with control group; ##P < 0.01 and ###P < 0.001, compared with LPS group (ANOVA with Tukey’s test).
The P2X receptors are members of ATP-gated cation channels, which include the P2X1–P2X7 seven subtypes. Because of the lack of selective antagonists to P2X2, P2X5, and P2X6 receptors, we used P2X1, P2X3, P2X4, and P2X7 receptor antagonists to interfere with LPS stimulation of neutrophils. It turned out that only the P2X1 receptor antagonist blocked the neutrophil stop signal both in the presence and absence of ATP-γ-S (Fig. 1B). Antagonists to the P2X3, P2X4, and P2X7 receptors failed to restore neutrophil chemotaxis (SI Appendix, Fig. S2D). Considering the pivotal role of P2X7 receptor in initiating and maintaining an inflammatory response, we further used P2X7 receptor agonist BzATP to stimulate neutrophils. Chemotaxis toward fMLP or IL-8 was not affected by BzATP (SI Appendix, Fig. S1K), which indicated that P2X7 receptor was not involved. HSP90 was identified as a P2X1 receptor-interacting protein and displayed the ability to reduce desensitization of the P2X1 receptor after activation (11). Inhibition of HSP90 achieved similar results to P2X1 inhibition (Fig. 1C).
Further findings revealed that LPS administration led to the neutrophil exocytosis of secretory vesicles, gelatinase, and specific granules, as demonstrated by a significant increase in the markers CD35, MMP9, and CD63, respectively (SI Appendix, Fig. S2E). Inhibition of the P2X1 receptor completely abolished LPS-induced neutrophil degranulation. Moreover, LPS-enhanced neutrophil phagocytosis was also inhibited by the P2X1 receptor antagonist (SI Appendix, Fig. S2F). The neutrophil MAPK pathways and AKT pathways are both indispensable for neutrophil activation (12, 13). The human phospho-MAPK proteome array showed that the P2X1 receptor antagonist markedly inhibited phosphorylation of the neutrophil MAPK pathways and AKT pathways (Fig. 1D). Therefore, we suggest that the P2X1 receptor is a key regulator that effectively increases the bactericidal functions of neutrophils by inhibiting neutrophil chemotaxis and promoting degranulation and phagocytosis.
ATP Is Released from LPS-Stimulated Neutrophils via Cx43 Hemichannels and Activates P2X1 Receptor in an Autocrine Manner.
Activation of the P2X1 receptor requires extracellular ATP. Unlike well-established mechanisms of platelets releasing ATP through degranulation of dense granule constituents, activated neutrophils release ATP primarily through membrane Cx43 hemichannels and gap junction protein pannexin 1 (Panx1) channels, as described in previous reports (14, 15). The present study demonstrated that LPS promoted neutrophils releasing ATP through the Cx43 hemichannels because the Cx43 inhibitor but not the Panx1 inhibitor decreased ATP release (Fig. 2A). This finding was consistent with the subsequent chemotaxis analyses that the Cx43 inhibitor but not the Panx1 inhibitor restored neutrophil chemotaxis after LPS stimulation (Fig. 2B and SI Appendix, Fig. S3A).
Fig. 2.
ATP is released from LPS-stimulated neutrophils via Cx43 hemichannels and activates the P2X1 receptor in an autocrine manner. (A) Neutrophils were pretreated with a Cx43 inhibitor (Gap19, 500 μmol/L), Panx1 inhibitor (10Panx, 500 μmol/L), or p38 inhibitor (SB 203580, 10 μmol/L). Then, cells were stimulated with LPS (1 μg/mL), and ATP release into the supernatant was determined. (B and C) Cx43 inhibitor- or p38 inhibitor-loaded neutrophils were treated with LPS. Neutrophil chemotaxis was assayed. (D) Neutrophils were loaded with a p38 inhibitor (10 μmol/L) followed by LPS (1 μg/mL). Dephosphorylation of Cx43 was observed by Western blot. (E) Neutrophils were pretreated with a Cx43 inhibitor (500 μmol/L) followed by LPS (1 μg/mL). Distributions of the P2X1 receptor (green) were observed by LSCM after the neutrophils were stimulated for 60 min. Images are representative of 20 cells. (Scale bar, 5 μm.) Data are representative of six (B and C), four (A and D), or three (E) independent experiments. Mean and SD are presented. ***P < 0.001, compared with control group; #P < 0.05 and ###P < 0.001, compared with LPS group (ANOVA with Tukey’s test).
Cx43 is continuously phosphorylated in resting cells. Dephosphorylation of Cx43 opens the channel to release ATP. MAPKs regulate the phosphorylation status and opening of Cx43 (16). By inhibiting one of three key MAPK signaling proteins (ERK1/2, p38, or JNK), we observed that only the p38 inhibitor succeeded in restoring the LPS-induced neutrophil chemotaxis stop signal (Fig. 2C and SI Appendix, Fig. S3 B and C). Western blots also showed that LPS-induced dephosphorylation of Cx43 was inhibited by the p38 inhibitor (Fig. 2D and SI Appendix, Fig. S4). These results suggested that LPS-stimulated neutrophils released ATP via Cx43 hemichannels to stop neutrophil chemotaxis.
MK2 is a downstream molecule of p38 and is critical for neutrophil migration toward fMLP (8). Cells cannot survive when p38 is knocked out, so the MK2-knockout cell model is generally applied for studying neutrophil chemotaxis (8). Considering the importance of the p38/MK2 pathway to neutrophil chemotaxis, we knocked out MK2 with the CRISPR-Cas9 system in neutrophil-like differentiated HL-60 (dHL-60) cells (SI Appendix, Fig. S5A). We observed that knockout of MK2 severely inhibited neutrophil chemotaxis toward fMLP or IL-8 but failed to restore LPS-induced neutrophil stop signal (SI Appendix, Fig. S5B). Next, we further verified the effect of MK2 in LPS-induced human neutrophil chemotaxis stop signal with a MK2 inhibitor. We found that neutrophil chemotaxis toward fMLP or IL-8 was partially inhibited by the MK2 inhibitor (SI Appendix, Fig. S5C). However, neutrophil chemotaxis in LPS-stimulated neutrophil cotreated with the MK2 inhibitor (SI Appendix, Fig. S5C) was unaffected. The results suggested that MK2 played an important role in neutrophil chemotaxis but MK2 might not be involved in the LPS-induced neutrophil stop signal.
Once activated by ATP, the P2X1 receptor is quickly desensitized and translocated from the plasma membrane into the cytoplasm (11). Owing to the presence of autocrine ATP, FCM analysis showed that LPS stimulation reduced the membrane expression of the P2X1 receptor (SI Appendix, Fig. S5D). However, when the Cx43 inhibitor was used, membrane P2X1 receptor levels were unchanged after LPS stimulation. Laser scanning confocal microscopy (LSCM) showed similar results to FCM. Membrane expression of the P2X1 receptor was reduced, but cytoplasm expression of P2X1 was increased after LPS stimulation (Fig. 2E).
LPS-Induced P2X1 Receptor Activation Promotes MLC Phosphorylation.
The P2X1 receptor functions as a ligand-gated cation channel with high calcium permeability. ATP-γ-S administration led to a rapid calcium influx that quickly declined in neutrophils, which was suppressed in the presence of a P2X1 receptor antagonist and completely abolished when using a calcium-free solution and EGTA (Fig. 3A and SI Appendix, Fig. S6A). ATP-γ-S–stimulated calcium influx was transient because once the P2X1 receptor was activated it became desensitized within seconds and recovery usually took 5–6 min (17). A gradually increased and sustained calcium influx was induced by LPS stimulation. EGTA completely abolished LPS-induced calcium influx but P2X1 receptor antagonist or Cx43 inhibitor partially inhibited LPS-induced calcium influx (Fig. 3B and SI Appendix, Fig. S6B). Combined with previous results showing that Cx43 inhibitor completely inhibited ATP release, we speculated that other ATP-independent calcium channels were involved in LPS-induced sustained calcium influx. Members of the transient receptor potential (TRP) family were demonstrated to be expressed in neutrophils and might be potential candidates (18). It was reported that LPS-induced entry of calcium into macrophages depended on TRPM2 by generation of H2O2 (18).
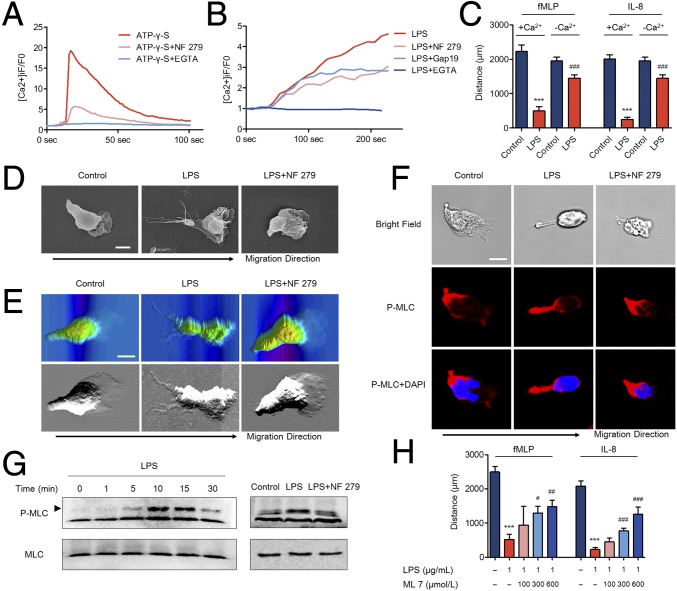
Fig. 3.
LPS-induced P2X1 receptor activation promotes MLC phosphorylation. (A and B) The P2X1 receptor antagonist (NF 279, 10 μmol/L) or Cx43 inhibitor (Gap19, 500 μmol/L) was preloaded. EGTA (2 mmol/L) was introduced to eliminate calcium in the medium. ATP-γ-S (1,000 μmol/L)- or LPS (1 μg/mL)-induced neutrophil calcium influx was assayed using LSCM with the fluorescence dye Fluo-4/AM. (C) Extracellular calcium in the under-agarose chemotaxis model was depleted by using a calcium-free solution and EGTA. Neutrophils were stimulated with LPS and migration was detected. ***P < 0.001, compared with +Ca2+ control group; ###P < 0.001, compared with +Ca2+ LPS group (ANOVA with Tukey’s test). (D and E) The P2X1 receptor antagonist (10 μmol/L) was preincubated, and LPS (1 μg/mL) was used to stimulate the neutrophils. Morphological characteristics of neutrophil migration toward fMLP were observed by SCM. Topographic and phase images of neutrophil migration toward fMLP were observed by AFM. Images are representative of 20 cells. (Scale bar, 5 μm.) (F) The P2X1 receptor antagonist was preincubated, and LPS was used to stimulate the neutrophils. The distribution of P-MLC (red) during neutrophil migration toward fMLP was observed by LCSM. Nuclei were counterstained with DAPI (blue). Images are representative of 20 cells. (Scale bar, 5 μm.) (G) Phosphorylation of MLC was determined by Western blot. (Left) LPS (1 μg/mL) was used to stimulate the neutrophils for indicated minutes. (Right) The P2X1 receptor antagonist (10 μmol/L) was preincubated, and LPS (1 μg/mL) was used to stimulate the neutrophils for 10 min. (H) A MLCK inhibitor (ML 7) was preincubated with neutrophils. Neutrophil chemotaxis distances were calculated. ***P < 0.001, compared with control group; #P < 0.05, ##P < 0.01, and ###P < 0.001, compared with LPS group (ANOVA with Tukey’s test). Data are representative of six (B, C, E, and H), four (A, D, and F), or three (G) independent experiments. Mean and SD are presented.
To gain further insight into the effects of calcium influx on neutrophil chemotaxis, we depleted the calcium in the under-agarose chemotaxis models using a calcium-free solution and EGTA. We observed that the inhibitory effects of LPS on neutrophil chemotaxis were impaired in the presence of calcium-depleted medium (Fig. 3C). Moreover, using a calcium ionophore ionomycin to stimulate neutrophils, a sustained calcium influx was observed and chemoattractant-induced neutrophil chemotaxis was dramatically inhibited (SI Appendix, Fig. S7A). The sustained calcium influx appeared to be required for initiating stop signal of neutrophil chemotaxis. Previous reports displayed that intracellular calcium was necessary for neutrophil migration (19). Therefore, we further clarified the effects of calcium on neutrophil migration. Different chemoattractant-induced calcium mobilization patterns were found to be inconsistent (20, 21). We noted that different from stimulation of IL-8, a rapid increase in intracellular calcium as well as a second extended increase in intracellular calcium were observed in fMLP-stimulated neutrophils (SI Appendix, Fig. S7 B and C). As previously demonstrated (21), the second extended increase in intracellular calcium was generated from extracellular calcium influx by mechanisms of store-operated calcium entry (SOCE) because depleting extracellular calcium with EGTA inhibited the second calcium mobilization but not the initial calcium flux peak (SI Appendix, Fig. S7B). A rapid increase in calcium without the second calcium mobilization was produced by IL-8 stimulation. EGTA had no effect on IL-8–induced calcium mobilization (SI Appendix, Fig. S7C). However, we observed that an endoplasmic reticulum (ER) inositol 1,4,5-triphosphate receptor (IP3R) channel inhibitor, 2-aminoethoxydiphenyl borate (2-APB), or a permeant Ca2+ chelator, BAPTA-AM, completely abolished neutrophil calcium mobilization stimulated by either fMLP or IL-8 (SI Appendix, Fig. S7 B and C), which indicated that intracellular calcium store release might be responsible for chemoattractant-induced calcium mobilization. Further under-agarose chemotaxis assays displayed that both 2-APB and BAPTA-AM dramatically stopped neutrophil migration (SI Appendix, Fig. S7D). These results demonstrated that intracellular calcium release but not calcium influx was necessary for maintaining neutrophil migration toward fMLP or IL-8.
As shown above, LPS-induced autocrine ATP release activated the P2X1 receptor to cause calcium influx, which inhibited neutrophil migration. However, the potential mechanisms were unclear. Scanning electron microscopy (SEM) enabled us to compare the delicate morphological characteristics between normal neutrophils and LPS-treated neutrophils. We observed that neutrophils were polarized with an anterior pseudopod at the leading edge and a thin posterior on the tail when migrating to fMLP (Fig. 3D). LPS administration had no impact on the initiation of neutrophil polarization but dramatically elongated the posterior on the tail of the polarized neutrophil, which was suppressed in the presence of the P2X1 receptor antagonist (Fig. 3D and SI Appendix, Fig. S8). Topographic and phase images captured by atomic force microscopy (AFM) showed similar results to SCM. LPS administration elongated the tail edge of chemotactic neutrophils (Fig. 3E). Posterior retraction was reported to be indispensable for cell migration through dense extracellular environments (22). Therefore, we considered that a defect in posterior retraction prevents the neutrophil from migrating through the dense interstitium formed by agarose gel. After decreasing the concentration of the agarose gel to form a less dense interstitium, neutrophil chemotaxis was partially recovered (SI Appendix, Fig. S9).
Because the dynamic phosphorylation and dephosphorylation cycle of MyoII regulates posterior retraction/formation, we next assessed MyoII activity by visualizing the distribution of phosphorylated myosin light chain (P-MLC) in chemotactic neutrophils. As previously reported, P-MLC was predominantly located at the posterior of polarized neutrophils (Fig. 3F). After stimulating with LPS, we observed enhanced MyoII activity indicated by the excessively elongated posterior overlapping with P-MLC (Fig. 3F). The P2X1 receptor antagonist inhibited the elongated posterior and distribution of P-MLC. Western blot analyses displayed consistent results with the immunofluorescence data. LPS resulted in phosphorylated MLC in neutrophils, but the P2X1 receptor antagonist inhibited P-MLC activation (Fig. 3G and SI Appendix, Fig. S10). There is a well-described relationship between MyoII and F-actin, where they are found to be mutually exclusive and inhibiting each other (23). Enhanced MyoII activity is known to decrease F-actin content and F-actin polymerization at the leading edge. F-actin staining data displayed a lower content of F-actin as well as inhibited F-actin polymerization at the leading edge in LPS-stimulated neutrophils, which were then reversed by the P2X1 antagonist (SI Appendix, Fig. S11). It provided further evidence that the P-MLC was activated.
MLCK and Rho-associated kinase (ROCK) are two major kinases that phosphorylate MLC, but their specific regulators and roles appear to be different (24). MLCK is a calcium/calmodulin-dependent kinase, and activation of MLCK relies on the intracellular Ca2+ concentration. ROCK-dependent regulation of MLC occurs through cAMP activation (22). Inhibiting MLCK, cAMP, or ROCK did not influence normal neutrophil chemotaxis using the under-agarose chemotaxis model (SI Appendix, Fig. S12). However, halting of LPS-induced chemotaxis was suppressed by inhibiting MLCK (Fig. 3H) but not cAMP or ROCK (SI Appendix, Fig. S12). This observation was consistent with the results above that calcium influx prevents neutrophil chemotaxis because MLCK activity is controlled by the intracellular calcium concentration. Taken together, these findings suggested that P2X1 promoted Ca/MLCK/MLC signaling and the formation of an elongated thin posterior that might play an important role in blocking neutrophil chemotaxis.
It is clear that the P2X1 receptor plays an important role in inhibiting LPS-stimulated neutrophil chemotaxis, but this is likely not the complete story. Because either block or knockout of the P2X1 receptor failed to completely abolish the LPS-induced stop signal, and extracellular ATP alone was unable to completely stop neutrophil chemotaxis. Neutrophil firm adhesion appears to be involved in a P2X1-independent neutrophil blocking mechanism, because we found that LPS significantly increased neutrophil adhesion but P2X1 receptor antagonist failed to suppress it (SI Appendix, Fig. S13A). To achieve persistent forward motility, neutrophils require formation of moderate adhesive contacts with the extracellular matrix (ECM) substrate. If the adhesion force is too weak, the cell will be unable to gain the traction necessary for motility. If the adhesion force is too strong, the cell cannot de-adhere and will remain attached to the substrate. The major cell surface receptors that mediate binding to ECM substrate belong to the integrin superfamily of adhesion molecules (25). β1 and β2 integrins are reported to be essential for chemotactic migration toward fMLP or IL-8 (26). In the present study, we found that fMLP or IL-8 increased membrane expression of β1 and β2 integrins (SI Appendix, Fig. S13B). By using blocking antibodies to β1 and β2 integrins, fMLP- and IL-8–induced neutrophil adhesion and migration were suppressed (SI Appendix, Fig. S13 C and D). Then, we set out to determine the effects of integrins on LPS-stimulated neutrophil adhesion and chemotaxis. We found that LPS increased neutrophil membrane expression of β1 and β2 integrins (SI Appendix, Fig. S13E). LPS-induced firm adhesion was also abolished by β1 and β2 integrins blocking antibodies (SI Appendix, Fig. S13F). However, β1 and β2 integrins blocking antibodies partially restored neutrophil chemotaxis in the stimulation of LPS (SI Appendix, Fig. S13D).
Effects of Knockout or Overexpression of P2X1 Receptor on LPS-Stimulated Differentiated HL-60 Cells.
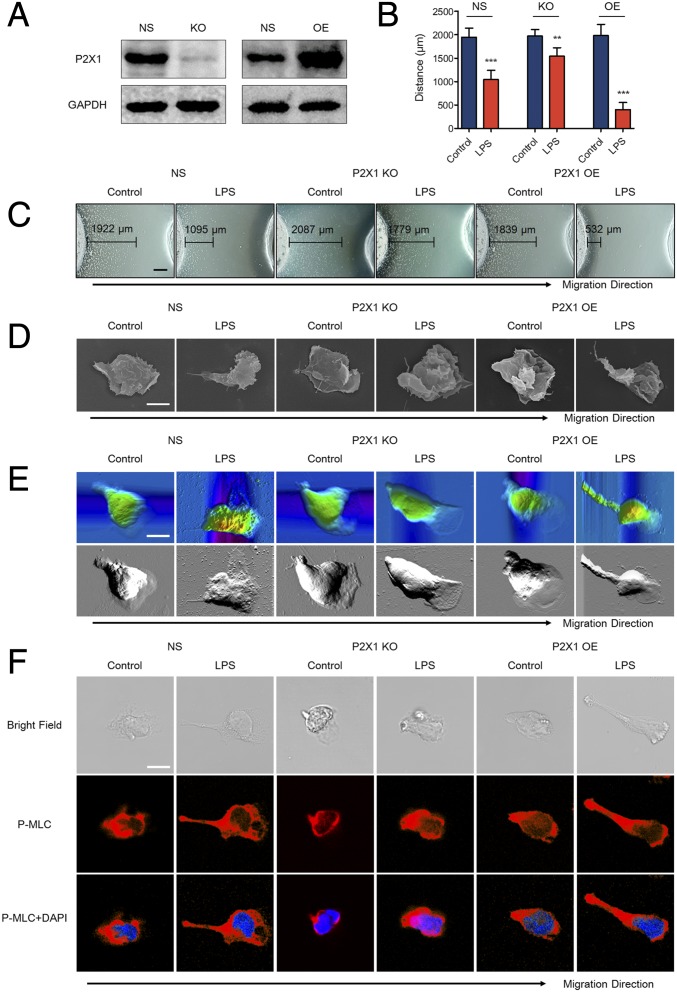
Previously, we have demonstrated that the P2X1 receptor was essential for inhibiting the migration of LPS-stimulated neutrophils from primary human blood. To reaffirm these findings, we generated neutrophil-differentiated HL-60 (dHL-60) cells with either knockout or overexpression of the P2X1 receptor (Fig. 4A and SI Appendix, Fig. S14A). Knockout of the P2X1 receptor with the CRISPR-Cas9 system impaired the inhibitory effects of LPS on neutrophil migrating to fMLP, whereas overexpression of P2X1 enhanced the inhibitory effects (Fig. 4 B and C). Meanwhile, SEM images showed that LPS markedly elongated the tail of P2X1-overexpressing dHL-60 cells but not the P2X1 knockout dHL-60 cells (Fig. 4D and SI Appendix, Fig. S14B). AFM images were obtained in parallel to the SEM images (Fig. 4E). LSCM also showed that the cell tail was elongated in P2X1-overexpressing dHL-60 cells and overlapped with P-MLC (Fig. 4F). On the contrary, P2X1 knockout did not affect the shape of the tail as much as it affected P-MLC distribution. The results of F-actin staining revealed that LPS inhibited polymerization at the leading edge of P2X1-overexpressing dHL-60 cells but not the P2X1-knockout dHL-60 cells (SI Appendix, Fig. S14C). These observations were consistent with that of human primary blood neutrophils, which indicated that the P2X1 receptor is required for the LPS-induced neutrophil chemotaxis stop signal.
Fig. 4.
Effects of knockout or overexpression of P2X1 receptor on LPS-stimulated differentiated HL-60 cells. (A) The P2X1 receptor was either knocked out (KO) or overexpressed (OE) in dHL-60 cells. The relevant empty lentivectors were used to induce control nonspecific (NS) expressing cells. Expression of the P2X1 receptor was detected by Western blot. (B and C) dHL-60 cells were treated with 10 μg/mL LPS, and chemotaxis toward fMLP was determined. (Scale bar, 500 μm.) (D) Approximately 10 μg/mL LPS was used to stimulate dHL-60 cells. The morphological characteristics of dHL-60 cell migration toward fMLP were observed by SCM. Images are representative of 20 cells. (Scale bar, 5 μm.) (E) Approximately 10 μg/mL LPS was used to stimulate dHL-60 cells. The topographic and phase images of dHL-60 cell migration toward fMLP were observed by AFM. Images are representative of 5 cells. (Scale bar, 5 μm.) (F) Approximately 10 μg/mL LPS was used to stimulate dHL-60 cells. The distribution of P-MLC (red) in chemotactic neutrophils was observed by LSCM. Nuclei were counterstained with DAPI (blue). (Scale bar, 5 μm.) Images are representative of 20 cells. Data are representative of six (B and C) or four (A and D–F) independent experiments. Mean and SD are presented. **P < 0.01 and ***P < 0.001, compared with control group (Student’s t test).
Discussion
The neutrophil recruitment cascade during infection has been well described, but how neutrophils precisely stop near pathogen foci to exert their bactericidal functions remains unclear. Here, we demonstrate that bacterial LPS-induced ATP release and autocrine feedback through the P2X1 receptor provide a strong signal for stopping human neutrophil chemotaxis toward both end-target chemoattractants and intermediary chemoattractants. Moreover, the bactericidal functions of neutrophils, including degranulation and phagocytosis, are also enhanced by the autocrine ATP signaling pathway (SI Appendix, Fig. S15).
p38 was reported to inhibit murine neutrophil chemotaxis toward macrophage inflammatory protein-2 (MIP-2) when stimulated with LPS (27). But the detailed mechanisms remain unknown. Our findings showed that p38 MAPK but not ERK1/2 or JNK was responsible for the LPS-induced chemotaxis stop signal. Moreover, we found that p38 activation led to dephosphorylation of Cx43 and ATP release. Thus, we suggest that LPS-induced p38 activation stops neutrophil chemotaxis by opening of Cx43 hemichannels and autocrine ATP release. It is worthwhile to note that p38 was also reported to sustain neutrophil chemotaxis toward fMLP by inhibiting internalization of FPR1 (8). Our observations did not contradict with this previous study because the concentrations of p38 inhibitor we used were insufficient to inhibit normal neutrophil chemotaxis. A 10-fold higher concentration of p38 inhibitor was found to obstruct neutrophil chemotaxis.
Except for the autocrine ATP activated by LPS, our results displayed that exogenous nonhydrolyzable ATP was also able to facilitate the halting of neutrophil chemotaxis at low LPS concentrations. It is interesting to note that application of hydrolyzable ATP elicits the opposite results. Low concentrations of hydrolyzable ATP recovered neutrophil chemotaxis after LPS stimulation. Neutrophil membrane ecto-ATPases contribute to these conflicting results because the products of ATP hydrolysis, AMP and ADO, sustained neutrophil chemotaxis after LPS stimulation. When the concentration of hydrolyzable ATP was elevated beyond the hydrolytic ability of ecto-ATPases, the inhibitory effects were restored because of the residual extracellular ATP. Moreover, chemokinesis (random cell migration) can be promoted by extracellular ATP through P2Y2 receptors (4). Therefore, given the complex environments of inflammatory foci, we believe that neutrophil chemotaxis is controlled synthetically by the interactions of bacterial LPS, extracellular ATP, and ecto-ATPases.
The dynamic phosphorylation and dephosphorylation cycling of MyoII regulates posterior retraction/formation and is required to overcome the resistance posed by the rigid nucleus in matrices with high densities (28). Morphological analyses showed that neutrophil posterior retraction was dramatically reduced after LPS stimulation. Considering the dense environment in the under-agarose chemotaxis assay, we speculate that the failed posterior retraction might be the cause of the LPS-induced neutrophil stop signal. After decreasing the concentration of agarose, the LPS-induced neutrophil stop signal was dampened. MyoII contractions are induced by P-MLC at the posterior. Next, we showed that the elongated posterior overlapped with P-MLC, which indicated the increased activation status of MyoII in LPS-stimulated neutrophils. By introducing specific inhibitors, we showed that a deficiency of LPS-induced chemotaxis was dependent on the MLCK pathway but not the ROCK pathway. The P2X1 receptor antagonist succeeded in inhibiting the formation of an elongated tail and the activation of P-MLC, which in turn restored the LPS-induced stop signal in neutrophil chemotaxis. Similar results were verified in neutrophil-like dHL-60 cell knockout or overexpressing of the P2X1 receptor. P2X1 overexpression enhanced the LPS-induced neutrophil stop signal and promoted the formation of an elongated tail and the activation of P-MLC. However, P2X1 knockout inhibited the LPS-induced neutrophil stop signal. MLCK is a calcium/calmodulin-dependent kinase, and its activation requires an increase in intracellular calcium. By blocking the P2X1 receptor, we observed that ATP- and LPS-stimulated calcium influx was reduced. Using a calcium-free solution and EGTA to deplete calcium influx, we found that neutrophil chemotaxis was restored in the presence of LPS. Moreover, sustained calcium influx and failed neutrophil migration were observed when neutrophils were treated with a calcium ionophore ionomycin. Therefore, we reason that the sustained calcium influx through P2X1 receptor is responsible for the activation of MLCK and stop signal of neutrophil chemotaxis.
In summary, these data identify a previously unknown function of LPS-induced autocrine ATP release in inhibiting neutrophil chemotaxis by enhancing myosin light chain phosphorylation, thus providing insight into the role of autocrine ATP signaling in the host innate immune response. Furthermore, this work provides important evidence that stoppage of neutrophil chemotaxis at infectious foci plays a key role for the defense against invading pathogens.
Materials and Methods
Venous blood was obtained from healthy volunteers. Neutrophils were isolated as previously described (9). Stably knocked out and overexpressing P2X1 cell lines were generated as previously described (9). The Medical Ethical Committee of Jiangsu University approved this study. After written informed consent was obtained, blood specimens were extracted from the cubital veins of healthy drug-free donors. Consent for the use of these samples was given by the Medical Ethical Committee of Jiangsu University.
Detailed materials and methods are described in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
This study was supported by the National Natural Science Foundation of China, 81071546, 81272148, and 81471903.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1616752114/-/DCSupplemental.
References
- 1.Junger WG. Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol. 2011;11:201–212. doi: 10.1038/nri2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trautmann A. Extracellular ATP in the immune system: More than just a “danger signal”. Sci Signal. 2009;2:pe6. doi: 10.1126/scisignal.256pe6. [DOI] [PubMed] [Google Scholar]
- 3.Eltzschig HK, Sitkovsky MV, Robson SC. Purinergic signaling during inflammation. N Engl J Med. 2013;368:1260. doi: 10.1056/NEJMc1300259. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, et al. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 5.Ayata CK, et al. Purinergic P2Y(2) receptors promote neutrophil infiltration and hepatocyte death in mice with acute liver injury. Gastroenterology. 2012;143:1620–1629 e1624. doi: 10.1053/j.gastro.2012.08.049. [DOI] [PubMed] [Google Scholar]
- 6.Lecut C, et al. ATP-gated P2X1 ion channels protect against endotoxemia by dampening neutrophil activation. J Thromb Haemost. 2012;10:453–465. doi: 10.1111/j.1538-7836.2011.04606.x. [DOI] [PubMed] [Google Scholar]
- 7.Maître B, et al. The P2X1 receptor is required for neutrophil extravasation during lipopolysaccharide-induced lethal endotoxemia in mice. J Immunol. 2015;194:739–749. doi: 10.4049/jimmunol.1401786. [DOI] [PubMed] [Google Scholar]
- 8.Heit B, et al. PTEN functions to ‘prioritize’ chemotactic cues and prevent ‘distraction’ in migrating neutrophils. Nat Immunol. 2008;9:743–752. doi: 10.1038/ni.1623. [DOI] [PubMed] [Google Scholar]
- 9.Liu X, et al. Bidirectional regulation of neutrophil migration by mitogen-activated protein kinases. Nat Immunol. 2012;13:457–464. doi: 10.1038/ni.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lokuta MA, Huttenlocher A. TNF-alpha promotes a stop signal that inhibits neutrophil polarization and migration via a p38 MAPK pathway. J Leukoc Biol. 2005;78:210–219. doi: 10.1189/jlb.0205067. [DOI] [PubMed] [Google Scholar]
- 11.Lalo U, Jones S, Roberts JA, Mahaut-Smith MP, Evans RJ. Heat shock protein 90 inhibitors reduce trafficking of ATP-gated P2X1 receptors and human platelet responsiveness. J Biol Chem. 2012;287:32747–32754. doi: 10.1074/jbc.M112.376566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dang PM, et al. A specific p47phox -serine phosphorylated by convergent MAPKs mediates neutrophil NADPH oxidase priming at inflammatory sites. J Clin Invest. 2006;116:2033–2043. doi: 10.1172/JCI27544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gardai SJ, et al. Phosphorylation of Bax Ser184 by Akt regulates its activity and apoptosis in neutrophils. J Biol Chem. 2004;279:21085–21095. doi: 10.1074/jbc.M400063200. [DOI] [PubMed] [Google Scholar]
- 14.Eltzschig HK, et al. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ Res. 2006;99:1100–1108. doi: 10.1161/01.RES.0000250174.31269.70. [DOI] [PubMed] [Google Scholar]
- 15.Bao Y, et al. Mitochondria regulate neutrophil activation by generating ATP for autocrine purinergic signaling. J Biol Chem. 2014;289:26794–26803. doi: 10.1074/jbc.M114.572495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riquelme MA, Burra S, Kar R, Lampe PD, Jiang JX. Mitogen-activated protein kinase (MAPK) activated by prostaglandin E2 phosphorylates Connexin 43 and closes osteocytic hemichannels in response to continuous flow shear stress. J Biol Chem. 2015;290:28321–28328. doi: 10.1074/jbc.M115.683417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 18.Di A, et al. The redox-sensitive cation channel TRPM2 modulates phagocyte ROS production and inflammation. Nat Immunol. 2011;13:29–34. doi: 10.1038/ni.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schaff UY, et al. Orai1 regulates intracellular calcium, arrest, and shape polarization during neutrophil recruitment in shear flow. Blood. 2010;115:657–666. doi: 10.1182/blood-2009-05-224659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Partida-Sánchez S, et al. Cyclic ADP-ribose production by CD38 regulates intracellular calcium release, extracellular calcium influx and chemotaxis in neutrophils and is required for bacterial clearance in vivo. Nat Med. 2001;7:1209–1216. doi: 10.1038/nm1101-1209. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto S, et al. TRPM2-mediated Ca2+influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat Med. 2008;14:738–747. doi: 10.1038/nm1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu L, Das S, Losert W, Parent CA. mTORC2 regulates neutrophil chemotaxis in a cAMP- and RhoA-dependent fashion. Dev Cell. 2010;19:845–857. doi: 10.1016/j.devcel.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson CA, et al. Myosin II contributes to cell-scale actin network treadmilling through network disassembly. Nature. 2010;465:373–377. doi: 10.1038/nature08994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Totsukawa G, et al. Distinct roles of ROCK (Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3T3 fibroblasts. J Cell Biol. 2000;150:797–806. doi: 10.1083/jcb.150.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jia W, Li H, He YW. The extracellular matrix protein mindin serves as an integrin ligand and is critical for inflammatory cell recruitment. Blood. 2005;106:3854–3859. doi: 10.1182/blood-2005-04-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rose DM, Alon R, Ginsberg MH. Integrin modulation and signaling in leukocyte adhesion and migration. Immunol Rev. 2007;218:126–134. doi: 10.1111/j.1600-065X.2007.00536.x. [DOI] [PubMed] [Google Scholar]
- 27.Khan AI, Heit B, Andonegui G, Colarusso P, Kubes P. Lipopolysaccharide: A p38 MAPK-dependent disrupter of neutrophil chemotaxis. Microcirculation. 2005;12:421–432. doi: 10.1080/10739680590960368. [DOI] [PubMed] [Google Scholar]
- 28.Wolf K, et al. Physical limits of cell migration: control by ECM space and nuclear deformation and tuning by proteolysis and traction force. J Cell Biol. 2013;201:1069–1084. doi: 10.1083/jcb.201210152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.