Significance
The parasitic weed Striga is the greatest biological constraint to production of many crops in Africa and parts of Asia. Genetic control is the most feasible means of crop protection from this pest. We report on identification of a gene regulating Striga resistance in sorghum and the associated change in strigolactone chemistry. Knowing this gene and its various natural alleles, sorghum breeders can design markers within it to facilitate its transfer into improved varieties providing farmers effective control of Striga in infested fields. The gene could also be used to potentially improve Striga resistance through genome editing in crops such as maize that evolved away from Striga, and hence have a paucity of Striga resistance genes.
Keywords: Striga, strigolactone, gene, sorghum, stereochemistry
Abstract
Striga is a major biotic constraint to sorghum production in semiarid tropical Africa and Asia. Genetic resistance to this parasitic weed is the most economically feasible control measure. Mutant alleles at the LGS1 (LOW GERMINATION STIMULANT 1) locus drastically reduce Striga germination stimulant activity. We provide evidence that the responsible gene at LGS1 codes for an enzyme annotated as a sulfotransferase and show that functional loss of this gene results in a change of the dominant strigolactone (SL) in root exudates from 5-deoxystrigol, a highly active Striga germination stimulant, to orobanchol, an SL with opposite stereochemistry. Orobanchol, although not previously reported in sorghum, functions in the multiple SL roles required for normal growth and environmental responsiveness but does not stimulate germination of Striga. This work describes the identification of a gene regulating Striga resistance and the underlying protective chemistry resulting from mutation.
Infestation by the parasitic weed Striga (Striga asiatica and Striga hermonthica) is a serious constraint to the production of sorghum (Sorghum bicolor), a staple cereal crop grown widely across sub-Saharan Africa and the Indian subcontinent. Global estimates of Striga’s human toll are lacking. An earlier report by the Food and Agriculture Organization of the United Nations focused on West Africa estimated that the livelihoods of 300 million people were negatively affected by the pest (1). Conservative extrapolation from a recent report on losses to Striga in rice (2) puts the economic impact on cereal production in sub-Saharan Africa at $1.2 billion annually with losses increasing by $177 million per year. Most of these losses are borne by subsistence farmers (3). Genetic resistance to this pest through low Striga germination stimulant activity provides control and permits economic production of this crop (4). Because it is an obligate root parasite, Striga seed will not germinate unless it receives a chemical cue from a potential host plant (5). Among chemicals identified in sorghum root exudates with Striga germination stimulant activity, the most potent are the strigolactones (SLs), a class of related compounds used by most terrestrial plants as hormones to regulate shoot (6) and root (7) branching. Their presence in root exudates is critical to symbiotic colonization by arbuscular mycorrhizal (AM) fungi (8). Associations with AM fungi greatly improve the performance of sorghum under nutrient and water deficits (9). Striga seems to have taken advantage of this signaling to detect its proximity to sorghum roots, germinating at the proper time and place to increase its chances of completing its life cycle on this preferred host. Sorghum produces several SLs and exudes them from its roots, particularly under conditions of limited phosphate and nitrogen, probably in attempt to promote mycorrhizal association (10). Among the SLs reported to be present in sorghum root exudates are sorgolactone, strigol, 5-deoxystrigol, and sorgomol (10–13) (Fig. 1). These compounds differ from each other by various substitutions on the A and B rings but share a common stereochemistry with respect to the β-orientation of their C rings (14). Striga is quite sensitive to these SLs, able to germinate at concentrations as low as 10−11M (15), depending on the particular SL (16).
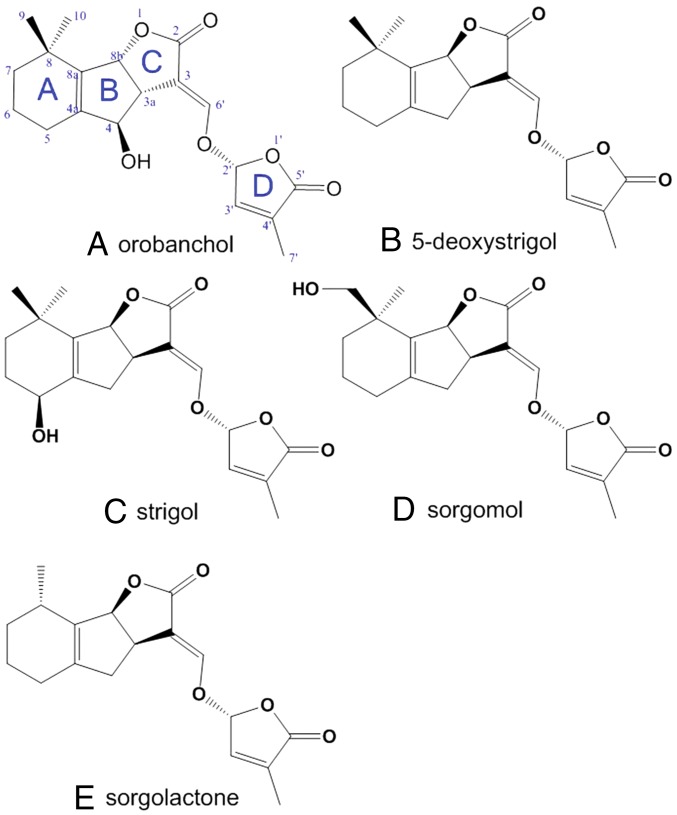
Fig. 1.
Chemical structures of SLs found in sorghum root exudates. Orobanchol (A) has not been previously reported in sorghum. Note the enantiomeric orientation of its C-ring (α-orientation) with respect to the other SLs (β-orientation), 5-deoxystrigol (B), strigol (C), sorgomol (D), and sorgolactone (E), previously reported in sorghum root exudates.
To facilitate the identification and characterization of resistance to Striga, our laboratory developed bioassays that allow observations of the parasitic association at its earliest stages, normally hidden below ground. Among these is the agar gel assay wherein the Striga germination stimulant activity of sorghum accessions can be quantified based on the distance between the sorghum root and germinating Striga seed in agar (17). This useful assay has resulted in the development and release of several Striga-resistant sorghum varieties with low germination stimulant activity (18). Although not all sorghum lines showing field resistance to Striga had low Striga germination stimulant activity, all low-stimulant sorghums that were field-tested showed Striga resistance (18). Low Striga germination stimulant activity has been an important resistance trait in sorghum improvement but less so in other crop hosts of Striga such as maize, millet, and rice (4). Genetic studies have shown that inheritance of low Striga germination stimulant activity in sorghum is through a mutant allele (lgs) expressed in homozygous recessive individuals (19). The Striga-resistant sorghum variety SRN39 carrying this mutation was mated with a Chinese landrace Shanqui Red, with high germination stimulant activity, to generate a genetic mapping population of 600 recombinant inbred lines (RILs). In a previous genotypic and phenotypic evaluation of 328 RILs by the agar gel assay we created a genetic map with 428 markers, placing the LGS1 (LOW GERMINATION STIMULANT 1) locus in a region near the tip of chromosome 5 with fine mapping that delimited it to a 30-gene region (20).
Much has been learned over the past decade about biosynthesis of SLs, particularly since their roles as growth regulators were discovered. The SLs are derived from β-carotene through a series of isomerization, cleavage, oxidation, and cyclization steps to form the four distinctive rings of the SLs (21, 22). Four enzymes have been identified to be involved in these steps: DWARF27 (D27), a carotenoid isomerase that converts all-trans-β-carotene to 9-cis-β-carotene that can be cleaved by CAROTENOID CLEAVAGE DIOXYGENASE 7 (CCD7) to form 9-cis-β-apo-10′-carotenal, which is converted by CAROTENOID CLEAVAGE DIOXYGENASE 8 (CCD8) to carlactone, which contains the A- and D-rings and is, in rice, subsequently oxidized by an ortholog of the Arabidopsis MORE AXILLARY BRANCHES 1 (MAX1), to the first canonical rice SL, 4-deoxyorobanchol (ent-2′-epi-5-deoxystrigol) (22). Mutant alleles at these loci were identified by plant growth phenotypes that affected shoot branching in model species. Less is known about the later steps of SL biosynthesis, particularly how the additions and/or modifications to functional groups on the member rings occurs. It has been assumed that 5-deoxystrigol is the proto-SL for the strigol-type SLs, having a β-oriented C-ring, and 4-deoxyorobanchol for the orobanchol-type SLs, with the C-ring in α-orientation (22, 23). Both groups have the D-ring in R configuration around the chiral center at C-2′ (21). A major SL in rice root exudates is orobanchol (Fig. 1A), and all other SLs present in this species share the same stereochemistry with respect to the spatial orientation of the C-ring (14, 24). Other plants, including tobacco (Nicotiana tabaccum), exude both types of SLs (14). For sorghum and many other plant species from which SLs have been described, the stereochemistries of their SLs have not always been determined. However, all SLs reported in the root exudates of sorghum (12), including 5-deoxystrigol, strigol, sorgomol, and sorgolactone, are of the strigol type (Fig. 1).
Because mutation at LGS1 causes a change in Striga germination stimulant activity, but without obvious changes to sorghum shoot architecture, we made quantitative and qualitative comparisons of SLs in the root exudates of mutant and WT lines.
Results
Striga Germination Stimulation and SLs.
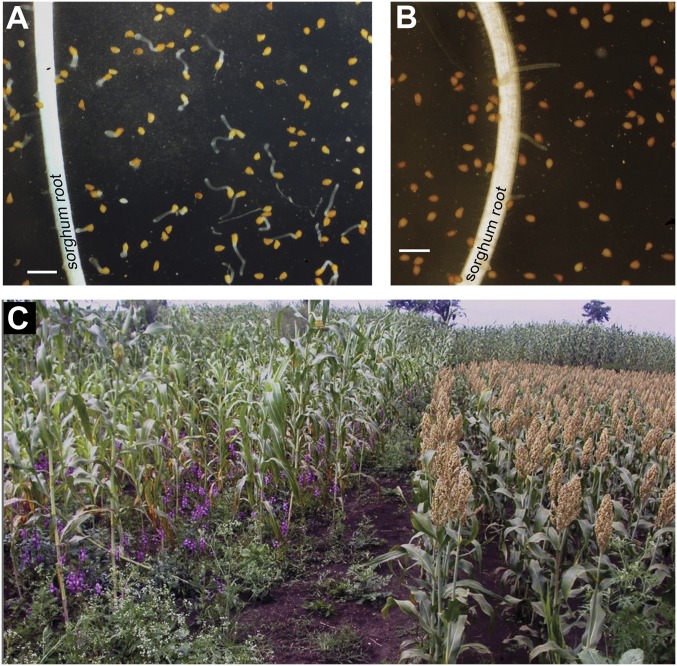
Diverse lines were classified for Striga resistance, based on the germination distance of Striga embedded in agar from the sorghum root as having high maximum germination distance (MGD ≥10 mm; Shanqui Red, Fig. 2A) or low (MGD <10 mm; SRN39, 555, IS7777, SC103, and Tetron) Striga germination stimulant activity with four Striga sources (Table 1). Low-stimulant genotype SRN39 (Fig. 2B), when crossed with high-germination stimulant lines, always result in F1 hybrids with high Striga germination stimulant activity, affirming the recessive nature of the lgs1 mutation. Complementation tests between SRN39 and all of the low germination stimulant lines in this study indicate that they all carry mutations at a common locus because no complementation occurs in their hybrids, that is, all such hybrid plants produce low Striga germination stimulant responses (Table 1). The difference in resistance between low- and high-stimulant varieties is also apparent when lines are cultivated under Striga infestation. SRN39 and its derivatives determined in the agar assay to have low Striga germination stimulant activity also support fewer parasites in field plots (Fig. 2C).
Fig. 2.
Striga resistance phenotypes of LGS1 variants. Sorghum seedlings with high Striga germination stimulant activity (A) will germinate conditioned S. asiatica seeds cocultured in agar, a centimeter or more from its root as the germination stimulant, 5-deoxystrigol, diffuses through the medium. Low-stimulant sorghum that exudes orobanchol instead of 5-deoxystrigol will not cause S. asiatica seeds to germinate in the agar gel assay, even very near its roots (B). (Scale bars, 1 mm.) The photograph (C) shows an LGS1 WT high-stimulant sorghum (left) growing next to a line (right) carrying the lgs1-1 allele in a field infested with S. hermonthica (purple flowers) in Ethiopia.
Table 1.
Measures of the Striga germination stimulant activity of sorghum lines and hybrids used for genetic mapping of LGS1
| Sorghum line or hybrid | S. asiatica (Derashe, Ethiopia) | S. asiatica (North Carolina) | S. hermonthica (Samanko, Mali) | S. hermonthica (Sinnar, Sudan) |
| Shanqui Red | 15.5 ± 5.0 | 19.8 ± 3.0 | 19.6 ± 5.2 | 21.2 ± 3.6 |
| SRN39 | 0 ± 0 | 1.9 ± 1.8 | 0.1 ± 0.2 | 0.1 ± 0.2 |
| 555 | 0 ± 0 | 0.5 ± 0.5 | 1.3 ± 2.2 | 0 ± 0 |
| IS7777 | 0 ± 0 | 0 ± 0 | 3.8 ± 1.7 | 2.1 ± 2.1 |
| Tetron | 1.2 ± 2.7 | 4.3 ± 1.5 | 1.4 ± 1.9 | 2.5 ± 3.9 |
| SC103 | 3.4 ± 2.7 | |||
| (SRN39 × Shanqui Red)F1 | 10.9 ± 2.3 | |||
| (SRN39 × 555)F1 | 0.2 ± 0.3 | |||
| (SRN39 × IS7777)F1 | 6.6 ± 2.8 | |||
| (SRN39 × Tetron)F1 | 7.1 ± 2.6 | |||
| (SRN39 × SC103)F1 | 3.1 ± 2.5 | |||
MGD (millimeters) as measured in the agar gel assay (discussed in the text); MGD >10 mm indicates high Striga germination stimulant activity; MGD <10 mm indicates low Striga germination stimulant activity. Values are means of measures from three plates ± one SD.
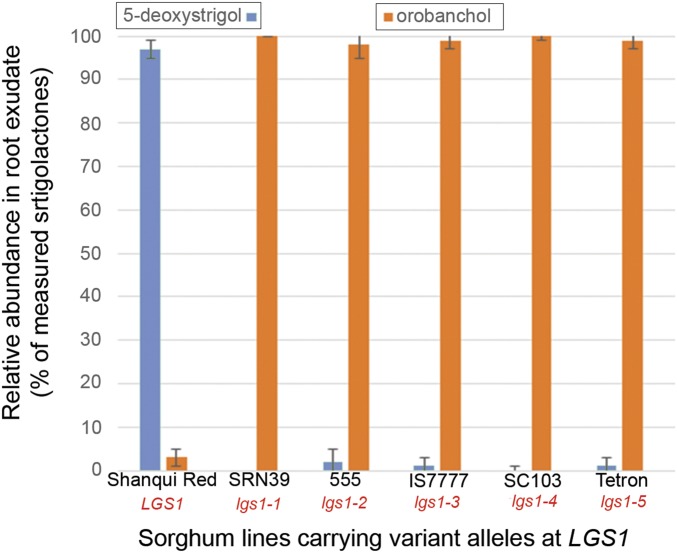
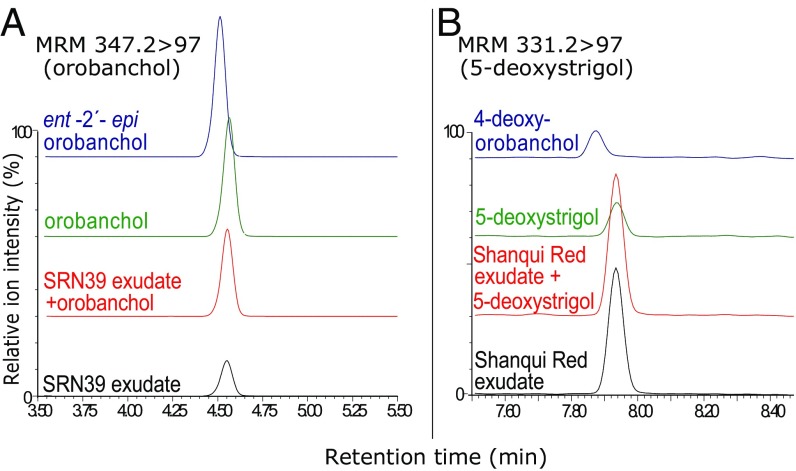
SL profiles of root exudates from lgs1 variants consistently display reduced 5-deoxystrigol and enhanced orobanchol levels relative to WT LGS1 root exudates (Fig. 3). Comparison of retention times and mass transitions of dominant SLs in sorghum root exudates with standards of known stereochemistry confirmed the β-orientation of the C-ring in 5-deoxystrigol of lines carrying LGS1 and α-orientation in orobanchol of those with lgs1 (Fig. 4). RILs with low germination stimulant activity have inherited the low 5-deoxystrigol/high orobanchol profile, whereas those with high germination stimulant activity always contain a threshold level of 5-deoxystrigol and do not accumulate orobanchol, confirming the identity of the gene and the link to this profile (Table S1).
Fig. 3.
Chemical phenotypes of LGS1 variants. SL profiles of root exudates from sorghum Shanqui Red (LGS1) with high Striga germination stimulant activity, and of five low-stimulant lines with mutant alleles at SRN39 (lgs1-1), 555 (lgs1-2), IS7777 (lgs1-3), SC103 (lgs1-4), and Tetron (lgs1-5) are shown. Specific SL quantifications are expressed in relative abundance (percent of total measured SLs) in each exudate. Although the absolute amount of the most abundant SL varies from run to run, typical values for 5-deoxystrigol in Shanqui Red or orobanchol in SRN39 are around 2,000 pmol per plant per 48 h. Values are averages of four measures from independent runs ± one SD.
Fig. 4.
UPLC-MS-MS determination of identity of major SLs in SRN39 (low Striga germination stimulant activity) and Shanqui Red (high Striga germination stimulant activity). Channels in the chromatograms monitor the mass transitions associated with loss of the D-ring (m/z 97) as SLs come off the UPLC column. The major SL in SRN39 root exudate (A) coelutes with authentic orobanchol, not its enantiomer, ent-2′-epi-orobanchol. The more typical sorghum SL with a β-orientation, 5-deoxystrigol, is the major one in Shanqui Red root exudate (B). This authentic standard is resolved from the α-oriented enantiomer, 4-deoxyorobanchol.
Table S1.
Major SLs in the root exudates of the 24 RILs derived from the cross of SRN39 and Shanqui Red used to fine-map the LGS1 region
| RIL ID | Striga germination stimulant activity | Relative abundance in root exudates, % measured SLs | |
| 5-deoxystrigol | Orobanchol | ||
| SSD#3–046 | High | 98 | 2 |
| SSD#3–047 | High | 98 | 2 |
| SSD#3–080 | High | 99 | 1 |
| SSD#3–113 | High | 100 | 0 |
| SSD#3–148 | High | 97 | 3 |
| SSD#3–246 | High | 100 | 0 |
| SSD#3–282 | High | 100 | 0 |
| SSD#3–521 | High | 98 | 2 |
| SSD#3–660 | High | 95 | 5 |
| SSD#3–816 | High | 99 | 1 |
| SSD#3–832 | High | 90 | 10 |
| SSD#3–177 | High | 100 | 0 |
| SSD#3–767 | High | 100 | 0 |
| SSD#3–061 | Low | 0 | 100 |
| SSD#3–070 | Low | 0 | 100 |
| SSD#3–128 | Low | 0 | 100 |
| SSD#3–158 | Low | 5 | 95 |
| SSD#3–162 | Low | 0 | 100 |
| SSD#3–307 | Low | 0 | 100 |
| SSD#3–535 | Low | 0 | 100 |
| SSD#3–683 | Low | 1 | 99 |
| SSD#3–809 | Low | 0 | 100 |
| SSD#3–302 | Low | 1 | 99 |
| SSD#3–320 | Low | 1 | 99 |
Values are average of three measures.
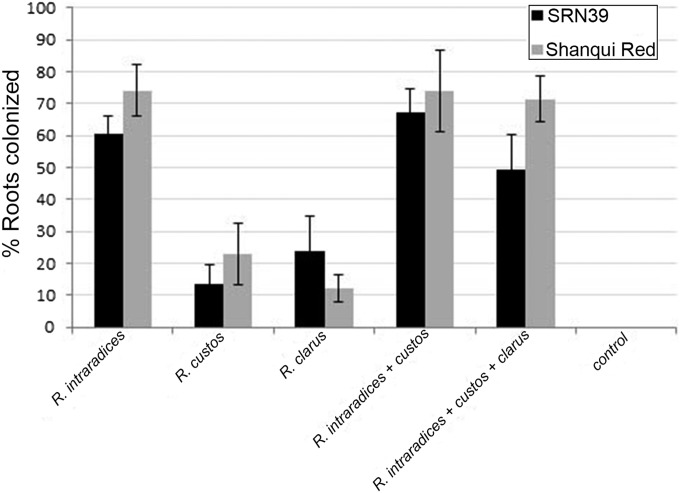
Because SLs serve other functions contributing to crop productivity (6–9), selecting for mutations that knock out SL production may have undesirable outcomes such as excessive shoot branching or impairment of mycorrhization. Sorghum lines examined in this study carrying lgs1 alleles all had similar SL exudation rates, typically around 2,000 pmol per plant over the 48-h collection period. Although the stereochemistry of the major SL in these exudates profoundly affected Striga germination stimulant activity, other SL functions seem to be unchanged by the mutation. Adult SRN39 plants on average have the same number of basal tillers (one) as Shanqui Red at 0.5-m spacing in a field row. The two lines also do not greatly differ in the degree to which their roots are colonized by three AM fungal species, Rhizophagus intraradices, Rhizophagus clarus, and Rhizophagus custos, alone or in combination (Fig. S1). Mutation at LGS1 results in both quantitative and qualitative changes in SL content of root exudates, effectively lowering Striga germination stimulant activity without negative productivity side effects.
Fig. S1.
Mycorrhization status (percent roots colonized) of Shanqui Red (LGS1) and SRN39 (lgs1) roots by three AM fungi Rhizophagus species 5 wk after inoculation. Plants were grown in sand with suboptimal phosphate. Values are averaged from seven plants ± one SD.
A search for polymorphisms in PCR products between the parents of the RILs contrasting for Striga germination stimulant activity, Shanqui Red and SRN39, allowed genotyping with eight new markers (Table S2) to refine the position of LGS1 on the sorghum genetic map. Polymorphisms resulting in PCR product size differences were scored by gel electrophoresis. Most (95%) polymorphic markers in the region cosegregated with the respective trait (RILs with Shanqui Red alleles had high Striga germination stimulant activity, whereas those with SRN39 alleles had low germination stimulant activity). The informative recombinants allowed us to rule out several gene candidates.
Table S2.
PCR primers used to identify LGS1 among the candidate genes on sorghum chromosome 5 and the qRT-PCR primers used to monitor its expression
| Primer name | Primer sequences (5′ → 3′) | Target position in reference genome* | Amplicon size | ||
| Forward | Reverse | ||||
| G2133 | TCAGGGAGTGCAGGAGAATC | CCGCATACTTATGATCAGACCTC | 60,340,583 | 60,340,934 | 351 |
| G2134 | GCTTCACAATCCCAGGTGTT | CTGTACCACACGGGCAATAA | 60,346,441 | 60,346,699 | 258 |
| PDstrigalgs4c | GACAGGCTCCATCTCATGGT | GGGAACTGAACAAAGGCCTAA | 60,366,129 | 60,366,722 | 593 |
| PDstrigalgs5b | CAAACCCATCGGACATCTTC | CAGCATGTCCTCGTACCTGA | 60,371,620 | 60,372,248 | 628 |
| PDstrigalgs6b | CTCTTCGGCGACGGGTACTA | TGCAGCAGTACGTCTCAGAACT | 60,376,824 | 60,377,456 | 632 |
| G2138 | AGATGGATCTCGCTTGCCTA | GGCAGTTGCTTCTGGAACTC | 60,393,288 | 60,393,558 | 270 |
| G2139 | ACTTCACGGAGGAGCCTGTA | AGCATGAGAGGCAAAAGCAT | 60,399,297 | 60,399,663 | 366 |
| 5bF + 2/3E3R | CAAACCCATCGGACATCTTC | CACGGATATGGAGTCGGCAA | 60,371,318 | 60,372,248 | 930 |
| qRT-PCR | TTCCATAGACGACAGCGAGC | AAAGCACTTCTTTGTGGAATAAAGG | 60,371,004 | 60,371,160 | 156 |
Phytozome, Sorghum bicolor v2.1 DOE-JGI.
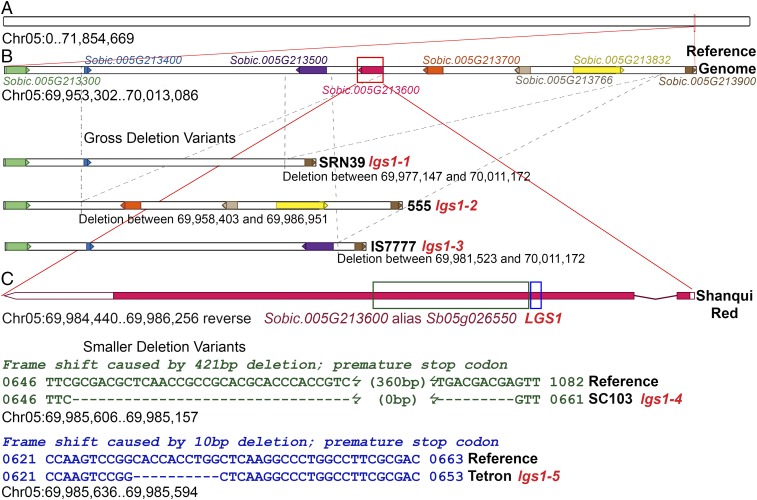
For a cluster of candidate genes from position 69,977,147–70,011,172 on the sorghum chromosome 5 physical map (Phytozome, Sorghum bicolor v3.1, DOE-JGI), a PCR product could not be obtained from SRN39, so the five genes predicted in this region (Sobic.005G213500 to Sobic.005G213832) could not be scored, except as a presence/absence polymorphism. Whole-genome sequencing of the parents revealed that this five-gene region is deleted in SRN39 (Fig. 5 and Table S3). The allele carried by SRN39 is given the designation lgs1-1. We also sequenced whole genomes of several unrelated low Striga germination stimulant lines in our collection. Examining genomic sequence of this region from these natural variants determined to be allelic to SRN39, we found that the allele in 555, lgs1-2 also has a large deletion here, but slightly shifted away from the chromosome tip, spanning the position 69,958,403–69,986,951, and therefore missing three predicted genes, Sobic.005G213400, Sobic.005G213500, and Sobic.005G213600. Its deletion overlapped with that of SRN39 for two genes, Sobic.005G213500 (Sb05g026540), coding for an uncharacterized protein with a functional domain similar to an iron/ascorbate oxidoreductase, and Sobic.005G213600 (Sb05g026550), whose uncharacterized product is predicted to have a sulfotransferase domain. A third allele, lgs1-3, with overlapping deletion occurs in IS7777, at Chr05:69,981,523..70,011,172, resulting in a loss of four genes, Sobic.005G213600, Sobic.005G213700, Sobic.005G213766, and Sobic.005G213832. The common deleted gene for all these alleles is Sobic.005G213600, which codes for the sulfotransferase. Sulfotransferases catalyze the transfer of a sulfate group from the universal donor 3′-phosphoadenosine 5′-phosphosulfate (PAPS) to a hydroxyl or amide group of its substrate (25). Several PCR primer pairs designed based on the reference genome sequence to amplify portions of Sobic.005G213600 were used to test a diverse collection of sorghum lines with high and low Striga germination stimulant activity. An amplicon was always present for the target locus among accessions with high Striga germination stimulant activity but missing in accessions with low Striga germination stimulant activity determined to be allelic to SRN39.
Fig. 5.
Schematic representation of the LGS1 locus and its identity based on mutant analysis. (A) Genetic mapping (20) indicated that LGS1 was near the tip of sorghum chromosome 5. (B) Fine mapping based on sequence polymorphisms indicated that the low Striga germination stimulant activity was always associated with a deleted region representing a five-gene loss in the low-stimulant parent of the mapping population, SRN39 (carrying allele lgs1-1). Comparing this region to other lines with low Striga germination stimulant activity determined to be allelic to SRN39, two other gross deletion variants were discovered with overlapping deletions, 555 (carrying allele lgs1-2) and IS7777 (carrying allele lgs1-3). The common deletion in these three is Sobic.005G213600. Further evidence comes from smaller deletion variants of this gene (C) in SC103 (lgs1-4), missing 421 bp in the second exon, and a 10-bp deletion near there in Tetron (lgs1-5), both predicted to cause frameshifts and severely truncated peptides without sulfotransferase function.
Table S3.
Summary of deletion variants in the LGS1 region
| Sorghum accession | Allele name | Deletion position* Chr05: | Sobic.005G213300† | Sobic.005G213400 | Sobic.005G213500 | Sobic.005G213600 | Sobic.005G213700 | Sobic.005G213766 | Sobic.005G213832 | Sobic.005G213900 |
| No annotated functional domains | No annotated functional domains | 2OG-Fe(II) oxygenase superfamily; nonhaem dioxygenase in morphine synthesis N terminal | Weakly similar to Os09g0555100 protein (sulfotransferase family) | Similar to epoxide hydrolase (alpha/beta hydrolase fold) | No annotated functional domains | UDP-glucose–glucose-phosphate glucosyltransferase | Gamma-thionin family | |||
| SRN39 | lgs1-1 | 69,977,147..70,011,172 | Intact | Intact | Deleted | Deleted | Deleted | Deleted | Deleted | Intact |
| 555 | lgs1-2 | 69,958,403..69,986,951 | Intact | Deleted | Deleted | Deleted | Intact | Intact | Intact | Intact |
| IS7777 | lgs1-3 | 69,981,523..70,011,172 | Intact | Intact | Intact | Deleted | Deleted | Deleted | Deleted | Intact |
| SC103 | lgs1-4 | 69,985,178..69,985,598 | Intact | Intact | Intact | 421-bp deletion; premature stop | Intact | Intact | Intact | Intact |
| Tetron | lgs1-5 | 69,985,617..69,985,626 | Intact | Intact | Intact | 10-bp deletion; premature stop | Intact | Intact | Intact | Intact |
Phytozome, Sorghum bicolor v3.1 DOE-JGI.
Gene model and annotation.
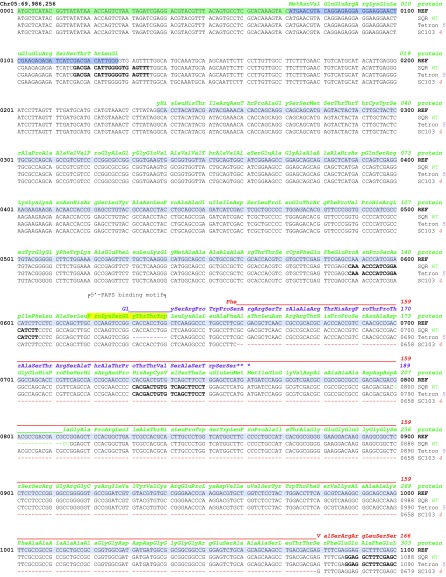
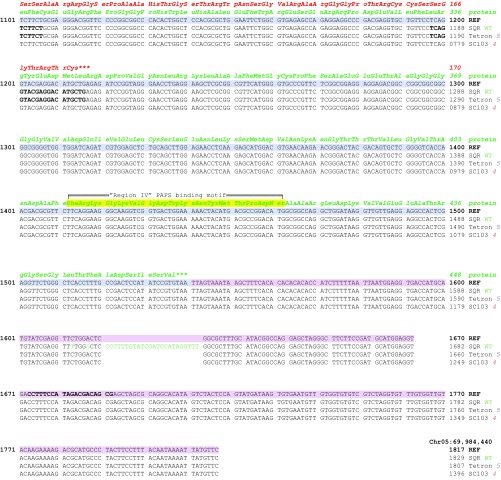
An exception to this association was observed in the allelic low-germination stimulant lines, SC103 and Tetron, in which at least some amplicons were obtained from PCR primers targeting this gene. Examining the genomic sequence from Sobic.005G213600 from these two accessions revealed deletions within the predicted coding region that cause frameshift mutations (Fig. 5, Table S3, and Fig. S2). The more obvious mutation is in SC103, which contains an allele, lgs1-4, having a 421-bp deletion in the second exon. This deletion not only results in a 137-aa residue loss in the predicted protein but also introduces a stop codon 46 residues downstream such that the resulting gene product, if it were translated, would be a protein 244 residues shorter than the WT protein. Tetron contains an allele, lgs1-5, with a 10-bp deletion 18 bp upstream of the deleted area of SC103 in the second exon, causing a frameshift that would introduce a stop codon after 39 aberrant residues beyond the deletion. A translated product of this mutant allele would therefore be missing 259 residues relative to the WT gene product (Fig. S2). Both of these mutations occur within the annotated sulfotransferase domain of the gene (residues 138–439). The one in Tetron destroys the 5′ PAPS binding motif (PKSGTTW, Fig. S2) highly conserved in all sulfotransferases (26). The conserved PAPS binding residues near the end of the protein (FRKGKVGDWKNYMTPDM) would be missing in both mutant peptides (Fig. S2). Therefore, all described lgs1 alleles would lack a functional sulfotransferase product from Sobic.005G213600.
Fig. S2.
Sequence comparison of WT Shanqui Red (LGS1) and mutant alleles from Tetron (lgs1-5) and SC103 (lgs1-4) with the sorghum reference genome, BTx623 for the reverse strand of Sobic.005G213600. Genomic sequences of Shanqui Red and the mutants are from consensus of Illumina reads. Variations from the reference DNA sequence are indicated by bases in green, purple, and red for Shanqui Red, Tetron, and SC103, respectively. The predicted WT LGS1 protein is shown in green in three-letter residue code above codons. The predicted variations in mutant peptides are shown in purple and red for Tetron and SC103, respectively. Residues highlighted in yellow are PAPS binding regions that are broadly conserved in sulfotransferases of all organisms. Chromosome positions according to Phytozome Sorghum bicolor v3.1, DOE-JGI.
Expression of Sobic.005G213600.
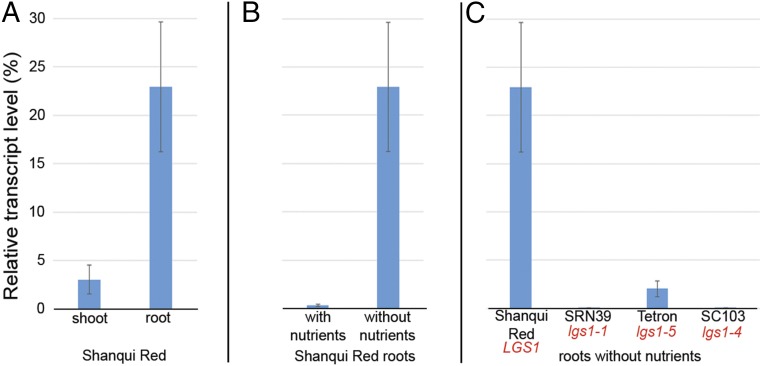
Publicly available expression profiles of Sobic.005G231600 based on ESTs from the sorghum reference, BTx623, from the Morokoshi Sorghum Transcriptome Database (sorghum.riken.jp/morokoshi/Data/Sobic.005G213600) and in the expression track of Phytozome Sorghum bicolor v3.1 (DOE-JGI) indicate that this gene is preferentially expressed in roots and under nitrogen deficiency, two qualities one would expect for genes involved in SL biosynthesis. We monitored the expression of this gene in Shanqui Red by qRT-PCR and confirmed that expression was significantly greater in roots versus shoots (Fig. 6). When seedlings of Shanqui Red were grown in sand for 1 month irrigated with tap water and compared with seedlings irrigated with nutrient solution (12:2:31) in a potting mix (peat and perlite), expression of this gene was approximately fivefold higher under the nutrient-leached conditions. LGS1 expression was significantly reduced in Tetron relative to Shanqui Red in sand (Fig. 6). The qRT-PCR primers targeting the transcript were nested in the 3′-UTR (Table S2). As expected for a completely deleted gene, no expression of this target was observed in SRN39 in either medium. The severe deletion in SC103 also knocked out expression of this gene.
Fig. 6.
Expression of sorghum LGS1. Expression of this gene is at least fivefold higher in roots than in shoots of Shanqui Red, carrying the WT allele LGS1 (A). RNA was extracted from 4-wk-old seedlings grown in sand without supplemental nutrients. Expression of this gene in roots under these conditions was greatly reduced compared with seedlings of the same age grown in potting mix and irrigated with nutrient solution (B). Little or no expression was observed in seedling roots grown in nutrient-leached sand of mutants missing all or part of this gene (C). Transcript levels were monitored by qRT-PCR comparing to actin (Materials and Methods for details). Values are averaged from three technical and three biological replicates ± one SD.
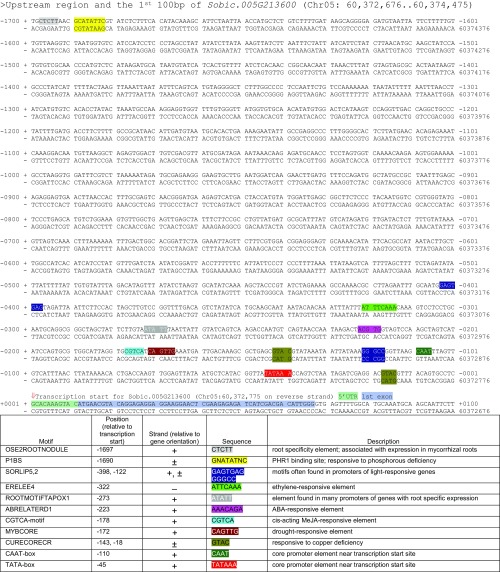
Motifs identified in silico using the PLACE database search tool (27) of the presumed promoter region of Sobic.005G213600 (Fig. S3) show some cis-acting regulatory elements (CAREs) that match other genes involved in SL biosynthesis, including root-specificity, drought, phytohormone, and nutrient deficiency responsive elements, including a phosphate deficiency response, P1BS. Most CAREs listed in Fig. S3 fall within a few hundred base pairs of the transcription start site, in the presumed core promoter.
Fig. S3.
Promoter sequence analysis indicating possible CAREs for Sobic.005G213600 based on in silico analysis using the PLACE database search tool. Chromosome positions according to Phytozome Sorghum bicolor v2.1, DOE-JGI.
Discussion
Mutation at LGS1 does not eliminate SL biosynthesis, but rather changes the type of SLs present in the root exudates. In a comparison of all possible stereoisomers of the SLs previously reported in sorghum root exudates, strigol, sorgolactone, sorgomol, and 5-deoxystrigol, it was shown that S. hermonthica germination was much higher when exposed to these SLs in their natural (β-oriented C-ring) form than when treated with their α-oriented enantiomers (16). Furthermore, Yoneyama et al. (28) predicted that SLs containing a hydroxyl group directly on the A- (e.g., strigol) or B-ring (e.g., orobanchol) would be prone to ring-destroying nucleophilic attack and therefore be less persistent in the soil. Together, these results explain why orobanchol-exuding sorghums, like the lgs1 mutants, would show low Striga germination stimulant activity in our laboratory agar assays, as well as when planted in farm fields infested with Striga.
We have presented compelling genetic evidence in the form of multiple mutant alleles at this locus that LGS1 is Sobic.005G213600, an uncharacterized gene with a sulfotransferase domain. Unfortunately, the substrates of sulfotransferases other than a few in Arabidopsis are largely unknown and cannot be accurately predicted by in silico modeling based on animal enzyme structures (25). Plant sulfotransferases resemble their better-studied counterparts in animals by the conserved motifs involved in binding PAPS, the universal donor of the sulfate group in the reactions that they catalyze (26). They sulfate a variety of substrates and are generally divided into two main classes: membrane-associated and cytosolic sulfotransferases. Only three of the former have been described in plants (Arabidopsis), all sulfating tyrosine residues in relatively small secreted peptides with growth-regulating activities, one that in turn stabilizes transcription factors (29). The larger class of cytosolic plant sulfotransferases sulfate low-molecular-weight substrates including flavonoids, coumarins and phytohormones such as brassinosteroids, salicylic acid, and jasmonates (25).
The lgs1 mutants preferentially make orobanchol, with an α-oriented C-ring over the common WT SL for sorghum, 5-deoxystrigol, lacking the hydroxyl group at position 4 and having a β-oriented C-ring. The biosynthesis of SLs from carotenoids through carlactone continues to be elucidated in model plants such as rice and likely involves hydroxylation of C-18 and carboxylation at C-19 (22, 23, 30). The orientation of the C-ring with respect to the B-ring must be determined when these rings form during the cyclization that follows oxidation by the sorghum MAX1 ortholog(s). We therefore assume that the sulfotransferase is involved in stereo-control of ring closure, perhaps by posttranslationally modifying proteins at the site where this occurs in such a way that it favors closure to β-orientation. Alternatively, the sulfotransferase may regulate, through sulfated phytohormone intermediates, which MAX1 ortholog or other enzymes metabolize carlactone, influencing the degree to which carlactone is oxidized and the catalytic environment in which its oxidized intermediate cyclizes to an SL. As some sulfotransferases do to other phytohormones, LGS1 might even sulfate the SL itself. The hydroxyl of orobanchol, perhaps formed at low levels in sorghum by an alternative pathway, could be sulfated and drive the production of 5-deoxystrigol, whereas accumulation of its unsulfated form, as occurs in lgs1 mutants, suppresses it. The mutant alleles described at LGS1 will be useful for further biochemical studies on how stereochemistry of SLs is determined or favored and help to establish precursor and product relationships among the various types of SLs in sorghum and other plant species.
Striga resistance based on low germination stimulant activity has been long known and successfully exploited in sorghum (17, 18) and improved varieties carrying this trait continue to show resistance to Striga populations from both East and West Africa (31). Its simple inheritance (19), particularly with molecular markers within the LGS1 locus, make it relatively simple to introgress into existing cultivars. Mutation at LGS1 does not knock out SLs in root exudates; it just changes the relative abundance of certain types, such that the other essential functions of SLs (ability for mycorrhizal colonization, favorable tillering, and root responsiveness to nutritional deficiencies) remain intact. Protection against Striga seems to be based on lack of responsiveness to orobanchol of those strains of the weed that parasitize sorghum and the loss of the known chemical cue 5-deoxystrigol. It should be noted, however, that rice, also parasitized by Striga, exudes orobanchol-type SLs from its roots (24). The nature of the protection offered by mutation at LGS1 might be extended to other cereal hosts of Striga for which resistance breeding lags behind, such as maize, which exudes a variety of noncanonical SLs (32), among which several of the strigol type have been reported (33).
Materials and Methods
Informative recombinants from the RIL population used to fine-map the LGS1 locus (20) were further genotyped and their SLs phenotyped by ultraperformance liquid chromatography tandem mass spectrometry (UPLC-MS-MS) with comparison with standards as previously described (32). In addition to these, four low-stimulant lines (555, IS7777, SC103, and Tetron) with reported Striga field resistance and their hybrids with SRN39 were used to establish allelic relationships of lgs1 mutants and verify the identity of LGS1 from among the gene candidates. The genotyping ultimately required Illumina sequencing of Shanqui Red, SRN39, 555, IS7777, and Tetron to an average depth of 10–27×. The raw reads from whole genome sequence for SC103 were downloaded from the National Center for Biotechnology Information (NCBI) short read archive (SRA). Sequence reads from the LGS1 region of Shanqui Red, SRN39, 555, IS7777, and Tetron have been deposited with NCBI-SRA under study accession no. SRP098704. Striga germination stimulant activity of the sorghum lines and hybrids were determined by the agar gel assay (17) using four sources of S. asiatica and S. hermonthica. Shoot branching of field-grown plants and mycorrhization of controlled-environment potted seedlings of Shanqui Red and SRN39 inoculated with three AM fungal species were compared for effects of variation at LGS1 on these phenotypes. Expression of LGS1 was monitored in seedlings of Shanqui Red, SRN39, Tetron, and SC103 grown for 4 wk in either sand without nutrients or potting mix to which nutrients were provided in the irrigation water. RNA was extracted separately from roots and shoots. Quantitative PCR was done with three technical and biological replicates comparing LGS1 with actin transcripts to determine their relative levels. Full details of all protocols used in this study are provided in SI Materials and Methods.
SI Materials and Methods
Sorghum Lines.
The same 328 RILs derived from SRN39 (a line with low Striga germination stimulant activity) and Shanqui Red (high Striga germination activity) used to fine-map the LGS1 locus to a 400-kb region near the tip of an arm of chromosome 5 (20) was used to further map its location. In addition to two parental lines, four additional sorghum lines with low Striga germination stimulant activity and reported field resistance to Striga (555, IS7777, SC103, and Tetron) were used to verify associations between phenotype and genotype outside the mapping population.
Striga Sources.
Seeds of S. hermonthica used to assay germination stimulant activity of sorghum exudates were collected from parasitic weeds in sorghum fields in Feddis, Ethiopia, Sinnar, Sudan, and Samanko, Mali. S. asiatica seed used was collected from Dereshe, Ethiopia and from North Carolina. Striga seed were surface-sterilized in 2.5% sodium hypochlorite and Metricide 28 (2.5% glutaraldehyde; Metrex Research Corp.). After soaking in a fungicide solution containing Benomyl (0.0014% methyl 1-[butylcarbamoyl]-2-benzimidazolecarbamate) for 3 d, the Striga seed was embedded in 0.7% agar in 100-mm Petri dishes at a density of ∼50 weed seeds per cm2 for an additional 7 d at 29 °C in darkness.
Striga Germination Stimulant Activity.
Striga germination stimulant activity of the sorghum lines were determined by the agar gel assay described previously (17) with slight modification. Sorghum accessions were surface-sterilized for 30 min in a 50% bleach solution (2.6% sodium hypochlorite) containing 0.2% Tween-20 (polyethylene glycol sorbitan monolaurate; Bio-Rad Corp.) and then imbibed overnight in a 5% aqueous slurry of Captan fungicide (active ingredient: N-trichloromethylthio-4-cyclohexene-1,2-dicarboximide, 48.9%; Arysta LifeScience North America LLC). Four imbibed sorghum seeds were planted per plate into the agar with embedded Striga seeds. These were thinned to one sorghum seedling per plate after 2 d in darkness at 29 °C. After 2–3 d more of incubation, MGD was determined by averaging the distance, in millimeters, of the three furthest germinated weed seeds from the sorghum root. The tabulated values are the average MGDs from three plates. Striga germination stimulant activity was called high if MGD was ≥10 mm and low if the MGD was below 10 mm. All lines except SC103 were measured in the agar gel assay with all four sources of Striga. SC103 and the hybrids were measured with the S. hermonthica collected from Sinnar, Sudan which showed of all four sources the best germinability (80% with 10−7 M GR24).
Complementation Tests Among lgs1 Mutants.
Shanqui Red, 555, IS7777, SC103, and Tetron were used to pollinate a male sterile version of SRN39. The male sterility used was genic based on recessive alleles at MS3. The Striga germination stimulant activity of the resulting F1 hybrids were determined by the agar gel assay with the Sudanese strain of S. hermonthica. Striga germination stimulant activity in the F1 hybrids with SRN39 was considered high if the MGD was ≥10 mm and low if the MGD was less than 10 mm.
SL Profiles of Exudates from Sorghum with Low and High Striga Germination Stimulant Activity.
For collection of root exudates, sorghum accessions were grown in a climate room with artificial lighting at 450 μmol⋅m−2⋅s−1 and controlled conditions [28 °C (day) 10 h and 25 °C (night) 14 h at 70% relative humidity] in Wageningen, The Netherlands. Sorghum seeds were surface-sterilized with 2% bleach for 30 min and germinated on moist filter paper in a Petri dish and incubated in darkness for 48 h. Germinated sorghum seeds were planted in 14-cm pots filled with sand and watered with half-strength modified Hoagland’s nutrient solution containing NH4NO3 (5.6 mM), K2HPO4 (0.4 mM), MgSO4 (0.8 mM), FeSO4 (0.18 mM), CaCl2 (1.6 mM), K2SO4 (0.8 mM), MnCl2 (4.5 µM), CuSO4 (0.3 µM), ZnCl2 (1.5 µM), and Na2MoO4 (0.1 µM). After 1 wk, the seedlings were thinned to three plants per pot. Nutrient solution was applied when needed (500 mL at ∼48-h intervals). After 4 wk, phosphorus deficiency was created in each pot to increase SL production. Hereto, 1 L phosphorus-deficient nutrient solution (half-strength Hoagland’s nutrient solution minus phosphate) was added to each pot and allowed to drain through the holes in the bottom of the pot to remove phosphorus from the sand. The plants were kept under phosphorus deficiency for 1 wk to increase SL production. After 1 wk the pots were drained once more with 1 L of phosphorus-deficient nutrient solution to remove any accumulated SLs. Subsequently, 48 h later, root exudates were collected in 1-L plastic bottles by passing 1 L of tap water through each pot. The collected root exudates were then run through an SPE C18 column (500 mg) and SLs were eluted with 4 mL acetone. The acetone was then evaporated to dryness and the sample redissolved in 4 mL hexane and loaded on a preconditioned 200-mg Silicagel Grace Pure SPE column for further purification. The columns were eluted with 2 mL of 10:90 hexane:ethyl acetate. The solvent was subsequently evaporated to dryness and the sample redissolved in 200 µl of 25% acetonitrile in water and filtered through a 0.45-µm Minisart SRP filter. Per sample 0.1nmol/mL of D6-5-deoxystrigol was added as internal standard for quantification.
SLs were analyzed using UPLC-MS/MS according to the method described by Kohlen et al. (34) with minor modifications. Chromatographic separation was achieved on an Acquity UPLC BEH C18 column (100 × 2.1 mm; 1.7 µm; Waters) by applying a water/acetonitrile gradient (containing 0.1% formic acid) to the column, starting from 5% (vol/vol) acetonitrile for 0.33 min and raised to 27% acetonitrile in 0.34 min, followed by a 4.33-min gradient to 40% acetonitrile, then rising to 65% acetonitrile in 3 min and maintained for 0.67 min, then raised to 90% acetonitrile in 0.2 min, which was maintained for 0.46 min before going back to 5% acetonitrile using a 0.2-min gradient, and maintained for 2.47 min to equilibrate the column before the next run. Operation temperature and flow rate of the column were 50 °C and 0.5 mL/min, respectively. The eluent of the column was introduced into the electrospray of the mass spectrometer (Xevo triple quadrupole tandem mass spectrometer; Waters) operating in positive mode. SLs were identified by comparing the MS/MS spectrum and retention time with eight authentic standards [5-deoxystrigol, ent-2-epi-5-deoxystrigol (or 4-deoxyorobanchol), orobanchol, ent-2′-epi-orobanchol, strigol, epi-strigol, sorgolactone, and sorgomol]. Multiple ion monitoring mode was used to quantify the SLs as described previously (32). Cone and desolvation gas flows were set to 50 L/h and 1,000 L/h, respectively. The capillary voltage was set at 3.0 kV, the source temperature was 150 °C, and the desolvation temperature was 650 °C. The cone voltage and collision energy were optimized for each standard compound using the Waters IntelliStart MS Console. Data acquisition and analysis were performed using MassLynx 4.1 (combined with TargetLynx) software (Waters). The proportion of the two major SLs per run (orobanchol and 5-deoxystrigol) was calculated from the quantities (picomoles per plant per 48 h) determined in four separate runs per sorghum line to construct Fig. 3.
Shoot Branching Observations.
Plants of SRN39 and Shanqui Red were grown under standard agronomic management for sorghum in West Lafayette, IN in two-row field plots with 1 m between rows. Plants were thinned to 0.5-m spacing within rows and the number of basal tillers was counted for each of 35 individuals every 20 d during the growing season.
Mycorrhization Observations.
Seedlings of Shanqui Red and SRN39 were planted as described for collecting exudates except they were initially watered with half-strength Hoagland’s for the first 10 d then the growing medium (sand) was flushed to remove adsorbed phosphorous. Seven plants (one per pot) arranged in a randomized complete block design for each treatment were then inoculated with 1,500 spores of AM fungi obtained from Mycovitro by mixing the spores with 1 mL of water and injecting the inoculum into the root zone with a syringe. The treatments were R. intraradices, R. clarus, and R. custos, a half/half mixture of R. intraradices and R. custos, and an equal-parts mixture of all three AM fungal species. Control treatments were inoculated with 1 mL of water containing heat-killed spores. Plants were grown an additional 5 wk, irrigating with modified half-strength Hoagland’s containing only 100 µM phosphate. Harvested roots were cut into 2-cm pieces fixed in 70% ethanol and stained according to the protocol of Brundrett et al. (35). Mycorrhization status was determined on 100 microscopic field views of sections taken at fixed intervals along the sampled roots at 400× magnification and percent colonization calculated based on those views (36).
Integration of the LGS1 Region Genetic Map onto Physical Map.
A high-resolution map was generated around the LGS1 locus by mapping all polymorphic markers in the 400-kb region including these new primers and the phenotypes (low or high Striga germination stimulant activity) collected from the 354 RILs of the mapping population (20). Informative recombinants in the high-resolution map were analyzed to locate the most likely candidate genes determining the Striga germination stimulant activity. SL profiles of exudates of these recombinants were also determined to establish their chemical phenotype and association of parental SL profile with Striga germination stimulant activity. Putative candidate gene markers, those that always cosegregated with the respective phenotype, were also checked against the set of sorghum lines with known Striga germination stimulant activity to see whether the associations held outside of the mapping population. The most likely candidate genes were sequenced in a set of low- and high-stimulating lines to characterize the nature of the mutation.
Genotype Confirmation by Sequencing.
The raw reads from whole genome sequence for SC103 were downloaded from the NCBI-SRA (https://www.ncbi.nlm.nih.gov/sra/?term=SC103-14E). High-molecular-weight genomic DNA from Shanqui Red, SRN39, 555, IS7777, and Tetron was extracted from young seedlings and used to construct indexed paired-end sequencing libraries. DNA samples were run on Illumina HiSeq2500 and generated an average of 100-bp sequence reads in fastq format. Adapter and poor quality (less than Phred-20) were removed from both 5′ and 3′ ends of the fragments then reads below a minimum length of 30 bases were discarded from the sequence dataset. The Bowtie2 sequence alignment software package was used for mapping to the reference genome determine the depth of coverage for each sample. The average depth of coverage for each sorghum line ranged from 10 to 27. The sequence data from all sorghum accessions were also mapped back to the reference genome with CLC Genomics Workbench 8.5.1 version for variant detection. Sequence reads from the LGS1 region of Shanqui Red, SRN39, 555, IS7777, and Tetron have been deposited with NCBI-SRA under study accession no. SRP098704.
Expression Analysis.
Surface-sterilized and imbibed sorghum seeds (as in the germination stimulant activity assays) were germinated at 30 °C on moistened sterile filter papers in Petri dishes and germinated seedlings were transferred to 1-dm3 pots filled with potting mix (milled peat and perlite) or sand. The seedlings in sand were grown for 4 wk and watered with tap water in which no nutrients were provided. Those in the potting mix were irrigated with a 12:2:31 (nitrogen:phosphorous:potassium) solution containing micronutrients (37). At harvest, the roots and shoots were frozen separately in liquid nitrogen and stored at −80 °C for RNA extraction. RNA extraction was done using the Spectrum Plant Total RNA kit (Sigma). One hundred micrograms of frozen root or leaf sample was ground to a fine powder in liquid nitrogen. Protocol A of the manufacturer’s instructions was followed for cell lysis and subsequent RNA purification steps. The On-Column DNase I Digestion Set (Sigma) was used according the manufacturer’s instructions to eliminate any genomic DNA contamination. One microgram RNA from each sample was reverse-transcribed using the RETROscript kit (Ambion). The template RNA in a 12-µL reaction volume was mixed with 2 µL oligo(dT) primer and heated for 3 min at 85 °C followed by brief spinning. The rest of the reaction components (2 µL 10× RT buffer, 4 µL dNTP, 1 µL RNase inhibitor, and 1 µL MMLV-RT reverse transcriptase) was added to the mix and incubated at 44 °C for 1 h. The reverse transcription reaction was inactivated by heating at 92 °C for 10 min. The resulting reverse transcribed product was diluted with double-deionized water to 100 µL and used as a template for following quantitative PCR. PCR primers targeting the 3′ UTR were designed using Primer3 software (https://www.ncbi.nlm.nih.gov/tools/primer-blast/). The iTaq Universal SYBR Green Supermix (Bio-Rad) was used for qPCR. Primers at a final concentration of 0.5 µM each, 20 ng template cDNA, 10 µL the 2× reaction mix, and double-deionized water to final 20-µL reaction volume were combined for qPCR. The reaction was carried out with three technical and biological replicates with the Mx3005P system. The PCR conditions were 2 min at 95 °C followed by 45 cycles of 95 °C for 15 s and 60 °C for 30 s. The plate values of the target gene and housekeeping gene (actin) (Sobic.001G112600) were used to determine the relative transcript level.
Acknowledgments
We thank K. Yoneyama (Weed Science Center, Utsunomiya University, Utsunomiya, Japan) and T. Asami (Department of Applied Biological Chemistry, The University of Tokyo, Tokyo, Japan) for supplying SL standards. This work was supported by Bill and Melinda Gates Foundation Grant OPP1006216 and Netherlands Organization for Scientific Research Vici Grant 865.06.002 and Equipment Grant 834.08.001 (to H.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Sequence reads from the LGS1 region of Shanqui Red, SRN39, 555, IS7777, and Tetron have been deposited with the National Center for Biotechnology Information Short Read Archive (accession no. SRP098704).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1618965114/-/DCSupplemental.
References
- 1.Mboob SS. A regional program for Striga control in West and Central Africa. In: Robson TO, Broad HR, editors. Striga – Improved Management in Africa. Food and Agriculture Organization; Rome: 1989. pp. 190–194. [Google Scholar]
- 2.Rodenburg J, Demont M, Zwart SJ, Bastiaans L. Parasitic weed incidence and related economic losses in rice in Africa. Agric Ecosyst Environ. 2016;235:306–317. [Google Scholar]
- 3.Ejeta G. The Striga scourge in Africa: A growing pandemic. In: Ejeta G, Gressel J, editors. Integrating New Technologies for Striga Control: Towards Ending the Witch-Hunt. World Scientific; Singapore: 2007. pp. 3–16. [Google Scholar]
- 4.Pérez-Vich B, Velasco L, Rich PJ, Ejeta G. Marker-assisted and physiology-based breeding for resistance to root parasitic Orobanchaceae. In: Joel DM, Gressel J, Musselman LJ, editors. Parasitic Orobanchaceae. Springer; Berlin: 2013. pp. 369–391. [Google Scholar]
- 5.Rich PJ, Ejeta G. Biology of host-parasite interactions in Striga species. In: Ejeta G, Gressel J, editors. Integrating New Technologies for Striga Control: Towards Ending the Witch-Hunt. World Scientific; Singapore: 2007. pp. 19–32. [Google Scholar]
- 6.Gomez-Roldan V, et al. Strigolactone inhibition of shoot branching. Nature. 2008;455:189–194. doi: 10.1038/nature07271. [DOI] [PubMed] [Google Scholar]
- 7.Rasmussen A, et al. Strigolactones suppress adventitious rooting in Arabidopsis and pea. Plant Physiol. 2012;158:1976–1987. doi: 10.1104/pp.111.187104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akiyama K, Matsuzaki K, Hayashi H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature. 2005;435:824–827. doi: 10.1038/nature03608. [DOI] [PubMed] [Google Scholar]
- 9.Sun X-G, Tang M. Effect of arbuscular mycorrhizal fungi inoculation on root traits and root volatile organic compound emissions of Sorghum bicolor. S Afr J Bot. 2013;88:373–379. [Google Scholar]
- 10.Jamil M, Van Mourik TA, Charnikhova T, Bouwmeester HJ. Effect of diammonium phosphate application on strigolactone production and Striga hermonthica infection in three sorghum cultivars. Weed Res. 2013;53:121–130. [Google Scholar]
- 11.Siame BA, Weerasuriya Y, Wood K, Ejeta G, Butler LG. Isolation of strigol, a germination stimulant for Striga asiatica, from host plants. J Agric Food Chem. 1993;41:1486–1491. [Google Scholar]
- 12.Awad AA, et al. Characterization of strigolactones, germination stimulants for the root parasitic plants Striga and Orobanche, produced by maize, millet and sorghum. Plant Growth Regul. 2006;48:221–227. [Google Scholar]
- 13.Motonami N, et al. The bioconversion of 5-deoxystrigol to sorgomol by the sorghum, Sorghum bicolor (L.) Moench. Phytochemistry. 2013;93:41–48. doi: 10.1016/j.phytochem.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 14.Xie X, et al. Confirming stereochemical structures of strigolactones produced by rice and tobacco. Mol Plant. 2013;6:153–163. doi: 10.1093/mp/sss139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cook CE, Whichard LP, Turner B, Wall ME, Egley GH. Germination of witchweed (Striga lutea Lour.): Isolation and properties of a potent stimulant. Science. 1966;154:1189–1190. doi: 10.1126/science.154.3753.1189. [DOI] [PubMed] [Google Scholar]
- 16.Nomura S, Nakashima H, Mizutani M, Takikawa H, Sugimoto Y. Structural requirements of strigolactones for germination induction and inhibition of Striga gesnerioides seeds. Plant Cell Rep. 2013;32:829–838. doi: 10.1007/s00299-013-1429-y. [DOI] [PubMed] [Google Scholar]
- 17.Hess DE, Ejeta G, Butler LG. Selecting sorghum genotypes expressing a quantitative biosynthetic trait that confers resistance to Striga. Phytochemistry. 1992;31:493–497. [Google Scholar]
- 18.Ejeta G. Striga resistance in sorghum: exploitation of the intricate host-parasite biology. Crop Sci. 2007;47:S216–S227. [Google Scholar]
- 19.Vogler RK, Ejeta G, Butler LG. Inheritance of low production of Striga germination stimulant in sorghum. Crop Sci. 1996;36:1185–1191. [Google Scholar]
- 20.Satish K, Gutema Z, Grenier C, Rich PJ, Ejeta G. Molecular tagging and validation of microsatellite markers linked to the low germination stimulant gene (lgs) for Striga resistance in sorghum [Sorghum bicolor (L.) Moench] Theor Appl Genet. 2012;124:989–1003. doi: 10.1007/s00122-011-1763-9. [DOI] [PubMed] [Google Scholar]
- 21.Alder A, et al. The path from β-carotene to carlactone, a strigolactone-like plant hormone. Science. 2012;335:1348–1351. doi: 10.1126/science.1218094. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, et al. Rice cytochrome P450 MAX1 homologs catalyze distinct steps in strigolactone biosynthesis. Nat Chem Biol. 2014;10:1028–1033. doi: 10.1038/nchembio.1660. [DOI] [PubMed] [Google Scholar]
- 23.Al-Babili S, Bouwmeester HJ. Strigolactones, a novel carotenoid-derived plant hormone. Annu Rev Plant Biol. 2015;66:161–186. doi: 10.1146/annurev-arplant-043014-114759. [DOI] [PubMed] [Google Scholar]
- 24.Cardoso C, et al. Natural variation of rice strigolactone biosynthesis is associated with the deletion of two MAX1 orthologs. Proc Natl Acad Sci USA. 2014;111:2379–2384. doi: 10.1073/pnas.1317360111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirschmann F, Krause F, Papenbrock J. The multi-protein family of sulfotransferases in plants: Composition, occurrence, substrate specificity, and functions. Front Plant Sci. 2014;5:556. doi: 10.3389/fpls.2014.00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein M, Papenbrock J. The multi-protein family of Arabidopsis sulphotransferases and their relatives in other plant species. J Exp Bot. 2004;55:1809–1820. doi: 10.1093/jxb/erh183. [DOI] [PubMed] [Google Scholar]
- 27.Higo K, Ugawa Y, Iwamoto M, Korenaga T. Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res. 1999;27:297–300. doi: 10.1093/nar/27.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoneyama K, Awad AA, Xie X, Yoneyama K, Takeuchi Y. Strigolactones as germination stimulants for root parasitic plants. Plant Cell Physiol. 2010;51:1095–1103. doi: 10.1093/pcp/pcq055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsubayashi Y. Post-translational modifications in secreted peptide hormones in plants. Plant Cell Physiol. 2011;52:5–13. doi: 10.1093/pcp/pcq169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abe S, et al. Carlactone is converted to carlactonoic acid by MAX1 in Arabidopsis and its methyl ester can directly interact with AtD14 in vitro. Proc Natl Acad Sci USA. 2014;111:18084–18089. doi: 10.1073/pnas.1410801111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bozkurt ML, Muth P, Parzies HK, Haussmann BIG. Genetic diversity of East and West African Striga hermonthica populations and virulence effects on a contrasting set of sorghum cultivars. Weed Res. 2015;55:71–81. [Google Scholar]
- 32.Jamil M, Kanampiu FK, Karaya H, Charnikhova T, Bouwmeester HJ. Striga hermonthica parasitism in maize in response to N and P fertilisers. Field Crops Res. 2012;134:1–10. [Google Scholar]
- 33.Yoneyama K, et al. Difference in Striga-susceptibility is reflected in strigolactone secretion profile, but not in compatibility and host preference in arbuscular mycorrhizal symbiosis in two maize cultivars. New Phytol. 2015;206:983–989. doi: 10.1111/nph.13375. [DOI] [PubMed] [Google Scholar]
- 34.Kohlen W, et al. Strigolactones are transported through the xylem and play a key role in shoot architectural response to phosphate deficiency in nonarbuscular mycorrhizal host Arabidopsis. Plant Physiol. 2011;155:974–987. doi: 10.1104/pp.110.164640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brundrett M, Bougher N, Dell B, Grove T, Malajczuk N. 1996. Working with mycorrhizas in forestry and agriculture (Australian Centre for International Agricultural Research, Bruce, Australia)
- 36.McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA. A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol. 1990;115:495–501. doi: 10.1111/j.1469-8137.1990.tb00476.x. [DOI] [PubMed] [Google Scholar]
- 37.Stewart GR. The regulation of nitrate reductase level in Lemna minor L. J Exp Bot. 1972;23:171–183. [Google Scholar]