Significance
Spread of bacteria via the bloodstream to vital organs causes most mortality due to bacterial infection. To exit the bloodstream and enter these organs, bacteria must be able to resist the forces generated by flowing blood so that they can adhere to the endothelial cells lining blood vessels without being washed away. This process is not yet understood for most disease-causing bacteria. Here, we show that the Lyme disease pathogen Borrelia burgdorferi exploits an abundant constituent of blood, plasma fibronectin, to form endothelial interactions that become stronger as forces due to blood-flow increase. The ability to recruit this highly conserved molecule may also be important for the vascular interaction mechanisms of other pathogens.
Keywords: catch bond, fibronectin, force, vascular, bacteria
Abstract
Bacterial dissemination via the cardiovascular system is the most common cause of infection mortality. A key step in dissemination is bacterial interaction with endothelia lining blood vessels, which is physically challenging because of the shear stress generated by blood flow. Association of host cells such as leukocytes and platelets with endothelia under vascular shear stress requires mechanically specialized interaction mechanisms, including force-strengthened catch bonds. However, the biomechanical mechanisms supporting vascular interactions of most bacterial pathogens are undefined. Fibronectin (Fn), a ubiquitous host molecule targeted by many pathogens, promotes vascular interactions of the Lyme disease spirochete Borrelia burgdorferi. Here, we investigated how B. burgdorferi exploits Fn to interact with endothelia under physiological shear stress, using recently developed live cell imaging and particle-tracking methods for studying bacterial–endothelial interaction biomechanics. We found that B. burgdorferi does not primarily target insoluble matrix Fn deposited on endothelial surfaces but, instead, recruits and induces polymerization of soluble plasma Fn (pFn), an abundant protein in blood plasma that is normally soluble and nonadhesive. Under physiological shear stress, caps of polymerized pFn at bacterial poles formed part of mechanically loaded adhesion complexes, and pFn strengthened and stabilized interactions by a catch-bond mechanism. These results show that B. burgdorferi can transform a ubiquitous but normally nonadhesive blood constituent to increase the efficiency, strength, and stability of bacterial interactions with vascular surfaces. Similar mechanisms may promote dissemination of other Fn-binding pathogens.
Dissemination of pathogens via the cardiovascular system is associated with most mortality due to bacterial infection, and is important for infection of many tissues, including the brain, heart, bone, joints, and visceral organs (1). Pathogens that travel via the cardiovascular system to sites distant from the original source of infection must be able to adhere to the inner endothelial lining of blood vessels to slow down and migrate out of the vasculature (extravasate) into extravascular tissues. Other pathogens do not exit the bloodstream, but can adhere tenaciously to structures such as heart valves and cardiac devices, causing life-threatening conditions, including endocarditis (2). Despite the importance of pathogen vascular adhesion and dissemination to human health, the mechanisms supporting these processes are largely uncharacterized for many microbes, including most bacterial pathogens.
The ability to overcome fluid shear stress caused by blood flow over endothelial surfaces is crucial for pathogens interacting with blood vessels. In the vasculature, interactions of circulating host cells such as leukocytes with endothelia are also subjected to fluid shear stress, and are stabilized by specialized force-resisting and force-strengthened mechanisms such as catch bonds and tethers (3, 4). Shock-absorbing structures such as pili and fimbriae can stabilize surface adhesion of bacteria subjected to external forces such as shear stress (5, 6). However, many bacteria, including spirochetes, do not form pili or fimbriae. We recently found that endothelial interactions of the Lyme disease spirochete Borrelia burgdorferi under physiological shear stress bear a remarkable biomechanical resemblance to the mechanisms supporting leukocyte rolling on the same surfaces and are stabilized by catch bonds and tethers, even though leukocytes and B. burgdorferi are genetically, morphologically, and physiologically distinct cells (7). Other bacterial pathogens also exploit or mimic strategies used by circulating host cells to adhere to and bypass endothelial barriers under vascular shear stress. For example, Neisseria meningitidis bypasses the blood–brain barrier by eliciting inflammatory responses and associated reorganization of brain endothelia following adhesion to a matrix metalloprotease regulator (8), and Staphylococcus aureus adheres to blood vessels walls by binding to fibrils of von Willebrand factor, a glycoprotein produced by endothelial cells that is crucial for adhesion of circulating platelets (9, 10). Thus, it appears that bacterial exploitation of ubiquitous host cell molecules and mimicry of common host cell shear-stress adhesion mechanisms are important for pathogen dissemination in this specialized and physically challenging environment.
One of the most abundant proteins in the cardiovascular system is fibronectin (Fn), which is part of the fibrous extracellular matrix supporting endothelial cells, and is also present in soluble form at high concentrations in blood [plasma Fn (pFn)] (11, 12). Fn interacts with integrins by a force-strengthened catch-bond mechanism, and Fn and integrins can mediate leukocyte adhesion to vascular surfaces under physiological shear stress (13–17). Therefore, Fn has the potential to support vascular interactions of disseminating pathogens. Consistent with this hypothesis, B. burgdorferi interacts with postcapillary venules (PCVs) in vivo by an Fn-dependent mechanism (7, 18, 19), and polymorphisms in an S. aureus adhesion protein (adhesin) that strengthen Fn binding are associated with infection of cardiac devices by these bacteria (20). Fn-binding sequences of the surface-localized B. burgdorferi vascular adhesin BBK32 are also important for PCV interactions of this pathogen (19).
Most invasive bacterial pathogens that infect vertebrates target Fn and can adhere to this highly conserved molecule via bacterial cell surface adhesion proteins (adhesins) collectively referred to as bacterial Fn-binding proteins (FnBPs) (12, 21, 22). FnBPs can adhere to the insoluble Fn matrix and soluble unpolymerized Fn produced by most host cells, and many FnBPs also bind pFn, which is produced by the liver and is important for hemostasis and wound healing in vertebrates (23, 24). The ability of bacterial pathogens to recruit soluble Fn to their surfaces can promote adhesion to and internalization by host cells, as well as bacterial aggregation during biofilm formation (12, 22). However, the specific pathogenic functions of bacterial pFn binding have not yet been defined.
We reported recently that the Fn-binding B. burgdorferi vascular adhesin BBK32 stabilizes bacterial–endothelial interactions at PCV shear stress by a catch-bond mechanism (7). BBK32, which binds to Fn by a mechanism similar to the binding mechanisms of S. aureus FnBPA and streptococcal FnBPs (21, 25), causes conformational changes in pFn that induce formation of an extended structure (26) and formation of superfibronectin, a highly cross-linked, exceptionally sticky, polymerized form of Fn (27, 28). Several other pathogens and FnBPs adhere to Fn under force conditions similar to the force conditions found in the vasculature, suggesting that the use of Fn for vascular adhesion may be widespread among microbes (29–32). The mechanisms by which Fn promotes bacterial interactions with surfaces in the cardiovascular system have not yet been defined.
Here, we investigated how Fn promotes B. burgdorferi-endothelial interactions, using a recently developed flow chamber system that reproduces B. burgdorferi–endothelial interaction properties in PCVs and real-time live cell imaging and particle tracking approaches for studying interaction biomechanics. We found that B. burgdorferi–endothelial interactions are dependent on bacterial recruitment of pFn, which forms polymerized Fn sheaths at bacterial ends that are part of mechanical load-bearing adhesion complexes in BBK32-expressing bacteria, and that pFn stabilizes BBK32-mediated interactions by a catch-bond mechanism. These results suggest that pFn polymerization and pFn-dependent catch bonds might also promote vascular interactions for other bacterial pathogens.
Results
B. burgdorferi–Endothelial Interactions Under Vascular Shear Stress Depend on Bacterial Recruitment of pFn.
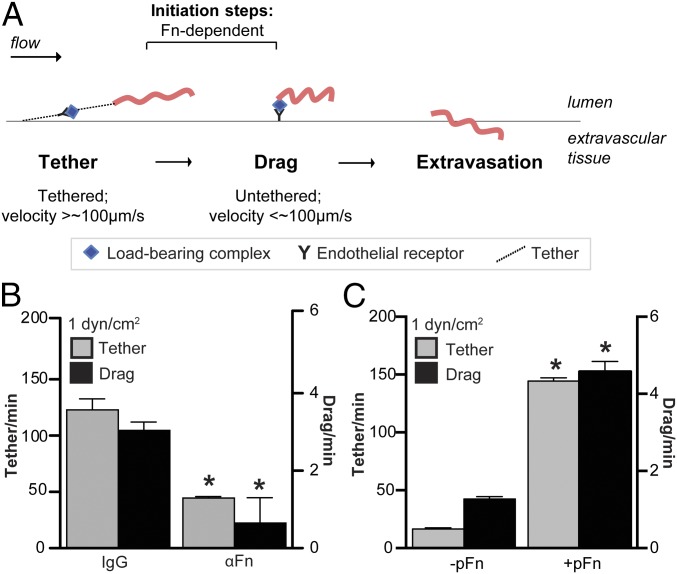
To determine how Fn promotes B. burgdorferi-vascular interactions, we studied its mechanistic contributions to bacterial interactions with postconfluent primary human umbilical vein endothelia under physiological shear stress in flow chambers. This flow chamber system recapitulates the major qualitative and quantitative properties of B. burgdorferi interactions with dermal PCVs in live mice (7), where the two major types of mobile B. burgdorferi-vascular interactions, tethering and dragging, are Fn-dependent (18, 33). Tethering and dragging are distinct initiation steps in the multistep B. burgdorferi-vascular interaction cascade and permit bacteria to slow down sufficiently to extravasate from blood vessels during dissemination (7, 18, 19, 33) (Fig. 1A). Tethering bacteria are stabilized by tethers anchoring bacteria to endothelia, move at least 50% more slowly under flow than control beads but faster than 100 μm⋅s−1, and pause briefly and repeatedly as they move over endothelial surfaces (7, 33) (Fig. 1A). Dragging bacteria are not stabilized by tethers, and move more slowly than 100 μm⋅s−1 (7, 33) (Fig. 1A). Tethering and dragging interactions can be measured by counting numbers of bacteria that pause (tether) or crawl/drag at a speed of less than 100 μm⋅s−1 in a 30 × 100-μm region of interest positioned in unbranched regions of PCVs in vivo (33) or at the center of flow chambers (7).
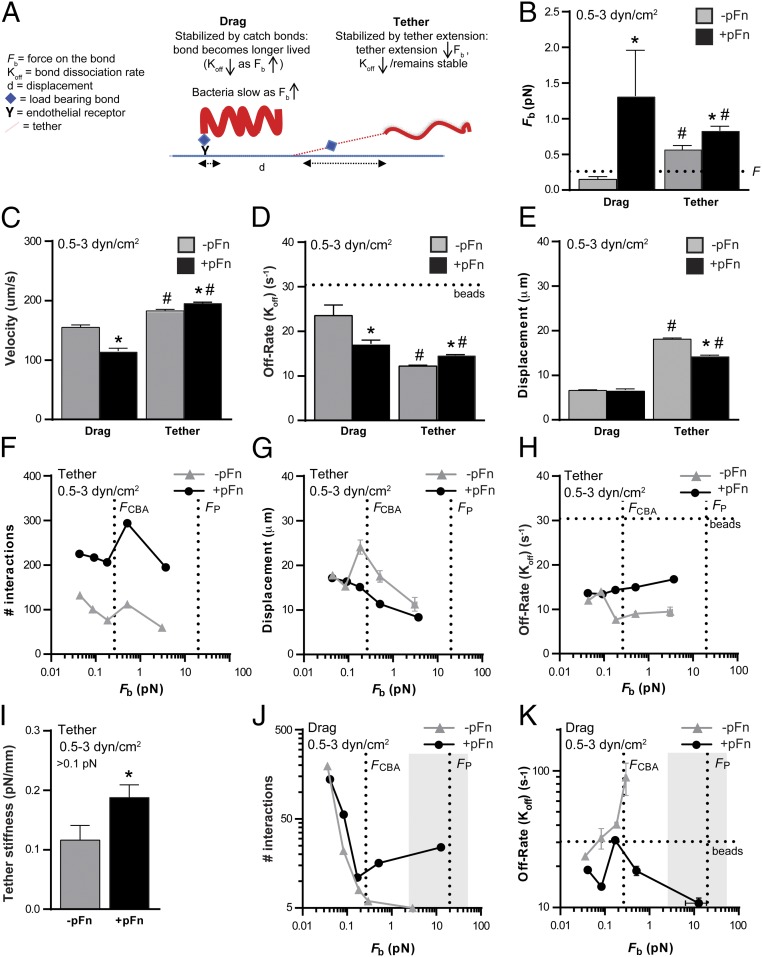
Fig. 1.
Dependence of B. burgdorferi–endothelial interactions under physiological shear stress on pFn. (A) Schematic illustrating initiation steps (tethering, dragging) of the B. burgdorferi–endothelial interaction cascade leading to bacterial transmigration across endothelial barriers into extravascular tissues. Tethering bacteria anchor to endothelial surfaces via tethers, pause repeatedly as they move over endothelial surfaces, but move faster than 100 μm⋅s−1. Dragging bacteria move more slowly (<100 μm⋅s−1) and are untethered. Both tethering and dragging are Fn-dependent in mouse PCVs (18). There are reduced numbers of B. burgdorferi tethering and dragging on primary human endothelial monolayers in flow chambers at typical PCV shear stress (1 dyn/cm2) following treatment with polyclonal anti-Fn antiserum (B) and depletion of pFn from serum in bacterial cultivation medium (C). Numbers of tethering and dragging GFP-expressing B. burgdorferi (strain GCB966) in the presence of nonspecific IgGs or polyclonal αFn IgGs were measured by manual counting. In B, GCB966 was cultivated in the presence of mouse blood before imaging. In C, bacteria were cultivated without mouse blood to eliminate all sources of pFn. In C, −pFn indicates pFn-depleted growth conditions; for +pFn samples, bacteria grown under pFn-depleted conditions were supplemented with human pFn (+pFn) to the concentration present in blood (0.3 mg/mL) before imaging. Summary values: mean ± SEM. Statistics: two-way ANOVA, Holm–Sidak posttest (n = 3 independent bacterial and endothelial cultures per group). *P < 0.05 vs. IgG (B) or −pFn (C) within the same interaction type.
To determine if B. burgdorferi–endothelial interactions in flow chambers were Fn-dependent, we treated bacteria with polyclonal IgGs to Fn from rabbit serum (Fig. 1B), which is a component of B. burgdorferi cultivation medium (34). As observed in mouse dermal PCVs (18), this treatment reduced tethering and dragging at a shear stress typical in PCVs (1 dyn/cm2; Fig. 1B), demonstrating that tethering and dragging interactions with human endothelia under physiological shear stress are also Fn-dependent.
In blood vessels, there are two types of Fn with the potential to support bacterial interactions with vascular surfaces. The first type is the insoluble Fn deposited on the luminal surface of endothelia lining blood vessels (Fig. S1 A and B). The second type is pFn, which is an abundant constituent of blood (∼0.3 mg/mL) and circulates in a soluble, nonadhesive, nonpolymerized state (11, 12). This pFn is ordinarily a compact, nonadhesive dimer in which most ligand-binding sites are buried. Preservation of the inert, nonadhesive properties of this molecule is important in blood, where constitutive exposure of pFn ligand-binding sites would affect blood flow, thrombosis, and movement and adhesion of circulating cells (11, 21). However, upon activation or force-induced stretching or conformational change induced by certain FnBPs, pFn undergoes a conformational change that exposes ligand-binding sites (11, 12, 26, 27, 35).
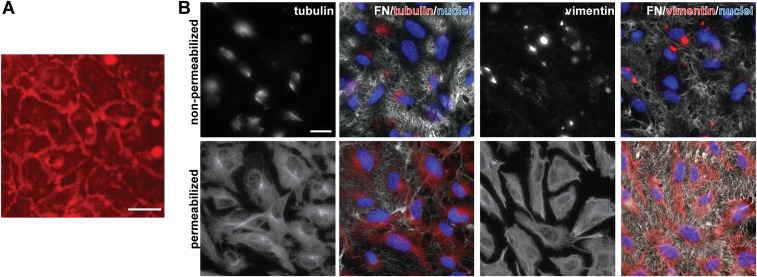
Fig. S1.
Visualization of apical and basolateral localization of Fn on HUVECs. (A) Representative 2-d postconfluent HUVEC monolayer visualized during live cell imaging of B. burgdorferi–endothelial interactions. The HUVECs are counterstained with live cell imaging plasma membrane dye (red). (Scale bar: 38 μm.) (B) Representative IF micrographs depicting Fn on apical (nonpermeabilized) and all (permeabilized) surfaces of 2-d postconfluent HUVECs. Antibodies against Fn (white), tubulin or vimentin (red: internal cellular structures), and DAPI-stained nuclei (blue) were used. (Scale bars: 20 μm.)
The observation that treating B. burgdorferi with IgGs against plasma Fn from the serum used to cultivate bacteria inhibited interactions (Fig. 1B) suggested that the form of Fn supporting B. burgdorferi–endothelial interactions was possibly pFn. To test this hypothesis, we cultivated bacteria in medium depleted of pFn (−pFn) (Fig. S2 A and B), followed by brief incubation with vehicle (−pFn) or with a physiological concentration of pFn (0.3 mg/mL) purified from human plasma (+pFn) (Fig. S2 C and D) before perfusion over endothelia (Movies S1–S4). B. burgdorferi growth in pFn-depleted and complete media was similar (Fig. S2B). Depletion of pFn from bacterial growth medium (Fig. 1C) reduced tethering and dragging as effectively as treatment with polyclonal αFn antibody (Fig. 1B), implying that the insoluble Fn present on surfaces of endothelial cells themselves was not sufficient to support normal levels of bacterial interaction. Conversely, supplementing bacteria grown in pFn-depleted medium with a physiological concentration of human pFn (0.3 mg/mL) restored interactions (Fig. 1C). Interactions were inhibited by treatment with monoclonal antibodies against sites in human Fn involved in Fn fibrillogenesis and interactions with Fn receptors and B. burgdorferi adhesins (Fig. S3 and Table S1). Under static (no-flow) conditions, brief coincubation of bacteria with pFn caused rapid lengthwise compression of bacterial cell shape (Fig. S4 A and B), and BBK32-expressing B. burgdorferi also induced pFn fibrillogenesis (Fig. S4C) and formation of foci of polymerized pFn at and extending from bacterial cell poles (Fig. S4D). We do not know if the polar sites of pFn foci are due to concentration of BBK32 to bacterial cell poles or to other factors, because the subcellular localization patterns of BBK32 have not yet been defined. Thus, bacteria induced widespread conformational changes in pFn that likely exposed ligand-binding sites in this molecule. Collectively, these results indicated that B. burgdorferi interactions with human endothelia under physiological shear stress were primarily dependent on bacterial recruitment of pFn.
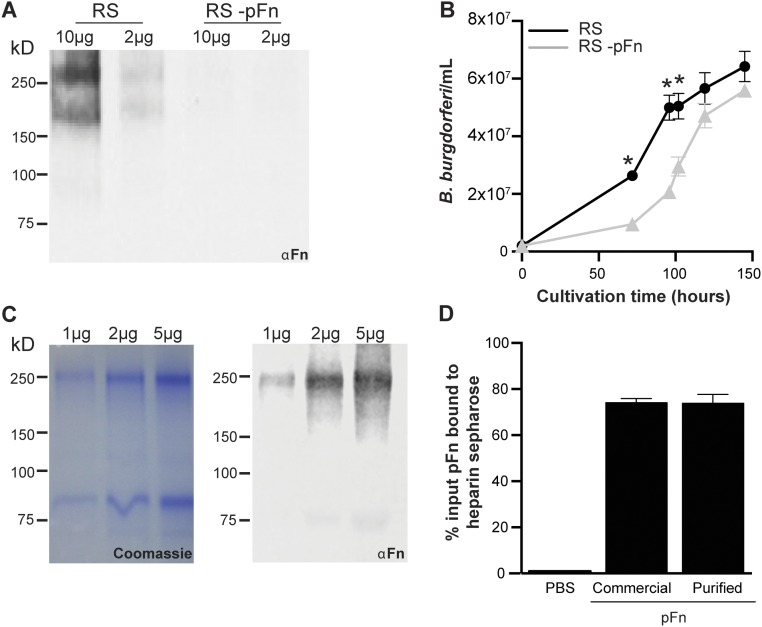
Fig. S2.
Depletion of pFn from rabbit serum used for B. burgdorferi cultivation and purification of pFn from human plasma. (A and B) Effect of depletion of pFn from rabbit serum used for B. burgdorferi cultivation on bacterial growth. (A) Immunoblot analysis of pFn in complete serum (RS) and serum depleted of pFn (−pFn). Values at the top indicate total protein quantities loaded. (B) Growth curves: GFP-expressing B. burgdorferi were inoculated at 2 × 105 cells per milliliter in BSK-II medium containing RS or RS −pFn, and the bacterial concentration was measured over 6 d. The strain used is GCB966. (C and D) Purification of pFn from human plasma and testing of functionality in heparin-binding assays. (C) Visualization of total eluted proteins (Coomassie-stained SDS/PAGE gel) and pFn (immunoblot: αFn) after fractionation of human plasma over protein A Sepharose and gelatin Sepharose columns. Values at the top indicate total protein quantities loaded. (D) Functionality of purified human pFn, tested by comparison with binding of commercial pFn to heparin Sepharose columns in fast protein liquid chromatography assays.
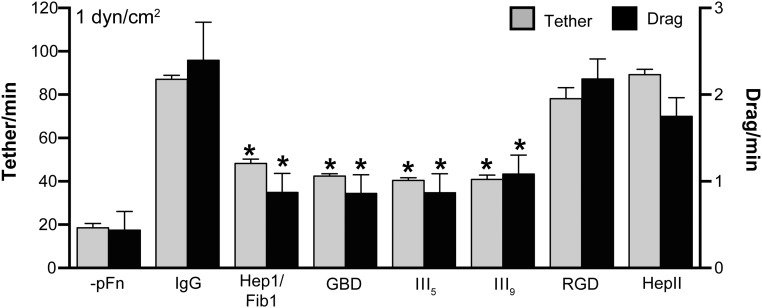
Fig. S3.
Mapping of Fn sites promoting B. burgdorferi–endothelial interactions under flow. We examined the effects of a panel of Fn domain-specific monoclonal antibodies and an Fn-derived Arg-Gly-Asp-Ser (RGDS) competitor peptide (Table S1) on endothelial tethering and dragging interactions of GCB966 B. burgdorferi grown in pFn-depleted medium (−pFn) and on bacteria incubated with 0.3 mg/mL human pFn for 1 h before incubation with equimolar concentrations of antibodies and peptide or nonspecific IgGs (IgG). Two of these antibodies recognize pFn regions [fibrin- and heparin-binding domain (Hepl/Fibl), collagen/gelatin-binding domains (GBD)] that also span the BBK32 interaction site: N-terminal HepI/FibI and an epitope in GBD that contain binding sites for B. burgdorferi FnBPs, including BBK32 and RevA (25, 26, 48), and that also play crucial roles in Fn fibrillogenesis (11). Unsurprisingly, these antibodies reduced bacterial–endothelial interactions under flow, implying that binding of B. burgdorferi FnBPs and/or pFn fibrillogenesis contributed to interactions. We also found that two antibodies directed against mechanosensitive sites in the cell-binding domain of pFn, Fn type III repeat 5 (FnIII5) and synergy site-containing type III repeat 9 (FnIII9), inhibited B. burgdorferi–endothelial interactions under physiological shear stress. FnIII5 interacts with α4β1 and α4β7 integrins in an Arg-Gly-Asp (RGD)-independent fashion and participates in Fn fibrillogenesis (11, 46, 50). FnIII9 and the integrin synergy site it contains are involved in fibrillogenesis and formation of RGD-mediated catch-bond interactions with integrin α5β1 (11, 13, 14, 16). The pFn-dependent bacterial–endothelial interactions were not inhibited by an RGDS peptide, similar to results reported for B. burgdorferi interactions with PCVs in mice (18), or by an antibody against the Fn heparin II (HepII) domain. Although this result suggested the possibility that interactions were not mediated by RGD-dependent integrins but were perhaps dependent on pFn fibrillogenesis itself or on one of the many known RGD-independent integrins (51), we could not exclude the possibility that RGD-dependent integrins were involved in interactions, because RGDS peptides have lower affinity for their targets than antibodies (52). Collectively, these data suggested that regions involved in Fn fibrillogenesis and/or mechanosensitive integrin-binding sites in pFn contributed to B. burgdorferi–endothelial interactions under physiological shear stress. Antibody and peptide details are provided in Table S1. Summary values: mean ± SEM. Statistics: two-way ANOVA, Holm–Sidak posttest (n = 3 independent bacterial and endothelial cultures). *P < 0.05 vs. IgG within the same interaction type.
Table S1.
Antibodies and peptides used in experiments
| Antibody targets, clones, and peptide | Source | Refs. |
| Nonspecific goat IgGs | Cappel/MP Biomedicals, catalog no. 64143 | |
| Goat anti-rabbit pFn IgG (polyclonal) | Cappel/MP Biomedicals, catalog no. 55632 | |
| Fn: N-terminal fibrin- and heparin-binding domain (HepI/FibI) (clone 2Q610) | US Biological, catalog no. 4215-90 | (54) |
| Fn: Epitope in gelatin-binding domain (GBD) (clone 2Q618) | US Biological, catalog no. 4215-90 | (55–57) |
| Fn: Fifth type III repeat (clone IST-4) | Sigma–Aldrich, catalog no. F0916 | (58) |
| Fn: Cell-binding fragment (CBD), ninth type III repeat (clone FN30-8) | Takara Bio, Inc., catalog no. AA11504Z | (59) |
| Fn: Heparin-binding domain II (HepII/FNIII12–14) (clone A32) | Thermo Fisher, catalog no. 005-32-02 | (60, 61) |
| Fn: Arg-Gly-Asp-Ser (linear RDGS peptide) | Sigma–Aldrich, catalog no. 9041 | (60–62) |
| Rabbit anti-human Fn (polyclonal) | Sigma–Aldrich, catalog no. F3648 | |
| Goat anti-rabbit IgG (Alexa Fluor 568) (polyclonal) | Life Technologies, catalog no. 1101 |
CBD, cell-binding domain; FNIII12–14, Fn type III repeats 12–14.
Fig. S4.
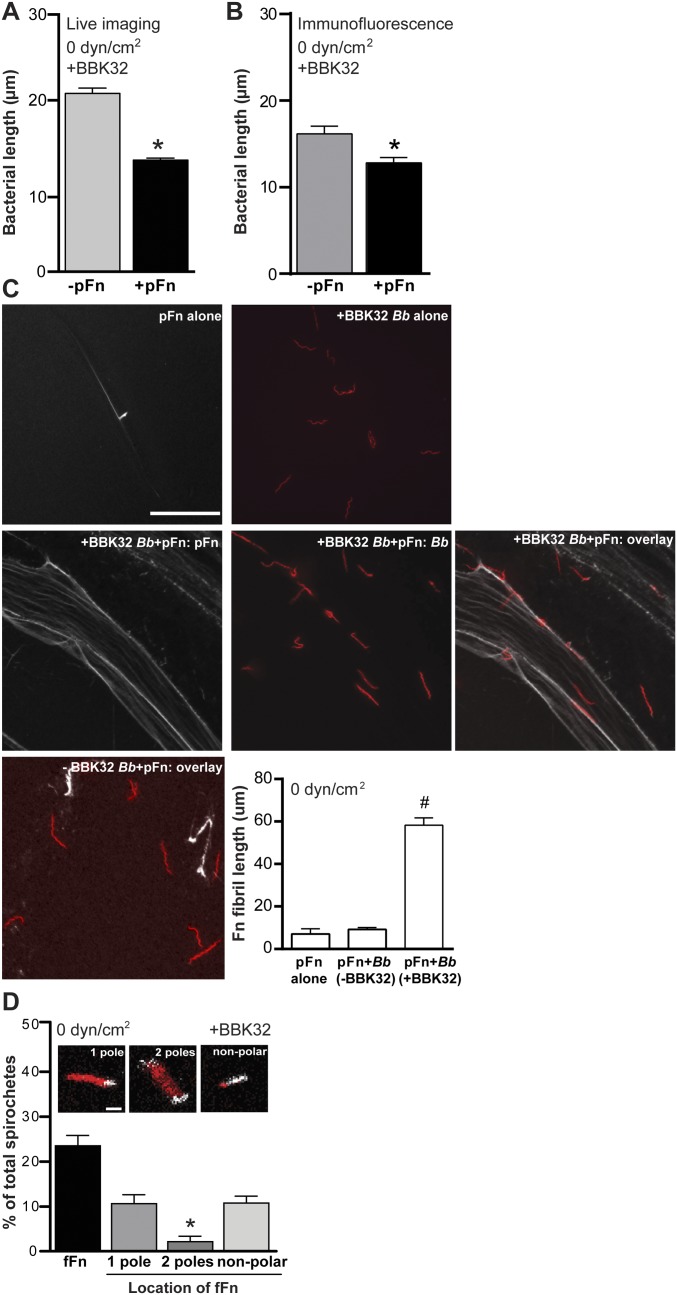
Induction of pFn fibrillogenesis and polymerization by BBK32-expressing B. burgdorferi. Under static conditions (0 dyn/cm2) and in IF experiments, coincubation of BBK32-expressing B. burgdorferi with pFn induces lengthwise bacterial compression (A and B), pFn fibrillogenesis (C), and accumulation of polymerized pFn at bacterial cell poles (D). The pFn fibrillogenesis and localization of polymerized pFn were visualized by IF for GCB966 or GCB971 coincubated with 0.3 mg/mL nonfluorescent pFn (white) in C, and by live cell imaging of Tomato-expressing BBK32-expressing GCB776 B. burgdorferi (red) coincubated with 0.3 mg/mL HiLyte-labeled fluorescent pFn (white) in D. GCB966 and GCB776 are isogenic but express different fluorescent proteins. (Scale bars: C, 20 μm; D, 5 μm.) Quantification in C shows mean ± 95% confidence interval (CI) fibril lengths of pFn alone, incubated with GC966 or GCB971. #P < 0.05 vs. pFn alone. Quantification in D shows the percentage of bacteria associated with polymerized fFn (fFn), and the percentage of bacteria for which polymerized fFn localizes to one pole, two poles, or in a nonpolar fashion (extends beyond poles or not at poles). *P < 0.05 vs. one pole. Summary values: mean ± 95% CI. Statistics: two-tailed unpaired t test (A and B), one-way ANOVA with Holm–Sidak posttest (C), and two-way ANOVA with Holm–Sidak posttest (D) (n = 3 independent bacterial cultures/experiment). Numbers of bacteria quantified: ≥163 per group (A), ≥48 per group (B), and 188 (D). Numbers of Fn fibrils measured in C: 19 (pFn alone) and 186 (BBK32-expressing B. burgdorferi + pFn).
Polymerized pFn Foci Formed on BBK32-Expressing B. burgdorferi Localize to Sites on Bacteria Where Adhesion Complexes Are Mechanically Loaded.
Conformational changes associated with pFn polymerization expose many ligand-binding sites that are cryptic in pFn (11). Because BBK32-expressing B. burgdorferi formed “caps” of polymerized pFn at and extending from bacterial cell poles (Fig. S4D), and because ligand-binding sites in pFn are more likely to be exposed in these foci, we investigated whether pFn foci colocalize with positions on bacteria that contact endothelial surfaces and support mechanical load during interactions (Fig. 2).
Fig. 2.
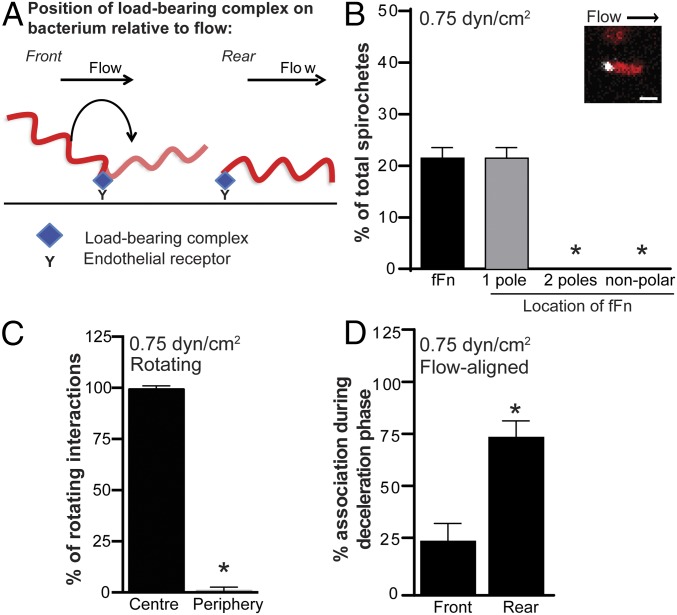
Localization of polymerized pFn to mechanically loaded adhesion sites on bacteria interacting with endothelia under shear stress. Under physiological shear stress, polymerized pFn localizes to the adhesion sites on bacteria that bear the mechanical load during endothelial interactions. (A) Schematic showing the positions on bacteria (front vs. rear, relative to flow direction) that are mechanically loaded when bacteria rotate during endothelial adhesion under flow (front-loaded) or are flow-aligned during adhesion (rear-loaded). B. burgdorferi that form adhesions at the front of the bacterial cell rotate/flip under flow. B. burgdorferi anchored to endothelia at the rear of bacteria align with flow. (B) Polymerized fFn (Inset, white) is redistributed exclusively to one cell pole of GCB776 bacteria (Inset, red) during endothelial interactions at 0.75 dyn/cm2 (n = 64 bacteria). *P < 0.05 vs. one pole. (Scale bar: 5 μm.) (C) Polymerized fFn localizes exclusively to the center of bacterial cell rotation (load-bearing adhesion site) for B. burgdorferi that rotate during endothelial interactions (n = 10 rotating bacteria). *P < 0.05 vs. localization at the center of rotation. (D) Polymerized fFn localizes primarily to the rear, load-bearing site when bacteria are decelerating (i.e., when they adhere to endothelia) (n = 17 flow-aligned bacteria). *P < 0.05 vs. localization at front. Summary values in all panels: mean ± 95% confidence interval (CI) (n = 3 independent bacterial and endothelial cultures per experiment). Statistics: two-way ANOVA, Holm–Sidak posttest (B) and two-tailed Wilcoxon matched pairs t tests (C and D).
B. burgdorferi interacts with endothelia by transferring mechanical load along a series of single adhesion complexes or tightly clustered and coordinated receptor–ligand complexes that dissociate with the kinetics of a single adhesion complex (7). As a result, only one physical site on a bacterium is involved in load-bearing interactions with endothelia at any given time, and that mechanical load is borne by only one receptor–ligand adhesion complex at this site or by a tightly spatially clustered and coordinated group of complexes at this site that behave kinetically as a single complex. On bacteria that flip as they adhere to endothelia under flow, the load-bearing site is located at the cell pole closest to the center of rotation (Fig. 2A). On bacteria that are aligned parallel to flow during endothelial interactions, the load-bearing site is typically located at the rear of bacteria that are decelerating (i.e., adhering to endothelia) (Fig. 2A). These properties permitted us to determine if foci of polymerized, fluorescent pFn visualized on bacteria by live cell imaging (Fig. 2B) colocalized with sites of mechanical loading on these bacteria during endothelial interactions under flow (Fig. 2 C and D). Although small “specks” of fluorescent pFn were observed at the poles of many bacteria under these conditions, to determine the localization of pFn foci unambiguously with respect to load-bearing adhesion sites, we confined our analysis to bacterial pFn foci with diameters >1 μm, which were observed on ∼20% of bacteria interacting with endothelia under flow (Fig. 2B). Experiments were performed at 0.75 dyn/cm2, a typical shear stress in dermal PCVs (33), because bacteria flip more frequently at this shear stress than at higher shear stresses. These analyses revealed that 99.2% of polymerized pFn foci localized to the load-bearing pole at the center of rotation in flipping bacteria (Fig. 2C), and that ∼75% of foci localized to the load-bearing rear of decelerating bacteria that interacted with endothelia in a flow-aligned fashion (Fig. 2D). These results implied that polymerized pFn localized nonrandomly to load-bearing adhesion sites on both flipping and flow-aligned bacteria, and was therefore likely part of load-bearing adhesion complexes.
The pFn Increases Mechanical Load Sustained by Adhesion Complexes Under Vascular Shear Stress.
Another way to determine if pFn is part of force-loaded adhesion complexes on B. burgdorferi interacting with endothelia is to determine if the force sustained by load-bearing bonds is altered by the presence of pFn (i.e., if pFn affects bond strength). To investigate the effect of pFn on bond strength, we grew B. burgdorferi in pFn-depleted conditions, incubated bacteria briefly with vehicle alone (−pFn) or with 0.3 mg/mL pFn (+pFn), and then perfused bacteria over endothelial monolayers at a range of increasing shear stresses found in PCVs (3) (0.5–3 dyn/cm2; Fig. 3). Interactions were measured from 0.5 to 3 dyn/cm2 because B. burgdorferi–endothelial interactions are progressively stabilized by a force-stimulated catch-bond mechanism as shear stress and force on load-bearing bonds increase over this range, especially from 0.5 to 1 dyn/cm2 (7). Force-resistant and force-stimulated adhesion mechanisms are particularly important for stabilizing the interaction of circulating cells with endothelial surfaces in the vasculature, where shear stress varies even within one blood vessel, and where small linear changes in shear stress cause conventional, force-sensitive adhesive bonds to fail at an exponential rate (Fig. 3A). Both tethering and dragging interactions were more numerous in the presence of pFn, particularly at 1 dyn/cm2 (Fig. 3 B–D), and +pFn interaction numbers increased at 1 dyn/cm2 (Fig. 3 B and C), a shear stress at which catch-bond properties of BBK32-dependent B. burgdorferi–endothelial adhesion complexes are activated (7).
Fig. 3.
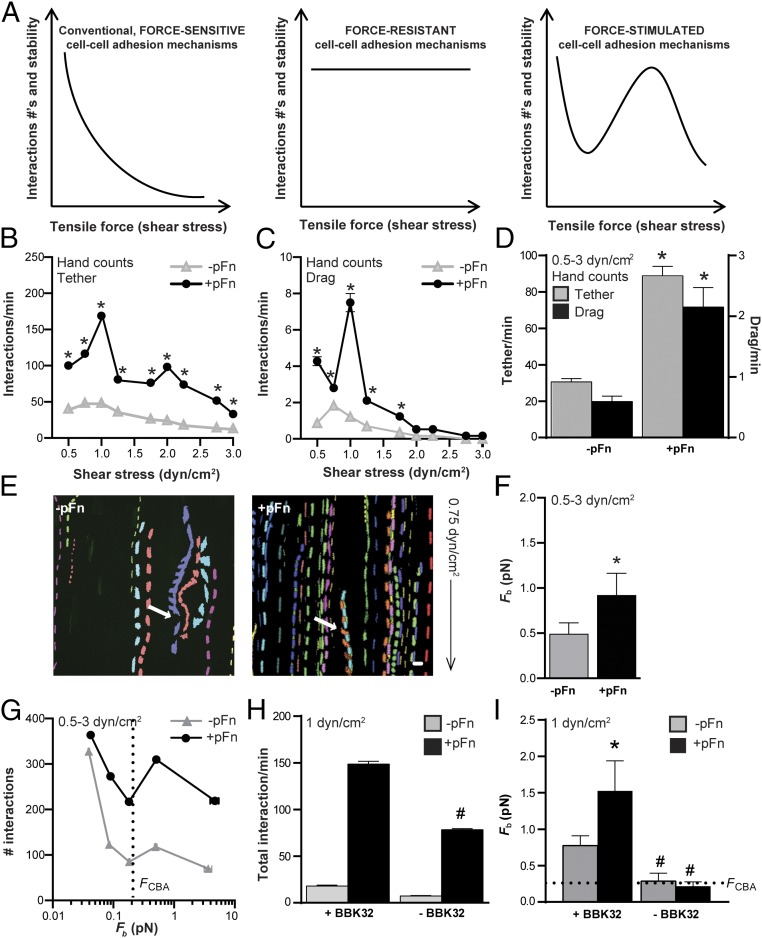
Effect of pFn on force sustained by load-bearing adhesion complexes. (A) Conventional, force-sensitive adhesion complexes fail at exponential rates with linear increases in tensile force such as shear stress (Left), whereas force-resistant complexes remain stable (Center) and force-stimulated complexes become more stable (longer lived) as tensile force increases above an activation threshold (Right) (43). Force-stimulated adhesion complexes eventually fail, but at a higher force than conventional complexes. In vascular environments, where shear stress varies, force-dependent adhesion complex strengthening promotes interaction of circulating cells with vascular surfaces over a broader shear stress range. pFn stimulates mean ± SEM. Tethering (B) and dragging (C) interactions of BBK32-expressing B. burgdorferi (GCB966) with endothelia over a typical PCV shear-stress range (0.5–3 dyn/cm2) are shown. (D) Global mean ± 95% CI interactions for all shear-stress conditions combined. *P < 0.05 vs. −pFn within interaction type (two-way ANOVA, Holm–Sidak posttest) (n = 6 independent bacterial cultures per shear-stress condition, for a total of 54 replicates per global mean). (E–I) Load-bearing adhesion complexes can withstand greater force in the presence of pFn and the pFn fibrillogenesis-inducing B. burgdorferi adhesin BBK32. (E) Sample time-lapse projections of interaction trajectories (tracks) for individual bacteria (depicted in different colors) captured by particle tracking over 2 min. White arrows indicate rotating B. burgdorferi interacting by one end. The black arrow indicates flow direction. (F) Effect of pFn on global mean (±95% CI) Fb for endothelial interactions of BBK32-expressing bacteria shown in D. (G) Numbers of tracked interactions at indicated mean ± SEM Fb values. Mean ± SEM total interactions per minute (H) and mean ± 95% CI bond forces (I) at 1 dyn/cm2 are shown for BBK32-expressing parental bacteria (+BBK32: GCB966) and an isogenic BBK32-deficient strain (–BBK32: GCB971) (n ≥ 724 individual interactions analyzed per group). Statistics: two-tailed unpaired Mann–Whitney test (E–I) and two-way ANOVA with Holm–Sidak posttests (H and I). *P < 0.05 vs. −pFn within group; #P < 0.05 vs. +BBK32 within group.
To determine if pFn affected the stability and amount of force sustained by load-bearing bonds supporting bacterial-endothelial interactions, we used particle-tracking methods for tracking and measuring the physical properties of individual B. burgdorferi interacting with endothelia (Fig. 3E) to estimate the average force imposed on load-bearing bonds during interactions (Fb), as described previously (3, 7, 36) and in Fig. S5. All −pFn and +pFn interactions exhibited dissociation kinetics characteristic of single force-loaded adhesion complexes (Fig. S5D), implying that although pFn at the load-bearing interaction site on each bacterium was polymerized (Fig. 2), each interaction was nevertheless dependent on loading of a single adhesion bond, or of closely physically clustered and coordinated complexes that behaved as a single bond. These analyses revealed that load-bearing bonds formed in the presence of pFn sustained significantly greater average force than load-bearing bonds formed under −pFn conditions (Fig. 3F). Furthermore, at all but the smallest bond forces, +pFn interactions were more abundant than −pFn interactions (Fig. 3G). The pFn-dependent stimulation of interactions and increased bond strength at 1 dyn/cm2 were dependent on the pFn-polymerizing, catch-bond–conferring adhesin BBK32 (Fig. 3 H and I and Fig. S4C). Thus, pFn-dependent interactions sustained more force on the load-bearing bond and promoted interactions at higher bond forces, indicating that pFn contributed to and strengthened load-bearing adhesion complexes.
Fig. S5.
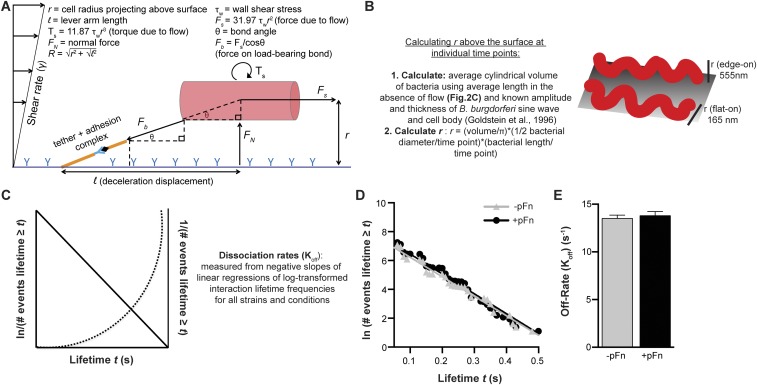
Estimation of forces on load-bearing bonds and bond dissociation rates. (A) Schematic depicting force and torque experienced by B. burgdorferi adhering to endothelia under flow and formulas used to estimate Fb (3). The spirochete bacterium was treated as a cylinder for calculations. (B) Schematic describing the method for calculating vertical projection in the z dimension [radius (r)] of B. burgdorferi above endothelial surfaces from 2D time-lapse imaging data. The B. burgdorferi planar sine wave can be oriented flat on or perpendicular to surfaces (edge-on) (53), as depicted. (C) Schematic depicting expected regressions for lifetimes (t) of the deceleration (load-bearing) phase of interactions if interactions are dependent on single or multiple adhesion complexes. If interactions are mediated by single adhesion complexes, they exhibit first-order dissociation kinetics, and log-transformed cumulative frequency distributions of interaction lifetimes decline in a linear fashion with increasing bond lifetime (36). If interactions are mediated by multiple adhesion complexes, plots of reciprocally transformed lifetimes are linear. Frequency distributions for B. burgdorferi interaction lifetimes decline in a linear fashion, increase exponentially for reciprocally transformed lifetimes, and are thus mediated by single adhesion complexes. (D) Linear regressions of log-transformed interaction lifetime cumulative frequency distributions for all B. burgdorferi interactions from 0.5 to 3 dyn/cm2. Bacteria were cultivated under pFn-depleted conditions and then coincubated with vehicle (−pFn) or 0.3 mg/mL pFn (+pFn) for 60 min before imaging. The strain used is GCB966. The R2 value for both curves is 0.97. (E) Bond dissociation rates for all interactions captured under −pFn and +pFn conditions, estimated from negative slopes and SDs of linear regressions of log-transformed lifetime frequency distributions (36).
pFn Promotes Tethering Interactions by Increasing Bond Formation Rates and Stabilizes Dragging by a Catch-Bond Mechanism.
Finally, we investigated how pFn promotes the two major types of mobile B. burgdorferi interactions with endothelia, tethering and dragging. Tethering and dragging are analogous to the interactions supporting leukocyte rolling on endothelial surfaces, which permit these cells to slow down and extravasate (7) (Fig. 1A). Leukocyte rolling on PCV surfaces is stabilized by force-strengthened selectin catch bonds that become longer lived (more stable) as bond force increases, and by elastic membrane-derived tethers associated with load-bearing adhesion complexes that anchor leukocytes to endothelia and stabilize load-bearing bonds by distributing (sharing/reducing) the force imposed on adhesion complexes (3). B. burgdorferi–endothelial interactions at PCV shear stress are also stabilized by BBK32-dependent catch-bond properties and by tethers, although the source and composition of tethers are unknown (7). Tethers stabilize B. burgdorferi-tethering interactions with endothelia by anchoring bacteria to surfaces and reducing the force imposed on load-bearing bonds, which reduces bond dissociation rates (Koffs) and prolongs bond lifetime (7) (Fig. 4A). B. burgdorferi-dragging interactions with endothelia are untethered, and they are subjected to larger bond forces and dissociate more rapidly than tethered interactions (7) (Fig. 4A). Although the bonds supporting dragging interactions are shorter lived than the bonds mediating tethered interactions, the velocity of dragging B. burgdorferi is considerably slower, because bacteria move forward in small, inchworm-like steps, whereas tethered interactions displace much further due to elongation of their anchoring tethers (7) (Fig. 4A). BBK32-dependent catch bonds are particularly important for increasing the number and stability of B. burgdorferi-dragging interactions with endothelia as shear stress increases (7).
Fig. 4.
Stabilization of pFn-dependent B. burgdorferi–endothelial interactions by a catch-bond mechanism. Tethered B. burgdorferi interactions are more numerous in the presence of pFn at all bond forces. Untethered dragging interactions become longer lived as force increases in the presence of pFn, indicating that pFn strengthens dragging interactions by a catch-bond mechanism. (A) Schematic summarizing differences in physical interaction properties of dragging and tethering B. burgdorferi (7). B. burgdorferi are shaped like a planar sine wave. Fbs and Koffs are greatest during dragging, when bacteria project above surfaces in an edge-on conformation (Fig. S5B). Tethering bacteria lie flatter against surfaces, and are anchored to endothelia by tethers, which reduce force on load-bearing bonds and increase bond lifetime. Global mean Fbs (B), velocity (C), Koffs (D), and displacement (E) are shown for tethering and dragging interactions. Tethering interaction numbers (F), displacement (G), and dissociation rates (H) are shown at indicated Fb values. (I) Estimated tether stiffness for tethered interactions with bond forces >0.1 pN. Dragging interaction numbers (J) and Koffs (K) are shown at indicated Fbs. Dotted lines: beads (Koff for negative control beads; bacterial interactions dissociating as fast or faster than beads are interacting nonspecifically), FCBA (bond force at BBK32-dependent catch bonds are activated) (7), and Fp (bond force at which proteins that are unanchored to cytoskeletal structures are plucked from lipid bilayers; indicates typical maximum bond force for BBK32-dependent interactions). Gray shading in J and K indicates the catch-bond force regime of Fn–α5β1 complexes, where bond dissociation slows as force increases. The strain used is GCB966 in all panels. Summary values: mean ± 95% CI. Statistics: two-way ANOVA, Holm–Sidak posttest. *P < 0.05 vs. −pFn within interaction type; #P < 0.05 vs. drag within group. Independent bacterial and endothelial cultures per group (n = 6 per shear stress condition for total of 54 replicates per group; n ≥ 481 and n ≥ 241 individual interactions analyzed per group for tethering and dragging, respectively).
We found that although bond forces sustained by tethered bacteria were somewhat greater in the presence of pFn compared with −pFn controls (Fig. 4B), tethering bacteria also moved faster (Fig. 4C) and bonds dissociated slightly more quickly (Fig. 4D) after shorter displacement distances (Fig. 4E). These observations suggested that in the presence of pFn, tethered interactions were less stable and that increased numbers of tethering interactions under +pFn conditions (Fig. 3D) were possibly due to increased bond formation rates. To test this hypothesis, we examined tethered interaction numbers over a range of bond forces, and found that interaction abundance profiles under −pFn and +pFn conditions were similar and that tethered interactions were consistently more abundant at all bond forces (Fig. 4F). These findings suggested that tethered interactions formed more readily in the presence of pFn. Examination of tether extension as a function of bond force also revealed that although force-dependent elongation of tethers at 0.1–0.2 pN was prominent under −pFn conditions, this elongation was absent in the presence of pFn (Fig. 4G), and that the slowing of bond dissociation observed for −pFn interactions at the tether extension threshold did not occur for +pFn interactions (Fig. 4H). Estimates of the stiffness of tethers anchoring bacteria to endothelia showed that tethers formed in the presence of pFn were also stiffer (Fig. 4I). Collectively, these data indicated that tether elongation and associated bond stabilization occurred less readily in the presence of pFn, and that stimulation of tethering interactions by pFn was likely due instead to increased rates of bond formation.
By contrast, in the presence of pFn, dragging interactions moved significantly more slowly (Fig. 4C), primarily due to slowing of bond dissociation (Fig. 4D), and also sustained much larger bond forces (Fig. 4B). Thus, pFn strengthened, stabilized, and slowed untethered interactions. These findings suggested the possibility that pFn promoted untethered interactions by a catch-bond mechanism. Catch bonds are bonds that become longer lived (dissociate more slowly) as bond force increases, and they are important for strengthening Fn–integrin interactions under tension and stabilizing selectin-mediated leukocyte rolling on PCVs under shear stress (4, 13, 14, 16, 17, 37–40), as well as BBK32-dependent B. burgdorferi–endothelial interactions under vascular shear-stress conditions (7). BBK32-dependent catch bonds are activated at a slightly higher bond force (∼0.25 pN) than the force threshold at which tether elongation occurs (7) (0.1–0.2 pN; Fig. 4G). To determine if pFn promoted untethered interactions by a catch-bond mechanism, we examined the abundance (Fig. 4J) and stability (Fig. 4K) of dragging interactions over a range of bond forces. In the absence of pFn, only tethering interactions (Fig. 4F), but not dragging interactions (Fig. 4J), were observed at bond forces greater than ∼0.2 pN, implying that without pFn, B. burgdorferi could not interact with endothelial cells at forces higher than this threshold unless interactions were stabilized by tethers. In the absence of pFn, interaction numbers also decreased exponentially and dissociated at exponentially faster rates, with linear increases in bond force above the BBK32 catch-bond activation threshold (∼0.25 pN; Fig. 4 J and K). Thus, without pFn, interactions exhibited kinetics characteristic of conventional slip bonds, which become less stable under increasing force. However, when pFn was present, dragging interactions exhibited kinetics characteristic of catch bonds at bond forces greater than ∼0.2 pN; that is, dragging interaction numbers progressively increased and bonds became progressively longer lived as force increased (Fig. 4 J and K). Together, these data indicated that B. burgdorferi could not interact with endothelial cells at bond forces greater than ∼0.2 pN in the absence of pFn unless interactions were stabilized by tethers, and that pFn stabilized untethered interactions above this force threshold by a catch-bond mechanism. The force regime over which +pFn-dependent catch bonds were formed was comparable to the catch-bond force regimes of BBK32 and Fn–α5β1 complexes, as well as leukocyte selectins (4, 7, 13, 16, 17, 37).
Discussion
These results show that B. burgdorferi exploits pFn, an abundant constituent of blood, to facilitate bacterial interactions with endothelia under vascular shear stress. pFn increased the shear-stress range of B. burgdorferi–endothelial interactions and the force sustained by load-bearing bonds in BBK32-expressing bacteria (Fig. 3), increased the abundance of all bacterial–endothelial interactions (Fig. 3), and stabilized and slowed dragging interactions by a catch-bond mechanism (Fig. 4). These effects may promote B. burgdorferi slowing and extravasation over a wider range of shear-stress conditions in the vasculature, and possibly in a wider range of tissues. It is also possible that pFn recruitment may promote endothelial interactions under vascular shear-stress conditions for other bacterial pathogens. Bacterial exploitation of pFn to facilitate endothelial interactions under flow may increase the risk of pathological infection outcomes such as endocarditis, colonization of cardiac and other devices, bacterial dissemination to secondary infection sites, and possibly immune evasion secondary to invasion of endothelial cells themselves (12, 20, 22).
BBK32 binds to the N-terminal fibrillogenesis region of Fn by the same tandem β-zipper mechanism exhibited by FnBPs from genetically distant staphylococcal and streptococcal bacteria, suggesting the possibility that FnBPs from other disseminating bacterial pathogens that also induce structural rearrangement of pFn (12, 21) have the potential to strengthen and stabilize bacterial-vascular interactions by a catch-bond mechanism. For one of these adhesins, S. aureus FnBPA, polymorphisms that increase the frequency and strength of Fn binding are associated with increased risk of infection of cardiac devices in patients (20, 29). It has not yet been determined if, like BBK32, other FnBPs are capable of inducing Fn fibrillogenesis or forming Fn-dependent catch bonds under mechanical force. This hypothesis warrants investigation, because any mechanism that strengthens bacterial–endothelial interactions under vascular shear stress conditions has the potential to facilitate bacterial adhesion to blood vessel surfaces in vascular beds of organs such as the heart and brain, where forces caused by blood flow are stronger than in other tissues. It has also been assumed that the form of Fn targeted by S. aureus that adheres to cardiac devices is Fn-deposited on the surfaces of these devices (20). However, our results suggest that bacterial recruitment of soluble pFn in the blood also has the potential to support these interactions, especially at sites without abundant deposits of insoluble Fn. Interestingly, BBK32-like FnBPs from streptococcal and staphylococcal species undergo structural unbinding when Fn fibers are stretched (41). It is unknown if BBK32 detaches from pFn fibrils when they are stretched, a property that could contribute to the faster dissociation of BBK32-dependent tethered interactions in the presence of pFn (Fig. 4).
We have not yet defined the molecular mechanisms by which pFn promotes B. burgdorferi–endothelial interactions under vascular shear stress. However, data presented here suggest that pFn likely facilitates interactions in multiple ways. The pFn increased interaction abundance for both BBK32-expressing and bbk32-null bacteria (Fig. 3H), indicating that even in the absence of BBK32-dependent pFn polymerization and interaction stabilization by catch bonds, pFn promotes B. burgdorferi–endothelial interactions under shear stress. This increase in interactions could be because pFn recruitment expands the range of endothelial cell surface molecules (e.g., heparin-sulfated GAGs, integrins, nonintegrin receptors) with which bacteria can interact (11, 21). It is also possible that force-driven Fn polymerization (42) promotes binding of pFn on bacterial surfaces to insoluble Fn deposited on endothelial surfaces. Conformational changes in pFn induced by Fn-binding adhesins, and possibly by forces induced by nonspecific contacts between pFn-coated bacteria and the endothelial glycocalyx/endothelial cells, are almost certainly required for pFn-dependent interactions, because many ligand-binding sites in globular pFn dimers are not exposed until the molecule undergoes conformational change (21).
For BBK32-expressing B. burgdorferi, polymerized pFn foci at bacterial cell poles localized nonrandomly and consistently to mechanically loaded adhesion sites on bacteria (Fig. 2), suggesting that polymerized pFn itself and/or conformational changes associated with pFn polymerization played key roles in BBK32-dependent endothelial interactions under flow. It is possible that pFn polymerization exposed cryptic sites in pFn facilitating binding to endothelial surface receptors, and/or directly strengthened and stabilized adhesion complexes after bond formation. Additionally, BBK32-Fn binding not only induces conformational elongation and polymerization of pFn but also structurally stabilizes a large intrinsically disordered region of BBK32 by a high-affinity tandem β-zipper mechanism (25–27). Thus, by stabilizing BBK32 conformation, pFn may improve this adhesin’s ability to withstand force. Finally, it is possible that BBK32/pFn-dependent interaction stabilization was not exclusively due to structural stabilization of BBK32–pFn complexes but also resulted from force-stimulated conformational changes and activation of high-affinity–binding sites in endothelial receptors targeted by these complexes (Fig. 5). Because Fn interactions with integrin α5β1 are stabilized by a force-activated catch-bond mechanism and by cyclic mechanical reinforcement (13, 14, 16, 17), it is plausible that endothelial receptors themselves also contribute to stabilization of mechanically loaded BBK32–pFn bacterial adhesion complexes. Our preliminary antibody-based mapping of pFn sites mediating interactions suggests that Fn receptors such as α5β1, α4β1, α4β7, and other Fn synergy site-dependent ligands that bind to sequences in Fn regions FnIII5 and synergy site-containing FnIII9 may contribute to interactions; however, because these regions are also involved in Fn fibrillogenesis, we cannot be sure that the effects of antibodies directed against these sites are not also due to disruption of pFn polymerization (Fig. S3). Despite the complexity of the potential molecular mechanisms underlying pFn-dependent B. burgdorferi-endothelial interactions, defining these mechanisms will be important for understanding dissemination mechanisms of B. burgdorferi and possibly other bacterial pathogens.
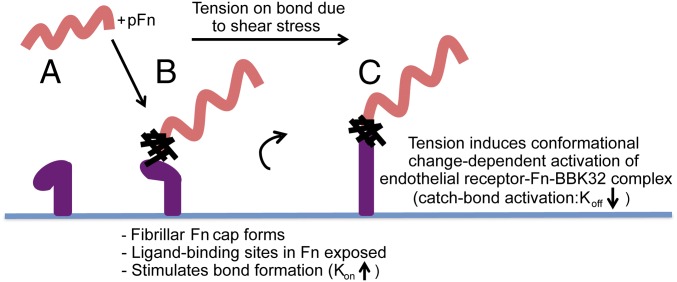
Fig. 5.
Proposed model: pFn-dependent mechanisms promoting B. burgdorferi–endothelial interactions under shear stress. BBK32-expressing B. burgdorferi (A) induces pFn conformational changes and polymerization, exposing binding sites for endothelial receptors (possibly integrins) (B). The exposure of ligand-binding sites increases interaction numbers by increasing the bond formation rate (Kon). (C) When the load-bearing adhesion complex is stretched as bacteria are pushed/pulled by shear stress, additional force-induced conformational changes in adhesion complex components (BBK32, pFn, and endothelial receptors) promote formation of a higher affinity, longer lived catch bond.
Cell–cell interactions in the vasculature depend on mechanically specialized interactions that can overcome shear stress due to blood flow. For disseminating bacteria, the mechanisms supporting these interactions have remained largely undefined for most pathogens. Most disseminating bacterial pathogens have been found to bind Fn, and many of these bacterial pathogens bind to pFn. The results of this study show that the ability to recruit pFn from blood provides a mechanical advantage to B. burgdorferi interacting with endothelial surfaces under physiological shear stress, and may thus facilitate bacterial escape from the vasculature and colonization of extravascular tissues. It is possible that similar exploitation of pFn may also prove important for the dissemination mechanisms of other pathogens.
Materials and Methods
Materials and methods for Figs. S1–S5 are discussed in SI Materials and Methods.
Ethics Statements.
This study conforms to the principles outlined in the most recent policies of The Canadian Council on Animal Care. Animal work was approved by the University of Toronto Animal Care Committee in accordance with institutional guidelines (Protocol 20011501). The authors declare no competing financial or other conflict of interest.
Endothelial Cell Cultivation and Labeling for Live Cell Imaging Flow Chamber Experiments.
As described elsewhere (7), all experiments were performed with low-passage primary human umbilical vein endothelial cells pooled from multiple donors (catalog no. CC-2519A; Lonza, Inc.). Endothelial cells (∼1.59 × 105 cells) were plated into each channel of an ibiTreat hydrophilic tissue culture-treated Ibidi μ-Slide VI0.4 (Ibidi GmbH), grown to 2 d postconfluence, and were labeled with CellMask Deep Red live cell imaging dye (Life Technologies) before imaging.
B. burgdorferi Strains, Cultivation, and Preparation for Live Cell Imaging.
Bacterial strains GCB966, GCB776, and GCB971 (Table S2) were grown in Barbour-Stonner-Kelly-II (BSK-II) medium containing 6% heat-inactivated whole or pFn-depleted rabbit serum and 100 μg/mL gentamicin, and were prepared for imaging by washing and resuspension to 1 × 108 cells per milliliter in HBSS as described elsewhere (7, 19). Bacteria used in polyclonal αFn antibody experiments were cultivated in 1% mouse blood, 100 μg/mL gentamicin, 20 μg/mL phosphomycin, 50 μg/mL rifampicin, and 2.5 μg/mL amphotericin for 48 h before preparation for imaging, as described elsewhere (33). Blood was obtained by cardiac puncture with heparinized needles in C57BL/6 mice (Charles River Laboratories) anesthetized with 10 mg/kg xylazine (MTC Pharmaceuticals) and 200 mg/kg ketamine hydrochloride (Rogar/STB). Bacterial cultures that were grown in rabbit serum depleted of pFn were reconstituted with vehicle (saline) or physiological concentrations (0.3 mg/mL) of pFn or HiLyte Fluor 488-labeled pFn (fFn; Cytoskeleton, Inc.) using 1 mg/mL purified pFn stocks, incubated at 4 °C for 1 h, stored on ice, and diluted to 1 × 108 cells per milliliter in HBSS before imaging. All flow chamber experiments were performed with bacteria resuspended in HBSS alone (no added serum), except the experiments reported in Fig. 1B, in which heat-inactivated FBS was added to a 10% final concentration. Perfusion of bacteria over endothelial monolayers at 0.5–3 dyn/cm2 was performed using a syringe pump, as described elsewhere (7). The average length of bacteria following incubation with pFn or vehicle was calculated from all measurements obtained during 1-min time-lapse video-recordings of bacteria adhering to endothelia under static conditions.
Table S2.
B. burgdorferi strains used in experiments
| Strain | Details | Refs. |
| GCB966 | B31 ML23-derived infectious strain transformed with GFP-expressing pJW201 (PflaB-GFP/bbe22) | (19, 63) |
| GCB776 | B31 5A4 NP1-derived infectious strain transformed with Tomato-expressing pTM201 (PflaB-Tomato) | (64) |
| GCB971 | B31 ML23-derived bbk32::strR bbk32 strain transformed with GFP-expressing pJW201(PflaB-GFP/bbe22), which does not express BBK32 | (19, 63) |
Live Cell Imaging Microscopy Conditions.
All flow chamber experiments except those experiments reported in Fig. 2 and Fig. S4 were performed as described elsewhere (7) on a Quorum Spinning Disk Confocal microscope (Quorum Technologies, Inc.) equipped with a Zeiss 25×/0.8-N.A. water immersion lens at the Hospital for Sick Children Imaging Facility (Toronto, ON, Canada). Images were acquired at 100% laser power and maximum sensitivity, at 14–15 fps, and at 0.375 μm per pixel, using Volocity software v.6.3.0 (Improvision/PerkinElmer). The experiments reported in Fig. 2 and Fig. S4 were performed by resonant scanner microscopy in a bidirectional resonant scanning mode (8,000 Hz), with an aperture size of 0.95, using a tandem resonant scanner TCS SP8 confocal microscope (Leica) equipped with argon, HeNe, and diode-pumped solid-state (DPSS) lasers; triple dichroic (TD) 488/561/633 excitation beam splitters; hybrid detector (HyD) spectral detectors; and an HCX IRAPO L 25×/0.95-N.A. water immersion objective (Leica). Time courses were acquired at 512 × 512 pixels, 0.61 μm per pixel, and ∼15 fps, with line averaging of 2. Excitation/emission wavelengths for fFn were 488 (argon laser 100% power) and 498–550 nm, respectively, and gain was set to 23. Excitation/emission wavelengths for Tomato-expressing B. burgdorferi were 561 (DPSS laser, 37.45% power) and 571–610 nm, respectively, with gain set to 10. Image acquisition software was Leica AF version 3.0.0, build 8139, and offline analysis was performed using Leica LAS software. Cells were kept warm during imaging with an infrared heat lamp.
Localization of Polymerized fFn on Live B. burgdorferi.
The percentage of Tomato-expressing B. burgdorferi associated with polymerized fFn (where fFn colocalized and moved with bacteria) in time-lapse video-recordings was counted manually, after coincubating bacteria grown under Fn-depleted conditions with 0.3 mg/mL fFn for 1 h at 4 °C. Bacteria for which the presence, absence, and/or localization of fFn could not be determined unambiguously were excluded from analysis. The following localization parameters were manually scored for B. burgdorferi-associated fFn at each time point in time-lapse series: unipolar, bipolar, or nonpolar localization; localization to the front or rear of bacteria relative to flow direction for bacteria aligned with flow during interactions; and localization to the center or periphery of rotation for bacteria that rotated or flipped as they moved. To determine whether bacteria were accelerating or decelerating, we scored whether bacteria at each time point were moving faster or slower than at immediately preceding time points, based on tracking-based velocity measurements.
Quantitative Image Analysis and Measurement of Biophysical Interaction Properties.
All images used for quantification were acquired under identical imaging conditions (identical among all groups reported in each experiment), and no postacquisition image processing was performed before quantification. Tethering and dragging interactions were manually counted in a 30 × 100-μm region of interest at the center of the field of view, as described elsewhere (7). As described in detail recently, bacterial–endothelial interaction trajectories were tracked offline using centroid-based particle-tracking methods and Volocity software. Tracks used for subsequent analyses were tracks with velocities <300 μm⋅s−1, which is <40% of the velocity of control beads in PCVs and flow chambers at similar shear-stress conditions (7, 33). As described in detail recently (7), the duration (lifetime), velocity, and displacement of bacterial interactions during adhesion to endothelia (deceleration phase of interactions), as well as changes in bacterial length and diameter during interactions, were measured for interaction tracks obtained from time courses and used to estimate interaction Koffs and the force sustained by load-bearing bonds (Fb). As described elsewhere (7, 36, 37), to estimate interaction Koff rates, natural logarithms of frequencies of individual adhesion events with lifetimes ≥t (lifetime duration) were plotted against t, and curves were fit with straight lines by nonlinear regression in GraphPad Prism (GraphPad Software). R2 values for lines were ≥0.9. Goodness of fit was evaluated by runs tests (P > 0.05 for all runs tests, indicating nonsignificant deviation from linearity). Interaction Koffs were estimated from negative slopes of lines. Fb estimates were obtained by methods described in detail previously (3, 7). Schematic illustrations of these methods and associated calculations are also provided in Fig. S5 A and B. Fb was calculated from Fb = Fs/cosθ, where force due to flow (Fs) = 31.97 τw r2, τw is wall shear stress, r is the radius of bacteria projecting above endothelial surfaces, and θ is bond angle. Bond angle (θ) was calculated from cosθ = l/R, where l = bacterial displacement during deceleration.
Statistical Analysis.
All statistical analysis was performed in GraphPad Prism. Specific statistical tests used are described in the figure legends. Statistical comparisons of differences in slopes for Koff calculations were compared using extra sum-of-square F tests.
SI Materials and Methods
Measurement of B. burgdorferi Growth Under pFn-Depleted Conditions.
Triplicate cultures of log-phase GCB966 were diluted to 2 × 106 cells per milliliter in BSK-II containing 6% complete or pFn-depleted rabbit serum in 96-well plates. Bacterial concentrations were counted over 48 h using a fluorescent plate reader (Fluostar Optima; BMG Labtech) by comparing fluorescence intensities of bacterial cultures with fluorescence intensities of standard wells containing dilution series of a GCB966 culture for which the bacterial concentration was determined by manual counting in Petroff–Hausser chambers and dark-field microscopy.
pFn Affinity Purification and Depletion.
Affinity purification of pFn from human plasma.
Similar to previously described conditions (44), 100 mL of human plasma (Equitech-Bio, Inc.) containing 5 mM EDTA and 0.5 mM PMSF was centrifuged at 10,000 × g for 15 min at room temperature (RT°C). The supernatant was precleared by flowing at 2 mL⋅min−1 RT°C over a 10-mL Sepharose 4B (GE Healthcare) column prepared and equilibrated in Tris-buffered saline [TBS; 0.15 M NaCl, 10 mM Tris⋅HCl (pH 7.4)] containing 5 mM EDTA. Flow-through was affinity-purified over a 5-mL gelatin Sepharose (catalog no.71-7094; GE Healthcare) column at 2 mL⋅min−1 after preparation and equilibration of resin in TBS containing 5 mM EDTA. The column was washed with 2 vol of 10 mM Tris⋅HCl (pH 7.4) and 0.5 M NaCl, and then with 3 vol of PBS. Bound pFn was eluted with 4 M urea in PBS, and buffer exchange to PBS was performed using Amicon 100-kDa Ultra Centrifugal Filters (Millipore Canada, Ltd). The pFn was diluted to 1 mg/mL and stored at −20 °C in 100-μL and 1-mL aliquots. The purity and identity of isolated pFn were tested by loading 1–5 μg of protein onto 7% SDS/PAGE gels, followed by electrophoresis and visualization of total proteins by gel fixation and staining in Coomassie blue or transfer and immunoblotting with a 1:400 dilution of polyclonal rabbit anti-human Fn antibody (catalog no. F3648; Sigma–Aldrich) in 5% skim milk, followed by washing and incubation with a 1:10,000 dilution of goat anti-rabbit secondary antibody conjugated to horseradish peroxidase (HRP; catalog no. ab6721; Abcam). Immunoblots were visualized using ECL Western blotting detection reagents (RPN 2106; GE Healthcare). Functionality of purified pFn was determined by comparing binding of 1 mL of 1 mg/mL plasma-purified and commercial human pFn (Sigma–Aldrich) with a 1-mL HiTrap Heparin Cartridge (catalog no. 17-0406-01; GE Healthcare) equilibrated with 10 bed volumes of buffer B [2.0 M NaCl, 10 mM sodium phosphate (pH 7.0)], followed by washing with buffer B until no protein remained in flow-through (5–10 bed volumes). The pFn was eluted in 5–10 mL of buffer A [150 mM NaCl, 10 mM sodium phosphate (pH 7.0)]. Binding assays were performed at RT°C and a flow rate of 4 mL⋅min−1 using an AKTA fast protein liquid chromatography system equipped with UNICORN system control software (catalog no. 18190026; GE Healthcare). Binding was expressed as the percentage of total input protein that was recaptured in the eluate.
pFn depletion from bacterial cultivation medium.
Fn was depleted from rabbit serum using the same protocol as for affinity purification of pFn from human plasma (discussed above), and flow-through was collected after serum was passed over gelatin-Sepharose. Rabbit serum depleted of pFn was filter-sterilized before addition to BSK-II medium. To ensure that the depletion was successful, immunoblotting was performed with rabbit serum depleted of pFn and complete rabbit serum. Due to the large amounts of protein in rabbit serum, a volume of 10 mL of rabbit serum depleted of pFn or complete rabbit serum was filtered through Amicon 100-kDa Ultra centrifigual filters to concentrate pFn and remove smaller proteins before SDS/PAGE electrophoresis. Immunoblotting conditions were a 1:400 dilution of polyclonal rabbit anti-human Fn antibody (Sigma) and a 1:10,000 dilution of HRP-conjugated goat anti-rabbit secondary antibody (Abcam).
Antibody and Peptide Interaction-Blocking Experiments.
A list of antibody and peptide sources and related references are provided in Table S1. Sodium azide was removed from antibodies before bacterial incubation by buffer exchange and resuspension to 1 mg/mL in PBS using Amicon 10-kDa Ultra centrifugal filters (Millipore).
Polyclonal αFn antibody experiments.
Washed bacteria grown for 48 h in 1% mouse blood (2.5 mL at 4 × 108 cells per milliliter in PBS) were incubated without agitation for 60 min at RT°C with 2.5 mg of nonspecific goat IgGs or polyclonal goat anti-rabbit pFn IgG. This solution was diluted to 1 × 108 bacteria per milliliter in HBSS with addition of heat-inactivated FBS to 10%, and then perfused over human umbilical vein endothelial cells (HUVECs) at 1 dyn/cm2.
Monoclonal αFn antibodies and Arg-Gly-Asp-Ser peptide experiments.
Washed bacteria grown in pFn-depleted rabbit serum were resuspended in PBS to 2.5 × 109 cells per milliliter, and 50 μL of this suspension was incubated for 60 min without agitation at 4 °C with 15 μg of purified human pFn (1 mg/mL stock), followed by 60 min of incubation without agitation at 4 °C with an equimolar quantity (5.1 μg) of nonspecific goat IgGs, monoclonal mouse anti-human Fn antibodies directed against different Fn regions, or 5.1 μg of linear Arg-Gly-Asp-Ser (RGDS) peptide. This solution was diluted to 1 × 108 bacteria per milliliter in HBSS without FBS, and perfused over HUVECs at 1 dyn/cm2.
B. burgdorferi-Dependent pFn Fibrillogenesis.
Triplicate GCB966 and GCB971 cultures grown to ∼7 × 106 cells per milliliter under pFn-depleted conditions were mixed, pooled, washed twice in PBS, and resuspended to 108 cells per milliliter Seventy microliters of bacteria was incubated for 1 h at 4 °C without agitation with 30 μg of pFn (final pFn concentration of 0.3 mg/mL), plated on coverslips, fixed for 10 min at RT°C in 3% paraformaldehyde (PFA), washed once with PBS at RT°C, and then stained immediately with rabbit anti-human Fn antibody in PBS/0.02% Triton X-100 (TX-100) at RT°C. Samples were washed three times for 10 min in PBS/0.02% TX-100, and then incubated with 1:100 goat anti-rabbit Alexa Fluor 568 and 0.05 mg/mL DAPI (Sigma) in PBS/0.02% TX-100 at RT°C in darkness, followed by three 10-min washes with PBS/0.02% TX-100. Controls for this experiment were incubated and processed as for GCB966 +pFn samples, and comprised B. burgdorferi alone (GCB966 stained with DAPI) and pFn alone (stained with anti-Fn antibodies only). Coverslips were mounted on Petri dishes with one drop of polyvinyl-alcohol (PVA) and dried overnight at RT°C in darkness. Immunofluorescence (IF) microscopy was performed using an upright Axio Imager M2 equipped with an AxioCam HRm camera; Plan Apochromat 40× objective; filter sets 49, 38 HE GFP, HE Cy3, and HE Cy5; and ZEN Pro software (Zeiss). Lengths of manually selected Fn fibrils were measured from IF micrographs using Volocity software. Thick, bundled fibers were measured as one fiber because individual fibril lengths within bundles were difficult to determine unambiguously. For polymerized Fn that did not form obvious fibrils, fibril length was measured as the cross-sectional area of Fn aggregates, measured along the longest axis. Fibrils were selected and measured from end to end by manual tracing. Bent or curved fibrils were traced using a series of lines that were combined to obtain total length. A total of four and 13 fields of view in micrographs contained measurable fibrils/polymerized Fn for pFn alone and pFn + B. burgdorferi conditions, respectively.
IF.
HUVECs (1.1 × 107 cells) were plated on 35-mm cell culture dishes without Fn or gelatin coating 48 h before fixation, which was performed for 10 min at RT°C in 1 mL of 3% PFA. Cells were permeabilized at RT°C for 5 min with 1 mL of 0.2% TX-100 in PBS (PBS alone for nonpermeabilized cells), washed once in RT°C PBS, and stained immediately. Monolayers were incubated with primary antibodies against polyclonal human Fn (rabbit, 1:400 dilution, catalog no. F3648; Sigma–Aldrich), monoclonal mouse vimentin (mouse, 1:200 dilution, catalog no. 0725; Dako Canada ULC), and monoclonal mouse tubulin (mouse, 1:500 dilution, catalog no. 9026; Sigma–Aldrich), followed by three 10-min washes with PBS/0.02% TX-100 (permeabilized cells) or PBS alone (nonpermeabilized cells). Cells were incubated at RT°C in darkness with PBS-diluted secondary antibodies: goat anti-mouse IgG Alexa 488 at a 1:250 dilution (catalog no. 210421; Life Technologies), goat anti-rabbit Alexa 647 at a 1:200 dilution (catalog no. 4414S; Cell Signaling Technology), and DAPI at a 1:50 dilution, followed by three 10-min washes with PBS/0.02% TX-100 for permeabilized cells and with PBS alone for nonpermeabilized cells. Coverslips were mounted with one drop of PVA (Sigma–Aldrich) on a glass slide and dried overnight at RT°C in darkness.
Associated Text.
Because several of the pFn sites implicated in B. burgdorferi–endothelial interactions are involved in pFn fibrillogenesis (Fig. S3), and because peptides derived from the Fn-binding B. burgdorferi vascular adhesin BBK32 induce pFn polymerization in vitro (27), we asked whether B. burgdorferi–endothelial interactions under shear stress depended on pFn fibrillogenesis. BBK32 is surface-expressed on B. burgdorferi (45), but it is not known if this protein localizes to specific regions of the bacterial surface. We first investigated whether these bacteria could induce pFn fibrillogenesis under static (no-flow) conditions, because the ability of live BBK32-expressing B. burgdorferi to induce pFn fibrillogenesis has not yet been examined. To perform this investigation, B. burgdorferi grown in the absence of pFn were coincubated with 0.3 mg/mL unlabeled human pFn for 1 h (Fig. S4 A–C), a period that is much shorter than the typical replication time for these bacteria (8–12 h). Live bacteria coincubated with pFn were 37% shorter than their −pFn counterparts (Fig. S4A), and cells were 21% shorter in the presence of pFn when cell length was measured by IF (Fig. S4B) (note that alignment of +pFn bacteria in an end-to-end fashion in the IF micrographs shown in Fig. S4C visually suggests that bacteria are longer than their −pFn counterparts; however, the length of individual bacteria, shown in Fig. S4B, was shorter in the presence of pFn). Although bacteria incubated without pFn were longer under live compared with IF conditions, presumably because movement of live bacteria increased their apparent cell length compared with fixed controls (P < 0.05; compare −pFn values in Fig. S4 A and B), no notable morphological changes suggestive of cell damage were evident in IF micrographs (Fig. S4C). We concluded that pFn induced a more compact conformation in bacteria. In addition, we found that pFn underwent prominent fibrillogenesis in the presence of B. burgdorferi and formed long (∼55 μm) fibrils that were not observed when bacteria were absent (Fig. S4C). Thus, B. burgdorferi induced rapid pFn fibrillogenesis, and fibrillogenesis was associated with a compressed/more compact bacterial conformation.
To ensure that B. burgdorferi-induced pFn fibrillogenesis was not an artifact of IF procedures such as sample fixation or processing, we also visualized polymerization of fFn on live bacteria under static conditions by live cell imaging. The fluorescent signal generated by fFn is too diffuse and weak to be visible until it has polymerized (46, 47). Thus, we expected that fFn would not be readily visible if B. burgdorferi bound fFn without inducing fibrillogenesis. Consistent with the conclusion derived from IF experiments, we found that fFn formed bright foci on ∼20% of live bacteria (Fig. S4D), implying that pFn polymerized on bacterial surfaces. Under these conditions, fluorescent foci of polymerized Fn are located at the poles of the bacteria, or in a nonpolar fashion encasing the bacteria. Fn colocalization does not truncate or cut the bacteria; alternatively, it seems to compress them, and this compression explains their shorter bacterial length. We did not observe individual fFn fibrils on or extending from live bacteria. However, it is possible that the pFn fibrils observed by IF were too thin to be readily distinguished under live cell imaging conditions, because bacteria were constantly motile, swam, and wriggled in and out of the focal plane, which blurred the images captured of bacteria themselves as well as associated fFn foci.
We also observed that under static conditions, fFn foci localized primarily to the poles of bacterial cells (polar localization), with some foci extending from the poles to encase nonpolar regions of the cell body (nonpolar localization) (Fig. S4D). Bacteria covered by polymerized fFn over much of their length were condensed and shortened compared with bacteria for which fFn localization was exclusively polar (Fig. S4D), suggesting that B. burgdorferi shortening following coincubation with pFn (Fig. S4 A and B) was due to formation of a polymerized pFn sheath that compressed bacteria. Collectively, these data implied that B. burgdorferi induced pFn fibrillogenesis and that pFn polymerization under static conditions occurred primarily at bacterial cell poles, with some spreading to nonpolar sites.
Supplementary Material
Acknowledgments
We thank the T.J.M laboratory members and J. Skare for review of the manuscript. We thank the Department of Comparative Medicine and Sick Kids Imaging Facility for technical support. We thank A. Eshghi and N. Zlotnikov for assistance with manuscript preparation. We thank G. Chaconas for providing bacterial strains. T.J.M was supported by funding from Canadian Institutes of Health Research (CIHR) Grants MOP-11959 and ICS-12398, Natural Sciences and Engineering Research Council (NSERC) Grant RGPIN 401938-11, Collaborative Health Research Projects (CHRP) Grants CIHR/NSERC 494968 and 494982, the Banting Research Foundation, and Canada Foundation for Innovation/Ontario Research Fund (CFI/ORF) Grant 27881. B.H. was supported by funding from CIHR Grants 210820, 286920, and 286720; CHRP Grants CIHR/NSERC 1004005 and 413783; and CFI/ORF Grant 26653. A.F.N. and R.E. were supported by a graduate scholarship from the University of Toronto and a Harron Fellowship. R.E. was supported by an Ontario Graduate Scholarship and a Faculty of Dentistry Undergraduate Summer Studentship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1615007114/-/DCSupplemental.
References
- 1.Lemichez E, Lecuit M, Nassif X, Bourdoulous S. Breaking the wall: Targeting of the endothelium by pathogenic bacteria. Nat Rev Microbiol. 2010;8:93–104. doi: 10.1038/nrmicro2269. [DOI] [PubMed] [Google Scholar]
- 2.Cahill TJ, Prendergast BD. Infective endocarditis. Lancet. 2016;387:882–893. doi: 10.1016/S0140-6736(15)00067-7. [DOI] [PubMed] [Google Scholar]
- 3.Sundd P, Pospieszalska MK, Cheung LS-L, Konstantopoulos K, Ley K. Biomechanics of leukocyte rolling. Biorheology. 2011;48:1–35. doi: 10.3233/BIR-2011-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marshall BT, et al. Direct observation of catch bonds involving cell-adhesion molecules. Nature. 2003;423:190–193. doi: 10.1038/nature01605. [DOI] [PubMed] [Google Scholar]
- 5.Beaussart A, et al. Nanoscale adhesion forces of Pseudomonas aeruginosa type IV Pili. ACS Nano. 2014;8:10723–10733. doi: 10.1021/nn5044383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Persat A, et al. The mechanical world of bacteria. Cell. 2015;161:988–997. doi: 10.1016/j.cell.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ebady R, et al. Biomechanics of Borrelia burgdorferi vascular interactions. Cell Reports. 2016;16:2593–2604. doi: 10.1016/j.celrep.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernard SC, et al. Pathogenic Neisseria meningitidis utilizes CD147 for vascular colonization. Nat Med. 2014;20:725–731. doi: 10.1038/nm.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pappelbaum KI, et al. Ultralarge von Willebrand factor fibers mediate luminal Staphylococcus aureus adhesion to an intact endothelial cell layer under shear stress. Circulation. 2013;128:50–59. doi: 10.1161/CIRCULATIONAHA.113.002008. [DOI] [PubMed] [Google Scholar]
- 10.Claes J, et al. Adhesion of Staphylococcus aureus to the vessel wall under flow is mediated by von Willebrand factor-binding protein. Blood. 2014;124:1669–1676. doi: 10.1182/blood-2014-02-558890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh P, Carraher C, Schwarzbauer JE. Assembly of fibronectin extracellular matrix. Annu Rev Cell Dev Biol. 2010;26:397–419. doi: 10.1146/annurev-cellbio-100109-104020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henderson B, Nair S, Pallas J, Williams MA. Fibronectin: A multidomain host adhesin targeted by bacterial fibronectin-binding proteins. FEMS Microbiol Rev. 2011;35:147–200. doi: 10.1111/j.1574-6976.2010.00243.x. [DOI] [PubMed] [Google Scholar]
- 13.Kong F, García AJ, Mould AP, Humphries MJ, Zhu C. Demonstration of catch bonds between an integrin and its ligand. J Cell Biol. 2009;185:1275–1284. doi: 10.1083/jcb.200810002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chakrabarti S, Hinczewski M, Thirumalai D. Plasticity of hydrogen bond networks regulates mechanochemistry of cell adhesion complexes. Proc Natl Acad Sci USA. 2014;111:9048–9053. doi: 10.1073/pnas.1405384111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sampaio ALF, et al. Inflammation-dependent alpha 5 beta 1 (very late antigen-5) expression on leukocytes reveals a functional role for this integrin in acute peritonitis. J Leukoc Biol. 2010;87:877–884. doi: 10.1189/jlb.1009670. [DOI] [PubMed] [Google Scholar]
- 16.Friedland JC, Lee MH, Boettiger D. Mechanically activated integrin switch controls α5β1 function. Science. 2009;323:642–644. doi: 10.1126/science.1168441. [DOI] [PubMed] [Google Scholar]
- 17.Kong F, et al. Cyclic mechanical reinforcement of integrin-ligand interactions. Mol Cell. 2013;49:1060–1068. doi: 10.1016/j.molcel.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Norman MU, et al. Molecular mechanisms involved in vascular interactions of the Lyme disease pathogen in a living host. PLoS Pathog. 2008;4:e1000169. doi: 10.1371/journal.ppat.1000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moriarty TJ, et al. Vascular binding of a pathogen under shear force through mechanistically distinct sequential interactions with host macromolecules. Mol Microbiol. 2012;86:1116–1131. doi: 10.1111/mmi.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Messina JA, Thaden JT, Sharma-Kuinkel BK, Fowler VG., Jr Impact of bacterial and human genetic variation on Staphylococcus aureus infections. PLoS Pathog. 2016;12:e1005330. doi: 10.1371/journal.ppat.1005330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maurer LM, Ma W, Mosher DF. Dynamic structure of plasma fibronectin. Crit Rev Biochem Mol Biol. 2015;51:213–227. doi: 10.1080/10409238.2016.1184224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hymes JP, Klaenhammer TR. Stuck in the middle: Fibronectin-binding proteins in gram-positive bacteria. Front Microbiol. 2016;7:1504. doi: 10.3389/fmicb.2016.01504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stoffels JMJ, Zhao C, Baron W. Fibronectin in tissue regeneration: Timely disassembly of the scaffold is necessary to complete the build. Cell Mol Life Sci. 2013;70:4243–4253. doi: 10.1007/s00018-013-1350-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Ni H. Fibronectin maintains the balance between hemostasis and thrombosis. Cell Mol Life Sci. 2016;73:3265–3277. doi: 10.1007/s00018-016-2225-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raibaud S, et al. Borrelia burgdorferi binds fibronectin through a tandem beta-zipper, a common mechanism of fibronectin binding in staphylococci, streptococci, and spirochetes. J Biol Chem. 2005;280:18803–18809. doi: 10.1074/jbc.M501731200. [DOI] [PubMed] [Google Scholar]
- 26.Harris G, Ma W, Maurer LM, Potts JR, Mosher DF. Borrelia burgdorferi protein BBK32 binds to soluble fibronectin via the N-terminal 70-kDa region, causing fibronectin to undergo conformational extension. J Biol Chem. 2014;289:22490–22499. doi: 10.1074/jbc.M114.578419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prabhakaran S, Liang X, Skare JT, Potts JR, Höök M. A novel fibronectin binding motif in MSCRAMMs targets F3 modules. PLoS One. 2009;4:e5412. doi: 10.1371/journal.pone.0005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morla A, Zhang Z, Ruoslahti E. Superfibronectin is a functionally distinct form of fibronectin. Nature. 1994;367:193–196. doi: 10.1038/367193a0. [DOI] [PubMed] [Google Scholar]
- 29.Lower SK, et al. Polymorphisms in fibronectin binding protein A of Staphylococcus aureus are associated with infection of cardiovascular devices. Proc Natl Acad Sci USA. 2011;108:18372–18377. doi: 10.1073/pnas.1109071108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sullan RMA, Li JK, Crowley PJ, Brady LJ, Dufrêne YF. Binding forces of Streptococcus mutans P1 adhesin. ACS Nano. 2015;9:1448–1460. doi: 10.1021/nn5058886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Müller NF, et al. Trimeric autotransporter adhesin-dependent adherence of Bartonella henselae, Bartonella quintana, and Yersinia enterocolitica to matrix components and endothelial cells under static and dynamic flow conditions. Infect Immun. 2011;79:2544–2553. doi: 10.1128/IAI.01309-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bustanji Y, et al. Dynamics of the interaction between a fibronectin molecule and a living bacterium under mechanical force. Proc Natl Acad Sci USA. 2003;100:13292–13297. doi: 10.1073/pnas.1735343100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moriarty TJ, et al. Real-time high resolution 3D imaging of the lyme disease spirochete adhering to and escaping from the vasculature of a living host. PLoS Pathog. 2008;4:e1000090. doi: 10.1371/journal.ppat.1000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barbour AG. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984;57:521–525. [PMC free article] [PubMed] [Google Scholar]
- 35.Marjenberg ZR, et al. Cooperative binding and activation of fibronectin by a bacterial surface protein. J Biol Chem. 2011;286:1884–1894. doi: 10.1074/jbc.M110.183053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alon R, Hammer DA, Springer TA. Lifetime of the P-selectin-carbohydrate bond and its response to tensile force in hydrodynamic flow. Nature. 1995;374:539–542. doi: 10.1038/374539a0. [DOI] [PubMed] [Google Scholar]
- 37.Sarangapani KK, et al. Low force decelerates L-selectin dissociation from P-selectin glycoprotein ligand-1 and endoglycan. J Biol Chem. 2004;279:2291–2298. doi: 10.1074/jbc.M310396200. [DOI] [PubMed] [Google Scholar]
- 38.Le Trong I, et al. Structural basis for mechanical force regulation of the adhesin FimH via finger trap-like beta sheet twisting. Cell. 2010;141:645–655. doi: 10.1016/j.cell.2010.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sauer MM, et al. Catch-bond mechanism of the bacterial adhesin FimH. Nat Commun. 2016;7:10738. doi: 10.1038/ncomms10738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas WE, Trintchina E, Forero M, Vogel V, Sokurenko EV. Bacterial adhesion to target cells enhanced by shear force. Cell. 2002;109:913–923. doi: 10.1016/s0092-8674(02)00796-1. [DOI] [PubMed] [Google Scholar]
- 41.Chabria M, Hertig S, Smith ML, Vogel V. Stretching fibronectin fibres disrupts binding of bacterial adhesins by physically destroying an epitope. Nat Commun. 2010;1:135. doi: 10.1038/ncomms1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baneyx G, Baugh L, Vogel V. Fibronectin extension and unfolding within cell matrix fibrils controlled by cytoskeletal tension. Proc Natl Acad Sci USA. 2002;99:5139–5143. doi: 10.1073/pnas.072650799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sokurenko EV, Vogel V, Thomas WE. Catch-bond mechanism of force-enhanced adhesion: Counterintuitive, elusive, but ... widespread? Cell Host Microbe. 2008;4:314–323. doi: 10.1016/j.chom.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Retta SF, Ferraris P, Tarone G. Purification of fibronectin from human plasma. Methods Mol Biol. 1999;96:119–124. doi: 10.1385/1-59259-258-9:119. [DOI] [PubMed] [Google Scholar]
- 45.Probert WS, Johnson BJ. Identification of a 47 kDa fibronectin-binding protein expressed by Borrelia burgdorferi isolate B31. Mol Microbiol. 1998;30:1003–1015. doi: 10.1046/j.1365-2958.1998.01127.x. [DOI] [PubMed] [Google Scholar]
- 46.Pankov R, Momchilova A. Fluorescent labeling techniques for investigation of fibronectin fibrillogenesis (labeling fibronectin fibrillogenesis) Methods Mol Biol. 2009;522:261–274. doi: 10.1007/978-1-59745-413-1_18. [DOI] [PubMed] [Google Scholar]
- 47.Pankov R, et al. Integrin dynamics and matrix assembly: tensin-dependent translocation of alpha(5)beta(1) integrins promotes early fibronectin fibrillogenesis. J Cell Biol. 2000;148:1075–1090. doi: 10.1083/jcb.148.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brissette CA, Bykowski T, Cooley AE, Bowman A, Stevenson B. Borrelia burgdorferi RevA antigen binds host fibronectin. Infect Immun. 2009;77:2802–2812. doi: 10.1128/IAI.00227-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Früh SM, Schoen I, Ries J, Vogel V. Molecular architecture of native fibronectin fibrils. Nat Commun. 2015;6:7275. doi: 10.1038/ncomms8275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moyano JV, et al. Fibronectin type III5 repeat contains a novel cell adhesion sequence, KLDAPT, which binds activated alpha4beta1 and alpha4beta7 integrins. J Biol Chem. 1997;272:24832–24836. doi: 10.1074/jbc.272.40.24832. [DOI] [PubMed] [Google Scholar]
- 51.Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010;339:269–280. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 53.Goldstein SF, Buttle KF, Charon NW. Structural analysis of the Leptospiraceae and Borrelia burgdorferi by high-voltage electron microscopy. J Bacteriol. 1996;178:6539–6545. doi: 10.1128/jb.178.22.6539-6545.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grant MB, et al. Matrix metalloproteinase expression in human retinal microvascular cells. Diabetes. 1998;47:1311–1317. doi: 10.2337/diab.47.8.1311. [DOI] [PubMed] [Google Scholar]
- 55.An B, et al. Definition of the native and denatured type II collagen binding site for fibronectin using a recombinant collagen system. J Biol Chem. 2014;289:4941–4951. doi: 10.1074/jbc.M113.530808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peters JH, et al. Preferential recognition of a fragment species of osteoarthritic synovial fluid fibronectin by antibodies to the alternatively spliced EIIIA segment. Arthritis Rheum. 2001;44:2572–2585. doi: 10.1002/1529-0131(200111)44:11<2572::aid-art438>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 57.Schor SL, et al. Migration-stimulating factor: A genetically truncated onco-fetal fibronectin isoform expressed by carcinoma and tumor-associated stromal cells. Cancer Res. 2003;63:8827–8836. [PubMed] [Google Scholar]
- 58.Mosher DF. Fibronectin. Prog Hemost Thromb. 1980;5:111–151. [PubMed] [Google Scholar]
- 59.Katayama M, et al. Isolation and characterization of two monoclonal antibodies that recognize remote epitopes on the cell-binding domain of human fibronectin. Exp Cell Res. 1989;185:229–236. doi: 10.1016/0014-4827(89)90051-7. [DOI] [PubMed] [Google Scholar]
- 60.Hubbard B, Buczek-Thomas JA, Nugent MA, Smith ML. Heparin-dependent regulation of fibronectin matrix conformation. Matrix Biol. 2014;34:124–131. doi: 10.1016/j.matbio.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Underwood PA, Dalton BA, Steele JG, Bennett FA, Strike P. Anti-fibronectin antibodies that modify heparin binding and cell adhesion: Evidence for a new cell binding site in the heparin binding region. J Cell Sci. 1992;102:833–845. doi: 10.1242/jcs.102.4.833. [DOI] [PubMed] [Google Scholar]
- 62.Pierschbacher MD, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984;309:30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- 63.Wu J, Weening EH, Faske JB, Höök M, Skare JT. Invasion of eukaryotic cells by Borrelia burgdorferi requires β(1) integrins and Src kinase activity. Infect Immun. 2011;79:1338–1348. doi: 10.1128/IAI.01188-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee W-Y, et al. An intravascular immune response to Borrelia burgdorferi involves Kupffer cells and iNKT cells. Nat Immunol. 2010;11:295–302. doi: 10.1038/ni.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.