Significance
The blood–brain barrier (BBB) poses a major obstacle for drug delivery to the central nervous system (CNS). This study revealed that genetic or pharmacological targeting of sphingosine 1–phosphate receptor-1 (S1P1) facilitates a small-molecule-selective BBB opening, without major signs of CNS inflammation or injury. This size-selective BBB opening could be attributed to changes in the cytoskeletal association of tight junction proteins. Importantly, BBB opening by pharmacological blockage of S1P1 was reversible, suggesting that targeting S1P1 may be a promising strategy for the safe delivery of therapeutic agents into the CNS to treat neurodegenerative and neuroinflammatory diseases and neurological cancers.
Keywords: blood–brain barrier, sphingosine 1–phosphate, endothelium, tight junction, drug delivery
Abstract
The vasculature of the central nervous system (CNS) forms a selective barrier termed the blood–brain barrier (BBB). Disruption of the BBB may contribute to various CNS diseases. Conversely, the intact BBB restricts efficient penetration of CNS-targeted drugs. Here, we report the BBB-regulatory role of endothelial sphingosine 1–phosphate (S1P) receptor-1, a G protein-coupled receptor known to promote the barrier function in peripheral vessels. Endothelial-specific S1pr1 knockout mice (S1pr1iECKO) showed BBB breach for small-molecular-mass fluorescence tracers (<3 kDa), but not larger tracers (>10 kDa). Chronic BBB leakiness was associated with cognitive impairment, as assessed by the novel object recognition test, but not signs of brain inflammation. Brain microvessels of S1pr1iECKO mice showed altered subcellular distribution of tight junctional proteins. Pharmacological inhibition of S1P1 function led to transient BBB breach. These data suggest that brain endothelial S1P1 maintain the BBB by regulating the proper localization of tight junction proteins and raise the possibility that endothelial S1P1 inhibition may be a strategy for transient BBB opening and delivery of small molecules into the CNS.
The blood–brain barrier (BBB) is a protective and regulatory interface that allows the entry of essential nutrients, while preventing harmful substances from entering the central nervous system (CNS) (1). BBB endothelial cells (ECs) contain abundant tight junction (TJ) proteins, which generate a paracellular seal. In addition, paucity of endocytotic vesicles in brain ECs results in low rates of transcytosis. Furthermore, brain ECs allow efficient transport of select molecules, such as glucose and amino acids, into the CNS (2). The capillary endothelium is surrounded by a defined basement membrane, pericytes, and astrocytic end-feet processes, constituting the neurovascular unit (NVU), which is essential for maintaining the homeostasis of the CNS (3). Disruption of the BBB is associated with various CNS diseases, including multiple sclerosis (MS), Alzheimer’s disease (AD), and ischemic stroke (1–3). Conversely, in the normal state, the BBB hinders pharmacotherapy of CNS diseases by limiting drug delivery (4). Among the 7,000 drugs analyzed in the Comprehensive Medical Chemistry database, only 5% showed efficient BBB permeability allowing transport into the CNS (5). Therefore, novel approaches for a regulated opening of the BBB may be desirable to facilitate efficient pharmacotherapy of CNS diseases.
Sphingosine 1–phosphate (S1P), a pleiotropic lipid mediator, interacts with five G protein-coupled receptors (GPCRs), S1P1–5, to regulate cell migration, adhesion, survival, and proliferation (6). Recently, an S1P1 modulator, FTY720 (fingolimod), was approved as the first-line oral drug for relapsing–remitting MS (7). Furthermore, S1P and its prototypic receptor S1P1 have been implicated in neurovascular diseases such as AD and ischemic stroke (8, 9). S1P activation of EC S1P1 is essential for vascular development and barrier function (10). S1P1 regulates Gi-dependent Rac activation, cytoskeletal reorganization, adherens junction (AJ) assembly, and focal adhesion formation, all of which are needed for enhancement of vascular barrier function (10). Indeed, genetic and pharmacological inhibition of S1P1 increased vascular permeability in various organs such as lung, retina, and colon (11–14). However, the role of EC S1P1 in CNS vasculature is unknown.
Here, we investigated the involvement of endothelial S1P1 in BBB integrity in vivo using EC-specific S1pr1 knockout mice (referred to as S1pr1iECKO mice). The results demonstrate that brain endothelial S1P1 regulates BBB integrity in a size-dependent manner. With protracted BBB leakiness, EC-specific S1pr1 knockout mice displayed cognitive impairment, but no sign of brain inflammation. Biochemical analysis on brain microvessels revealed that subcellular localization of TJ proteins was altered in S1pr1iECKO mice, which provides a molecular explanation for the size-selective BBB leakiness in these mice. Transient pharmacological inhibition of S1P1 led to increased CNS penetration of small molecules, suggesting that targeting S1P1 may be a promising strategy for the safe delivery of therapeutic agents into the CNS.
Results
Size-Selective BBB Opening in S1pr1iECKO Mice.
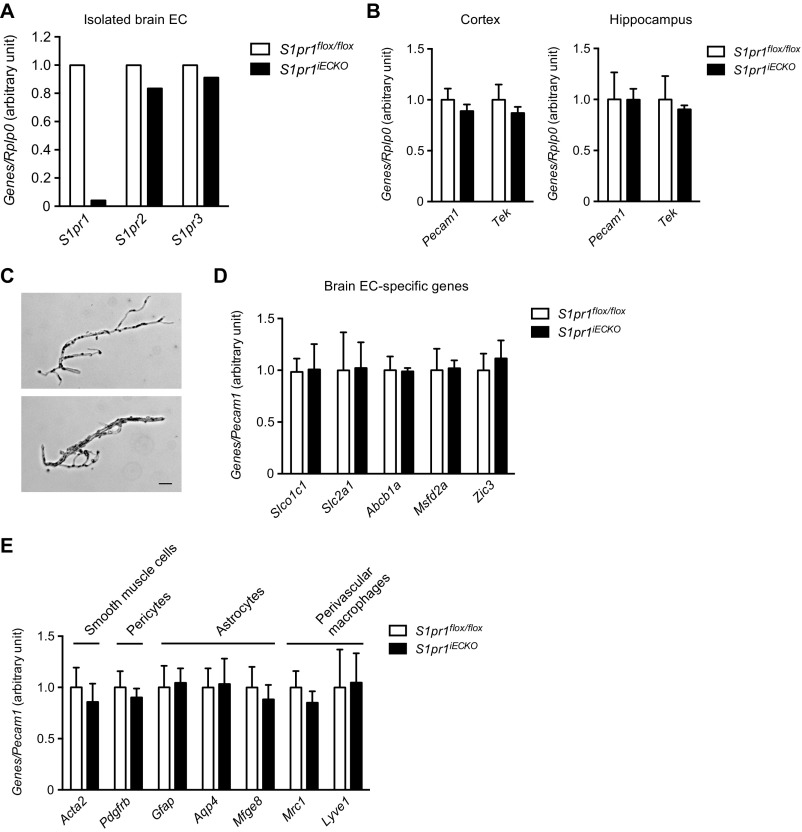
To assess the role of endothelial S1P1 in brain vasculature, we used a mouse model in which S1P1 is deleted in an EC-specific manner (S1pr1flox/flox Cdh5–Cre–ERT2; referred to as S1pr1iECKO) (12, 15–17). Mice were treated with tamoxifen for the first 3 d after birth and allowed to develop into adulthood. Quantitative PCR (qPCR) analysis of RNA from adult brain ECs showed >95% reduction of S1pr1 expression (Fig. S1A). Brain ECs did not show compensatory up-regulation of S1pr2 and S1pr3 (Fig. S1A).
Fig. S1.
Gene expression analysis for S1P receptors, pan-EC, brain EC, and cells of the NVU. (A) qPCR analysis on the mRNA expression for major endothelial S1P receptors, S1pr1, S1pr2, and S1pr3 in isolated brain ECs from control (S1pr1flox/flox) and S1pr1iECKO mice (n = 1). (B) qPCR analysis on the mRNA expression for pan-EC maker genes, Pecam1 and Tek in cerebral cortex and hippocampus from control and S1pr1iECKO mice (n = 5). (C) Brightfield images of representative microvessels isolated from whole brain. Microvessels ranged from 4 to 15 μm in diameter. (Scale bar: 20 μm.) (D and E) qPCR analysis on the mRNA expression of genes specific for brain-EC (D), and other cell types constituting NVU; smooth muscle cells, pericytes, astrocytes, and perivascular macrophages (E) in brain microvascular fragments from control and S1pr1iECKO mice (n = 4).
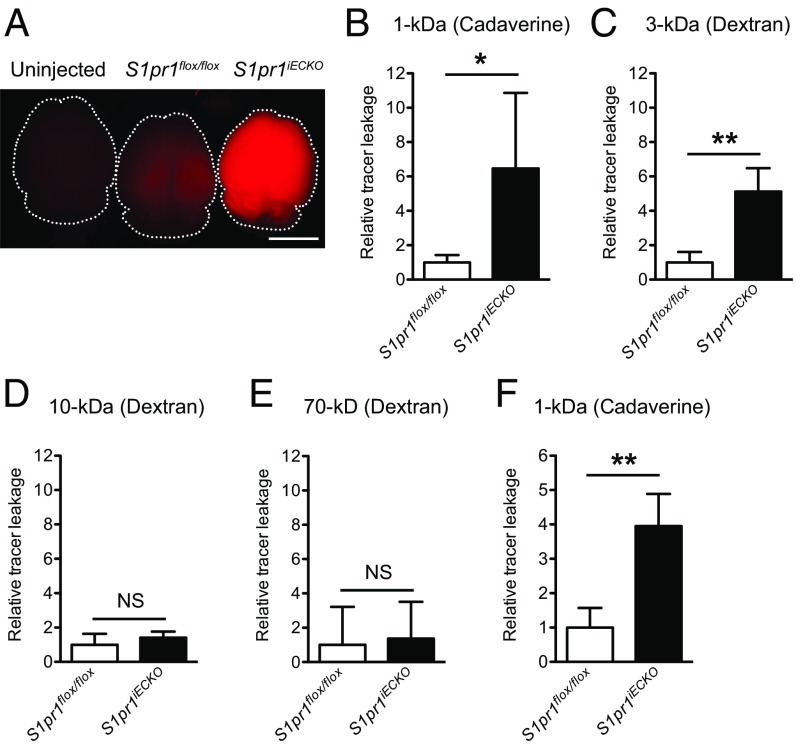
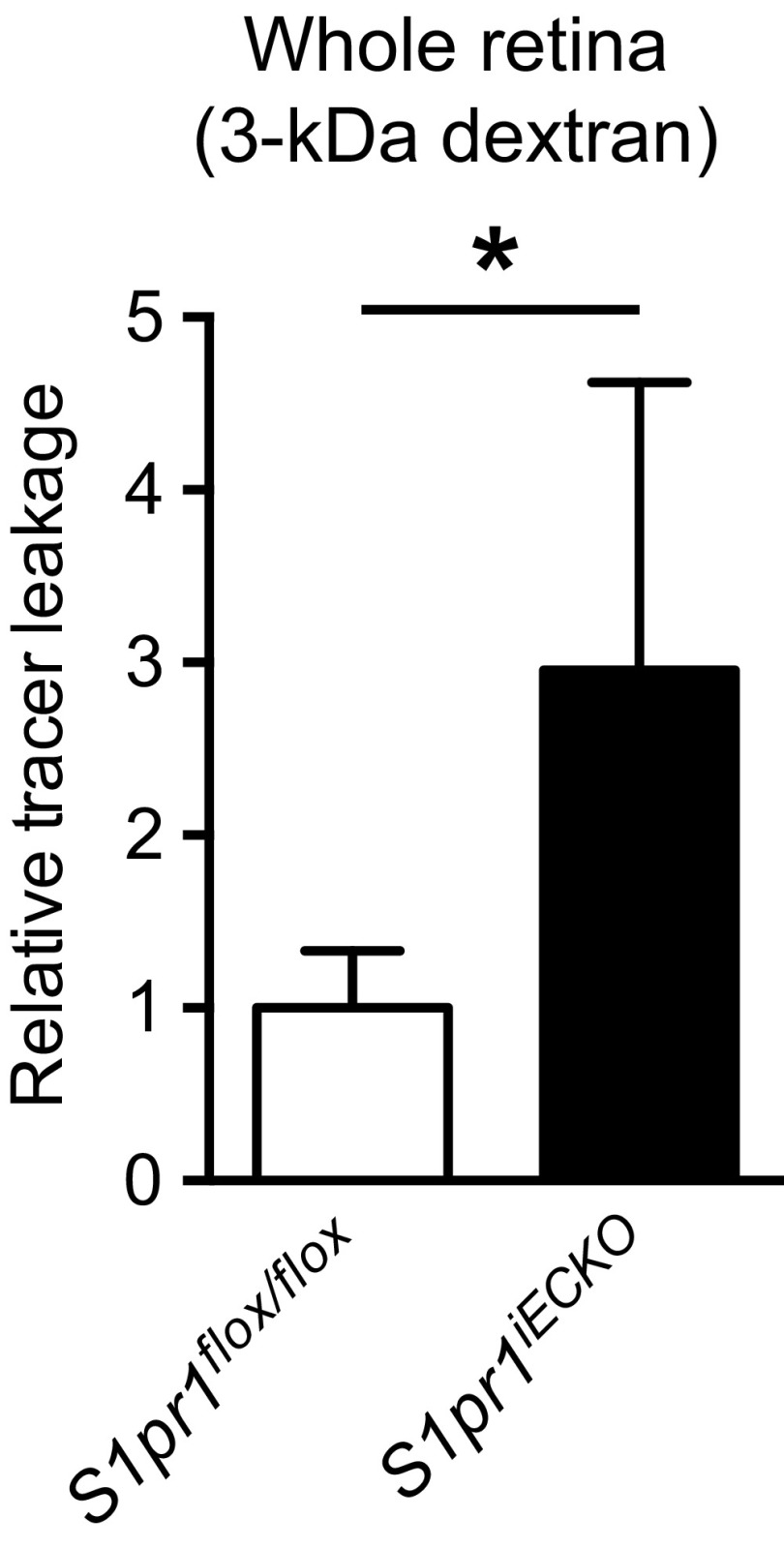
To address the role of endothelial S1P1 in BBB function, we injected 1-kDa fluorescent tracer, Alexa Fluor 555–cadaverine, and examined its distribution in brain. As shown in Fig. 1A, S1pr1iECKO brains showed enhanced tracer accumulation in the parenchyma by approximately fivefold compared with control mice (Fig. 1B), suggesting that CNS vasculature is leaky. The tracer accumulation into brain parenchyma was also observed when a 3-kDa dextran–tetramethylrhodamine (TMR) was administered (Fig. 1C). However, when the mice were injected with 10- and 70-kDa dextran–TMR, there was no significant difference in tracer accumulation between control and S1pr1iECKO brains (Fig. 1 D and E). The blood–retina barrier, which is similar to the BBB, is tight and restrictive (18). Similar to brain, the enhanced leakage of 3-kDa dextran–TMR was also observed in S1pr1iECKO retinas (Fig. S2).
Fig. 1.
Tracer extravasation into the brain is increased in S1pr1iECKO mice. S1pr1 deletion was induced after birth in A–E and in the adult in F. (A) Whole brain images taken after injection of 1-kDa Alexa Fluor 555–cadaverine. (Scale bar: 5 mm.) (B–E) Quantification of extravasated 1-kDa Alexa Fluor 555–cadaverine (B), 3-kDa dextran–TMR (C), 10-kDa dextran–TMR (D), and 70-kDa dextran–TMR (E) in control (S1pr1flox/flox) and S1pr1iECKO mice (n = 3 or 4). (F) Quantification of extravasated cadaverine in control and S1pr1iECKO mice when the deletion was induced in the adult (n = 3). Data are expressed as mean ± SD. *P < 0.05; **P < 0.01 (Student’s t test). NS, nonsignificant.
Fig. S2.
S1pr1iECKO mice showed increased 3-kDa dextran–TMR tracer extravasation into retina. Data are expressed as mean ± SD. n = 5. *P < 0.05 (Student’s t test).
CNS vasculature undergoes angiogenesis and remodeling at early postnatal stages (19, 20), alterations of which can affect subsequent BBB properties (2, 21). To determine whether S1P1 regulates the BBB directly or indirectly via vascular developmental defects (2, 21), we deleted endothelial S1pr1 in adult mice (>8 wk) and analyzed them for BBB function 4 wk after gene deletion. As in the case of early postnatal deletion, adult deletion of S1pr1 enhanced BBB permeability to the 1-kDa Alexa Fluor 555–cadaverine by approximately fourfold (Fig. 1F). Collectively, these results indicate that endothelial S1P1 directly regulates BBB integrity in a size-selective manner.
Normal Brain Vascular Structure in S1pr1iECKO Mice.
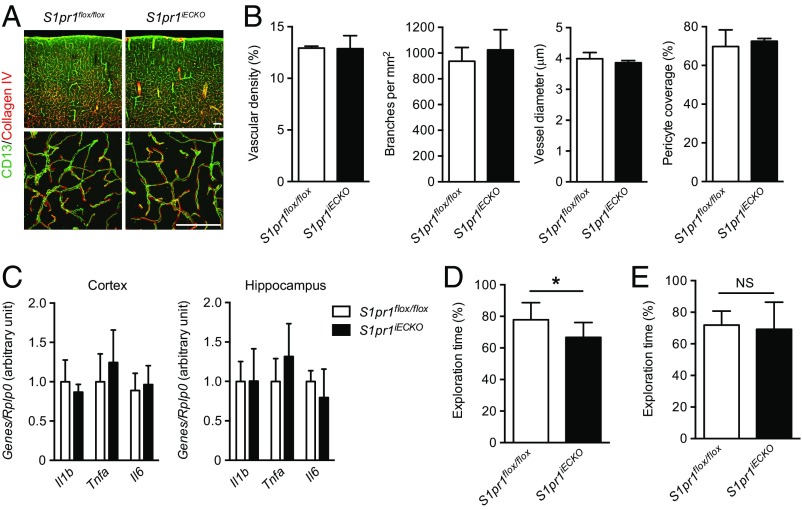
Because S1P1 is a key regulator of sprouting angiogenesis (12, 22, 23), we next examined whether deletion of endothelial S1pr1 affects CNS vascular development, maturation, and pattern formation. No abnormalities were detected in brain vascular density, diameter, and branch points in S1pr1iECKO mice (Fig. 2 A and B). Because endothelial S1P1 regulates pericyte investment during embryonic development (24), we assessed pericyte coverage in S1pr1iECKO brain vasculature. Immunohistochemical staining with the pericyte marker CD13 did not show any anomalies in pericyte coverage or positioning relative to the ECs of the capillary wall (Fig. 2 A and B). Expression of pan-EC marker genes such as Pecam1 and Tek in whole cortical or hippocampal tissues was not altered (Fig. S1B). Furthermore, isolated microvascular fragments (Fig. S1C) from S1pr1iECKO brain displayed normal mRNA expression of brain EC-specific genes (Slco1c1, Slc2a1, Abcb1a, Mfsd2a, and Zic3) (ref. 21; Fig. S1D) or other components of the NVU, smooth muscle cells (Acta2), pericytes (Pdgfrb), astrocytes (Gfap, Aqp4, and Mfge8), and perivascular macrophages (Mrc1 and Lyve1) (ref. 25; Fig. S1E). Together, these results show that S1pr1iECKO brain ECs undergo normal development, CNS-specific EC differentiation, and NVU development.
Fig. 2.
Endothelial deletion of S1pr1 in the neonatal period did not affect brain vascular development or induce inflammation, but is associated with cognitive impairment. (A) Representative confocal images of cerebral cortex from control (S1pr1flox/flox) and S1pr1iECKO mice stained with collagen IV (basement membrane) and CD13 (pericyte). A, Lower shows high-power magnification images. (Scale bars: 100 μm.) (B) Quantification of cortical vascular density, branching, diameter, and pericyte coverage (n = 3). (C) qPCR analysis on the expressions for Il1b, Il6, and Tnfa in cerebral cortex and hippocampus (n = 5). (D and E) Assessment of recognition memory performance on control and S1pr1iECKO mice by NOR task when the deletion starts after birth (D) or in the adult (E). The exploration time of the novel objects was expressed in percent of both novel and familiar objects (n = 11, D; n = 7, E). Data are expressed as mean ± SD. *P < 0.05 (Student’s t test). NS, nonsignificant.
Inflammatory Gene Expression and Cognitive Function in Early Postnatally Deleted S1pr1iECKO Mice.
An increase in BBB permeability has been associated with neuroinflammation (26). For example, the mRNA expression of inflammatory cytokine genes including Il1b, Tnfa, and Il6 increased (up to 40- to 60-fold) in multiple animal stroke models (27). We next examined inflammatory gene expression in the cortex and hippocampus of control and S1pr1iECKO mice. However, there was no significant difference in the expression level of Il1b, Tnfa, and Il6 between S1pr1iECKO mice and controls (Fig. 2C). In addition, S1pr1iECKO brains had no signs of perivascular reactive astrogliosis (3) (Fig. S3).
Fig. S3.
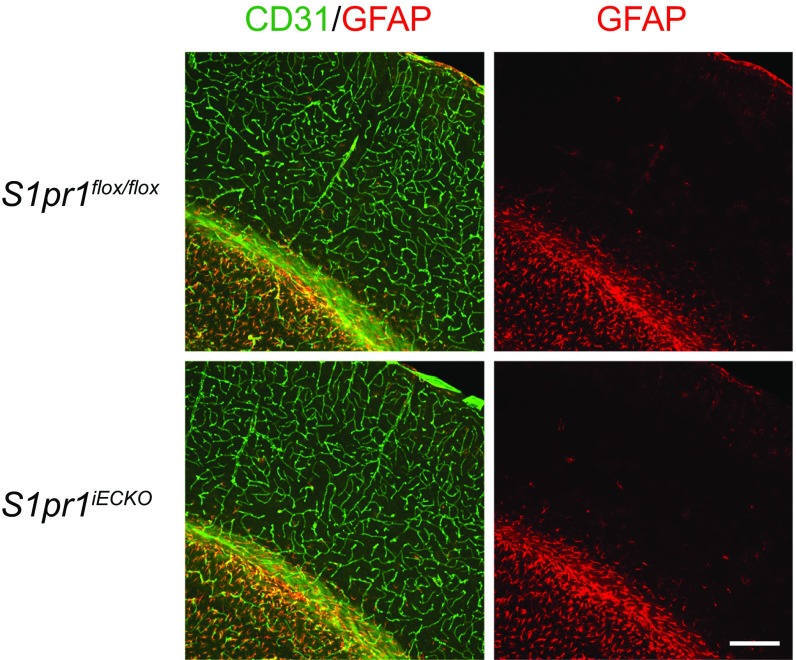
S1pr1iECKO mice had no signs of perivascular reactive astrogliosis. Representative confocal images of cerebral cortex from control (S1pr1flox/flox) and S1pr1iECKO mice stained with CD31 and GFAP are shown. (Scale bar: 200 μm.)
BBB disruption has also been associated with cognitive impairments (28, 29). Of note, chronic, but not acute, hypertension increases BBB leakage for small molecules, which causes cognitive impairment by enhancing exposure of angiotensin II to perivascular macrophage (30). Therefore, we asked whether deletion of endothelial S1pr1 and after BBB opening can affect recognition memory. For this purpose, control and S1pr1iECKO mice were tested for the novel object recognition (NOR) task, a paradigm that is commonly used to investigate recognition memory performance (31). In this behavioral test, we found that S1pr1iECKO mice spent significantly less time in exploring a novel object than control mice (Fig. 2D), although the extent of the impairment was milder than the murine chronic hypertension models (10% vs. 20% reduction in novel object exploration time). Notably, S1pr1iECKO mice did not show memory defects when endothelial S1pr1 deletion was induced in the adult (Fig. 2E). Therefore, chronic defects in endothelial S1P1 signaling that began at early postnatal period led to deficits in cognition memory.
Altered Subcellular Distribution of TJ Protein in S1pr1iECKO Brain Microvessels.
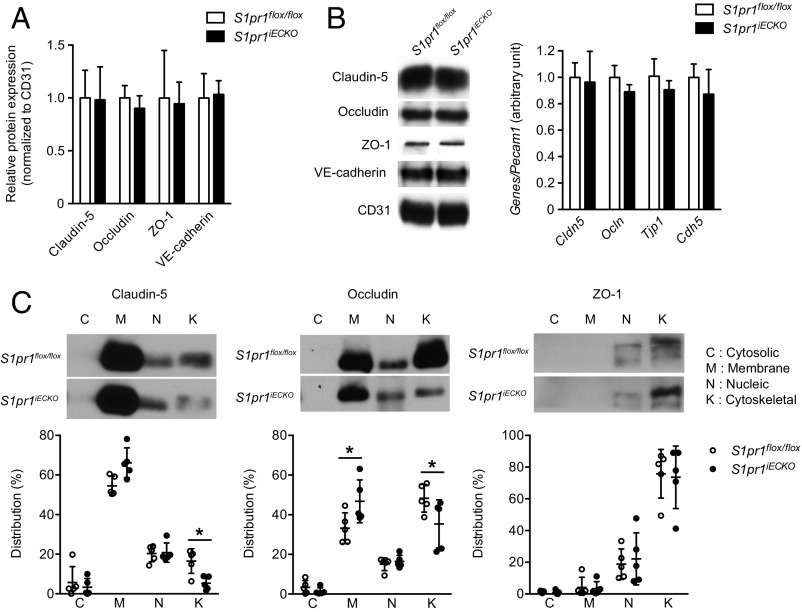
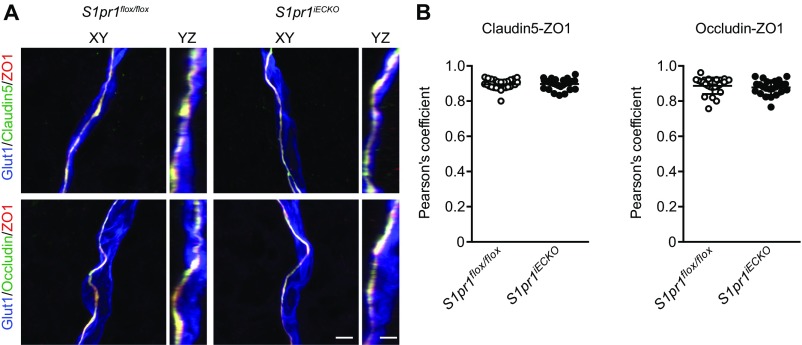
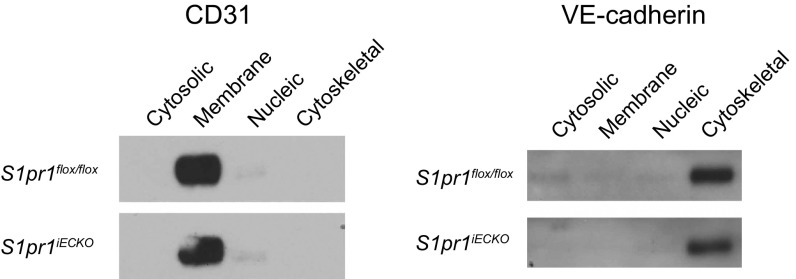
BBB is characterized by highly specialized TJs sealing the paracellular space between adjacent ECs and a low rate of transcytosis from the vessel lumen to the brain parenchyma (1–3). Size-selective BBB opening has been observed in mouse models where TJ genes, including the Cldn5 encoding claudin-5 and/or the Ocln encoding occludin, were deleted or knocked down (32–34). Therefore, we sought to determine whether BBB opening in S1pr1iECKO mice is associated with TJ protein modulation. To this end, we first compared the expression levels of major TJ proteins, claudin-5, occludin, and ZO-1, together with AJ protein, VE–cadherin, between control and S1pr1iECKO mice. Examination of mRNA and protein expression levels in purified brain microvessels revealed that S1pr1iECKO mice showed normal expression levels for these TJ and AJ proteins (Fig. 3 A and B). TJs and AJs are linked to the cortical actin cytoskeleton, which controls the subcellular distribution and functions of these adhesive proteins (35). Because S1P1 is a key regulator of endothelial actin cytoskeleton (10), we further examined the subcellular distribution of TJ and AJ proteins in the brain vasculature. For this purpose, subcellular distribution of TJ proteins was examined by immunofluorescence confocal microscopy. However, we did not observe obvious differences in the TJ protein distribution in microvessels from control and S1pr1iECKO brains (Fig. S4A). Next, we performed subcellular fractionation experiments using purified microvessels from the brain of control and S1pr1iECKO mice. Irrespective of the genotypes, CD31 and VE–cadherin were exclusively detected in the membrane and cytoskeletal fractions, respectively (Fig. S5). Meanwhile, claudin-5 and occludin were detected in the membrane, nuclear, and cytoskeletal fractions. Higher expression of these TJ proteins was observed in the membrane fraction, but less expression was detected in actin cytoskeletal fraction in S1pr1iECKO microvascular fragments compared with control mice (Fig. 3C). However, immunofluorescence confocal microscopy did not detect altered subcellular distribution of TJ proteins (Fig. S4B). These results suggest that the loss of S1pr1 reduces cytoskeletal association of TJ proteins without gross changes in their expression level or localization in brain ECs, which may facilitate an increase in BBB permeability to small molecules.
Fig. 3.
Altered subcellular distribution of TJ proteins in brain microvessels of S1pr1iECKO mice. (A) qPCR analysis on the mRNA expression for TJ proteins Cldn5, Ocln, and Tjp1 and AJ protein Cdh5 in microvascular fragments from control (S1pr1flox/flox) and S1pr1iECKO mice. Relative expression levels normalized to pan-EC marker Pecam1 are shown (n = 4). (B) Representative immunoblot image (Left) with quantification (Right) of TJ proteins Claudin-5, Occludin, and ZO-1 and AJ protein VE-cadherin in control and S1pr1iECKO brain microvessels. Anti-CD31 antibody was used as a loading control and for the normalization in quantification (n = 3). (C) Subcellular distribution of TJ proteins Claudin-5, Occludin, and ZO-1 in control and S1pr1iECKO brain microvessels (n = 5). Representative immunoblot images (C, Upper) with quantification (C, Lower) are shown. The distribution of TJ proteins in each fraction represents percent of total (C, Lower). Data are expressed as mean ± SD. *P < 0.05 (one-way analysis of variance).
Fig. S4.
Immunohistochemical analysis of TJ proteins. (A) Representative high-resolution confocal images of brain capillary from control (S1pr1flox/flox) and S1pr1iECKO mice stained with Glut1 (EC), and TJ proteins claudin-5, occludin and ZO-1. The 3D reconstructions of z-stack (XY projection or YZ projection) are presented. (Scale bars: 3 μm.) (B) Quantification of the colocalization coefficient between claudin-5 or occludin and ZO-1 via Pearson’s analysis. Data are expressed as mean ± SD (n = 21–30).
Fig. S5.
Endothelial deletion of S1pr1 did not affect subcellular distribution of CD31 and VE-cadherin. Representative immunoblot images of CD31 and VE-cadherin in each subcellular fraction from control (S1pr1flox/flox) and S1pr1iECKO brain microvessels are shown. Note that CD31 or VE-cadherin is exclusively detected in cell membrane or actin cytoskeletal fractions, respectively.
Reversible and Size-Selective Opening of BBB by Pharmacological Targeting of S1P1.
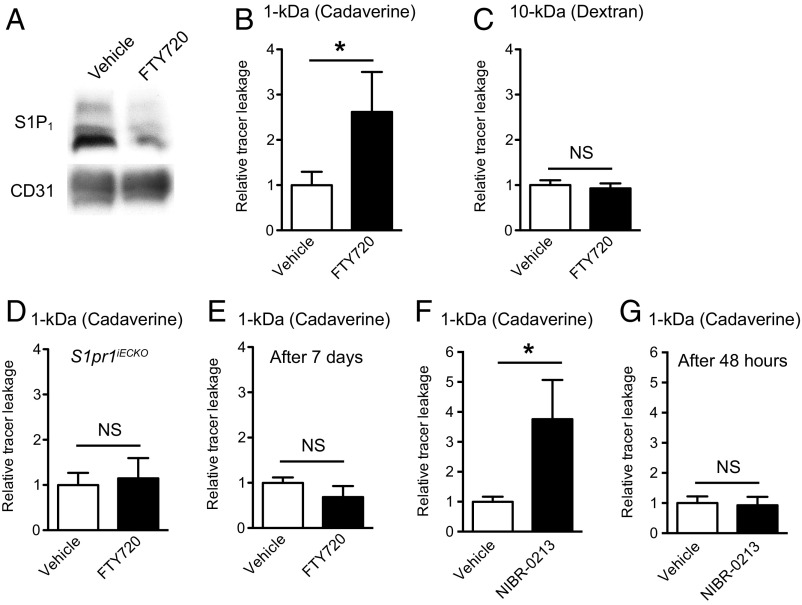
Because S1pr1 deletion induced a size-selective BBB opening, we next investigated whether S1P1 inhibitors can open the BBB. For this purpose, we used the FDA-approved drug FTY720 (fingolimod), which targets all S1P receptors except S1P2 (7). FTY720 is a prodrug that is phosphorylated in vivo to form FTY720-P, which works as functional antagonist for the S1P1 by inducing its internalization and degradation (11, 36, 37). Indeed, three consecutive days of treatment with FTY720 successfully reduced the expression of S1P1 in microvascular fragments (Fig. 4A). FTY720-treated wild-type mice showed higher brain accumulation of 1-kDa Alexa Fluor 555–cadaverine tracers, but not of the 10-kDa tracer, recapitulating the S1pr1iECKO mice phenotype (Fig. 4 B and C). However, FTY720 treatment did not show any enhancement of 1-kDa Alexa Fluor 555–cadaverine extravasation to brain parenchyma in S1pr1iECKO mice, indicating that the effect is S1P1-dependent (Fig. 4D). Importantly, the BBB opening induced by FTY720 was reversible and was not observed at 7 d after the injection (Fig. 4E). Treatment with the S1P1-selective functional antagonist NIBR-0213 (38) showed fivefold increase of 1-kDa Alexa Fluor 555–cadaverine accumulation in brain parenchyma at 6 h after injection, but not at 48 h after injection of NIBR-0213 (Fig. 4 F and G). Collectively, these results indicate that pharmacological targeting of S1P1 reversibly increases the BBB permeability in a size-selective manner.
Fig. 4.
Pharmacological targeting of S1P1 increased brain tracer extravasation in a size-selective and reversible manner. (A) Immunoblot analysis of S1P1 in microvascular fragments from vehicle- or FTY720-treated (5 mg/kg for three consecutive days) mice. CD31 expression is shown as a loading control. (B and C) Quantification of extravasated 1-kDa Alexa Fluor 555–cadaverine (B) and 10-kDa dextran–TMR (C) in vehicle- or FTY720-treated mice. (D) Quantification of 1-kDa Alexa Fluor 555–cadaverine brain leakage in vehicle- or FTY720-treated S1pr1iECKO mice. (E) Quantification of extravasated 1-kDa Alexa Fluor 555–cadaverine in vehicle- or FTY720-treated mice at 7 d after treatment. (F and G) Quantification of extravasated 1-kDa Alexa Fluor 555–cadaverine in vehicle-treated or NIBR-0213–treated (30 mg/kg) mice at 6 h (F) or 48 h (G) after the treatment. Data are expressed as mean ± SD. n = 3 or 4. *P < 0.05 (Student’s t test). NS, nonsignificant.
Discussion
The BBB is essential for the maintenance of brain homeostasis, while BBB dysfunction has been associated with numerous CNS diseases (1–3). Therefore, maintenance and restoration of normal BBB function is thought to be a potential therapeutic approach. Conversely, BBB is an impediment to drug delivery into the CNS. Therefore, a detailed understanding of the development and maintenance of BBB is of critical importance for the development of novel therapeutic approaches for many CNS diseases (4). In the present study, we examined the role of endothelial S1P1 on BBB function using S1pr1iECKO mice. We found that S1pr1iECKO mice exhibit a small-molecule-selective BBB opening, without major signs of CNS inflammation or injury. Such a size-selective BBB opening could be attributed to changes in the subcellular localization of TJ proteins. Furthermore, we succeeded in reversibly and size-selectively opening the BBB by pharmacological blockage of S1P1.
Numerous studies have examined BBB development or maintenance (2); however, there is limited evidence of size-selective disruption of BBB in mice. For example, Cldn5 knockout (32), as well as knockdown of both Cldn5 and Ocln (33, 34), results in enhanced BBB permeability to small molecules. These reports have highlighted that small-molecule transport through the BBB may occur via the paracellular route. Interestingly, the molecular size threshold for increased permeability observed in S1pr1iECKO mice (3–10 kDa) is similar to that in Cldn5 and Ocln double knockdowns (34). Thus, inhibition of S1P1-regulated TJ protein function in brain EC may allow small molecules to enter the brain parenchyma.
Very recently, two other molecules, lipolysis-stimulated lipoprotein receptor (39) and GPR116 (40), were shown to regulate BBB permeability in a size-selective manner. Although TJ morphology did not show major alterations in these models (39, 40), it is still conceivable that S1P1 may regulate BBB function by cooperating with these molecules. Indeed, S1P1 and GPR116 are both EC-enriched GPCRs, and may contribute to BBB maintenance through common mechanisms.
As a barrier-enhancing GPCR, S1P1 has been reported to regulate AJ by regulating VE–cadherin distribution in cultured ECs (10, 41). Although our biochemical approach did not show any changes in VE–cadherin subcellular distribution in S1pr1iECKO mice, alteration of the AJ structure or function may also be involved in the increased BBB leakage in these mice.
The molecular mechanism by which loss of S1P1 alters the localization of TJ proteins in brain ECs is still unknown. One possibility is that continuous activation of S1P1 might be essential for cortical actin formation in brain ECs, which could directly facilitate proper maintenance of TJ protein complexes (35). Importantly, the S1P1/Rac pathway strongly induces cortical actin rearrangement by regulating actin-associated proteins, cortactin and myosin light chain kinase, in cultured ECs (10). Another possibility is that S1P1 regulates phosphorylation of TJ proteins. Multiple phosphorylation sites have been reported in claudin-5, occludin, and ZO-1 (42), which have been linked to both increases and decreases in their interaction to actin cytoskeleton (43). Notably, these phosphorylation sites are targets of c-Src, FAK, PI3Ks, Rho kinases, and PKCs (42), which can be modulated by S1P1 signaling. Further studies are needed to examine whether TJ phosphorylation is altered in S1pr1iECKO mice, and, if so, to assess which signaling pathways are used.
Size-selective leakage of BBB observed in S1pr1iECKO mice may impact initiation or progression of CNS diseases. High levels (∼1 μM) of S1P are found in blood, and more than half of plasma S1P is associated with high-density lipoprotein (HDL) (44). Importantly, lower plasma concentrations of HDL were reported to be associated with dementia (45). Considering that BBB leakage for small-size molecules (angiotensin II) can lead to cognitive defects (30), attenuated HDL/S1P1-dependent tightening of the BBB may be a risk factor for dementia. A recent study also demonstrated that BBB disruption is selective for small-size molecules at the early phase of murine ischemic brain injury (46). This initial size-selective BBB disruption was attributed to actin cytoskeletal changes (46), which is a likely mechanism of BBB disruption in S1pr1iECKO mice, as discussed above. Importantly, S1P1 agonists may be protective in ischemic stroke (9), and an anticoagulant protein S displayed a protective role in hypoxic/ischemic BBB disruption in a S1P1-dependent manner (47). Together, these observations raise the possibility that brain endothelial S1P1 may have a protective role in ischemic brain injury or dementia.
Endothelial S1P1 may be one of the key regulators of homeostatic BBB function. Tonic activation of S1P1 in brain ECs from the luminal side may tighten the paracellular barrier and promote BBB function. In addition, S1P1 activity may also be regulated from the abluminal side by circulating S1P. In the lymphoid organs, multiple perivascular cellular components maintain local perivascular S1P gradients, which are important for immune cell trafficking (44). Recent studies suggest the highly dynamic nature of BBB during the sleep–awake cycle (48). The plasticity of the BBB seems to be important to facilitate both the protection of brain from blood-derived toxins and the elimination of deleterious byproducts of brain metabolism. Because brain capillary endothelium is surrounded by other cellular components of NVU, the potential perivascular local supply of S1P might facilitate S1P1-dependent BBB maintenance by cooperating with circulatory S1P.
Our study also suggests that targeting endothelial S1P1 could be a promising approach for the delivery of therapeutic agents to the CNS. Many attempts have been made to bypass the BBB for CNS drug delivery (4). However, massive disruption of the BBB could have damaging consequences for the CNS (4). Therefore, reversible and limited opening of the BBB would be an ideal strategy for CNS drug delivery. Indeed, using FTY720 and NIBR-0213, we were able to reversibly open the BBB. The size-selective property of BBB opening by targeting S1P1 would also offer the safe CNS delivery of compounds by avoiding severe brain inflammation or edema that could be driven by the transfer of blood-borne macromolecules into the brain parenchyma, such as fibrinogen. This notion is further supported by the observation that S1pr1iECKO mice did not show any signs of inflammation or gliosis in brain. Indeed, S1pr1iECKO mice did not show memory defects if endothelial S1pr1 was deleted at adult stages.
Notably, it is reported that S1P1-agonism reduces P-glycoprotein (P-gp) transport activity in rodent in situ perfusion experiments (49). In that study, FTY720 was shown to increase brain accumulation of the drugs by its agonistic activity. Furthermore, the authors found unaltered brain distribution of sucrose by either S1P1 agonist or antagonist, suggesting that TJ-mediated paracellular BBB permeability may not be regulated by S1P1. Although the reasons for this discrepancy remain unclear, differences in chemical properties of tracers (cadaverine or dextran vs. sucrose), model system (awake mouse vs. in situ perfusion), or ligand administration route (gavage vs. carotid infusion) may have played a role. Future studies testing different classes of tracers including P-gp substrates on S1pr1iECKO mice would help provide a better understanding of the different factors determining the BBB functional characteristics.
In summary, we have demonstrated that endothelial S1P1 regulates BBB permeability in a size-dependent manner. Furthermore, we have shown that pharmacological targeting of S1P1 induces a reversible opening of the BBB, which may be of potential therapeutic value for the safe delivery of drugs into the CNS. Further investigations into the contribution of S1P1 in other NVU components on the development and maintenance of BBB would yield important information for the future therapeutic application of S1P1 modulators on a variety of CNS diseases.
Materials and Methods
Mouse Strains.
Mice were housed in individual ventilated cages in a temperature-controlled facility with a 12-h light/dark cycle at Weill Cornell Medical College. EC-specific S1pr1 knockout mice (S1pr1flox/flox Cdh5–Cre–ERT2; S1pr1iECKO) were generated as described (12, 13, 16, 17). Mice were treated with tamoxifen by oral gavage (50 μg/d) for the first 3 d after birth and used for the experiments at the age of 10–16 wk. For the adult deletion experiments, 8-wk-old mice were treated with tamoxifen by oral gavage (100 μg per gram of body weight) for three consecutive days and used for the experiments 4 wk after last injection of tamoxifen. S1pr1flox/flox mice without the Cdh5–Cre–ERT2 gene were treated with tamoxifen in the same way as S1pr1iECKO mice and used as control mice (S1pr1flox/flox). All experiments were performed in male mice. All animal experiments were approved by the Weill Cornell Institutional Animal Care and Use Committee.
Tracer Injection Experiments.
Brain tracer leakage experiments were performed as described (40, 50). Mice (10–12 wk of age) were injected in the tail vein with Alexa Fluor 555–cadaverine (6 μg/g; Invitrogen), 3-kDa dextran–TMR (10 μg/g; Invitrogen), 10-kDa dextran–TMR (15 μg/g; Invitrogen), or 70-kDa dextran–TMR (100 μg/g; Invitrogen) dissolved in saline. After 2 h (for Alexa Fluor 555–cadaverine or 3-kDa dextran–TMR), 4 h (for 10-kDa dextran–TMR), or 16 h (for 70-kDa dextran–TMR), mice were anesthetized and perfused for 7 min with ice-cold PBS (pH 7.4), and brains were removed. After dissection, the cortex was weighed and homogenized with 1% Triton X-100 in PBS. Cortical lysates were centrifuged at 12,000 × g for 20 min at 4 °C, and the supernatant was used to quantify fluorescence (excitation/emission 540/590 nm; SpectraMax M2e; Molecular Devices). The relative fluorescence values were normalized with cortical weights.
Retinal tracer leakage experiments were performed as described (51). Under deep isoflurane anesthesia, 3-kDa dextran–TMR (50 μg/g) was injected intravenously. After 10 min, the chest cavity was opened, and mice were perfused for 7 min with ice-cold PBS. After perfusion, retinas were removed and homogenized in distilled water. The extract was processed through a 30,000-molecular-weight filter (Amicon Ultra-0.5 mL; Millipore) at 13,000 × g for 10 min. The fluorescence in each 300-μL sample was measured (excitation/emission 540/590 nm; SpectraMax M2e).
Brain Microvascular Fragments.
Brain microvascular fragments were prepared as described (52, 53) with minor modifications (see details in SI Materials and Methods).
qPCR Analysis, Immunoblot Analysis, and Immunohistochemistry.
RNA isolation, RT-qPCR analysis, preparation of total lysates or subcellular fractions, Western blotting, and immunofluorescence experiments were performed as described in SI Materials and Methods.
NOR.
The NOR task was conducted as described (30) (see details in SI Materials and Methods).
Drug Treatment.
FTY720 and NIBR-0213 are kind gifts from Novartis. To examine the effect of FTY720, mice were administered 5 mg⋅kg−1⋅d−1 FTY720 or vehicle (water) via oral gavage continuously for three consecutive days. These mice were used for the tracer injection experiments 1 or 7 d after last injection of FTY720. For NIBR-2013 experiments, mice were treated with 60 mg/kg NIBR-0213 or vehicle [30% (vol/vol) PEG/phosphate buffer (pH 7.4)] via oral gavage. The mice were used for the tracer injection experiments 6 or 48 h after NIBR-0213 injection.
Statistical Analysis.
Statistical analysis was performed by using two-tailed Student’s t test or one-way analysis of variance with Bonferroni’s multiple comparison test as described using Prism software (GraphPad). P values < 0.05 were considered statistically significant. All results are expressed as the mean ± SD.
SI Materials and Methods
Brain Microvascular Fragments.
Brain microvascular fragments were prepared as described (52, 53) with minor modification. Brains were collected and rinsed in MCDB131 medium (Corning) supplemented with 0.5% fatty acid-free BSA (Fisher). Cerebella, brainstem, white matter, and leptomeninges were removed on ice by macroscopic dissection, and cortices were homogenized in 8 mL of PBS with a 7-mL loose-fit Dounce tissue grinder with 10 strokes. The homogenates were centrifuged at 2,000 × g for 5 min at 4 °C. The pellet was suspended in 15% (wt/vol) 70-kDa dextran (Sigma; catalog no. 31390) in PBS and centrifuged at 10,000 × g for 15 min at 4 °C. The pellet (containing the microvessel fragment) was resuspended in MCDB131 supplemented with 0.5% fatty acid-free BSA, trapped in a 40-μm cell strainer (Corning), washed with 5 mL of PBS, and collected with 5 mL of MCDB131 supplemented with 0.5% fatty acid-free BSA into a 50-mL falcon tube. The microvascular fragments were washed with PBS and used for the protein extraction or fractionation. The fresh microvessel preparation in its entirety was also applied to glass slides (in 200 μL of medium per slide), covered with a glass coverslip, and immediately imaged by using a brightfield microscope (Observer.Z1; Zeiss). Images were taken with a 10× lens by using AxioCam MRm (Zeiss) and edited by using ImageJ software to improve contrast of the unstained tissue.
qPCR Analysis.
Total RNA was extracted from cortex, hippocampus, or brain microvessels by using the RNeasy Mini Kit (Qiagen) according to the manufacturer’s instructions. Reverse transcription was carried out by using SuperScript III FirstStrand Synthesis Kit (Invitrogen) with random primers or qScript XLT cDNA SuperMix (Quanta). Real-time PCR was performed on a 7500 real-time PCR system (Applied Biosystems) by using PerfeCTa SYBR Green FastMix Low ROX (Quanta). Primers used for gene expression are listed below (F, forward; R, reverse):
S1pr1 F: ATGGTGTCCACTAGCATCCC
R: CGATGTTCAACTTGCCTGTGTAG
S1pr2 F: ATGGGCGGCTTATACTCAGAG
R: GCGCAGCACAAGATGATGAT
S1pr3 F: GCCTAGCGGGAGAGAAACCT
R: CCGACTGCGGGAAGAGTGT
Pecam1 F: TTGAGCCTCACCAAGAGAACGGAA
R: AATCCAGGAATCGGCTGCTCTTCT
Tek F: AGGAAGAAAAGCGAGGGAAATG
R: CAGGTGAGGAGCAGAGTTGAGAG
Slco1c1 F: GTCCAGCAGTCCATCACCAAG
R: CCCAGGAAGACATAAACCCACA
Slc2a1 F: GAGAGCCCATCCCATCCAC
R: GAGAAGCCCATAAGCACAGCA
Abcb1a F: GCCAACATAGCGCGTTCC
R: TCCCAGTCCCACCCCTCT
Mfsd2a F: CGTGCGGGAGCAGAGAGA
R: AAGCCAGGGAGGTAAAAAGGAAG
Zic3 F: ACCCTTCCCGTGTCCCTTC
R: GGGCTTGTCCGAGGTGTG
Acta2 F: CGGGAGAAAATGACCCAGA
R: CCAGAGTCCAGCACAATACCA
Pdgfrb F: TCTTGGCTCTGGGGCTTTC
R: GCTTCTCGCTACTTCTGGCTGT
Gfap F: GTTGAATCGCTGGAGGAGGA
R: CGCTGTGAGGTCTGGCTTG
Aqp4 F: GCTTCTCGCTACTTCTGGCTGT
R: CCACATCAGGACAGAAGACATACTC
Mfge8 F: GAGGAGCAAGGAAGCAGCAA
R: TGCGGTTATGCCAGGACAC
Mrc1 F: CCCATTTATCATTCCCTCAGCA
R: CCCCACCCTCCTTCCTACAA
Lyve1 F: TGGGAAGAATGGCAAAGGTG
R: TCCAACACGGGGTAAAATGTG
Il1b F: CTCTCCACCTCAATGGACAGA
R: TTTTGTCGTTGCTTGGTTCTC
Il6 F: ATGGATGCTACCAAACTGGAT
R: TGAAGGACTCTGGCTTTGTCT
Tnfa F: TTGGAGTCATTGCTCTGTGAA
R: GGGTCAGAGTAAAGGGGTCAG
Cldn5 F: CCAGAGCAGAGGCACCAGA
R: AGACACAGCACCAGACCCAGA
Ocln F: AGATTCCTCTGACCTTGAGTGTGG
R: TCCTGCTTTCCCCTTCGTG
Tjp1 F: GATTTACCCGTCAGCCCTTCT
R: TCGCAAACCCACACTATCTCC
Cdh5 F: TAGCAAGAGTGCGCTGGAGATTCA
R: ACACATCATAGCTGGTGGTGTCCA
Rplp0 F: CTGAGATTCGGGATATGCTGTTG
R: AAAGCCTGGAAGAAGGAGGTCTT.
Preparation of Total Lysates and Subcellular Fractions from Brain Microvessels.
Microvascular fragments were resuspended in 200 μL of 2× SDS sample buffer after two freeze/thaw cycles. The lysates were then sonicated and centrifuged at 12,000 × g for 5 min, and the supernatants (10 μL each) were used for the immunoblot analysis as described below. Four subcellular fractions, including cytosolic, membrane, nuclear, and cytoskeletal fractions, were extracted from snap-frozen microvascular fragments by using the ProteoExtract Subcellular Proteome Extraction Kit (Calbiochem) according to the manufacturer’s instructions. A total of 200 μL of Extraction Buffer I/II or 100 μL of Extraction Buffer III/IV was used for the fractionation, and 20 or 10 μL of lysates was used for the immunoblot analysis, respectively.
Immunoblot Analysis.
Total extracts or fractionated protein samples were separated into 8%, 12%, or 15% (wt/vol) polyacrylamide gel and transferred on poly(vinylidene difluoride) membrane. Transferred proteins were blocked with 5% (wt/vol) skim milk and probed with primary antibodies against claudin-5 (1:5,000; Invitrogen, catalog no. 35-3500), occludin (1:2,000; Invitrogen, catalog no. 331500), ZO-1 (1:1,000; Invitrogen, catalog no. 617300), VE-cadherin (1:500; R&D, catalog no. AF1002), CD31 (1:2,000; Dianova, catalog no. SZ31), and S1P1 (1:1,000; Santa Cruz, catalog no. sc-25489). Relative band intensities were obtained by densitometric analysis of images using ImageJ software.
Immunohistochemistry.
Anesthetized mice were perfused with ice-cold PBS followed by ice-cold 4% (wt/vol) paraformaldehyde (PFA) in PBS. The brain was fixed overnight with 4% PFA in PBS at 4 °C, then thoroughly washed with PBS. The fixed brain was sectioned at 50 μm on a vibratome. Sections were permeabilized and blocked overnight in blocking buffer (1% BSA and 0.5% Triton X-100 in PBS), followed by incubation with primary antibodies, Collagen IV (1:300; Serotec, catalog no. 2150-1470), CD13 (1:300; Serotec, catalog no. MCA2183EL), CD31 (1:100; Dianova, catalog no. SZ31), and GFAP (1:400; Invitrogen, catalog no. 180063) diluted in blocking buffer at 4 °C at constant agitation. After 2 d of incubation with primary antibodies, the sections were washed for 6 h in washing buffer (0.5% Triton X-100 in PBS), incubated with fluorescent secondary antibodies (1:200; Alexa Fluor dyes; Invitrogen) diluted in blocking buffer for 24 h at 4 °C. Sections were then washed for 6 h with washing buffer, mounted in ProLong Gold Antifade Mountant (Life Technologies), and imaged by using an Olympus FluoView FV10i confocal microscope. All presented images are 3D reconstructions of z-stack. Image processing and quantification was performed by using Adobe Photoshop, ImageJ, or Fiji software (National Institutes of Health). Collagen IV- or CD13-positive immunofluorescent signals were subjected to threshold processing, and areas occupied by their signals were quantified by using the Fiji software. The pericyte coverage was determined by normalizing the CD13-positive area to the collagen IV-positive area. Vascular density was determined by normalizing collagen IV-positive area to total area of cortex. Branch point number was quantified on skeletonized collagen IV signal and normalized to the total area of cortex. Total vascular length was quantified on skeletonized collagen IV signal, and vessel diameter was determined by dividing collagen IV-positive area by total vascular length. Mean ± SD from three independent animals for each genotype were presented.
Immunohistochemical staining on TJ proteins was performed on methanol-fixed fresh-frozen brain sections (30 μm). Floating sections were permeabilized and blocked overnight in blocking buffer (1% BSA and 0.5% Triton X-100 in PBS), followed by overnight incubation with the following primary antibodies: claudin-5 (1:400; mouse IgG1, Alexa Fluor 488-conjugated; Invitrogen, catalog no. 352588) or occludin (1:400; mouse IgG1, Alexa Fluor 488-conjugated, Invitrogen, catalog no. 331588), and ZO-1 (1:300; Invitrogen, catalog no. 617300) at 4 °C at constant agitation. Sections were washed for 3 h in washing buffer (0.5% Triton X-100 in PBS) and incubated overnight with fluorescent secondary antibodies (1:300; donkey anti-rabbit IgG, Alexa Fluor 568-conjugated; Invitrogen) at 4 °C to visualize ZO-1. Sections were washed for 3 h and followed by immunostaining for Glut1 (1:400; rabbit IgG; catalog no. 07-1401, Millipore) with fluorescent secondary antibody (1:400; goat anti-rabbit IgG, Alexa Fluor 405-conjugated; Invitrogen) as described above. To avoid cross-reaction between anti–ZO-1 and anti-Glut1 antibodies, sections were incubated with donkey anti-rabbit Fab fragments (1:100; Jackson Laboratories) and fixed with 4% PFA before incubation with anti-Glut1 antibodies. Sections were mounted on ProLong Gold Antifade Mountant and imaged by using a Zeiss LSM 800 with Airyscan confocal microscope. The 3D reconstructions of z-stack (XY projection or YZ projection) images are shown. For colocalization analysis between claudin-5 or occludin and ZO-1, immunofluorescent signals were subjected to threshold processing and analyzed for Pearson’s colocalization by using the ZEN software (Zeiss) in a blinded manner. Images (21–30) from three independent animals for each genotype were analyzed, and results are presented as mean ± SD.
NOR.
The NOR task was conducted under dim light in a plastic box measuring 29- × 47- × 30-cm high as described (30). Stimuli consisted of plastic objects that varied in color and shape, but had similar size. A video camera mounted on the wall directly above the box was used to record the testing session for off-line analysis. Mice (16 wk of age) were acclimated to the testing room and chamber for 1 d before testing (30 min in the testing room and 5 min to explore the empty box). At 24 h after habituation, mice were placed in the same box in the presence of two identical sample objects and were allowed to explore for 5 min. After an intersession interval of 1 h, mice were placed in the same box, but one of the two objects was replaced by a novel object. Mice were allowed to explore for 5 min. Exploratory behavior was later assessed manually by an experimenter blinded to the treatment group. Exploration of an object was defined as the mouse sniffing the object or touching the object while looking at it (31). A minimal exploration time for both objects (total exploration time) during the test phase (∼10 s) was used. The amount of time taken to explore the novel object was expressed as percentage of the total exploration time and provides an index of recognition memory.
Acknowledgments
We thank Steven Swendeman, Akira Ito, Nichole Chang (Weill Cornell Medicine), Christer Betsholtz, Bongnam Jung (Uppsala University), Annika Keller, and Josephin Wagner (University Hospital Zurich) for technical help and advice. This work is supported by NIH Grants HL89934, HL117798, and HL135821 (to T.H.), NS89323, NS95441, and NS100447 (to C.I.), and HL094465 (to T.S.); a Fondation Leducq Transatlantic Network Grant (to T.H., C.I., and T.S.); and American Heart Association Grants GIA12GRNT12050110 (to T.S.) and 15SDG22760007 (to G.F.). K.Y. was supported by the Japan Society for the Promotion of Science.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1618659114/-/DCSupplemental.
References
- 1.Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV. Establishment and dysfunction of the blood-brain barrier. Cell. 2015;163:1064–1078. doi: 10.1016/j.cell.2015.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chow BW, Gu C. The molecular constituents of the blood-brain barrier. Trends Neurosci. 2015;38:598–608. doi: 10.1016/j.tins.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med. 2013;19:1584–1596. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banks WA. From blood-brain barrier to blood-brain interface: New opportunities for CNS drug delivery. Nat Rev Drug Discov. 2016;15:275–292. doi: 10.1038/nrd.2015.21. [DOI] [PubMed] [Google Scholar]
- 5.Pardridge WM. The blood-brain barrier: Bottleneck in brain drug development. NeuroRx. 2005;2:3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Proia RL, Hla T. Emerging biology of sphingosine-1-phosphate: Its role in pathogenesis and therapy. J Clin Invest. 2015;125:1379–1387. doi: 10.1172/JCI76369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brinkmann V, et al. Fingolimod (FTY720): Discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov. 2010;9:883–897. doi: 10.1038/nrd3248. [DOI] [PubMed] [Google Scholar]
- 8.Brunkhorst R, Vutukuri R, Pfeilschifter W. Fingolimod for the treatment of neurological diseases-state of play and future perspectives. Front Cell Neurosci. 2014;8:283. doi: 10.3389/fncel.2014.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanchez T. Sphingosine-1-phosphate signaling in endothelial disorders. Curr Atheroscler Rep. 2016;18:31. doi: 10.1007/s11883-016-0586-1. [DOI] [PubMed] [Google Scholar]
- 10.Xiong Y, Hla T. S1P control of endothelial integrity. Curr Top Microbiol Immunol. 2014;378:85–105. doi: 10.1007/978-3-319-05879-5_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oo ML, et al. Engagement of S1P1-degradative mechanisms leads to vascular leak in mice. J Clin Invest. 2011;121:2290–2300. doi: 10.1172/JCI45403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jung B, et al. Flow-regulated endothelial S1P receptor-1 signaling sustains vascular development. Dev Cell. 2012;23:600–610. doi: 10.1016/j.devcel.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montrose DC, et al. S1P1 localizes to the colonic vasculature in ulcerative colitis and maintains blood vessel integrity. J Lipid Res. 2013;54:843–851. doi: 10.1194/jlr.M034108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christensen PM, et al. Impaired endothelial barrier function in apolipoprotein M-deficient mice is dependent on sphingosine-1-phosphate receptor 1. FASEB J. 2016;30:2351–2359. doi: 10.1096/fj.201500064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allende ML, Yamashita T, Proia RL. G-protein-coupled receptor S1P1 acts within endothelial cells to regulate vascular maturation. Blood. 2003;102:3665–3667. doi: 10.1182/blood-2003-02-0460. [DOI] [PubMed] [Google Scholar]
- 16.Blaho VA, et al. HDL-bound sphingosine-1-phosphate restrains lymphopoiesis and neuroinflammation. Nature. 2015;523:342–346. doi: 10.1038/nature14462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galvani S, et al. HDL-bound sphingosine 1-phosphate acts as a biased agonist for the endothelial cell receptor S1P1 to limit vascular inflammation. Sci Signal. 2015;8:ra79. doi: 10.1126/scisignal.aaa2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.London A, Benhar I, Schwartz M. The retina as a window to the brain-from eye research to CNS disorders. Nat Rev Neurol. 2013;9:44–53. doi: 10.1038/nrneurol.2012.227. [DOI] [PubMed] [Google Scholar]
- 19.Whiteus C, Freitas C, Grutzendler J. Perturbed neural activity disrupts cerebral angiogenesis during a postnatal critical period. Nature. 2014;505:407–411. doi: 10.1038/nature12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wälchli T, et al. Quantitative assessment of angiogenesis, perfused blood vessels and endothelial tip cells in the postnatal mouse brain. Nat Protoc. 2015;10:53–74. doi: 10.1038/nprot.2015.002. [DOI] [PubMed] [Google Scholar]
- 21.Ben-Zvi A, et al. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature. 2014;509:507–511. doi: 10.1038/nature13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaengel K, et al. The sphingosine-1-phosphate receptor S1PR1 restricts sprouting angiogenesis by regulating the interplay between VE-cadherin and VEGFR2. Dev Cell. 2012;23:587–599. doi: 10.1016/j.devcel.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Ben Shoham A, et al. S1P1 inhibits sprouting angiogenesis during vascular development. Development. 2012;139:3859–3869. doi: 10.1242/dev.078550. [DOI] [PubMed] [Google Scholar]
- 24.Paik JH, et al. Sphingosine 1-phosphate receptor regulation of N-cadherin mediates vascular stabilization. Genes Dev. 2004;18:2392–2403. doi: 10.1101/gad.1227804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeisel A, et al. Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science. 2015;347:1138–1142. doi: 10.1126/science.aaa1934. [DOI] [PubMed] [Google Scholar]
- 26.Engelhardt B, Ransohoff RM. Capture, crawl, cross: The T cell code to breach the blood-brain barriers. Trends Immunol. 2012;33:579–589. doi: 10.1016/j.it.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Lambertsen KL, Biber K, Finsen B. Inflammatory cytokines in experimental and human stroke. J Cereb Blood Flow Metab. 2012;32:1677–1698. doi: 10.1038/jcbfm.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sagare AP, Bell RD, Zlokovic BV. Neurovascular dysfunction and faulty amyloid β-peptide clearance in Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a011452. doi: 10.1101/cshperspect.a011452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faraco G, et al. Perivascular macrophages mediate the neurovascular and cognitive dysfunction associated with hypertension. J Clin Invest. 2016;126:4674–4689. doi: 10.1172/JCI86950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leger M, et al. Object recognition test in mice. Nat Protoc. 2013;8:2531–2537. doi: 10.1038/nprot.2013.155. [DOI] [PubMed] [Google Scholar]
- 32.Nitta T, et al. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161:653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campbell M, et al. RNAi-mediated reversible opening of the blood-brain barrier. J Gene Med. 2008;10:930–947. doi: 10.1002/jgm.1211. [DOI] [PubMed] [Google Scholar]
- 34.Keaney J, et al. Autoregulated paracellular clearance of amyloid-β across the blood-brain barrier. Sci Adv. 2015;1:e1500472. doi: 10.1126/sciadv.1500472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spindler V, Schlegel N, Waschke J. Role of GTPases in control of microvascular permeability. Cardiovasc Res. 2010;87:243–253. doi: 10.1093/cvr/cvq086. [DOI] [PubMed] [Google Scholar]
- 36.Gräler MH, Goetzl EJ. The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G-protein-coupled receptors. FASEB J. 2004;18:551–553. doi: 10.1096/fj.03-0910fje. [DOI] [PubMed] [Google Scholar]
- 37.Oo ML, et al. Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J Biol Chem. 2007;282:9082–9089. doi: 10.1074/jbc.M610318200. [DOI] [PubMed] [Google Scholar]
- 38.Quancard J, et al. A potent and selective S1P(1) antagonist with efficacy in experimental autoimmune encephalomyelitis. Chem Biol. 2012;19:1142–1151. doi: 10.1016/j.chembiol.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 39.Sohet F, et al. LSR/angulin-1 is a tricellular tight junction protein involved in blood-brain barrier formation. J Cell Biol. 2015;208:703–711. doi: 10.1083/jcb.201410131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niaudet C, et al. Gpr116 receptor regulates distinctive functions in pneumocytes and vascular endothelium. PLoS One. 2015;10:e0137949. doi: 10.1371/journal.pone.0137949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee MJ, et al. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell. 1999;99:301–312. doi: 10.1016/s0092-8674(00)81661-x. [DOI] [PubMed] [Google Scholar]
- 42.González-Mariscal L, Tapia R, Chamorro D. Crosstalk of tight junction components with signaling pathways. Biochim Biophys Acta. 2008;1778:729–756. doi: 10.1016/j.bbamem.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 43.Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: Molecular organization and role in vascular homeostasis. Physiol Rev. 2004;84:869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- 44.Yanagida K, Hla T. Vascular and immunobiology of the circulatory sphingosine 1-phosphate gradient. Annu Rev Physiol. 2017;79:67–91. doi: 10.1146/annurev-physiol-021014-071635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vitali C, Wellington CL, Calabresi L. HDL and cholesterol handling in the brain. Cardiovasc Res. 2014;103:405–413. doi: 10.1093/cvr/cvu148. [DOI] [PubMed] [Google Scholar]
- 46.Shi Y, et al. Rapid endothelial cytoskeletal reorganization enables early blood-brain barrier disruption and long-term ischaemic reperfusion brain injury. Nat Commun. 2016;7:10523. doi: 10.1038/ncomms10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu D, et al. Protein S controls hypoxic/ischemic blood-brain barrier disruption through the TAM receptor Tyro3 and sphingosine 1-phosphate receptor. Blood. 2010;115:4963–4972. doi: 10.1182/blood-2010-01-262386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keaney J, Campbell M. The dynamic blood-brain barrier. FEBS J. 2015;282:4067–4079. doi: 10.1111/febs.13412. [DOI] [PubMed] [Google Scholar]
- 49.Cannon RE, Peart JC, Hawkins BT, Campos CR, Miller DS. Targeting blood-brain barrier sphingolipid signaling reduces basal P-glycoprotein activity and improves drug delivery to the brain. Proc Natl Acad Sci USA. 2012;109:15930–15935. doi: 10.1073/pnas.1203534109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Armulik A, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 51.Muranaka K, et al. Effects of peroxisome proliferator-activated receptor gamma and its ligand on blood-retinal barrier in a streptozotocin-induced diabetic model. Invest Ophthalmol Vis Sci. 2006;47:4547–4552. doi: 10.1167/iovs.05-1432. [DOI] [PubMed] [Google Scholar]
- 52.Biegel D, Spencer DD, Pachter JS. Isolation and culture of human brain microvessel endothelial cells for the study of blood-brain barrier properties in vitro. Brain Res. 1995;692:183–189. doi: 10.1016/0006-8993(95)00511-n. [DOI] [PubMed] [Google Scholar]
- 53.Boulay AC, Saubaméa B, Declèves X, Cohen-Salmon M. Purification of mouse brain vessels. J Vis Exp. 2015;(105):e53208. doi: 10.3791/53208. [DOI] [PMC free article] [PubMed] [Google Scholar]