Abstract
Common sites for metastatic spreading from breast cancer are bones, lungs and liver, the skeletal muscle being an unusual site. Although rare, when skeletal muscle metastases occur they are associated with a poor prognosis. These metastases are clinically difficult to diagnose since they can be found without pain symptoms. Radiologically, magnetic resonance imaging has been considered better than computed tomography for imaging of the muscles and has been the first procedure to use in case of muscle metastasis suspicion. In the last years, positron emission tomography (PET) with 18Fluorine-2-fluoro-2-deoxy-d-glucose (18F-FDG) has emerged as the main imaging tool. We here report a case of a hormone receptor-positive/human epidermal growth factor receptor 2-negative patient who presented with a recurrent infiltrating ductal carcinoma and diffuse skeletal muscle metastases detected by 18F-FDG-PET. The treatment of the patient with exemestane and everolimus led to a durable complete response.
INTRODUCTION
Cancer metastases in skeletal muscle are rare. In all malignancies, the incidence may vary between 0.8% and 16% according to autopsy series performed >30 years ago [1]. However, this is an approximate value since serial sectioning of the entire muscle in autopsies is not feasible.
Although uncommon, skeletal muscle metastases from breast cancer have been described by few recently reports [2, 3]. The increasing number of papers addressing this issue may be in line with the expanding use of modern imaging technologies to manage breast cancer patients.
The lower incidence of cancer metastases in the skeletal muscle is a well-known clinical phenomenon [4]. However, the molecular mechanisms underlying it are not completely understood. Several factors have been suggested as possible reasons to explain the resistance of the muscle to metastases. Both high production and capacity of the muscle to metabolize lactic acid [5], the activation of purinergic receptors [4] and the accumulation of low molecular weight factors [6] can be cited.
When skeletal muscle metastasis occurs, it is associated with a poor prognosis. Indeed, most patients diagnosed with muscle metastases die within a few months despite different treatment strategies [7]. Thus, therapeutic interventions at this stage are mainly palliative to control pain. We here report a case of a breast cancer patient with diffuse muscle metastases diagnosed using 18Fluorine-2-fluoro-2-deoxy-d-glucose-positron emission tomography (18F-FDG-PET) who is in complete response 1 year after treatment.
CASE PRESENTATION
In September 2001, a 56-year-old woman, with no particular medical background except Graves’ disease was diagnosed with infiltrating ductal carcinoma (pT3N+(8/19)M0). She underwent a mastectomy of the right breast with right axillary lymph nodes dissection. The patient was staged with histological Scarff–Bloom–Richardson Grade I.
Immunohistochemistry analysis showed positive staining for oestrogen receptors (ER), <10% of cells staining for progesterone receptors (PR) and no expression of the human epidermal growth factor receptor 2 (HER2). She received adjuvant chemotherapy consisting of six cycles of FEC-100 (5-fluorouracil, epirubicin, cyclophosphamide) every 3 weeks, followed by radiotherapy of the right chest wall and 5 years of endocrine therapy based on tamoxifene followed by anastrozol when menopause occurred.
During surveillance, 14 years later (February 2015), the patient presented with a tumefaction in the exofacial part of the left parotid gland. Following magnetic resonance imaging (MRI) and a biopsy, which confirmed the malignancy, the lesion was surgically removed. A left cervical lymphadenectomy was also performed (revealing non metastatic lymph nodes).
Histological examination of the tumour showed a high grade adenocarcinoma measuring 1 cm with vascular emboli and perineural invasion. At that time, we suspected of a salivary carcinoma. However, tumour cells did not express the thyroid transcription factor-1, a neuroendocrine cell marker that is frequently expressed in the normal epithelium and in neoplasms of the thyroid gland and lungs, but also in atypical neuroendocrine tumours such as salivary glands. Hormone receptors status were ER+, PR+ and HER2−.
Considering the expression of ER, the absence of HER2 and their concordance with the primary breast cancer, we diagnosed a relapse of the patient. A complete imaging assessment was then undertaken and included a PET using 18F-FDG. Tumour activity measured by 18F-FDG-PET revealed persistent hypermetabolism (SUV max 6.4) in the left parotid area apparently from inflammatory origin. Other distant metastases were identified in the lungs, bones and vertebrae (L5, S3, T10). Also, multiple and diffuse muscle metastases were observed. On physical examination, there was no palpable mass but the patient reported mild muscle pain. These muscle metastases corresponded to multiple round or oval masses with the density of soft tissue in computed tomography (CT) scan.
One month later (April 2015), an ultrasound-guided breast biopsy of a mass that appeared in the wall of the upper outer quadrant of the right reconstructed breast attested a local relapse of the infiltrating ductal carcinoma. Again, histological analysis revealed ER+, PR+ and HER2−. In May 2015, a systemic treatment associating exemestane (25 mg per day) and everolimus (10 mg per day) was started. One month later, we noticed a partial metabolic response followed by a complete metabolic response observed after 6 months on treatment. A persistent complete response was observed after 1 year (Fig. 1). The treatment was well tolerated and the only side effect experienced by the patient was a Grade II mucositis.
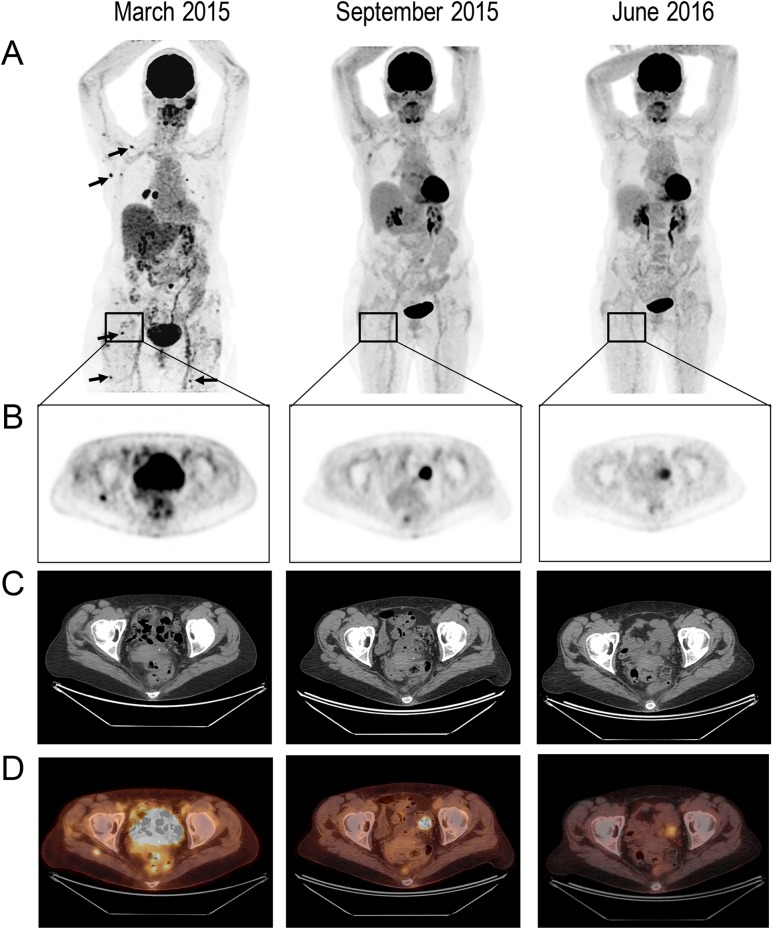
Figure 1:
Response to treatment in a breast cancer patient with diffuse skeletal muscle metastases.(A) 3D MIP (maximum intensity projection) 18F-FDG-PET (anterior views) performed before treatment (March 2015), 4 months after treatment initiation (September 2015) and 1 year on treatment (June 2016). Pretreatment images show multifocal hypermetabolic foci (arrows), from which the most intense and prominent are one in the external part of the right pectoralis major muscle and one in the right lower area of the periscapular; (B) Selected transaxial slice of a right gluteal muscle mass with increased FDG uptake; (C) the selected region slice on CT and (D) fused PET/CT.
DISCUSSION
Skeletal muscle metastases are rare and difficult to diagnose clinically and radiologically. They generally manifest as a painful mass in the involved area or as mild muscular pain in the case of diffuse lesions. However, skeletal muscle metastasis can also be found without symptoms. Our patient who had diffuse skeletal muscle metastases complained of mild muscular pain.
The pattern of skeletal muscle metastases also varies. According to Nocuń et al. 2015 [8], three patterns exists. The first pattern is multiple skeletal muscle metastasis generally accompanied by other metastases in lymph nodes and organs. Second, skeletal muscle metastasis can also be found as single lesions, associated or not to other metastatic lesions. The latter is less common and more difficult to define since this pattern of lesions can also be observed in other malignant and benign conditions. Indeed, some benign processes such as intramuscular abscesses, myositis ossificans and systemic sclerosis have manifestations similar to metastases on CT or MRI. Thus, these cases may require a biopsy.
In our institution, since 18F-FDG-PET has been used in routine staging and to follow-up patients with different cancers it has added a great benefit of diagnosing extent of metastases. Thus, by providing whole body scan information, 18F-FDG-PET was a powerful imaging tool for the detection of muscle metastases in our patient. The superiority of 18F-FDG-PET over CT to detect skeletal muscle metastases is also related to its capacity to distinguish some lesions (which may appear hypodense or isodense in other imaging modalities) from the surrounding muscle.
For comparison, soft tissue resolution of the MRI has been considered better than CT for imaging of the muscles [9] and generally remain the first specific procedure to use in case of muscle metastasis suspicion. Nevertheless, MRI is not commonly used in daily oncologic practice because of its costs compared with CT scan and the poor awareness of physicians dealing with muscular pain in metastatic situations.
Finally, to treat our patient, we aimed to enhance the efficacy of the endocrine therapy using everolimus. Based on the results of BOLERO-2 trial, everolimus in combination with exemestane has been approved for patients with advanced hormone receptor-positive/HER2-negative breast cancer who progressed on prior non-steroidal aromatase inhibitor [10]. Following patients’ treatment with everolimus plus exemestane, all muscle nodules regressed in parallel to lung metastases and a complete response was achieved. This successful clinical effect might be mechanistically explained because acquired resistance to endocrine therapies in HR-positive breast cancer has been liked to an aberrant signalling through the phosphatidylinositol 3-kinase-AKT-mammalian target of rapamycin (mTOR) pathway [11]. Indeed, within this pathway, a kinase called S6 (mTOR/S6K) contributes to oestrogen-independent phosphorylation of ER alpha, resulting in receptor activation. Downregulation of mTOR pathway should then attenuate glucose uptake and metabolism resulting in significant reduced glucose avidity on 18F-FDG-PET.
DISCLOSURE
The authors declare no conflict of interest.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
There was no funding for this report.
ETHICAL APPROVAL
No ethical approval required.
CONSENT
This report has been writing with the approval of the patient. An informed written consent was obtained prior to the submission.
GUARANTOR
Professor Jean-Pierre Lotz.
REFERENCES
- 1.Pearson CM. Incidence and type of pathologic alterations observed in muscle in a routine autopsy survey. Neurology 1959;9:757–66. [DOI] [PubMed] [Google Scholar]
- 2.Salemis NS. Skeletal muscle metastasis from breast cancer: management and literature review. Breast Dis 2015;35:37–40. [DOI] [PubMed] [Google Scholar]
- 3.Emmering J, Vogel WV, Stokkel MPM. Intramuscular metastases on FDG PET-CT: a review of the literature. Nucl Med Commun 2012;33:117–20. [DOI] [PubMed] [Google Scholar]
- 4.Bar-Yehuda S, Barer F, Volfsson L, Fishman P. Resistance of muscle to tumor metastases: a role for a3 adenosine receptor agonists. Neoplasia 2001;3:125–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seely S. Possible reasons for the high resistance of muscle to cancer. Med Hypotheses 1980;6:133–7. [DOI] [PubMed] [Google Scholar]
- 6.Djaldetti M, Sredni B, Zigelman R, Verber M, Fishman P. Muscle cells produce a low molecular weight factor with anti-cancer activity. Clin Exp Metastasis 1996;14:189–96. [DOI] [PubMed] [Google Scholar]
- 7.Molina-Garrido MJ, Guillén-Ponce C. Muscle metastasis of carcinoma. Clin Transl Oncol 2011;13:98–101. [DOI] [PubMed] [Google Scholar]
- 8.Nocuń A, Chrapko B. Multiple and solitary skeletal muscle metastases on 18F-FDG PET/CT imaging. Nucl Med Commun 2015;36:1091–9. [DOI] [PubMed] [Google Scholar]
- 9.Arpaci T, Ugurluer G, Akbas T, Arpaci RB, Serin M. Imaging of the skeletal muscle metastases. Eur Rev Med Pharmacol Sci 2012;16:2057–63. [PubMed] [Google Scholar]
- 10.Hortobagyi GN. Everolimus plus exemestane for the treatment of advanced breast cancer: a review of subanalyses from BOLERO-2. Neoplasia 2015;17:279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schiff R, Massarweh SA, Shou J, Bharwani L, Mohsin SK, Osborne CK. Cross-talk between estrogen receptor and growth factor pathways as a molecular target for overcoming endocrine resistance. Clin Cancer Res 2004;10:331S–6S. [DOI] [PubMed] [Google Scholar]