Abstract
Background
In acute coronary syndrome (ACS), potassium levels <3.5 mEq/L are associated with ventricular arrhythmias. Current guidelines therefore recommend a potassium target >4.0 mEq/L in ACS. Our study evaluated the association between potassium levels, cardiac arrhythmias, and cardiovascular death in patients with non-ST-segment elevation myocardial infarction or unstable angina.
Methods
Potassium levels were measured in 6515 patients prior to randomization to receive either ranolazine or a placebo in the MERLIN-TIMI 36 trial. A seven-day continuous electrocardiographic assessment was obtained to determine the incidence of non-sustained ventricular tachycardia (NSVT) and ventricular pauses. The association between potassium levels and cardiovascular death was evaluated using a Cox proportional hazards regression model with multivariable adjustment.
Results
NSVT lasting for at least eight consecutive beats occurred more frequently at potassium levels <3.5 mEq/L than at potassium levels ⩾5 mEq/L (10.1 vs. 4.5%, p=0.03 for trend), whereas the inverse pattern was observed for ventricular pauses >3 s, which occurred more frequently at potassium levels ⩾5 mEq/L than at potassium levels <3.5 mEq/L (5.9 vs. 2.0%, p=0.03 for trend). There was a U-shaped relationship between the potassium level at admission and both early and late risk of cardiovascular death. Compared with patients with potassium levels of 3.5 to <4 mEq/L, a potassium level <3.5 mEq/L was associated with an increased risk of cardiovascular death at day 14 (2.4 vs. 0.8%, HRadj 3.1, p=0.02) and at one year (6.4 vs. 3.0%, HRadj 2.2, p=0.01). The risk of cardiovascular death at one year was also significantly increased at potassium levels ⩾4.5 mEq/L and a similar trend was noted at potassium levels ⩾5 mEq/L.
Conclusions
The lowest risk of cardiovascular death was observed in patients with admission potassium levels between 3.5 and 4.5 mEq/L. Both lower and higher levels of potassium were associated with tachyarrhythmias and bradyarrhythmias, suggesting a potential mechanistic explanation for the increased risk of cardiovascular death at the extremes of potassium homeostasis.
Keywords: Arrhythmias, acute coronary syndrome, potassium levels, myocardial infarction, cardiovascular death, ventricular tachycardia
Introduction
Potassium has an essential role in maintaining myocardial electrical stability. Hypokalemia was first noted to decrease the fibrillation threshold in rabbit hearts over 60 years ago.1 In the setting of myocardial ischemia, adrenergic stimulation activates the Na–K ATPase pump, lowering plasma potassium levels.2 Several small, observational studies have suggested that in the setting of acute coronary syndrome (ACS), hypokalemia, typically defined as potassium levels <3.5 mEq/L, is associated with ventricular arrhythmias.3–9 As a result, both professional guidelines and experts have recommended a target potassium level of at least 4.0 mEq/L in the setting of ACS.10–12 However, the body of evidence behind this recommendation predates several advances in the management of ACS that are known to reduce the risk of ventricular arrhythmias, specifically the routine use of β blockers and early reperfusion strategies. Two contemporary retrospective cohort studies of patients post-myocardial infarction (MI) observed the lowest in-hospital and three-year mortality rates in patients whose potassium levels were between 3.5 and 4.5 mEq/L.13,14 These studies therefore challenge prior recommendations of a potassium target >4.0 mEq/L in the setting of ACS.15 To further explore the risks of hypokalemia, we evaluated the association between potassium levels, mortality, and the incidence of arrhythmias in a well-characterized cohort of patients with non-ST-segment elevation ACS (NSTE-ACS), defined as patients with non-ST-segment elevation MI (NSTEMI) or unstable angina (UA).
Methods
Patient population
The design and primary results of the Metabolic Efficiency With Ranolazine for Less Ischemia in Non-ST-Elevation Acute Coronary Syndromes – Thrombolysis In Myocardial Infarction 36 (MERLIN-TIMI 36) trial have been reported previously.16 Patients eligible for enrollment had at least 10 minutes of ischemic symptoms at rest and presented with one of the following additional risk indicators: elevated levels of biomarkers of myonecrosis, ST-segment depression ⩾0.1 mV, a history of diabetes mellitus, or an intermediate to high (⩾3) TIMI risk score. Of the 6560 patients enrolled in MERLIN-TIMI 36, 4252 patients had their current generation sensitive troponin I (TnI-Ultra, Seimens) levels drawn. NSTEMI was defined as TnI-Ultra levels above the 99th percentile, ⩾0.04 μg/L. Patients with troponin I levels <0.04 μg/L were categorized as UA. Patients were excluded if they had end-stage renal disease requiring dialysis, cardiogenic shock, or a life expectancy of less than one year. Patients were randomized in a 1:1 ratio to receive either ranolazine or placebo. The protocol was approved by the institutional review boards of our institution and written consent was obtained from all patients.
Serum potassium measurements and endpoints
Potassium levels obtained prior to randomization were used for the analysis. Patients were categorized into five different potassium groups: <3.5; 3.5 to <4, 4 to <4.5; 4.5 to <5; and ⩾5 mEq/L. As described previously, continuous electrocardiographic (cECG) Holter monitors (Lifecard CF, Delmar Reynolds, Irvine, CA, USA) were applied to patients on randomization and remained in place for seven days.16,17 All cECG recordings were analyzed at the TIMI Electrocardiographic Core Laboratory (Boston, MA, USA). Arrhythmias were identified using a commercially available arrhythmia software program (Pathfinder, Spacelabs Healthcare) that used a combined automated and interactive detection technique. Non-sustained ventricular tachycardia (NSVT) was defined as at least four consecutive ventricular beats with a rate >100 beats per minute. Episodes were further categorized according to a pre-specified analysis plan as lasting at least eight consecutive beats. Ventricular pauses were defined as any absence of a QRS complex for at least 3 s. An independent committee that was unaware of the treatment assignment adjudicated all deaths as cardiovascular or non-cardiovascular.
Statistical analysis
Of the 6560 patients enrolled in MERLIN-TIMI 36, 6515 (99%) patients had the requisite Holter and potassium data and were included in this analysis. The baseline characteristics for this patient cohort stratified by potassium level were compared using the Kruskal–Wallis test for continuous variables and the χ2 test for categorical variables. The association between the incidence of arrhythmias (NSVT or pauses) and potassium levels was analyzed in the total population by the Cochran–Armitage test. In addition, the following subgroups were analyzed for the incidence of arrhythmias and potassium levels: (1) patients with NSTEMI and UA; (2) patients with a prolonged QTc interval defined as ⩾450 ms; and (3) patients with ischemia detected on cECG monitoring. These analyses were also assessed using the Cochran–Armitage test. The comparison of the incidence of arrhythmias (NSVT or pauses) between the ranolazine and placebo groups was assessed using the χ2 test. Event rates at 14 days and one year were calculated by the Kaplan–Meier method. The associations between each group of potassium level and 14-day and one-year mortality were evaluated using a Cox proportional hazards regression model presented as hazards ratios and adjusted for the variables in the TIMI risk score18 (age ⩾65 years of age, documented coronary artery disease, recent severe angina, ST-segment deviation >0.5 mm, prior treatment with aspirin, positive cardiac biomarkers, at least three cardiac risk factors (diabetes mellitus, current cigarette smoking, hypertension, hypercholesterolemia, and family history of coronary artery disease)) and also for creatinine clearance, history of congestive heart failure, and coronary angiography at the index hospitalization. Analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA). p<0.05 (two-tailed) was considered to indicate statistical significance. The authors had full access to, and take full responsibility for, the integrity of the data.
Results
Patient characteristics and potassium levels
The baseline patient characteristics by potassium category are presented in Table 1. Potassium levels were collected prior to randomization, which occurred on average <24 h after the onset of ischemic symptoms and thus represent an early assessment of potassium levels. The median and mean potassium levels were 4.2 mEq/L (interquartile range (IQR) 3.9–4.5) and 4.22 mEq/L (SD 0.51), respectively. The distribution of potassium levels was normally distributed (Figure 1). Patients with hyperkalemia (⩾5.0 mEq/L) were more likely to have diabetes, heart failure, reduced creatinine clearance, and prior MI (p<0.001 for all comparisons; Table 1). Patients with potassium <3.5 mEq/L were more likely to have NSTEMI than UA, to have received glycoprotein IIb/IIIa inhibitors, and to have had a coronary angiogram during hospitalization (p<0.001 for all comparisons).
Table 1.
Baseline characteristics of patients in MERLIN-TIMI 36 by potassium level.
| Characteristic | Potassium levels (mEq/L)
|
|||||
|---|---|---|---|---|---|---|
| <3.5 | 3.5 to<4.0 | 4.0 to <4.5 | 4.5 to <5.0 | ⩾5.0 | p (trend) | |
| Female sex | 114 (44.4) | 626 (36.9) | 855 (31.9) | 489 (35.5) | 196 (39.2) | 0.3933 |
| White ethnicity | 230 (89.5) | 1598 (94.2) | 2562 (95.5) | 1335 (96.9) | 475 (95.0) | <0.0001 |
| Diabetes mellitus | 81 (31.5) | 522 (30.8) | 891 (33.2) | 503 (36.5) | 210 (42.0) | <0.0001 |
| Hypertension | 217 (84.4) | 1234 (72.8) | 1874 (69.8) | 1044 (75.8) | 407 (81.4) | <0.0001 |
| Hyperlipidemia | 167 (64.9) | 1046 (61.7) | 1704 (63.5) | 830 (60.2) | 281 (56.2) | <0.0001 |
| Current smoker | 54 (21.0) | 423 (24.9) | 722 (26.9) | 335 (24.3) | 129 (25.8) | <0.0001 |
| Prior myocardial infarction | 78 (30.4) | 485 (28.6) | 903 (33.6) | 524 (38.0) | 211 (42.2) | <0.0001 |
| Prior coronary revascularization | 68 (26.5) | 447 (26.4) | 741 (27.6) | 367 (26.6) | 109 (21.8) | <0.0001 |
| Prior heart failure | 33 (12.8) | 238 (14.0) | 385 (14.3) | 289 (20.9) | 143 (28.6) | <0.0001 |
| Estimated creatinine clearance <60 mL/min | 51 (19.8) | 318 (18.8) | 524 (19.5) | 342 (24.8) | 160 (32.0) | <0.0001 |
| Unstable angina | 120 (46.7) | 758 (44.7) | 1190 (44.3) | 695 (50.4) | 291 (58.2) | <0.0001 |
| Non-ST-elevation myocardial infarction | 133 (51.8) | 900 (53.1) | 1419 (52.9) | 656 (47.6) | 203 (40.6) | <0.0001 |
| Other | 4 (1.6) | 38 (2.2) | 75 (2.8) | 27 (1.9) | 6 (1.2) | <0.0001 |
| TIMI risk score 0–2 | 63 (24.5) | 511 (30.1) | 765 (28.5) | 314 (22.8) | 95 (19.0) | <0.0001 |
| TIMI risk score 3–4 | 143 (55.6) | 876 (51.7) | 1378 (51.3) | 772 (56.0) | 264 (52.8) | <0.0001 |
| TIMI risk score 5–7 | 51 (19.8) | 309 (18.2) | 541 (20.2) | 292 (21.2) | 141 (28.2) | <0.0001 |
| Coronary angiography during the index hospitalization | 166 (64.6) | 1106 (65.2) | 1636 (60.9) | 713 (51.7) | 222 (44.4) | <0.0001 |
| β blocker | 232 (90.3) | 1537 (90.6) | 2391 (89.1) | 1210 (87.8) | 443 (88.6) | 0.0234 |
| Aspirin | 249 (96.9) | 1634 (96.3) | 2574 (95.9) | 1316 (95.5) | 488 (97.6) | 0.9236 |
| Heparin | 229 (89.1) | 1570 (92.6) | 2440 (90.9) | 1218 (88.4) | 440 (88.0) | 0.0003 |
| Glycoprotein IIb/IIIa receptor inhibitor | 38 (14.8) | 281 (16.6) | 404 (15.1) | 172 (12.5) | 57 (11.4) | 0.0005 |
| Thienopyridine | 166 (64.6) | 1138 (67.1) | 1804 (67.2) | 814 (59.1) | 260 (52.0) | <0.0001 |
| ACE inhibitor or angiotensin II receptor blocker | 205 (79.8) | 1283 (75.7) | 2083 (77.6) | 1114 (80.8) | 412 (82.4) | 0.0004 |
| Statin | 228 (88.7) | 1467 (86.5) | 2290 (85.3) | 1066 (77.4) | 315 (63.0) | <0.0001 |
| Ranolazine | 126 (49.0) | 848 (50.0) | 1333 (49.7) | 700 (50.8) | 245 (49.0) | 0.8750 |
| Age (years) | 63.00±11.2 | 62.97±11.1 | 63.05±11.1 | 64.38±10.9 | 65.27±10.6 | 0.061 |
| Weight (kg) | 84.18±18.5 | 82.06±15.7 | 82.80±15.9 | 81.94±15.8 | 80.92±17.1 | 0.069 |
| Body mass index | 29.62±5.4 | 28.69±4.9 | 28.92±5.4 | 28.86±4.7 | 28.52±5.2 | 0.053 |
Data are presented as n (%) or mean±SD values.
Figure 1.
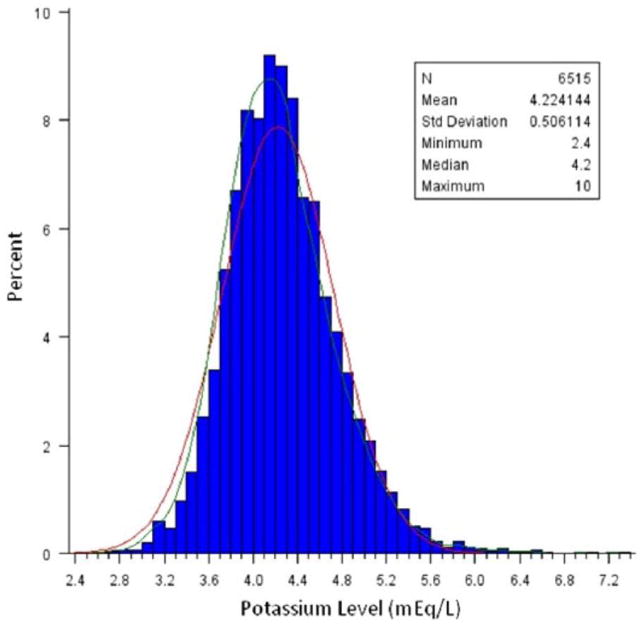
Distribution of potassium levels at admission.
Serum potassium levels and arrhythmias
The median number of days between admission potassium level and the detected of arrhythmia on cECG was 2.5 (IQR 1.4–4.4) for NSVT ⩾8 beats and 2.3 days (IQR 1.2–4.3) for pauses ⩾3 s. Among the entire cohort, there was a stepwise increase in the rate of NSVT lasting at least eight beats in patients with lower levels of potassium, ranging from a rate of 4.5% in patients with a potassium level ⩾5.0 mEq/L to 10.1% in patients with a level <3.5 mEq/L (p=0.03 for trend) (Figure 2(a)). There was no relationship between shorter episodes of NSVT and levels of potassium (data not shown). The pattern of an inverse relationship between potassium levels and NSVT ⩾8 beats was similar in patients with NSTEMI and in patients with UA, although the relationship did not reach statistical significance in either subgroup due to the smaller sizes (Table S1). In addition, a similar overall pattern between potassium levels and NSVT ⩾8 beats was noted in patients with both normal (<450 ms) and prolonged (⩾450 ms) QTc, and in patients with and without ischemia noted on cECG monitoring (Table S1).
Figure 2.
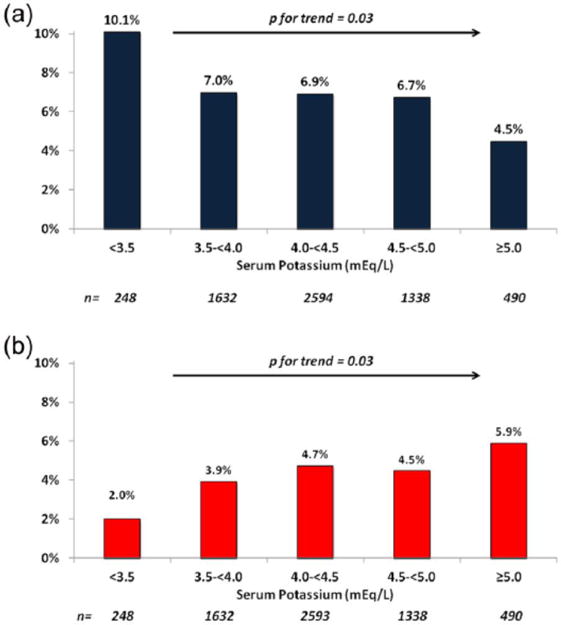
(a) Incidence of ventricular tachycardia (⩾8 beats) and (b) ventricular pauses ⩾3 s based on potassium levels.
The trend for pauses ⩾3 s followed the opposite pattern to that of ventricular arrhythmias. The lowest rate of pauses (2.0%) was in patients with potassium levels <3.5 mEq/L and the highest (5.9%) was in patients with potassium levels ⩾5.0 mEq/L (p=0.03 for trend) (Figure 2(b)). The pattern was similar in all subgroup analyses, although several trends did not reach statistical significance based on smaller sample sizes (Table S2).
The relationship between potassium levels and arrhythmias was similar in patients treated with ranolazine or with the placebo. Overall, patients treated with ranolazine tended to have fewer episodes of tachyarrhythmias or bradyarrhythmias than patients treated with placebo, regardless of the baseline levels of potassium (Figure 3(a) and 3(b)). This relationship was true for both patients with NSTEMI and patients with UA (data not shown).
Figure 3.
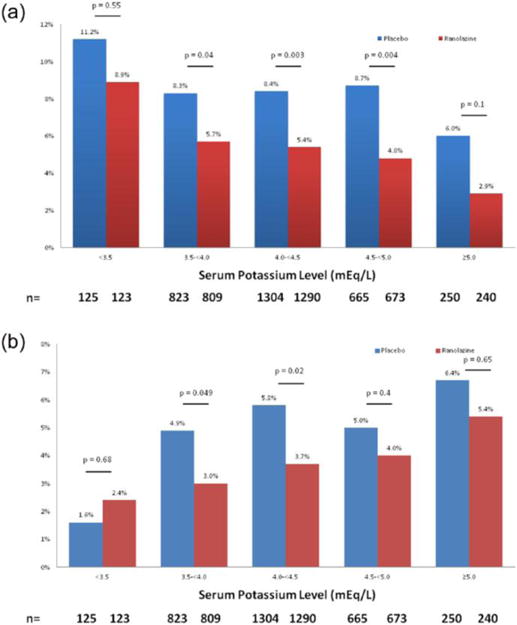
(a) Incidence of ventricular tachycardia ⩾8 beats and (b) ventricular pauses ⩾3 s in patients treated with ranolazine and a placebo.
Serum potassium levels and cardiovascular death
There was an overall U-shaped relationship between potassium levels and cardiovascular death at day 14 (Figure 4(a)). Compared with the reference group (3.5 to <4 mEq/L), the mortality rates were significantly higher for patients with potassium levels <3.5 mEq/L (0.8 vs. 2.4%, HRadj 3.1, 95% confidence interval (CI) 1.2–8.1, p=0.02). There was no significant difference in cardiovascular death between the reference group (3.5 to <4 mEq/L) and the remaining groups (Figure 4(a)). The one-year cardiovascular death rates followed a similar U-shaped relationship across potassium levels. Compared with the reference group (3.5 to <4 mEq/L), mortality rates were significantly higher for patients with potassium levels between 4.5 and 5 mEq/L (3.0 vs. 6.2%, HRadj 1.7, 95% CI 1.1–2.4, p=0.01), and even higher for those with potassium levels <3.5 mEq/L (Figure 4(b)). Patients with potassium levels ⩾5.0 mEq/L had a trend toward increased risk of one-year cardiovascular death compared with the reference group (HRadj, 1.6, 95% CI 0.99–2.5, p=0.06).
Figure 4.
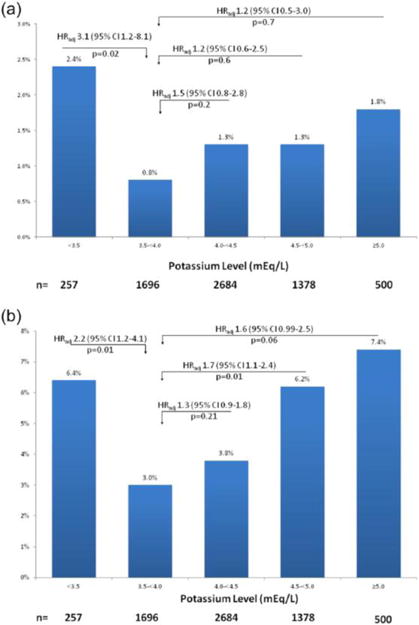
(a) Cardiovascular death at 14 days and (b) one-year based on potassium levels.
Discussion
In this analysis of over 6500 patients with NSTE-ACS, we found that, after adjustment for baseline clinical characteristics, there was a U-shaped relationship between levels of potassium and short- and long-term risk of cardiovascular death. The lowest risk of death was observed in patients with potassium levels between 3.5 and 4.5 mEq/L, whereas the highest risk was seen in patients with either hypokalemia (<3.5 mEq/L potassium) or hyperkalemia (⩾4.5 mEq/L potassium). Interestingly, the risk of ventricular tachycardia and bradycardic events was associated with potassium levels, but were inversely related to each other, suggesting arrhythmogenic instability at either end of the potassium range and thus identifying 3.5–4.5 mEq/L as the most electrically stable range. This bimodal distribution of arrhythmic risk may explain the U-shaped relationship between potassium levels and early cardiovascular death. Treatment with ranolazine, an anti-angina drug with anti-arrhythmic properties, reduced the risk of both tachyarrhythmias and bradyarrhythmias across the range of potassium levels.
Most studies reporting an association between hypokalemia and arrhythmic death in ACS predate the era of reperfusion and widespread use of β blockers and, in general, included relatively small patient populations.3–9 Nonetheless, these studies, together with animal models, were the basis for the common practice of targeting potassium levels of 4.0–5.0 mEq/L in the setting of ACS.10,11 Since these recommendations were published, two retrospective cohort studies have challenged the benefit of consistently targeting potassium levels >4.0 mEq/L. Goyal et al.13 noted a lower in-hospital mortality in a cohort of over 30,000 patients post-MI with potassium levels of 3.5–4.5 mEq/L. Subsequently, Choi et al.14 noted similar findings in a cohort of over 2000 patients in South Korea. Our present study of over 6000 patients supports the conclusion that potassium levels >4.0 mEq/L are associated with increased cardiovascular risk. In addition to the increased risk of in-hospital mortality noted by Goyal et al.,13 our study also suggested that potassium levels >4.5 mEq/L are associated with increased long-term cardiovascular mortality at one year, even after adjusting for other risk factors. In theory, the optimum potassium repletion strategy would be identified via a randomized controlled trial; however, such trials are unlikely to be feasible given the cost and complexity of an adequately powered trial.
This study provides new insights into the different arrhythmic complications associated with hypo- and hyperkalemia. Consistent with prior studies, lower levels of potassium were clearly associated with higher rates of longer episodes of NSVT. Not surprisingly, the presence of a prolonged QTc interval and hypokalemia were associated with the highest incidence of NSVT. Conversely, elevated levels of potassium were associated with an increased the risk of significant bradyarrhythmias. Hyperkalemia is known to decrease ventricular excitability and precipitate complete heart block and sinus arrest.19 Emerging treatments with novel potassium binders may improve the management of hyperkalemia in the acute setting.20,21 The proximity in timing between potassium levels at admission and arrhythmias further substantiates the arrhythmic potential at either end of the potassium range. It is possible that arrhythmic complications at either end of the potassium range account for the U-shaped curve of cardiovascular mortality.
Management of NSTE-ACS has advanced substantially over the past 20 years. With the broad utilization of β blockers in ACS, the rates of ventricular arrhythmias and mortality have decreased significantly, probably through the suppression of hypokalemia-induced ventricular arrhythmias.2,22–24 In addition to β blockers, reperfusion with percutaneous coronary intervention, robust antiplatelet treatments, and the creation of cardiac care units have substantially decreased the risk of malignant ventricular arrhythmias and death. In some studies, the risk of ventricular fibrillation in the setting of ACS has decreased by at least one-third over this time period.25,26 As a result, the magnitude of potassium repletion required to prevent adverse events may not be as substantial. Although it is not possible to determine a causal relationship from our study, our findings raise the possibility that aggressive potassium repletion may even be associated with increased risk.
There are several limitations to our study. As a selected population enrolled in a clinical trial, these results may not be generalizable to all patients with NSTE-ACS. Potassium repletion and serial potassium levels during hospitalization were not captured and thus we cannot evaluate the relationships between supplemental potassium or serial potassium levels and outcomes. Determining whether a particular clinical parameter associated with poor outcomes is part of the “causal” pathway or simply a marker of an overall poor prognosis is often difficult. In this study, the association between potassium levels and cardiac arrhythmias provides a potential “causal” link between potassium levels and increased short-term outcomes. In contrast, the admission potassium level may be more reflective of underlying comorbidities when evaluating the relationship with long-term outcomes. However, even with this limitation, it remains significantly associated with one-year mortality events after extensive modeling. Drugs, such as anti-arrhythmic drugs and diuretics, were not used in the multivariable model. In addition, cardiovascular deaths were not sub-classified as arrhythmic or non-arrhythmic in MERLIN-TIMI 36, thus preventing the further characterization of deaths. Further research is necessary to determine whether our results are applicable to patients with ST-elevation MI.
Our findings have demonstrated a relationship between potassium concentration and the risk of ventricular tachycardia and bradycardic events, which were inversely related to each other, suggesting that arrhythmogenic instability at either end of the potassium range and identifying 3.5–4.5 mEq/L as the most electrically “stable” range.
Acknowledgments
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Footnotes
Conflict of interest
Ravi B Patel, Sara Tannenbaum, Ana Viana-Tejedor, Jianping Guo, and KyungAh Im have nothing to disclose.
Benjamin M Scirica reports grants from Gilead, during the conduct of the study; grants from AstraZeneca, grants from Bristol-Myers Squibb, grants from Daichi-Sankyo, grants from Johnson and Johnson, grants from Bayer Healthcare, grants from Gilead, grants from Eisai, grants from Merck, personal fees from Gilead, personal fees from Lexicon, personal fees from Arena, personal fees from Eisai, personal fees from St Jude’s Medical, personal fees from Boston Clinical Research Institute, fees from University of Calgary, personal fees from Elsevier Practice Update Cardiology, personal fees from Forest Pharmaceuticals, personal fees from Boehringer Ingelheim, personal fees from Merck, personal fees from GlaxoSmithKline, personal fees from GE Healthcare, and personal fees from BiogenIdec, outside the submitted work.
References
- 1.Grumbach L, Howard JW, Merrill VI. Factors related to the initiation of ventricular fibrillation in the isolated heart; effect of calcium and potassium. Circ Res. 1954;2:452–459. doi: 10.1161/01.res.2.5.452. [DOI] [PubMed] [Google Scholar]
- 2.Brown MJ, Brown DC, Murphy MB. Hypokalemia from beta2-receptor stimulation by circulating epinephrine. N Engl J Med. 1983;309:1414–1419. doi: 10.1056/NEJM198312083092303. [DOI] [PubMed] [Google Scholar]
- 3.Hulting J. In-hospital ventricular fibrillation and its relation to serum potassium. Acta Med Scand Suppl. 1981;647:109–116. doi: 10.1111/j.0954-6820.1981.tb02646.x. [DOI] [PubMed] [Google Scholar]
- 4.Kafka H, Langevin L, Armstrong PW. Serum magnesium and potassium in acute myocardial infarction. Influence on ventricular arrhythmias Arch Intern Med. 1987;147:465–469. [PubMed] [Google Scholar]
- 5.Solomon RJ, Cole AG. Importance of potassium in patients with acute myocardial infarction. Acta Med Scand Suppl. 1981;647:87–93. doi: 10.1111/j.0954-6820.1981.tb02643.x. [DOI] [PubMed] [Google Scholar]
- 6.Madias JE, Shah B, Chintalapally G, et al. Admission serum potassium in patients with acute myocardial infarction: its correlates and value as a determinant of in-hospital outcome. Chest. 2000;118:904–913. doi: 10.1378/chest.118.4.904. [DOI] [PubMed] [Google Scholar]
- 7.Nordrehaug JE, Johannessen KA, von der Lippe G. Serum potassium concentration as a risk factor of ventricular arrhythmias early in acute myocardial infarction. Circulation. 1985;71:645–649. doi: 10.1161/01.cir.71.4.645. [DOI] [PubMed] [Google Scholar]
- 8.Friedensohn A, Faibel HE, Bairey O, et al. Malignant arrhythmias in relation to values of serum potassium in patients with acute myocardial infarction. Int J Cardiol. 1991;32:331–338. doi: 10.1016/0167-5273(91)90295-z. [DOI] [PubMed] [Google Scholar]
- 9.Siscovick DS, Raghunathan TE, Psaty BM, et al. Diuretic therapy for hypertension and the risk of primary cardiac arrest. N Engl J Med. 1994;330:1852–1857. doi: 10.1056/NEJM199406303302603. [DOI] [PubMed] [Google Scholar]
- 10.Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction) Circulation. 2004;110:e82–292. [PubMed] [Google Scholar]
- 11.Cohn JN, Kowey PR, Whelton PK, et al. New guidelines for potassium replacement in clinical practice: a contemporary review by the National Council on Potassium in Clinical Practice. Arch Intern Med. 2000;160:2429–2436. doi: 10.1001/archinte.160.16.2429. [DOI] [PubMed] [Google Scholar]
- 12.Macdonald JE, Struthers AD. What is the optimal serum potassium level in cardiovascular patients? J Am Coll Cardiol. 2004;43:155–161. doi: 10.1016/j.jacc.2003.06.021. [DOI] [PubMed] [Google Scholar]
- 13.Goyal A, Spertus JA, Gosch K, et al. Serum potassium levels and mortality in acute myocardial infarction. JAMA. 2012;307:157–164. doi: 10.1001/jama.2011.1967. [DOI] [PubMed] [Google Scholar]
- 14.Choi JS, Kim YA, Kim HY, et al. Relation of serum potassium level to long-term outcomes in patients with acute myocardial infarction. Am J Cardiol. 2014;113:1285–1290. doi: 10.1016/j.amjcard.2014.01.402. [DOI] [PubMed] [Google Scholar]
- 15.Scirica BM, Morrow DA. Potassium concentration and repletion in patients with acute myocardial infarction. JAMA. 2012;307:195–196. doi: 10.1001/jama.2011.2003. [DOI] [PubMed] [Google Scholar]
- 16.Morrow DA, Scirica BM, Karwatowska-Prokopczuk E, et al. Effects of ranolazine on recurrent cardiovascular events in patients with non-ST-elevation acute coronary syndromes: the Merlin-TIMI 36 randomized trial. JAMA. 2007;297:1775–1783. doi: 10.1001/jama.297.16.1775. [DOI] [PubMed] [Google Scholar]
- 17.Morrow DA, Scirica BM, Karwatowska-Prokopczuk E, et al. Evaluation of a novel anti-ischemic agent in acute coronary syndromes: design and rationale for the metabolic efficiency with ranolazine for less ischemia in non-ST-elevation acute coronary syndromes (Merlin)-TIMI 36 trial. Am Heart J. 2006;151:1186.e1181–1189. doi: 10.1016/j.ahj.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA. 2000;284:835–842. doi: 10.1001/jama.284.7.835. [DOI] [PubMed] [Google Scholar]
- 19.Podrid PJ. Potassium and ventricular arrhythmias. Am J Cardiol. 1990;65:33E–44E. doi: 10.1016/0002-9149(90)90250-5. discussion 52E. [DOI] [PubMed] [Google Scholar]
- 20.Weir MR, Bakris GL, Bushinsky DA, et al. Patiromer in patients with kidney disease and hyperkalemia receiving raas inhibitors. N Engl J Med. 2015;372:211–221. doi: 10.1056/NEJMoa1410853. [DOI] [PubMed] [Google Scholar]
- 21.Packham DK, Rasmussen HS, Lavin PT, et al. Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med. 2015;372:222–231. doi: 10.1056/NEJMoa1411487. [DOI] [PubMed] [Google Scholar]
- 22.A randomized trial of propranolol in patients with acute myocardial infarction. I. Mortality results. JAMA. 1982;247:1707–1714. doi: 10.1001/jama.1982.03320370021023. [DOI] [PubMed] [Google Scholar]
- 23.The Norwegian Multicenter Study Group. Timolol-induced reduction in mortality and reinfarction in patients surviving acute myocardial infarction. N Engl J Med. 1981;304:801–807. doi: 10.1056/NEJM198104023041401. [DOI] [PubMed] [Google Scholar]
- 24.Huikuri HV, Tapanainen JM, Lindgren K, et al. Prediction of sudden cardiac death after myocardial infarction in the beta-blocking era. J Am Coll Cardiol. 2003;42:652–658. doi: 10.1016/s0735-1097(03)00783-6. [DOI] [PubMed] [Google Scholar]
- 25.Goldberg RJ, Yarzebski J, Spencer FA, et al. Thirty-yearcpatients with acute myocardial infarction complicated by ventricular fibrillation. Am J Cardiol. 2008;102:1595–1601. doi: 10.1016/j.amjcard.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Avezum A, Piegas LS, Goldberg RJ, et al. Magnitude and prognosis associated with ventricular arrhythmias in patients hospitalized with acute coronary syndromes (from the Grace Registry) Am J Cardiol. 2008;102:1577–1582. doi: 10.1016/j.amjcard.2008.08.009. [DOI] [PubMed] [Google Scholar]


