Abstract
Aggressive NK-cell leukemia (ANKL) is an exceedingly rare form of leukemia and carries a poor prognosis with a median survival of only 2-months. Using the Center for International Blood and Marrow Transplant Research database, we evaluated outcomes of allogeneic hematopoietic cell transplantation (alloHCT) in patients with ANKL. Twenty-one patients with a centrally confirmed diagnosis of ANKL were included. Median patient age was 42-years and 15 patients (71%) were Caucasian. Fourteen patients (67%) were in complete remission (CR) at the time of alloHCT, while 5 patients had active disease. Median follow-up of survivors was 25 months (range: 12–116). The 2-year estimates of non-relapse mortality, relapse/progression, progression-free (PFS) and overall survival (OS) were 21%, 59%, 20% and 24%, respectively. The 2-year PFS of patients in CR at the time of alloHCT was significantly better than that of patients with active disease at transplantation (30% vs. 0%; p=0.001). The 2-year OS in similar order was 38% vs. 0% (p<0.001). In conclusion, this registry analysis that included majority non-Asian patient population shows that alloHCT can provide durable disease control in a subset of ANKL patients. Achieving CR before transplantation appears to be a prerequisite for successful transplantation outcomes.
Keywords: Aggressive NK-cell leukemia, myeloablative, allogeneic transplant, reduced intensity conditioning
Introduction
Aggressive NK-cell leukemia (ANKL) is an exceedingly rare form of leukemia and comprises less than 0.1% of all lymphoid neoplasms[1]. It is more prevalent among Asians than other ethnic populations[2]. The median age at diagnosis is 42 years. Little is known about the etiology of this aggressive leukemia, but the strong association with Epstein-Barr virus (EBV) suggests a pathogenetic role of the virus. However, rare EBV negative ANKL cases have been reported[3]. The neoplastic cells are CD2+, surface CD3-, cytoplasmic CD3ε+, CD56+ and positive for cytotoxic molecules. CD16 is frequently positive, while CD57 is usually negative. The most commonly involved sites at diagnosis are bone marrow and peripheral blood, but hepatomegaly (64%), splenomegaly (55%) and lymphadenopathy (41%) are frequently observed[4]. Contrary to extranodal NK/T-cell lymphomas, nasal type (ENKL), cutaneous or nasal involvement is uncommon at presentation. In further distinction from ENKL, genome-wide array-based comparative genomic hybridization studies show more frequent gains of chromosome 1q23.1–24.2 and 1q31.3-q44 and loss of chromosome 7p15.1-p22.3 and 17p13.1 in patients with ANKL[5]. ANKL carries a grim prognosis with a median survival ranging from only 2 to 6 months[4,6–9]. Anecdotal reports and small case series (each with 6 or fewer patients) exclusively from Asian populations, have hinted at durable disease control in ANKL following allogeneic hematopoietic cell transplantation (alloHCT)[6,7,10–12], but larger multicenter data are not available. Using the Center for International Blood and Marrow Transplant Research (CIBMTR) database (for details see Supplemental Appendix), we report here the largest series evaluating alloHCT outcomes of patients with ANKL.
Methods
Data source
The Center for International Blood and Marrow Transplant Research (CIBMTR) is a working group of more than 500 transplantation centers worldwide that contribute detailed data on HCT to a statistical center at the Medical College of Wisconsin (MCW). Participating centers are required to report all transplantations consecutively and compliance is monitored by on-site audits. Computerized checks for discrepancies, physicians’ review of submitted data, and on-site audits of participating centers ensure data quality. Observational studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. The MCW and National Marrow Donor Program, Institutional Review Boards approved this study.
Patients
Adult (≥18 years) ANKL patients undergoing alloHCT between 2000–2014 and reported to CIBMTR were eligible. Central review of biopsy reports by an expert hematopathologist (HO) to confirm the diagnosis according to the WHO classification was required for inclusion in the study. Reporting centers were contacted to obtain detailed baseline patient-, disease- and transplant-related data.
Definitions and Study endpoints
Complete remission (CR) was defined as the complete resolution of all known areas (of nodal and extranodal) disease on radiographic imaging along with a negative bone marrow aspiration and biopsy. Overall survival (OS) was defined as the interval from the date of transplantation to the date of death or last follow-up. Surviving patients were censored at last contact. For progression-free survival (PFS), a patient was considered a treatment failure at the time of disease progression/relapse or death from any cause. Probabilities of OS and PFS were calculated using the Kaplan-Meier estimate. Cumulative incidence of non-relapse mortality (NRM) and disease progression/relapse were calculated while accounting for competing risks. Neutrophil recovery was defined as the first of 3 successive days with absolute neutrophil count (ANC) ≥500/μL after post-transplantation nadir. Platelet recovery was defined as achieving platelet counts ≥20,000/μL for at least 3 days, unsupported by transfusion. For neutrophil and platelet recovery, death without the event was considered a competing risk. Acute GVHD[13] and chronic GVHD[14] were graded using standard criteria. The intensity of conditioning regimens was defined using consensus criteria[15]. All statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC).
Results
Twenty one patients (from 19 transplantation centers) with a centrally confirmed diagnosis of ANKL were included in the final analysis. Cases where a biopsy report was not available for central review, or ones where the central review did not confirm ANKL diagnosis, were excluded. The baseline patient-, disease- and transplantation-related characteristics are shown in Table 1. Median patient age was 42 years (range 18–67) and 15 patients (71%) were Caucasian. SMILE (dexamethasone, methotrexate, ifosfamide, L-asparaginase and etoposide) was the most common frontline therapy and 17 patients (81%) received an asparaginase-containing regimen before alloHCT. Median interval between diagnosis and alloHCT was six months (range 2–31 months). Nine patients (42%) underwent alloHCT after 1st line therapy (upfront alloHCT), while 10 patients (48%) received more than one therapy line before transplantation (late alloHCT). Fourteen patients (67%) were in CR at the time of alloHCT, while 5 patients had active disease. The majority of patients received myeloablative conditioning regimens (n=14).
Table 1.
Baseline characteristics of patients with Aggressive NK-cell Leukemia
| Variable | N=21 (%) |
|---|---|
| Median age at HCT (range) | 42 years (18–67) |
| Male sex | 15 (71) |
| Karnofsky performance score before HCT | |
| 80–100% | 13 (62) |
| < 80% | 4 (19) |
| Unknown | 4 (19) |
| HCT-CI | |
| 0 | 9 (43) |
| 1–2 | 4 (20) |
| ≥3 | 4 (19) |
| Not collected before 2007 | 4 (19) |
| Race | |
| Caucasian | 15 (71) |
| Asian | 4 (19) |
| Unknown | 2 (10) |
| History of prior autologous HCT | 1 (5) |
| Median interval from diagnosis to HCT, months (range) | 6 (2–31) |
| <1 year | 17 (81) |
| ≥1 year | 4 (19) |
| Elevated Lactate Dehydrogenase at diagnosis | 8 (38) |
| Unknown | 11 (52) |
| CNS involvement any time prior to HCT | 2 (10) |
| First line of therapy | |
| SMILE-like | 12 (57) |
| DeVIC ± asparaginase | 3 (14) |
| Anthracycline-based | 4 (19) |
| Unknown | 2 (10) |
| Response to first line of therapy | |
| Complete remission | 13 (62) |
| Refractory disease | 5 (24) |
| Unknown | 3 (14) |
| Median (range) lines of therapy before HCT | 2 (1–5) |
| Timing of transplantation | |
| Upfront Transplantation (after first line of therapy) | 9 (42) |
| Late HCT (>1 line of therapy prior to HCT) | 10 (48) |
| Unknown | 2 (10) |
| Received asparaginase containing therapy (any time before HCT) | 17 (81) |
| Received gemcitabine containing therapy (any time before HCT) | 3 (14) |
| PET/CT-scan status before HCT | |
| Negative | 7 (33) |
| Positive | 5 (24) |
| Not done or unknown | 9 (43) |
| Remission status prior to HCT | |
| Complete remission | 14 (67) |
| Active disease | 5 (24) |
| Unknown | 2 (10) |
| Donor type | |
| Matched related donor | 9 (43) |
| Unrelated donor | 11 (52) |
| Haploidentical related donor | 1 (5) |
| Conditioning regimen intensity | |
| Non-myeloablative/Reduced-intensity conditioning | 7 (33) |
| Myeloablative conditioning | 14 (67) |
| Total body irradiation in conditioning | 13 (62) |
| Graft Source | |
| Bone marrow | 3 (14) |
| Peripheral blood | 18 (86) |
| GVHD prophylaxis | |
| Post-transplant cyclophosphamide-based | 1 (5) |
| Calcineurin inhibitor + mycophenolate mofetil | 2 (10) |
| Calcineurin inhibitor + methotrexate ± others2 | 13 (62) |
| Calcineurin inhibitor ± others3 | 4 (19) |
| Missing | 1 (5) |
| Donor/recipient CMV status | |
| Either or both positive | 15 (72) |
| Both negative | 3 (14) |
| Missing | 3 (14) |
| Median follow-up of survivors (range), months | 25 (12–116) |
Abbreviations: CMV=cytomegalovirus; CNS=central nervous system; DeVIC=dexamethasone, etoposide, ifosfamide and carboplatin; HCT=hematopoietic cell transplantation; HCT-CI= hematopoietic cell transplantation-comorbidity index; SMILE=dexamethasone, methotrexate, ifosfamide, L-asparaginase, and etoposide.
CNI/MTX alone (n=11), CNI/MTX/steroids (n=1), CNI/MTX/Sirolimus(n=1)
CNI alone (n=3), CNI/Sirolimus (n=1)
Median follow-up of survivors was 25 months (range: 12–116). The cumulative incidence of neutrophil recovery at day-28 and platelet recovery at day-100 was 100% and 88% respectively. The cumulative incidence of grade II–IV acute GVHD at day-180 and chronic GVHD at 1-year was 29% and 27%, respectively. The 2-year estimates of NRM, disease relapse/progression, PFS and OS were 21% (95%CI=6–42), 59% (95%CI=37–79), 20% (95%CI=5–41) and 24% (95%CI=8–46), respectively (Figure 1a–b). The 2-year PFS of patients in CR at the time of alloHCT was significantly better than that of patients with active disease at transplantation (30% vs. 0%; p=0.001, Figure 1c). The 2-year OS in similar order was 38% vs. 0% (p<0.001; Figure 1d). Among patients receiving upfront vs. late alloHCT, the 2-year rates of relapse/progression, PFS and OS were 44% vs. 90% (p=0.02), 17% vs. 0% (p=0.24) and 17% vs. 13% (p=0.86), respectively. There was no significant difference between the 1-year outcomes of patients receiving myeloablative vs. reduced-intensity conditioning (RIC) regimens in terms of disease relapse (50% vs. 57%; p=0.76), NRM (14% vs. 14%; p=1.00), PFS (36% vs. 29%; p=0.74) and OS (50% vs. 29%; p=0.32). In patients receiving RIC regimens, generating 2-year survival estimates was not feasible as no follow-up data were available at 2-years or beyond. The 2-year PFS and OS of patients receiving myeloablative conditioning was 21% and 29%, respectively. The 2-year PFS and OS of patients receiving SMILE (n=12) as first line chemotherapy was 11% and 20%, respectively. At last follow up, 16 patients (76%) had died (Table 2). The most common cause of death was disease relapse (n=11).
Figure 1.
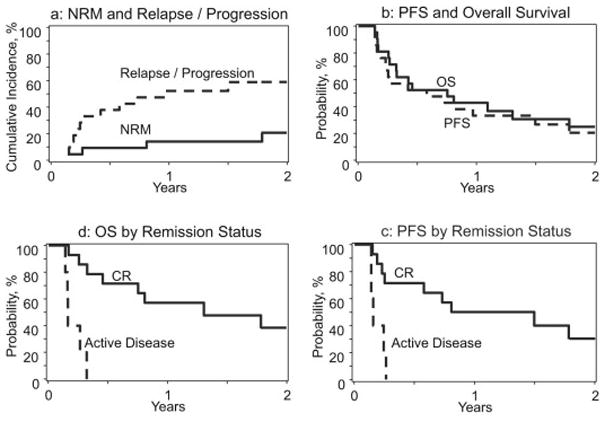
Post allogeneic transplantation rates of NRM (1a), disease relapse/progression (1a), progression-free survival (PFS) (1b) and overall survival (OS) (1b) in patients with aggressive NK-cell leukemia (ANKL). Post allogeneic transplantation outcomes of ANKL patients in complete remission vs. those with active disease: PFS (1c) and OS (1d).
Table 2.
Causes of death for Aggressive NK-cell leukemia.
| Cause of death | |
|---|---|
| Number of deaths | 16 |
| Infection | 2 (13) |
| Graft-versus-host disease | 1 (6) |
| Primary disease | 11 (69) |
| Organ failure | 1 (6) |
| Unknown | 1 (6) |
Discussion
This series is the largest assessment of alloHCT in this exceedingly rare leukemia. It is also the only report comprised primarily of non-Asian patients, with 71% being of Caucasian race[6,7,10–12]. An additional strength is the central expert hematopathology review of reports, to confirm diagnosis of included cases. Our analysis shows that about 20% of ANKL patients can achieve a durable remission after alloHCT. This is encouraging considering the fact that the median survival in this disease without transplantation is only ~2 months[4,6,9]. In a prior case series by Ishida et al[6] the median survival of ANKL patients undergoing alloHCT (n=6) was ~9months, while all non-transplanted subjects (n=26) died due to progressive disease. Similarly in a recent series by Jung et al[7] durable remissions were limited only to patients undergoing alloHCT (n=6). In our current report, upfront transplantation was associated with lower rates of disease relapse/progression. Achievement of a CR appeared to be the key determinant of successful outcome of alloHCT. While none of the patients in this analysis with active disease at alloHCT survived long-term, 30% of the patients in CR before alloHCT were alive and disease-free at 2-years post alloHCT and potentially cured of their disease. ANKL have dismal outcomes with standard anthracycline based inductions[16]. This poor response might be due to very high P-glycoprotein concentrations in normal NK-cells, a property that is retained in NK/T-cell lymphomas and leukemias, resulting in a multidrug resistance (MDR) phenotype[17]. Therefore, non–MDR-dependent drugs (e.g. L-asparaginase, gemcitabine) are now incorporated in protocols specifically designed for ANKL. Majority of patients in the current analysis received L-asparaginase containing regimens pre alloHCT (n=17).
In conclusion, this multicenter CIBMTR report that included majority non-Asian patient population shows that alloHCT can provide durable disease control in a subset of ANKL patients. Achieving CR before transplantation appears to be a prerequisite for successful transplantation outcomes, underscoring the need for more effective remission-inducing therapies for this group of patients. Early referral for transplantation and donor search should be strongly considered in all such cases. Monitoring for residual disease or early relapse (e.g. with serial EBV DNA PCR) post alloHCT warrant investigation to optimize peri-HCT disease control.
Highlights.
Largest series of allo-HCT in Aggressive NK-cell leukemia.
All included cases had central pathology review.
Achievement of CR, key to successful allo-HCT in ANKL
Acknowledgments
Morgan Geronime for administrative Support
CIBMTR Support List
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-13-1-0039 and N00014-14-1-0028 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals; Allos Therapeutics, Inc.; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; *Blue Cross and Blue Shield Association; *Celgene Corporation; Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Fresenius-Biotech North America, Inc.; *Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.;*Gentium SpA; Genzyme Corporation; GlaxoSmithKline; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; Jeff Gordon Children’s Foundation; Kiadis Pharma; The Leukemia & Lymphoma Society; Medac GmbH; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; *Milliman USA, Inc.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Perkin Elmer, Inc.; *Remedy Informatics; *Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; St. Baldrick’s Foundation; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; *Tarix Pharmaceuticals; *TerumoBCT; *Teva Neuroscience, Inc.; *THERAKOS, Inc.; University of Minnesota; University of Utah; and *Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
Footnotes
Corporate Members
Author contributions:
Conception and design: Abraham S. Kanate and Mehdi Hamadani.
Collection and assembly of data: Alyssa DiGilio and Mehdi Hamadani.
Data analysis: Kwang W. Ahn, Alyssa DiGilio and Mehdi Hamadani.
Interpretation: All authors.
Manuscript writing: First draft prepared by Mehdi Hamadani. All authors helped revise the manuscript.
Final approval of manuscript: All authors
Disclosure of conflict of interest: No disclosures to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Suzuki R. Treatment of advanced extranodal NK/T cell lymphoma, nasal-type and aggressive NK-cell leukemia. Int J Hematol. 2010;92:697–701. doi: 10.1007/s12185-010-0726-2. [DOI] [PubMed] [Google Scholar]
- 2.Ruskova A, Thula R, Chan G. Aggressive Natural Killer-Cell Leukemia: report of five cases and review of the literature. Leuk Lymphoma. 2004;45:2427–2438. doi: 10.1080/10428190400004513. [DOI] [PubMed] [Google Scholar]
- 3.Nicolae A, Ganapathi KA, Pham TH, et al. EBV-negative Aggressive NK-cell Leukemia/Lymphoma: Clinical, Pathologic, and Genetic Features. AmJSurgPathol. 2016 doi: 10.1097/PAS.0000000000000735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suzuki R, Suzumiya J, Nakamura S, et al. Aggressive natural killer-cell leukemia revisited: large granular lymphocyte leukemia of cytotoxic NK cells. Leukemia. 2004;18:763–770. doi: 10.1038/sj.leu.2403262. [DOI] [PubMed] [Google Scholar]
- 5.Nakashima Y, Tagawa H, Suzuki R, et al. Genome-wide array-based comparative genomic hybridization of natural killer cell lymphoma/leukemia: different genomic alteration patterns of aggressive NK-cell leukemia and extranodal Nk/T-cell lymphoma, nasal type. Genes Chromosomes Cancer. 2005;44:247–255. doi: 10.1002/gcc.20245. [DOI] [PubMed] [Google Scholar]
- 6.Ishida F, Ko YH, Kim WS, et al. Aggressive natural killer cell leukemia: therapeutic potential of L-asparaginase and allogeneic hematopoietic stem cell transplantation. Cancer Sci. 2012;103:1079–1083. doi: 10.1111/j.1349-7006.2012.02251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jung KS, Cho SH, Kim SJ, Ko YH, Kang ES, Kim WS. L-asparaginase-based regimens followed by allogeneic hematopoietic stem cell transplantation improve outcomes in aggressive natural killer cell leukemia. JHematol Oncol. 2016;9:41-016-0271-4. doi: 10.1186/s13045-016-0271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ito T, Makishima H, Nakazawa H, et al. Promising approach for aggressive NK cell leukaemia with allogeneic haematopoietic cell transplantation. EurJ Haematol. 2008;81:107–111. doi: 10.1111/j.1600-0609.2008.01090.x. [DOI] [PubMed] [Google Scholar]
- 9.Song SY, Kim WS, Ko YH, Kim K, Lee MH, Park K. Aggressive natural killer cell leukemia: clinical features and treatment outcome. Haematologica. 2002;87:1343–1345. [PubMed] [Google Scholar]
- 10.Murashige N, Kami M, Kishi Y, et al. Allogeneic haematopoietic stem cell transplantation as a promising treatment for natural killer-cell neoplasms. BrJ Haematol. 2005;130:561–567. doi: 10.1111/j.1365-2141.2005.05651.x. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki R, Suzumiya J, Nakamura S, et al. Hematopoietic stem cell transplantation for natural killer-cell lineage neoplasms. Bone Marrow Transplant. 2006;37:425–431. doi: 10.1038/sj.bmt.1705244. [DOI] [PubMed] [Google Scholar]
- 12.Ichikawa S, Fukuhara N, Yamamoto J, et al. Successful allogeneic hematopoietic stem cell transplantation for aggressive NK cell leukemia. Intern Med. 2010;49:1907–1910. doi: 10.2169/internalmedicine.49.3814. [DOI] [PubMed] [Google Scholar]
- 13.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 14.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. AmJ Med. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 15.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamaguchi M, Kita K, Miwa H, et al. Frequent expression of P-glycoprotein/MDR1 by nasal T-cell lymphoma cells. Cancer. 1995;76:2351–2356. doi: 10.1002/1097-0142(19951201)76:11<2351::aid-cncr2820761125>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 17.Tse E, Kwong YL. How I treat NK/T-cell lymphomas. Blood. 2013;121:4997–5005. doi: 10.1182/blood-2013-01-453233. [DOI] [PubMed] [Google Scholar]


