PET/CT that includes a diagnostic-quality CT examination increases the sensitivity of diagnostic CT significantly in both the abdomen and the pelvis.
Abstract
Purpose
To assess the diagnostic accuracy of fluorine 18 fluorodeoxyglucose (FDG) positron emission tomography (PET) combined with diagnostic contrast material–enhanced computed tomography (CT) in detecting lymph node (LN) metastasis in high-risk endometrial cancer.
Materials and Methods
This prospective multicenter HIPAA-compliant study had institutional review board approval, and all participants gave written informed consent. Data were accrued between January 2010 and June 2013. Patients underwent PET/CT and pelvic and abdominal lymphadenectomy. Two hundred seven of 215 enrolled patients had PET/CT and pathologic examination results for the abdomen and pelvis. Mean patient age was 62.7 years ± 9.6 (standard deviation). Data in all 23 patients with a positive abdominal examination and in 26 randomly selected patients with a negative abdominal examination were used for this central reader study. Seven independent blinded readers reviewed diagnostic CT and PET/CT results in different sessions 1 month apart. Accuracy was calculated at the participant level, correlating abdominal (right and left para-aortic and common iliac) and pelvic (right and left external iliac and obturator) LN regions with pathologic results, respecting laterality. Reader-average sensitivities, specificities, and areas under the receiver operating characteristic curve (AUCs) of PET/CT and diagnostic CT were compared. Power calculation was for sensitivity and specificity in the abdomen.
Results
Sensitivities of PET/CT versus diagnostic CT for the detection of LN metastasis were 0.65 (95% confidence interval [CI]: 0.57, 0.72) versus 0.50 (95% CI: 0.43, 0.58) (P = .01) in the abdomen and 0.65 (95% CI: 0.57, 0.72) versus 0.48 (95% CI: 0.41, 0.56) (P = .004) in the pelvis. Corresponding specificities were 0.88 (95% CI: 0.83, 0.92) versus 0.93 (95% CI: 0.89, 0.96) (P = .11) and 0.93 (95% CI: 0.86, 0.96) versus 0.89 (95% CI: 0.82, 0.94) (P = .27), and AUCs were 0.78 (95% CI: 0.66, 0.89) versus 0.74 (95% CI: 0.63, 0.86) (P = .39) and 0.82 (95% CI: 0.71, 0.92) versus 0.73 (95% CI: 0.63, 0.84) (P = .02).
Conclusion
FDG PET/CT has satisfactory diagnostic accuracy in the detection of abdominal LN metastasis in high-risk endometrial cancer. Compared with diagnostic CT alone, addition of PET to diagnostic CT significantly increased sensitivity in both the abdomen and pelvis while maintaining high specificity.
© RSNA, 2017
Introduction
Endometrial cancer is the most common gynecologic cancer in the United States, and more than 54 000 women are diagnosed each year (1). Involvement of both pelvic and para-aortic lymph nodes (LNs) predicts poorer outcomes. In the 1988 International Federation of Gynecology and Obstetrics, or FIGO, staging of endometrial cancer, patients with LN metastasis were all classified as having stage IIIC disease. In the 2009 staging system, patients with pelvic LN metastases were classified as having stage IIIc1 disease and those with para-aortic LN metastases were classified as having stage IIIc2 disease because of differences in outcome (2). Therefore, accurate diagnosis of LN metastasis, including location, is now required. Currently, an accurate noninvasive test is not available for the detection of LN metastases. Surgical staging is therefore the most accurate and standard method to determine LN involvement and is considered the reference standard. Although controversy continues over the necessity of surgical staging to evaluate the status of LNs in patients with early-stage endometrial cancer, the risk of extrauterine disease is sufficiently high in high-risk patients to justify surgical staging (3). Patients with high-risk disease include women with grade 3 endometrioid, serous papillary, clear cell, or carcinosarcoma endometrial cancer; as well as patients who have deep myometrial invasion or cervical stromal involvement (4,5). Lymphatic drainage of the uterus is complex, and multiple lymphatic chains could be involved, including obturator, iliac (external, internal, and common), paracaval, para-aortic, as well as parametrial and presacral, LNs. Therefore, unlike in cervical carcinoma, para-aortic and para-caval LNs may be involved directly without involvement of pelvic LNs. The reported sensitivity and specificity of computed tomography (CT) and magnetic resonance (MR) imaging in the detection of LN metastasis in endometrial cancer on the basis of a short-axis diameter of 8 or 10 mm are 18%–66% and 73%–99%, respectively (6–11).
The 2008 annual report of the United States National Oncology PET Registry indicated that, out of 81 951 positron emission tomography (PET) studies performed at 1368 U.S. facilities, 8362 studies (10.2%) were performed for gynecologic tumors (12). Therapeutic strategies were changed in 38% of the cases on the basis of PET results, indicating the importance of PET in diagnosis and treatment. Small cohort data are available on the accuracy of PET diagnostic contrast material–enhanced CT with fluorine 18 fluorodeoxyglucose (FDG) for the detection of LN metastasis in endometrial cancer (13–19). To our knowledge, there is no published multicenter study on the accuracy of PET for detecting LN metastasis in patients with a diagnosis of endometrial cancer and no study on the comparison of PET combined with contrast-enhanced diagnostic CT and contrast-enhanced CT alone.
ACRIN 6671/GOG 0233 is a prospective multicenter clinical trial conducted by the American College of Radiology Imaging Network (ACRIN) and the Gynecologic Oncology Group (GOG) designed to evaluate the efficacy of PET/CT in the evaluation of LN metastasis in women with high-risk endometrial cancer and local-regionally advanced cervical cancer. Here, we report the results in the high-risk endometrial cancer cohort; the results from the advanced cervical cancer cohort will be reported separately. The primary objective of the study was to assess the diagnostic accuracy of FDG PET/CT in detecting LN metastasis in high-risk endometrial cancer. The secondary objectives were to determine the sensitivity, specificity, and areas under the receiver operating characteristic (ROC) curve (AUCs) in the pelvis and abdomen and pelvis combined and to compare the diagnostic accuracy values of FDG PET/CT with those of diagnostic CT.
Materials and Methods
Study Population
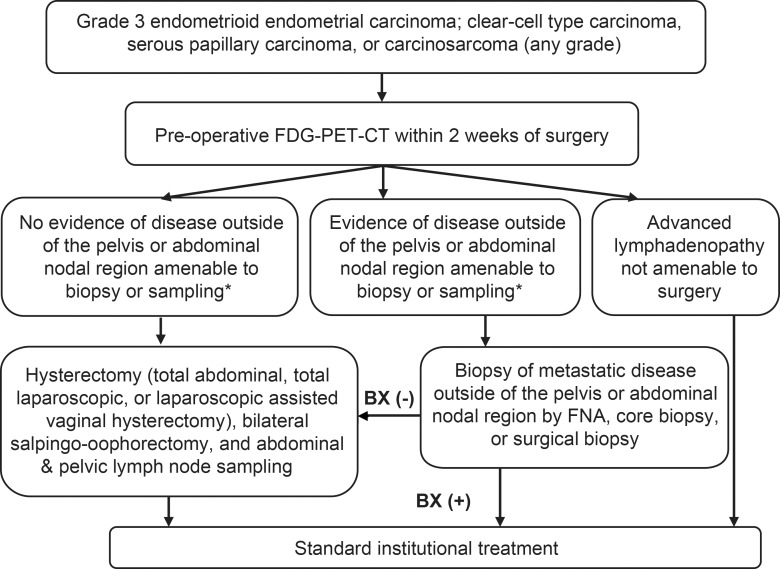
ACRIN 6671/GOG 0233 was a Health Insurance Portability and Accountability Act–compliant nonrandomized multicenter trial performed by ACRIN and GOG. All patients provided written consent to participate in the study prior to enrollment. This trial had institutional review board approval at all participating centers. Patients with primary, previously untreated, histologically confirmed, high-risk cancer (grade 3 endometrioid, clear-cell, serous papillary, or carcinosarcoma endometrial cancer; or grade 1 or 2 endometrioid endometrial carcinoma with overt cervical stromal involvement [at clinical examination or confirmed by means of endocervical curettage]) who were appropriate surgical candidates were eligible. Patients underwent FDG PET/CT followed by hysterectomy and bilateral salpingo-oophorectomy and pelvic and abdominal LN sampling within 2 weeks. The trial schema is shown in Figure 1. Patients who had known metastasis outside of the pelvis or abdominal LNs, patients who had undergone prior pelvic radiation therapy or prior pelvic or abdominal lymphadenectomy, and patients who had had other invasive malignancies within the last 5 years were excluded.
Figure 1:
Study schema. BX = biopsy. *That is, intrahepatic or pulmonary metastasis; bone involvement; suprarenal, thoracic, or supraclavicular lymphadenopathy; or lymphadenopathy above the renal hilum at PET/CT.
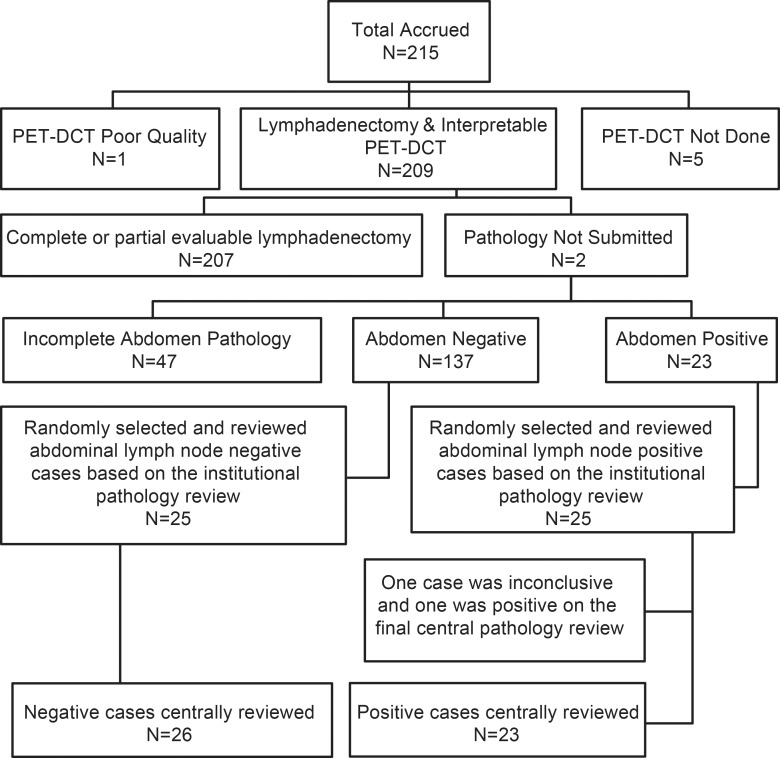
Two hundred fifteen women were accrued to the trial between January 2010 and June 2013 at 22 accruing institutions. Fifty-five patients were excluded because of poor quality PET/CT (n = 1), PET/CT not done (n = 5), pathologic results not submitted (n = 2), or incomplete pathologic results (n = 47) (Fig 2). In the 47 patients with incomplete pathologic results, lymphadenectomy had not been performed in one or more regions in the abdomen, with the other regions being negative, resulting in an incomplete reference standard. Incomplete lymphadenectomy was generally related to technical reasons. Therefore, 160 patients had complete reference standard results available for the abdomen, and 168 had them available for the pelvis. The prevalence of LN metastasis was 14% (23 of 160) in the abdomen and 20% (33 of 168) in the pelvis.
Figure 2:
Patient flowchart. DCT = diagnostic CT.
Imaging Protocol
All patients underwent FDG PET/CT prior to surgery. Patients fasted for at least 4 hours and had a blood glucose level of less than 200 mg/dL prior to FDG injection. For participation in this study, all PET/CT scanners were qualified by the ACRIN PET Core Laboratory. The details of the qualification process have been described previously (20).
Combined abdomen and pelvis PET imaging was scheduled to begin 60 minutes (±10 minutes) after 10–20 mCi (370–740 MBq) (0.14–0.21 mCi [5.18–7.77 MBq]/kg) of FDG was administered. The region imaged extended from the base of the skull to the upper thighs. The PET data were corrected for dead time, random coincidence events, and attenuation by using standard algorithms provided by the scanner manufacturers. Depending on the site’s preferred workflow, a low-dose CT or contrast-enhanced diagnostic CT study was performed that covered the same axial field of view. If low-dose CT was performed prior to the PET emission scan, diagnostic CT was performed immediately after the PET scan without moving or repositioning the patient. For diagnostic CT, patients received oral contrast material and nonionic intravenous contrast material when there was adequate intravenous access and no contradictions to the contrast agent. Five patients could not receive intravenous contrast material, and one patient received no oral contrast material. The placement of a Foley catheter and administration of 20 mg of furosemide were allowed. Among the patients in the reader study, none received diuretics and one had a Foley catheter placed for imaging.
Image reconstruction was dependent on the scanner manufacturer. The difference between the trial and the standard of care was to mandate that both examinations be done concurrently (a more detailed description of the imaging protocol, FDG dosage, time from injection to imaging, and duration of the scans is given in Appendix E1 [online]).
Surgery
Lymphadenectomy was performed in eight nodal regions: right and left obturator, right and left external iliac, right and left common iliac, and right para-aortic (para-caval and aorto-caval) and left para-aortic LNs. The upper extent of the abdominal aortic region was the inferior mesenteric artery origin. Common iliac LNs extended from the aortic bifurcation to the common iliac artery bifurcation. All LNs caudal to the common iliac bifurcation were considered to be pelvic LNs. External iliac LNs were those LNs anterior or medial to the external iliac vessels. Obturator LNs were anterior to the obturator nerve and posterior to the external iliac vessels. LNs at the boundary of two regions were considered to belong to the more cephalad region. Surgeons were required to perform a complete lymphadenectomy for each of the eight regions independently or to confirm LN metastasis in a region by intraoperative biopsy if malignant LNs were not resectable. Common iliac and para-aortic LN regions were considered to be in the abdominal region, and external iliac and obturator LNs were considered to be in the pelvic region, for data analysis. A patient was considered to have pelvic or abdominal malignant lymphadenopathy if at least one of the four regions in the pelvis or abdomen was involved.
Surgeons were given the results of FDG PET/CT to encourage them to remove an LN that was positive at PET/CT that could potentially be left behind, such as LNs posterior to the common iliac vessels or high in the apex of the obturator fossa. This was to prevent a potential false-positive diagnosis for PET/CT.
Pathologic Analysis
Pathologic review was performed at the primary institution of the accrued patients, and results were confirmed at the semiannual GOG meeting by a team of pathologists. Institutional pathologists were all experienced in gynecologic tumors. If the LNs were smaller than 10 mm in the long axis, they were bisected into two halves. LNs 10 mm or larger were sliced at 5-mm intervals parallel to the short axis. The total number of LNs per region, the presence or absence of LN metastasis, and the size of the largest focus of involvement in each region were recorded. The latter information was collected because the size of the focus of involvement is one of the factors that determine detectability at PET.
Missing reference standard results occurred when some LN regions were not surgically removed. In some patients, LN dissection was not performed in all regions because of a technical reason (eg, problem with the laparoscopic equipment or poor visualization) or the presence of retroperitoneal fibrosis.
Central Reader Study Design and Power Analysis
The primary objective of the study was to determine the diagnostic sensitivity and specificity of FDG PET/CT in identifying metastases to abdominal (common iliac, para-aortic, and paracaval) LNs in participants with high-risk endometrial cancer. The secondary objectives were to determine the sensitivity and specificity in the pelvis and the abdomen and pelvis combined and to compare the sensitivity and specificity values of FDG PET/CT and those of diagnostic CT. The analysis for the primary and secondary aims utilized imaging review data obtained in the central reader study, in which all radiologists interpreted all FDG PET/CT studies independently and were blinded to pathologic examination results. Readers were not aware of the case mix.
The sample size calculation was adapted from the formula used in the multireader multicase study design (21) in which the study power is dependent on both the number of cases and the number of readers. To achieve maximum power, the use of an equal number of positive and negative cases is the most efficient design. Our calculation determined that a central reader study with 36 positive cases and 36 negative cases, each case read by seven central readers, would provide adequate precision for the estimates of mean sensitivity and specificity of FDG PET/CT for abdominal LN assessment. Assuming an estimate of sensitivity or specificity of 0.7 for CT, such a sample size would provide an expected half-length of 0.14 of the 95% confidence interval (CI) for sensitivity or specificity.
Assuming that approximately 20% of the enrolled women will have positive nodes in the abdomen and that 15% of the accrued patients will not undergo lymphadenectomy owing to the finding of biopsy-proven metastatic disease outside the pelvic or abdominal LN regions, we calculated that a total of 215 patients with endometrial cancer would be required to provide 36 patients with positive abdominal LNs.
Readers were asked to score LNs of a region from 1 to 6, with a score of 1 indicating definitely negative and a score of 6 indicating definitely positive to allow us to create an ROC curve. The latter was dichotomized into negative (scores 1–3) and positive (scores 4–6) to allow us to compute estimates of sensitivity and specificity. Readers were informed about the threshold for positivity that would be used in the analysis. Individual readers’ sensitivity and specificity and the mean sensitivity and specificity across all readers were calculated.
Four of the seven PET/CT readers had previous experience in review of PET/CT trials, and three underwent an initial didactic training session for the trial, as well as three test cases, before reviewing study PET/CT examinations to familiarize them with the forms. The individual readers’ experience in reading PET/CT studies was as follows: 2 years for reader 1, 8 years for readers 2 and 3, 12 years for readers 4 and 5, 14 years for reader 6, and 30 years for reader 7. Readers included four radiologists and three nuclear physician specialists. One of the radiologists (S.I.L.) and one of the nuclear physician specialists (F.D.) are authors of this article. PET/CT readers were blinded to the clinical information and final pathologic results, and reviews were performed independently. Reviewers were chosen from centers that were not involved in the accrual of patients. The central review was performed at the ACRIN headquarter PET laboratory by using the same workstation. The review process was arranged in two steps: (a) all diagnostic CT examinations were reviewed alone without knowledge of PET findings; and (b) after at least a 1-month washout period, both PET and diagnostic CT images were reviewed together.
Imaging Review
An LN was considered positive at diagnostic CT if the short axis was larger than 8 mm for an ovoid LN (short axis > half the long axis) or was larger than 10 mm for an elongated LN (short axis < half the long axis) in all regions.
PET images were interpreted qualitatively in standard clinical fashion. For an enlarged LN (>10-mm short axis), abnormal FDG uptake was defined as moderate to markedly increased uptake relative to the uptake in comparable normal structures or surrounding tissues, with the exclusion of physiologic bowel and urinary activity, and for normal-size LNs (<10-mm short axis), mildly increased uptake relative to the uptake in comparable normal structures or surrounding tissues was considered abnormal.
Involved LNs in the abdomen or pelvis outside eight regions were recorded. A pelvic LN was considered to be outside the four pelvic regions if it was located in the internal iliac or presacral region. A para-aortic or para-caval LN above the inferior mesenteric artery was considered to be an abdominal LN outside the four abdominal LN regions.
Statistical Analysis
Analysis was performed at the patient level by using pathologic data as the reference standard. The abdomen and pelvic regions were analyzed separately. The abdomen or pelvic region was considered positive if at least one of the corresponding four regions had a positive LN and was considered negative if all four regions were negative according to the reference standard results. In the latter case, if any region was missing, the corresponding abdomen or pelvic data were considered nonanalyzable.
For the reader to get credit for making a correct diagnosis of a positive LN, the positive node identified at imaging needed to be on the same side as at pathologic examination. Ipsilateral para-aortic/paracaval and common iliac LNs were combined at both PET/CT and pathologic examination for data analysis. We did the same for external iliac and obturator LNs. The reason was that, despite the anatomic instructions for regional identification of LNs provided to the surgeons, there was a possibility of miscategorization of location owing to the proximity of the involved LN to the adjacent region.
The paired t test was used to compare the size of the largest positive focus at pathologic examination between LNs from the abdomen and those from the pelvis. The linear mixed model was used to compare the average number of LNs identified at PET/CT with the number of positive LNs identified at pathologic examination.
For each reader, 95% CIs for the estimates of sensitivity and specificity were presented as the exact CIs (Clopper-Pearson). Ninety-five percent CIs for differences in sensitivity and specificity were calculated by using the method of Fleiss (22). P values for the sensitivity and specificity comparisons were based on the McNemar test. Estimates of 95% CIs and P values related to the AUC were derived by using the method of DeLong et al (23) for empiric ROC curve. P < .05 was considered to indicate a statistically significant difference.
Comparison of the seven-reader-average sensitivity and specificity between diagnostic CT alone and PET/CT was performed with a generalized linear mixed model with a random-reader effect. Comparison of the average empiric AUCs between diagnostic CT and PET/CT, from all seven readers, was performed by using the Obuchowski method (23). PET/CT diagnoses of LN metastasis (yes or no) from the seven readers were compared by using Fleiss κ statistics, assuming all observations were independent, for diagnostic CT and PET/CT and for abdominal, pelvic, and combined abdominal and pelvic regions.
We performed a post-hoc analysis to compare the sensitivity of PET/CT with that of diagnostic CT in the subset of patients whose largest positive focus was either above or below the median value.
Results
Patient Characteristics
Because of the lower-than-expected prevalence rate, we could not accrue the 36 expected cases with positive LNs in the abdomen from among the 215 cases we accrued. The central review cases were selected to achieve the balance of positive and negative cases in the abdomen that included all accrued 23 patients with positive abdominal LNs and 26 randomly selected patients with negative abdominal LNs. The reason for the discrepancy between the number of positive and negative cases was that two of the 25 cases initially selected as positive at initial institutional review were identified as either negative or inconclusive at final pathologic review. All 49 patients had complete pathologic results in the abdomen, and 47 of the 49 patients had complete pathologic results in the pelvis (23 had positive results and 24 had negative results).
Patient ages ranged from 36 to 81 years (mean age, 62.7 years ± 9.6). Cancer stages were as follows: I in 30 patients, II in 14, and III in five. Sixteen cancers were endometrioid, 17 were serous, six were carcinosarcoma or malignant mixed Müllerian tumor, six were mixed epithelial carcinoma, three were unspecified adenocarcinoma, and one was heterologous carcinosarcoma. The 16 endometrioid cancers included five grade 2 cancers and 11 grade 3 cancers. Four of the five grade 2 endometrioid cancers were stage 2 or higher, and one was stage 1B, in the hysterectomy specimens.
Sensitivity, Specificity, and AUC of PET/CT in the Abdomen and Pelvis
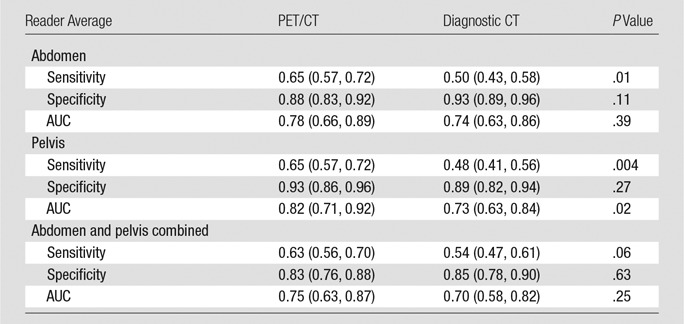
In the abdomen, the sensitivities of PET/CT ranged from 0.52 to 0.74 (Figs 3, 4), and the specificities ranged from 0.85 to 0.92 (Tables 1, 2; Figs 5, 6). The difference between PET/CT and diagnostic CT reader-average sensitivity to detect LN metastasis in the abdomen was significant (0.65 vs 0.50, P = .01, Table 1). The average specificity of PET/CT was not significantly different from that of diagnostic CT in the abdomen (0.88 vs 0.93, P = .11), and the same held true for average AUC (0.78 vs 0.74, P = .39) (Tables 1, 2).
Figure 3a:
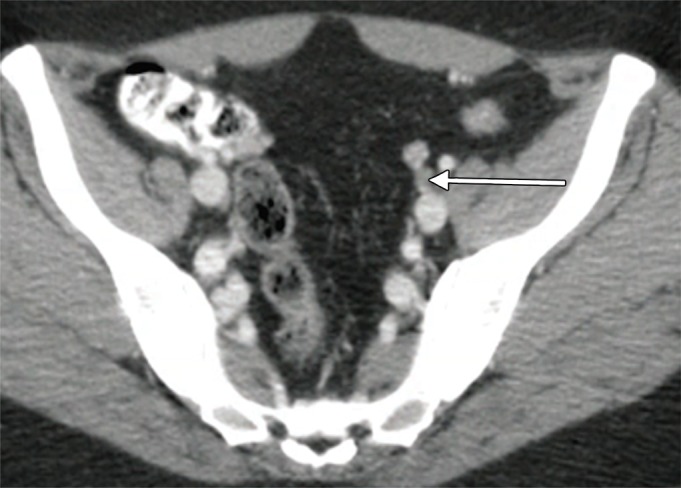
An example of a true-positive PET/CT. (a) Axial contrast-enhanced CT scan. A small left external iliac LN is present (arrow). (b) Axial fused PET/CT image. The LN is FDG PET avid (arrow). Pathologic findings in the left external iliac region were positive.
Figure 4a:
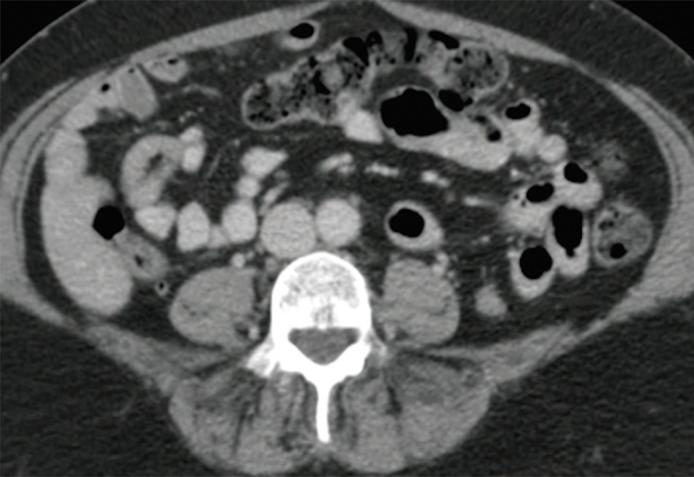
An example of a false-negative PET/CT. (a) Axial contrast-enhanced CT scan. There is no enlarged LN. (b) Axial fused PET/CT image. There is no FDG PET–avid LN. Pathologic findings were positive for the left para-aortic region.
Table 1.
Reader-Average Accuracy Values for PET/CT and Diagnostic CT
Note.—Data in parentheses are 95% CIs.
Table 2.
Individual Reader Accuracy Values for PET/CT and Diagnostic CT
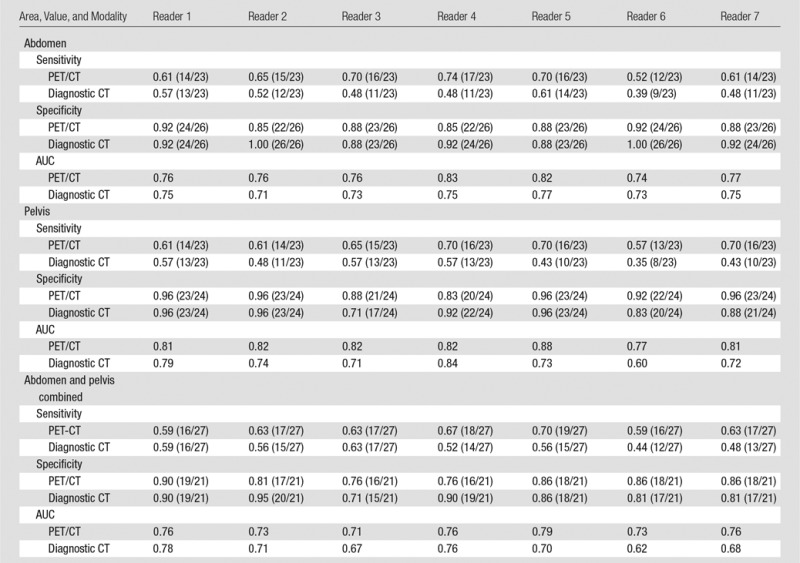
Figure 5a:
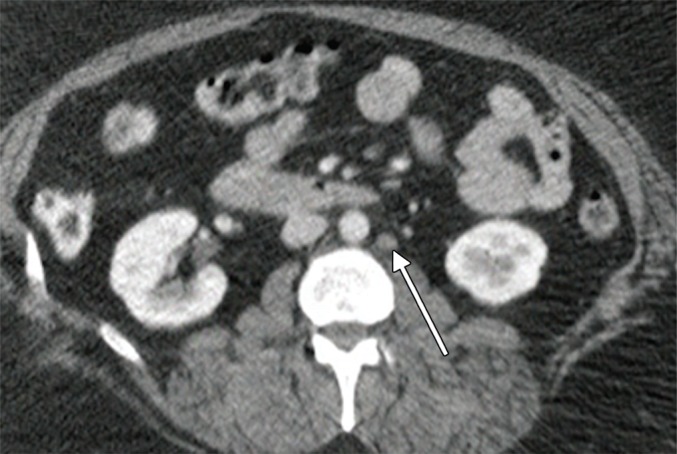
An example of a true-negative PET/CT study. (a) Axial contrast-enhanced CT scan. Prominent left para-aortic LN is present (arrow). (b) Axial fused PET/CT image. The LN is not FDG PET avid. Pathologic findings in the left para-aortic region were negative.
Figure 6a:
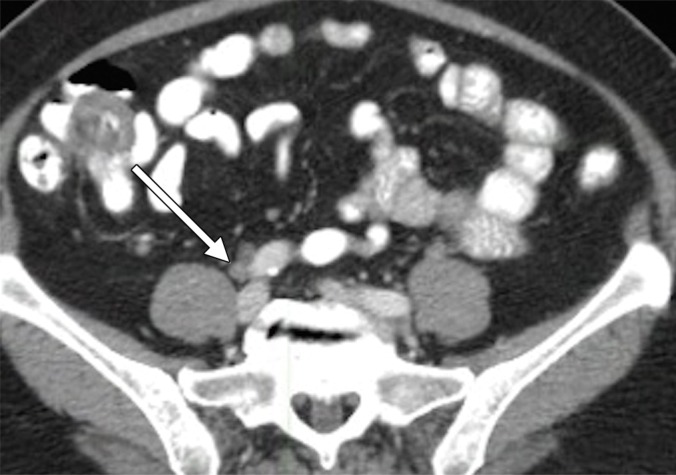
An example of a false-positive PET/CT. (a) Axial contrast-enhanced CT scan. A rounded right common iliac LN is present (arrow). (b) Axial fused PET/CT image. The LN is FDG PET avid (arrow). Pathologic findings were negative.
Figure 3b:
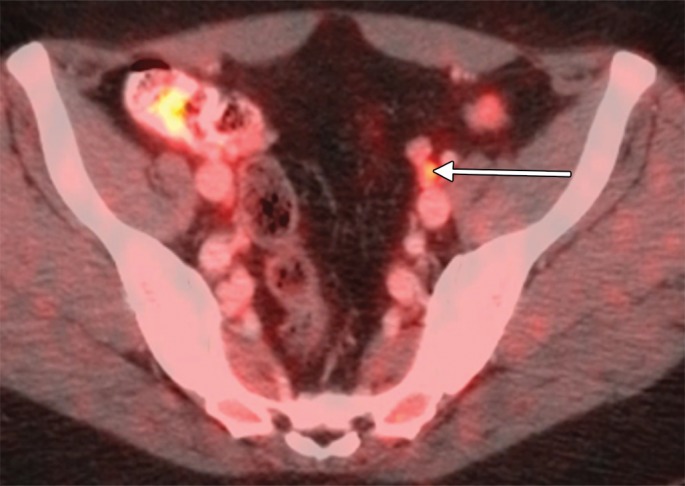
An example of a true-positive PET/CT. (a) Axial contrast-enhanced CT scan. A small left external iliac LN is present (arrow). (b) Axial fused PET/CT image. The LN is FDG PET avid (arrow). Pathologic findings in the left external iliac region were positive.
Figure 4b:
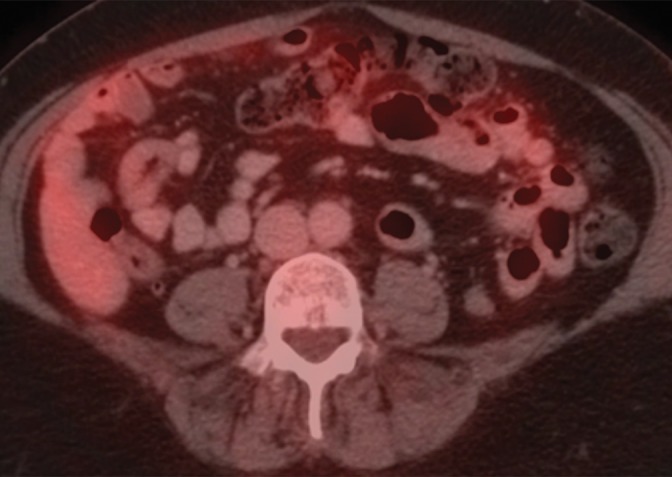
An example of a false-negative PET/CT. (a) Axial contrast-enhanced CT scan. There is no enlarged LN. (b) Axial fused PET/CT image. There is no FDG PET–avid LN. Pathologic findings were positive for the left para-aortic region.
Figure 5b:
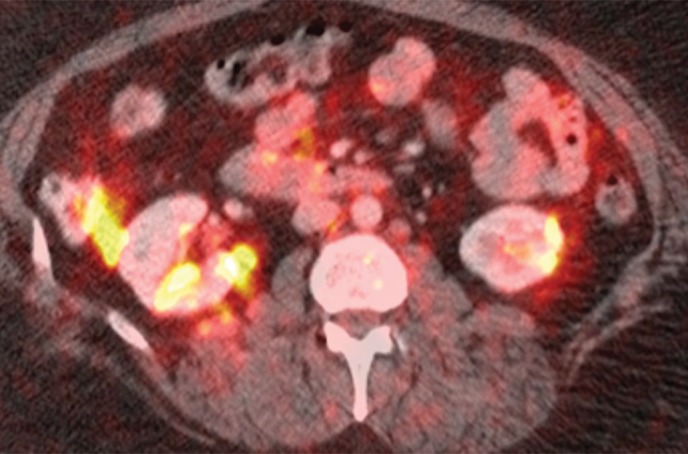
An example of a true-negative PET/CT study. (a) Axial contrast-enhanced CT scan. Prominent left para-aortic LN is present (arrow). (b) Axial fused PET/CT image. The LN is not FDG PET avid. Pathologic findings in the left para-aortic region were negative.
Figure 6b:
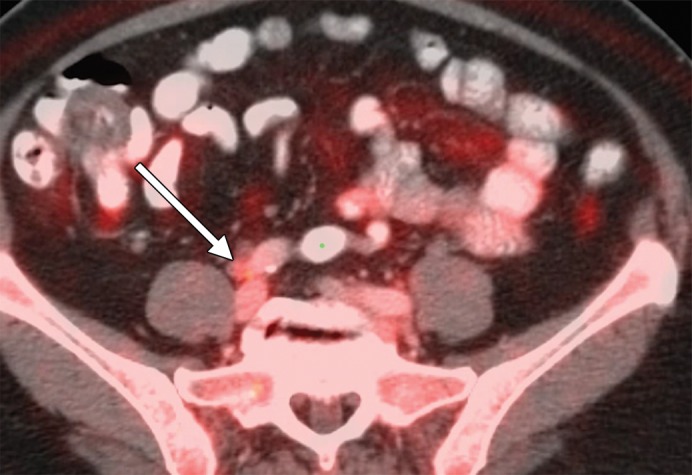
An example of a false-positive PET/CT. (a) Axial contrast-enhanced CT scan. A rounded right common iliac LN is present (arrow). (b) Axial fused PET/CT image. The LN is FDG PET avid (arrow). Pathologic findings were negative.
The average sensitivity of PET/CT in the pelvis was significantly higher than that of diagnostic CT (0.65 vs 0.48, P = .004). The same held true for average AUC (0.82 vs 0.73, P = .02). The average specificity of PET/CT and diagnostic CT were comparable in the pelvis (0.93 vs 0.89, P = .27).
The average sensitivity of PET/CT was not significantly higher than that of diagnostic CT when the abdomen and pelvis were combined, but this might have been influenced by low patient numbers (0.63 vs 0.54, P = .06). The average specificities and AUCs of PET/CT and diagnostic CT in the abdomen and pelvis combined were not significantly different (0.83 vs 0.85 [P = .63] for specificity and 0.75 vs 0.70 [P = .25] for AUC).
LNs Outside of Eight Lymphadenectomy Regions
Among 49 cases selected for central review, there were six reported as having positive LNs outside of the eight lymphadenectomy regions at PET/CT by the majority of reviewers (four or more out of seven)—three in the abdomen above the inferior mesenteric artery, one in the other pelvic regions, and two in the other abdominal and pelvic regions. All cases had positive reference standard results at pathologic examination in the corresponding abdominal or pelvic lymphadenectomy regions, but LNs were not removed because they were outside standard lymphadenectomy regions.
Interreader Variability
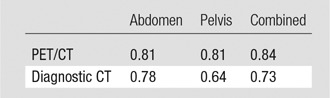
The agreement between the seven readers for PET/CT was excellent (κ between 0.81 and 0.84), and that for diagnostic CT alone was substantial (κ between 0.64 and 0.78, Table 3).
Table 3.
κ Values for Interobserver Agreement between Seven Readers for PET/CT and Diagnostic CT
Pathologic Results in the Removed LNs
Each participant had an average of 26.7 nodes ± 13.3 (3.6 ± 5.6 positive and 23.1 ± 14.0 negative) removed. The average number of positive LNs in the abdomen was 2.0 nodes ± 3.7 and that in the pelvis was 1.6 nodes ± 2.5 at pathologic examination. Considering the seven-reader average, the number of positive LNs identified at PET/CT was 1.2 nodes ± 2.4 (range, 0–13.1 nodes) in the abdomen and 0.8 node ± 1.5 (range, 0–8 nodes) in the pelvis, all of which were significantly fewer than what was identified at pathologic examination in the abdomen, pelvis, and abdomen and pelvis combined (P = .015, .0018, and .0007, respectively).
The size of the largest positive focus at pathologic examination was 16.3 mm ± 16.1 in the abdomen (median, 8 mm) and 17.7 mm ± 12.9 in the pelvis (median, 13 mm). The sizes were comparable (P = .77).
Sensitivity of PET/CT in the Subset of Patients with a Small Positive Focus of Disease
In the subset of patients whose largest positive focus of disease in the LNs of the abdomen and pelvis was below the median value, PET/CT had significantly better sensitivity than diagnostic CT in the abdomen (0.47 vs 0.25, P = .005) and pelvis (0.69 vs 0.39, P = .0006). This was not true for the subset of patients whose largest positive focus of disease was above the median values (0.89 vs 0.87 [P = .80] in the abdomen and 0.76 vs 0.70 [P = .42] in the pelvis).
Discussion
With the 2009 International Federation of Gynecology and Obstetrics, or FIGO, staging system, the presence and extent of LN metastasis have important roles in the treatment of patients with endometrial cancer (2). The 5-year survival rate for disease limited to the uterus is 96%. The survival rate decreases to 57% with pelvic LN metastasis and to 49.4% with abdominal LN metastasis (24).
The reader-average PET/CT sensitivity was 0.65 in both the abdomen and pelvis, which was significantly higher than that for diagnostic CT (P = .01 and .004, respectively). Both PET/CT and CT had high specificity, ranging from 0.88 to 0.93, with no significant difference between the two in the abdomen or pelvis (P = .11 and .27, respectively). Our results are similar to those of a meta-analysis published in 2012 by Chang et al (25), which reported a sensitivity of 0.63 and a specificity of 0.95. However, our sensitivity is slightly lower than that in another meta-analysis, which showed a sensitivity of 0.72 (95% CI: 0.64, 0.80) (26). Our results show a significantly higher sensitivity of PET/CT as compared with diagnostic CT in nodes smaller than the median size of the involved LNs; this could be the result of PET/CT being more sensitive than diagnostic CT. Sironi et al (27) and Kitajima et al (15) have shown that the sensitivity of PET/CT depends on the size of the involved LN, with LNs smaller than 5 mm showing lower sensitivity. The AUC of PET/CT was significantly higher in the pelvis than that of diagnostic CT (P = .02).
All patients with positive LNs at PET/CT outside standard lymphadenectomy regions also had positive LNs in their corresponding abdominal/pelvic lymphadenectomies. Interobserver agreement for the interpretation of PET/CT was more than 0.8 in the abdomen and pelvis, indicating excellent agreement.
We want to emphasize that PET/CT examinations in this study included diagnostic CT that was performed concurrently with PET. We recommend the use of diagnostic CT along with PET both to increase the diagnostic value of PET and to eliminate the need for a separate diagnostic CT, as is suggested in a previous report (28).
Study limitations included a missing reference standard in some regions in some patients. However, this missing reference standard likely resulted in LN metastases different from the rate expected from the literature. Furthermore, some patients found to have intraperitoneal disease at the time of surgery did not undergo LN dissection. Because the central reader review did not review all negative cases, the effect of bias toward negative cases is not known. Another limitation was that we were not able to accrue the full sample size calculated at the outset of the study. A larger sample size would have made CIs tighter and would have potentially yielded more statistically significant findings in the subanalysis. Ideally, we should have performed node-for-node comparison, but this was not done. In conclusion, with respect to LN metastasis, PET/CT can improve detection of LN metastasis in the abdomen as compared with the current standard of contrast-enhanced diagnostic CT. Sensitivity is moderate and cannot replace surgical sampling.
Advances in Knowledge
■ PET combined with contrast-enhanced diagnostic CT significantly increases the reader-average sensitivity of contrast-enhanced diagnostic CT in the detection of lymph node (LN) metastasis in the abdomen (0.65 vs 0.50, P = .01) and pelvis (0.65 vs 0.48, P = .004) in high-risk endometrial cancer.
■ The reader-average specificities of PET/CT and diagnostic CT in detection of LN metastasis were comparable in both the abdomen (0.88 vs 0.93, P = .11) and pelvis (0.93 vs 0.89, P = .27).
■ The reader-average area under the receiver operating characteristic curve of PET/CT was significantly higher than that of diagnostic CT in the pelvis (0.82 vs 0.73, P = .02) but was comparable in the abdomen (0.78 vs 0.74, P = .39).
■ The κ value for interpretation of PET/CT studies for all readers was excellent (>0.80).
Implication for Patient Care
■ PET/CT can improve detection of LN metastasis in the abdomen compared with the current standard of contrast-enhanced diagnostic CT.
APPENDIX
Received February 1, 2016; revision requested March 30; revision received August 24; accepted September 29; final version accepted October 17.
The American College of Radiology Imaging Network (U10 CA80098 and U10 CA079778) and the Gynecologic Oncology Group (U10CA27469 and U10 CA37517) receive funding through the National Cancer Institute.
Disclosures of Conflicts of Interest: M.A. disclosed no relevant relationships. Z.Z. disclosed no relevant relationships. F.D. disclosed no relevant relationships. S.I.L. disclosed no relevant relationships. H.M. disclosed no relevant relationships. S.A. disclosed no relevant relationships. W.J.K. disclosed no relevant relationships. R.S.M. disclosed no relevant relationships. P.D. disclosed no relevant relationships. S.A.K. disclosed no relevant relationships. M.P. disclosed no relevant relationships. X.C.Z. disclosed no relevant relationships. M.P. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a surgical consultant for Ethicon; receives royalties from VitaTex. Other relationships: disclosed no relevant relationships. K.M.M. disclosed no relevant relationships. M.G. disclosed no relevant relationships.
Abbreviations:
- ACRIN
- American College of Radiology Imaging Network
- AUC
- area under the ROC curve
- CI
- confidence interval
- FDG
- fluorine 18 fluorodeoxyglucose
- GOG
- Gynecologic Oncology Group
- LN
- lymph node
- ROC
- receiver operating characteristic
References
- 1.American Cancer Society . Cancer Facts & Figures. http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2015/index. Published 2015.
- 2.Plataniotis G, Castiglione M; ESMO Guidelines Working Group. Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21(Suppl 5):v41–v45. [DOI] [PubMed] [Google Scholar]
- 3.Mariani A, Webb MJ, Keeney GL, Haddock MG, Aletti G, Podratz KC. Stage IIIC endometrioid corpus cancer includes distinct subgroups. Gynecol Oncol 2002;87(1):112–117. [DOI] [PubMed] [Google Scholar]
- 4.Creasman WT, Morrow CP, Bundy BN, Homesley HD, Graham JE, Heller PB. Surgical pathologic spread patterns of endometrial cancer: a Gynecologic Oncology Group Study. Cancer 1987;60(8 Suppl):2035–2041. [DOI] [PubMed] [Google Scholar]
- 5.Creasman WT, DeGeest K, DiSaia PJ, Zaino RJ. Significance of true surgical pathologic staging: a Gynecologic Oncology Group Study. Am J Obstet Gynecol 1999;181(1):31–34. [DOI] [PubMed] [Google Scholar]
- 6.Hricak H, Rubinstein LV, Gherman GM, Karstaedt N. MR imaging evaluation of endometrial carcinoma: results of an NCI cooperative study. Radiology 1991;179(3):829–832. [DOI] [PubMed] [Google Scholar]
- 7.Sugiyama T, Nishida T, Ushijima K, et al. Detection of lymph node metastasis in ovarian carcinoma and uterine corpus carcinoma by preoperative computerized tomography or magnetic resonance imaging. J Obstet Gynaecol (Tokyo 1995) 1995;21(6):551–556. [DOI] [PubMed] [Google Scholar]
- 8.Connor JP, Andrews JI, Anderson B, Buller RE. Computed tomography in endometrial carcinoma. Obstet Gynecol 2000;95(5):692–696. [DOI] [PubMed] [Google Scholar]
- 9.Manfredi R, Mirk P, Maresca G, et al. Local-regional staging of endometrial carcinoma: role of MR imaging in surgical planning. Radiology 2004;231(2):372–378. [DOI] [PubMed] [Google Scholar]
- 10.Rockall AG, Sohaib SA, Harisinghani MG, et al. Diagnostic performance of nanoparticle-enhanced magnetic resonance imaging in the diagnosis of lymph node metastases in patients with endometrial and cervical cancer. J Clin Oncol 2005;23(12):2813–2821. [DOI] [PubMed] [Google Scholar]
- 11.Rockall AG, Meroni R, Sohaib SA, et al. Evaluation of endometrial carcinoma on magnetic resonance imaging. Int J Gynecol Cancer 2007;17(1):188–196. [DOI] [PubMed] [Google Scholar]
- 12.Hillner BE, Siegel BA, Shields AF, et al. Relationship between cancer type and impact of PET and PET/CT on intended management: findings of the national oncologic PET registry. J Nucl Med 2008;49(12):1928–1935. [DOI] [PubMed] [Google Scholar]
- 13.Park JY, Kim EN, Kim DY, et al. Comparison of the validity of magnetic resonance imaging and positron emission tomography/computed tomography in the preoperative evaluation of patients with uterine corpus cancer. Gynecol Oncol 2008;108(3):486–492. [DOI] [PubMed] [Google Scholar]
- 14.Kitajima K, Murakami K, Yamasaki E, Kaji Y, Sugimura K. Accuracy of integrated FDG-PET/contrast-enhanced CT in detecting pelvic and paraaortic lymph node metastasis in patients with uterine cancer. Eur Radiol 2009;19(6):1529–1536. [DOI] [PubMed] [Google Scholar]
- 15.Kitajima K, Murakami K, Yamasaki E, et al. Accuracy of 18F-FDG PET/CT in detecting pelvic and paraaortic lymph node metastasis in patients with endometrial cancer. AJR Am J Roentgenol 2008;190(6):1652–1658. [DOI] [PubMed] [Google Scholar]
- 16.Horowitz NS, Dehdashti F, Herzog TJ, et al. Prospective evaluation of FDG-PET for detecting pelvic and para-aortic lymph node metastasis in uterine corpus cancer. Gynecol Oncol 2004;95(3):546–551. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki R, Miyagi E, Takahashi N, et al. Validity of positron emission tomography using fluoro-2-deoxyglucose for the preoperative evaluation of endometrial cancer. Int J Gynecol Cancer 2007;17(4):890–896. [DOI] [PubMed] [Google Scholar]
- 18.Nogami Y, Iida M, Banno K, et al. Application of FDG-PET in cervical cancer and endometrial cancer: utility and future prospects. Anticancer Res 2014;34(2):585–592. [PubMed] [Google Scholar]
- 19.Crivellaro C, Signorelli M, Guerra L, et al. Tailoring systematic lymphadenectomy in high-risk clinical early stage endometrial cancer: the role of 18F-FDG PET/CT. Gynecol Oncol 2013;130(2):306–311. [DOI] [PubMed] [Google Scholar]
- 20.Scheuermann JS, Saffer JR, Karp JS, Levering AM, Siegel BA. Qualification of PET scanners for use in multicenter cancer clinical trials: the American College of Radiology Imaging Network experience. J Nucl Med 2009;50(7):1187–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Obuchowski NA. Hypothesis testing of diagnostic accuracy for multiple readers and multiple tests: an ANOVA approach with dependent observations. Commun Stat Simul Comput 1995;24:285–308. [Google Scholar]
- 22.Fleiss DJ. Diagnostic tests for gastrocnemius tightness. J Bone Joint Surg Am 2003;85-A(4):760; author reply 760. [DOI] [PubMed] [Google Scholar]
- 23.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44(3):837–845. [PubMed] [Google Scholar]
- 24.Lewin SN, Herzog TJ, Barrena Medel NI, et al. Comparative performance of the 2009 international Federation of gynecology and obstetrics’ staging system for uterine corpus cancer. Obstet Gynecol 2010;116(5):1141–1149. [DOI] [PubMed] [Google Scholar]
- 25.Chang MC, Chen JH, Liang JA, Yang KT, Cheng KY, Kao CH. 18F-FDG PET or PET/CT for detection of metastatic lymph nodes in patients with endometrial cancer: a systematic review and meta-analysis. Eur J Radiol 2012;81(11):3511–3517. [DOI] [PubMed] [Google Scholar]
- 26.Kakhki VR, Shahriari S, Treglia G, et al. Diagnostic performance of fluorine 18 fluorodeoxyglucose positron emission tomography imaging for detection of primary lesion and staging of endometrial cancer patients: systematic review and meta-analysis of the literature. Int J Gynecol Cancer 2013;23(9):1536–1543. [DOI] [PubMed] [Google Scholar]
- 27.Sironi S, Buda A, Picchio M, et al. Lymph node metastasis in patients with clinical early-stage cervical cancer: detection with integrated FDG PET/CT. Radiology 2006;238(1):272–279. [DOI] [PubMed] [Google Scholar]
- 28.Prabhakar HB, Kraeft JJ, Schorge JO, Scott JA, Lee SI. FDG PET-CT of gynecologic cancers: pearls and pitfalls. Abdom Imaging 2015;40(7):2472–2485. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.