Abstract
Purpose: To evaluate the relative cost-effectiveness of percutaneous radiofrequency (RF) ablation versus nephron-sparing surgery (NSS) in patients with small (≤4-cm) renal cell carcinoma (RCC), given a commonly accepted level of societal willingness to pay.
Materials and Methods: A decision-analytic Markov model was developed to estimate life expectancy and lifetime costs for 65-year-old patients with a small RCC treated with RF ablation or NSS. The model incorporated RCC presence, treatment effectiveness and costs, and short- and long-term outcomes. An incremental cost-effectiveness analysis was performed to identify treatment preference under an assumed $75 000 per quality-adjusted life-year (QALY) societal willingness-to-pay threshold level, within proposed ranges for guiding implementation of new health care interventions. The effect of changes in key parameters on strategy preference was addressed in sensitivity analysis.
Results: By using base-case assumptions, NSS yielded a minimally greater average quality-adjusted life expectancy than did RF ablation (2.5 days) but was more expensive. NSS had an incremental cost-effectiveness ratio of $1 152 529 per QALY relative to RF ablation, greatly exceeding $75 000 per QALY. Therefore, RF ablation was considered preferred and remained so if the annual probability of post–RF ablation local recurrence was up to 48% higher relative to that post-NSS. NSS preference required an estimated NSS cost reduction of $7500 or RF ablation cost increase of $6229. Results were robust to changes in most model parameters, but treatment preference was dependent on the relative probabilities of local recurrence after RF ablation and NSS, the short-term costs of both, and quality of life after NSS.
Conclusion: RF ablation was preferred over NSS for small RCC treatment at a societal willingness-to-pay threshold level of $75 000 per QALY. This result was robust to changes in most model parameters, but somewhat dependent on the relative probabilities of post–RF ablation and post-NSS local recurrence, NSS and RF ablation short-term costs, and post-NSS quality of life, factors which merit further primary investigation.
© RSNA, 2008
Keywords: ICER = incremental cost-effectiveness ratio, NSS = nephron-sparing surgery, QALY = quality-adjusted life-year, RCC = renal cell carcinoma, RF = radiofrequency
Renal cell carcinoma (RCC) accounts for more than 80% of kidney cancers, which are predicted to have resulted in over 12 890 deaths in the United States in 2007 (1–3). A continual rise in RCC incidence is attributed largely to increased detection, with more than 60% of RCCs being discovered incidentally at radiologic imaging (4).
Small (defined as ≤4 cm) RCCs account for the majority of increased detection rates and carry a favorable prognosis (5,6). However, despite increased detection rates and surgical treatment, RCC mortality has not decreased, suggesting tumor indolence (5). These trends underscore the need to reassess RCC treatment paradigms, particularly the effects of less aggressive management on outcomes.
Small RCC treatment has evolved from radical nephrectomy toward nephron-sparing surgery (NSS), a shift driven by lower morbidity and improved long-term renal function and quality of life (7,8). In recent years, the feasibility of percutaneous radiofrequency (RF) ablation for small RCC has been established (9–15). RF ablation confers advantages of a nephron-sparing procedure but is better tolerated and less expensive than surgery (16).
Microscopic multifocal disease is often present close to the primary RCC identified at imaging (17). Because the treatment zone is more limited in RF ablation than with NSS, RF ablation may be associated with a higher local recurrence rate. This higher risk may or may not be associated with a survival difference. When NSS is compared with radical nephrectomy for small RCC, an analogous comparison, the minimally higher local recurrence rate after NSS does not confer decreased survival (18–20).
Local recurrence rates following RF ablation have not been shown to exceed those of NSS for small RCC (9–12,21). However, long-term follow-up data are not yet available for RF ablation. Therefore, RF ablation currently is favored primarily in subpopulations in which risks of an increased local recurrence rate are outweighed by benefits of a percutaneous, localized approach, such as patients with multiple comorbidities, reduced life expectancy, or reduced renal reserve (12).
Before RF ablation can be routinely advocated for small RCC, its risks, benefits, and long-term consequences in a general population must be carefully evaluated and compared with NSS. To perform a definitive comparison, large randomized clinical trials would be necessary. A large number of patients would be required to detect small differences in outcomes, as would a long follow-up period to determine true recurrence rates. Such trials would be challenging and costly; more important, it is unclear that equipoise between trial arms could be offered without a more rigorous evaluation of current published data.
Decision analysis provides an ideal initial method with which to assess treatment paradigms for the management of small RCC, enabling efficient incorporation of the relative risks and benefits of each strategy considered and of numerous other factors that influence long-term outcomes. In this study, RF ablation and NSS were compared by using decision-analytic techniques. A Markov model was developed to estimate life expectancy and lifetime costs associated with each intervention for a simulated cohort of patients with small RCC. Preference of RF ablation versus NSS was determined on the basis of a commonly accepted level of societal willingness to pay (22–25). The stability of results to changes in key parameters, including the probability of local RCC recurrence after RF ablation, was evaluated in sensitivity analysis.
MATERIALS AND METHODS
Cost-effectiveness Analysis Overview
Cost-effectiveness analysis is a decision analysis method that enables assessment of the incremental value of health care interventions by comparing differences in cost and quality-adjusted life expectancy afforded by each intervention. This evidence-based method carries advantages of efficiency and versatility and therefore helps assess new technologies before they transition to use in standard practice. In this study, we used cost-effectiveness analysis to compare RF ablation with NSS for the treatment of small RCC.
Cost-effectiveness analysis was performed under guidelines issued by the Panel on Cost-effectiveness in Health and Medicine (26). The analysis was performed from a quasi-societal perspective; that is, costs of disease management were included, regardless of who incurred them. Time costs to the patient, however, were not included. Life expectancy and lifetime costs were calculated for RF ablation and NSS. Life expectancy was expressed in quality-adjusted life-years (QALYs), with use of health state–specific utilities to weight quality of life. A utility is an accepted metric of quality-of-life weighting in cost-effectiveness analysis that consists of a fraction value between 0 (equivalent to death) and 1 (equivalent to perfect health) (26). Relevant utilities were elicited from the literature and used to calculate QALYs (utility × life-years = QALYs) for each health state in our analysis (27–29). An annual discount rate of 3% was applied to future costs and QALYs.
Strategies were compared in an incremental cost-effectiveness analysis (26). If one was associated with fewer QALYs and greater costs than the other, it was considered as dominated and therefore eliminated. If not, strategies were aligned in order of increasing QALYs and costs, and an incremental cost-effectiveness ratio (ICER) was calculated as their difference in cost divided by their difference in QALYs. A $75 000 per QALY societal willingness-to-pay threshold level was used to determine strategy preference. If the computed ICER was below $75 000 per QALY, then the strategy with the higher life expectancy and cost was considered preferred from a cost-effectiveness standpoint. If not, then the strategy with the lower life expectancy and cost was considered preferred.
The use of a single willingness-to-pay threshold level for policy decisions is not standard practice in the United States (30–32). ICERs for currently funded health care interventions differ widely and are dependent on multiple factors, including the specific disease process and populations at risk. The $75 000 per QALY threshold level was prospectively chosen within commonly accepted levels of societal willingness to pay ($50 000–$100 000 per QALY) (22–25). This threshold level range is linked to reported ICERs for hemodialysis, an intervention that is widely considered a benchmark for societal willingness to pay (22–25). In this study, use of the $75 000 per QALY threshold level to evaluate RF ablation and NSS is not intended for patient-level decisions; instead, it is provided to generate a general economic framework to facilitate population-level cost-effectiveness evaluation.
Decision Tree
A decision-analytic Markov model was developed to estimate life expectancy and lifetime costs for 65-year-old men with unilateral RCCs 4 cm or smaller. The primary (base-case) analysis incorporated specified best available model input estimates (Tables 1, 2). Stability of results over changes in model estimates was evaluated in secondary (sensitivity) analysis.
Table 1.
Parameter Estimates for Base-Case Analysis and Sensitivity Analysis
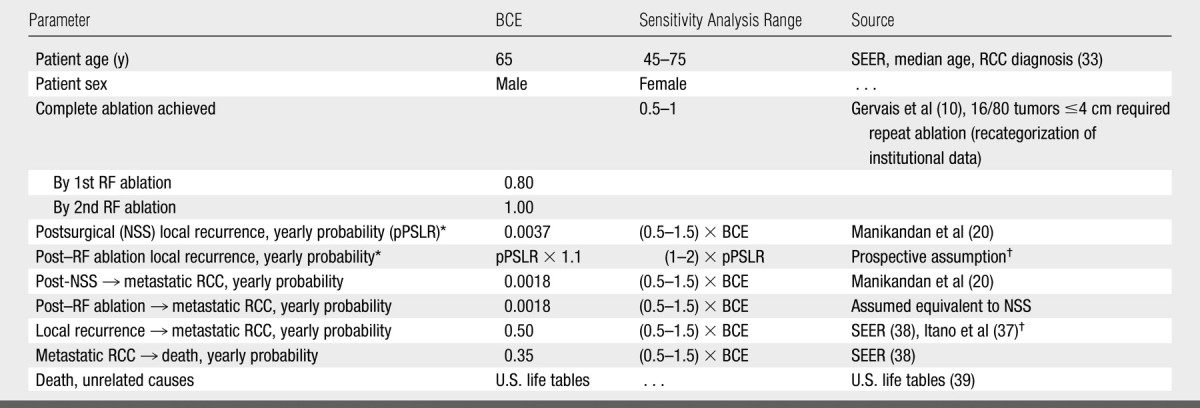
Note.—BCE = base-case estimate, pPSLR - yearly probability of postsurgical local recurrence.
The yearly probability of post–RF ablation local RCC recurrence was modeled to be 10% higher relative to that after NSS. For sensitivity analysis, this value was varied from (1–2) × pPSLR (from equivalent to double that post-NSS).
See Model Data and Data Sources (Probability estimates) section for further detail.
Table 2.
Costs and Utilities for Base-Case Analysis and Sensitivity Analysis
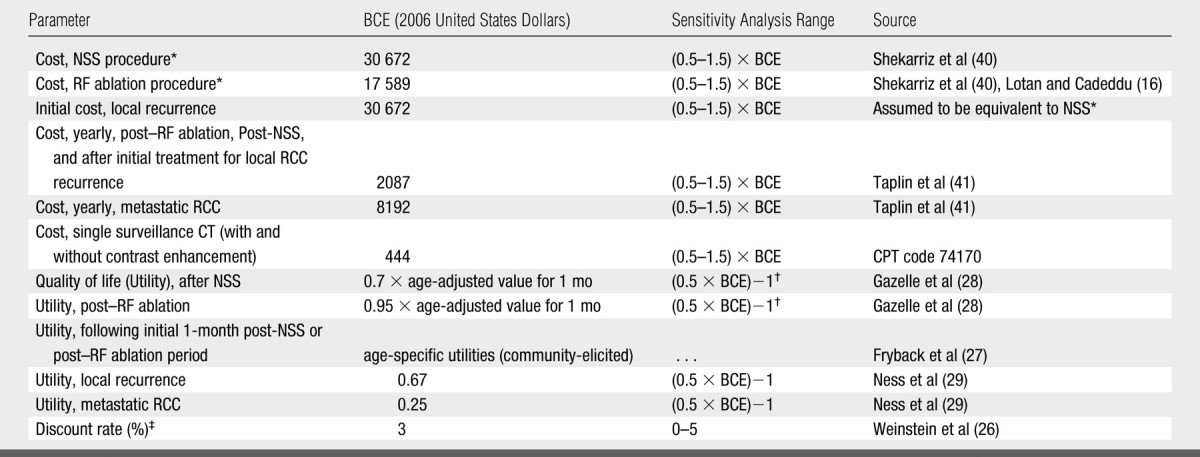
Note.—BCE = base-case estimate.
See Model Data and Data Sources (Costs and utilities) section for further detail.
Utilities of 0.7 and 0.95, were varied from 0.35 to 1, and from 0.48 to 1, respectively, in sensitivity analysis.
A discount rate was applied to all future costs and QALYs as per guidelines from reference 26.
The age of 65 years was used, given the national median age of RCC diagnosis (33). Men were considered in the base-case analysis because of greater RCC incidence (33). The cohort was assigned as follows: normal contralateral kidneys and renal function, exophytic or parenchymal tumors easily amenable to a percutaneous RF ablation approach (this would include >70% of tumors [10]), and good operative candidacy.
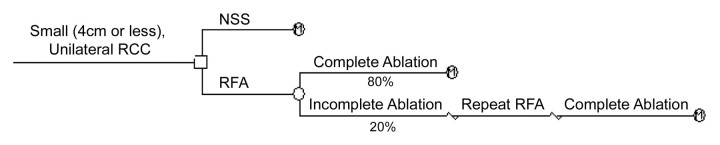
The model decision tree was constructed and analyzed by using software (TreeAge Pro, 2005; TreeAge Software, Williamstown, Mass) (Fig 1). We assumed that patients could have no more than one incomplete RF ablation; further ablation requirements were made on the basis of a report on renal tumor RF ablation from our institution and recategorized for tumors 4 cm or smaller (10) (Table 1). Specifically, all tumors were considered completely ablated after two sessions: 80% (64 of 80 tumors) required one session only and 20% (16 of 80 tumors) required two sessions. NSS and RF ablation procedural and operative mortality were not incorporated as they are reported to have comparable, low rates (8,21). Morbidity was incorporated by including short-term RF ablation and NSS costs, including estimated costs of patients with complications, and short-term quality of life decrements (discussed below). Patients requiring repeat RF ablation incurred twice the RF ablation cost and quality-of-life penalty of patients requiring RF ablation only once.
Figure 1:
Decision tree for treatment of small (≤4-cm) unilateral RCC with NSS versus RF ablation. Therapeutic strategies are shown after decision node (to right of □). Probabilistic outcome is shown after chance node (to right of ○). Terminal nodes (M) signify that Markov model informed by therapeutic effectiveness defines ensuing pathway. We assumed that 80% of tumors could be successfully ablated in one session, and that 20% of tumors would require two sessions; all tumors were considered completely ablated following two sessions. These estimates were made on basis of report of renal tumor RF ablation from our institution (10), recategorized for 4-cm or smaller RCCs.
Markov Model
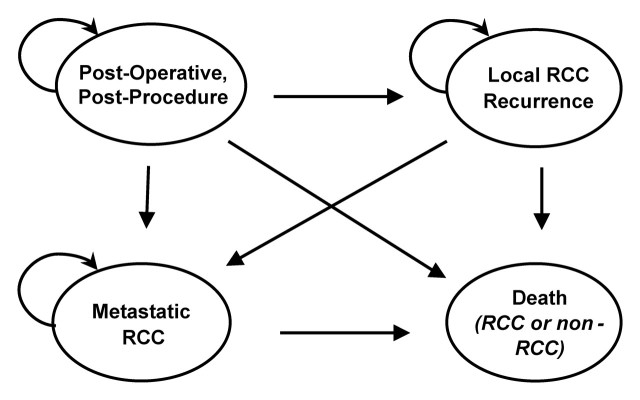
Following treatment, the cohorts' lifetime course was modeled by using a four-state Markov model with a 1-month-long cycle (Fig 2). We constructed this model in keeping with widely accepted methods for Markov modeling in medicine (34).
Figure 2:
Markov model simulates outcomes for 65-year-old cohort following RCC treatment with NSS or RF ablation. Simulated cohort enters “Post-Operative” (post-NSS) or “Post-Procedure” (post–RF ablation) health state. During each 1-month cycle, part of cohort transits to states of “Local RCC Recurrence,” “Metastatic RCC,” and “Death” based on constant transition probabilities (Table 1), until all patients die of RCC or non-RCC causes. Cumulative incurred time and expenses in each health state can be summed, enabling calculation of strategy-specific life expectancy and lifetime costs.
Patients entered the model after either NSS or RF ablation, facing probable development of local RCC recurrence or metastatic disease each month. Patients with local recurrence were subject to development of metastatic disease and patients with metastatic disease were subject to death (RCC related or not). Patients in all other states were also subject to non-RCC mortality.
Model Data and Data Sources
Probability estimates.—Markov health state transition probabilities are included in Table 1. For NSS, the yearly local recurrence probability (0.37%) was derived from a meta-analysis by using an exponential assumption to convert reported mean follow-up time and recurrence rate estimates to a probability (20). Specifically, we used the standard assumption of an exponential relationship between an event probability (p) and hazard rate (r) over a specified time period (t), which can be expressed as p = 1−e−rt (35,36). For the base-case analysis, it was assumed that the yearly probability of local recurrence was 10% higher for RF ablation than for NSS (relative difference). This estimate was made under the presumption that RF ablation may have higher rates of local recurrence in future long-term follow-up studies. This may have created bias against RF ablation—to date, no studies compare RF ablation and NSS directly to show a significant difference in effectiveness.
The probability of direct progression to metastatic disease following tumor treatment also was elicited from the meta-analysis (20) and was assumed to be the same for both. The probability of progression from local recurrence to metastatic RCC was calibrated, given reported cancer-specific mortality estimates associated with local recurrence (37) and metastatic RCC (38). Non-RCC mortality was elicited from United States life tables (2000) (39).
Costs and utilities.—All cost estimates are included in Table 2. Costs were converted to 2006 United States dollars with the use of the medical care component of the Consumer Price Index. A detailed comparison of RF ablation and NSS costs (including operating/procedure room time, personnel, and hospital stay and services) was identified in Lotan and Cadeddu (16); however, this study did not include patients with complications. In a separate study, a comprehensive estimate of NSS inpatient costs, including patients with and without postoperative complications, was reported (40). For our analysis, anticipated RF ablation costs were scaled to this NSS estimate (40) given the relative ratio of uncomplicated RF ablation and NSS costs (16). This yielded an RF ablation cost estimate that accounted for patients with and without complications.
Computed tomographic (CT) surveillance was incorporated at 1-, 3-, and 6-month intervals after RF ablation, and then yearly for 10 years. For NSS, the cost of a CT scan in the first postoperative month was included, and then yearly CT studies were incorporated for 5 years. For years 5–10, CT surveillance occurred every 20 months. Surveillance patterns were modeled according to institutional experience.
Because RCC posttreatment health state costs and utilities have not been well studied to date, the costs and utilities for Markov health states were derived by using colon cancer as a proxy (28,29,41). “Post-Operative, Post-Procedure” and “Metastatic RCC” health state costs were derived from comparable states reported for colon cancer (41). When entering the “Local RCC Recurrence” health state, patients incurred a one-time treatment cost equivalent to that of NSS (40); subsequent costs were the same as the “Post-Procedure, Post-Operative” state (41).
All utility estimates are included in Table 2. Post–RF ablation and post-NSS utilities were scaled to reflect underlying age-specific quality of life (27). Age-specific utility values were elicited from a widely accepted, large community-based study and were specific to sex and ranged from 0.80 to 0.84 for our study population (27). Utilities in the first month following RF ablation and NSS were further scaled to incorporate expected short-term compromises in quality of life. These adjustments were made based on short-term post–RF ablation (0.95) and postsurgical (0.7) utilities reported for modeling RF ablation and surgical treatments of colorectal cancer metastases in the liver (28). For example, a 65-year-old man with an age-specific utility of 0.84 who underwent RF ablation would have a utility of 0.80 (0.84 × 0.95) for the 1 month following RF ablation. “Local RCC Recurrence” and “Metastatic RCC” utilities were determined on the basis of comparable health states for colon cancer (0.67 and 0.25, respectively [29]) (Table 2).
Sensitivity Analysis
Sensitivity analysis was performed to evaluate the effect of model assumptions and parameters on results; sensitivity ranges for each parameter tested are included in Tables 1 and Tables 2. Of note, the probability of local recurrence after RF ablation was varied to determine the threshold level below which RF ablation would be preferred over NSS, given a societal willingness to pay of $75 000 per QALY. Sensitivity analysis included a comparison of costs and quality-adjusted life expectancies associated with RF ablation and NSS when the need for repeat RF ablation was decreased to 0%. Results were considered stable to parameter changes if the preferred strategy remained constant across the range considered. If the preferred strategy changed or became dominated, results were considered sensitive to that parameter.
Model Validation
To assess the validity of the four-state Markov model, a separate Markov model was constructed with the use of independent data (6), and postsurgical life expectancy estimates from the two models were compared. Specifically, a 96.5% 5-year survival probability for RCC tumors 4 cm or smaller reported in one of the largest studies of RCC outcomes, Frank et al (6), was converted to a yearly hazard rate by using the previously described assumption of an exponential relationship between an event probability and hazard rate over a specified time (t) (35,36). This rate was applied to yearly age-dependent all-cause mortality rates for a 65-year-old male cohort to develop a model that yielded a comparison life expectancy estimate.
RESULTS
Cost-effectiveness Analysis Results
Base-case analysis results are summarized in Table 3. The NSS strategy yielded a minimally greater life expectancy (2.5 days), with greater lifetime costs than RF ablation. The ICER of NSS relative to RF ablation ($1 152 529 per QALY) far exceeded assumed societal willingness to pay ($75 000 per QALY).
Table 3.
Base-Case Results for Cost-Effectiveness Analysis: NSS versus RF Ablation
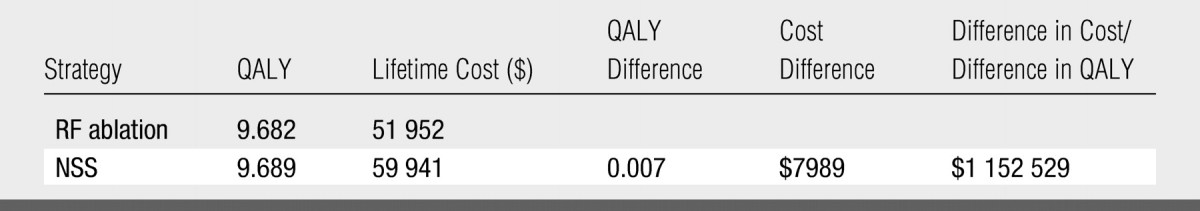
Note.—A discount rate was applied to all future costs and QALYs, as per guidelines from reference 26.
Sensitivity Analysis Results
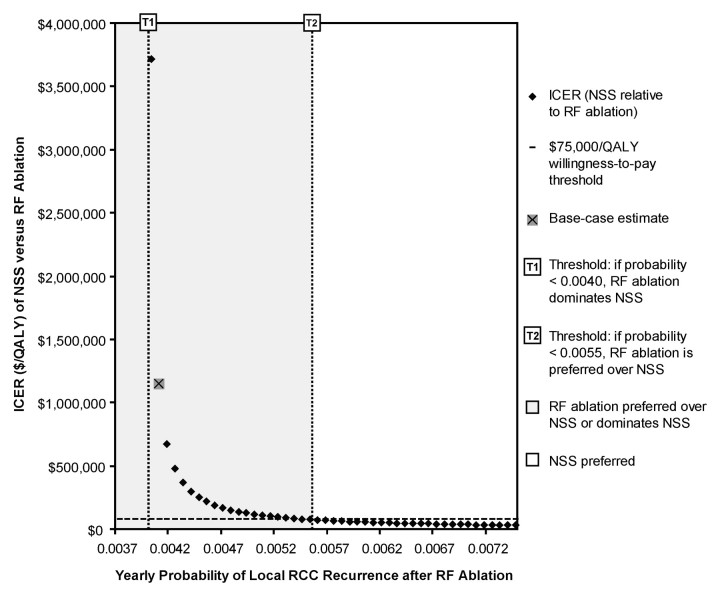
Below an annual post–RF ablation local recurrence probability of 0.0055 (0.55%), RF ablation was preferred over NSS at the $75 000 per QALY threshold level (Fig 3). This probability was 48% higher relative to that associated with NSS (0.0037 [0.37%]) (20). RF ablation strongly dominated NSS below an annual post–RF ablation local recurrence probability of 0.0040 (0.4%), affording a greater quality-adjusted life expectancy at a lower cost (Fig 3).
Figure 3:
ICER of NSS relative to RF ablation versus yearly probability of local RCC recurrence after RF ablation. Given $75 000 per QALY willingness-to-pay threshold level, RF ablation was preferred at base-case estimate (probability = 0.0041 [10% higher relative to NSS]), and for probabilities less than 0.0055 (48% higher relative to NSS). For probabilities less than 0.0040 (7.1% higher relative to NSS), RF ablation dominated NSS and was associated with greater QALYs and lower expense as compared with NSS. For probabilities of 0.0055 or higher, NSS was preferred. Probability range shown corresponds to that tested in sensitivity analysis (Table 1).
RF ablation was preferred for men and women across the age range considered (45–75 years) (Fig 4). For men 70 years and older and women 73 years and older, RF ablation was less expensive and afforded greater quality-adjusted life expectancy, and therefore dominated NSS. These effects were driven by decreased life expectancy with increasing age, resulting in (a) fewer years of (higher) post–RF ablation surveillance costs and (b) increased effect of a lower quality of life immediately after surgery. For each age considered, the ICER of NSS relative to RF ablation for men exceeded that for women, an effect driven by greater life expectancy in women.
Figure 4:
ICER of NSS relative to RF ablation versus age. RF ablation was considered preferred for men and women aged 45–75 years. For men aged 45–69 years and women aged 45–72 years, ICERs of NSS relative to RF ablation exceeded $75 000 per QALY willingness-to-pay threshold level. For men aged 70 years and older and women aged 73 years and older, RF ablation afforded higher quality-adjusted life expectancy at lower cost, and therefore dominated NSS.
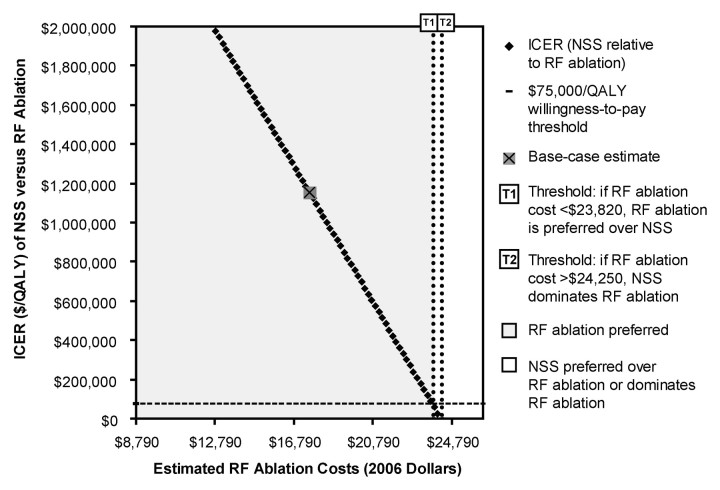
Results were sensitive to short-term RF ablation and NSS costs, and to post-NSS utilities. RF ablation was considered preferred, for RF ablation costs of less than $23 818, at the $75 000 per QALY willingness-to-pay threshold level (Fig 5). For higher RF ablation costs, NSS was preferred; for RF ablation costs of more than $24 251, NSS dominated RF ablation. RF ablation was preferred if NSS costs were more than $23 172. For lower NSS costs, NSS was preferred. If NSS costs were less than $22 650, NSS dominated RF ablation. To summarize, for NSS to be preferred over RF ablation, an estimated NSS cost reduction of $7500 or RF ablation cost increase of $6229 was necessary (relative to NSS and RF ablation base-case cost estimates).
Figure 5:
ICER of NSS relative to RF ablation versus estimated RF ablation costs. RF ablation is considered preferred at both base-case estimate of RF ablation cost ($17 589) and for estimated RF ablation cost (<$23 818). For RF ablation costs $23 818 or higher, NSS is preferred at $75 000 per QALY willingness-to-pay threshold level; for RF ablation costs of more than $24 251, NSS dominates RF ablation. Probability range shown corresponds to that tested in sensitivity analysis (Table 2). Estimated RF ablation costs are in constant 2006 dollars.
RF ablation was preferred for the range of 1-month post-NSS utilities considered. If the 1-month post-NSS utility factor was less than 0.60 (not age adjusted), then RF ablation dominated NSS. Results were stable to changes in short-term post–RF ablation utilities across the range considered.
Results were stable to changes in the percentage of patients who underwent complete tumor ablation (by using imaging criteria) in one RF ablation attempt, across the sensitivity range considered. When the need for repeat RF ablation was reduced from 20% (base-case estimate) to 0%, the ICER of NSS compared with RF ablation increased to $1 699 235 per QALY, increasing the favorability of RF ablation. With a first-attempt RF ablation success rate of 100%, lifetime costs for the RF ablation strategy decreased from $51 952 to $48 401, driving the further increase in the ICER of NSS as compared with RF ablation. The difference in quality-adjusted life expectancy between RF ablation and NSS remained nearly identical (decreasing by <1 day).
Results also were stable to changes in the following parameters: costs and utilities for all long-term (Markov) health states, probabilities of developing metastatic disease from the “Post-Operative/Post-Procedure” or “Local RCC Recurrence” states, the probability of RCC-related death with metastatic disease, and CT costs.
Model Validation
A life expectancy of 15.34 years after NSS was calculated with the four-state Markov model used in this study (without discounting or quality adjustment). A life expectancy of 15.13 years following RCC surgery was calculated by using the Markov model generated from a validation source (6). This difference was 2.6 months (estimates were within 1.4% of each other) (6). For the purpose of this study, this difference was considered adequate for validation of the four-state Markov model.
DISCUSSION
With improvements in both the ability to predict tumor behavior and the range of treatment options available, there is an unparalleled need to readdress cancer treatment paradigms to better match tumor biologic parameters and treatment aggressiveness. For small RCC, often an indolent tumor (5), RF ablation represents a better tolerated, less expensive alternative to NSS (12,16,21). The primary limitation of RF ablation is that long-term outcomes are unknown, including RCC local recurrence rates and long-term survival (12,21). In this study, the best available estimates for RCC outcomes and costs were integrated to compare RF ablation and NSS from a cost-effectiveness standpoint.
For 65-year-old men with small RCC, RF ablation was preferred over NSS at a societal willingness-to-pay threshold level of $75 000 per QALY; the ICER of NSS relative to RF ablation, $1 152 529 per QALY, far exceeded the $75 000 per QALY threshold level. The preference of RF ablation to NSS over the majority of the assumptions and populations considered was driven by the combination of a large lifetime cost discrepancy between the two strategies ($7989) and a relatively minimal difference in quality-adjusted life expectancy (2.5 days). When considering these results, it is important to note that these differences are averaged over a population. In reality, some patients would have no benefit or reduced benefit from the choice of NSS over RF ablation, and others would have a quality-adjusted life expectancy benefit that is higher than 2–3 days.
Life expectancy following RF ablation was calculated with the initial assumption that the annual probability of local recurrence following RF ablation was 10% higher than that of NSS (relative difference). This assumption may have created bias against RF ablation—to date, no studies have directly compared RF ablation and NSS to demonstrate a difference in long-term effectiveness. In sensitivity analysis, RF ablation remained the preferred treatment if the annual post–RF ablation local recurrence probability was up to 48% higher relative to that after NSS. If the post–RF ablation local recurrence probability was within 7% of that after NSS, then RF ablation was both less expensive and yielded higher quality-adjusted life expectancy—the decrease in life expectancy conferred by a minimally higher post–RF ablation recurrence probability was outweighed by superior short-term quality of life after RF ablation.
A few key circumstances could change the relative preference of RF ablation over NSS from a cost-effectiveness standpoint. First, future long-term follow-up studies could find RF ablation to be substantially less effective than NSS. Given current literature, this is unlikely for small, peripheral RCC, a tumor subset easily amenable to RF ablation (10,12,21). Furthermore, as a newly developed procedure, methods of improving RF ablation effectiveness are continually evolving. For example, concurrent tumor RF ablation and subselective renal arterial embolization may improve future effectiveness in selected settings; however, further research to better define potential additive effects of RF ablation and arterial embolization is necessary (42,43).
Second, future RF ablation and NSS costs could converge. In this analysis, results were sensitive to short-term RF ablation and NSS costs, and therefore to the relative difference in NSS and RF ablation costs. A dedicated cost analysis that incorporates both NSS and RF ablation immediate costs and their associated complications has not been reported to date and will be essential for further related cost-effectiveness research. However, as further RF ablation experience is gained, costs will likely decrease, owing to expected decreased equipment costs and reduced hospital stays. As such, convergence of RF ablation and NSS costs is unlikely.
Third, future reports of RF ablation complications may be greater than predicted, which would influence both RF ablation costs and effectiveness. This possibility is not supported by relevant literature to date (7,21,44). A multi-institutional review of NSS reported a complication rate of 13.7% (155 of 1129 cases) (7,21). A separate multi-institutional review of RF ablation reported a complication rate of 8.3% (11 of 132 cases) (21,44). To date, patients who have undergone RF ablation have carried higher morbidity than have typical patients with small RCC, because poor operative candidacy has remained a major determinant of RF ablation referral. It is more likely that for a typical population of patients with small RCC, the complication rate will be even lower.
The primary limitation of this study relates to the requisite use of simplifications to reduce the complexity of human disease into a biologically and clinically plausible model. This limitation has been addressed in two ways: (a) inclusion of the most relevant patient and cohort characteristics, health states, and outcomes; and (b) evaluation of uncertainty inherent in model inputs and assumptions through sensitivity analysis.
Results were robust to substantial variability in the majority of model parameters, including long-term health state costs and utilities, and estimates extrapolated from the colon cancer literature (29,41). Results were sensitive to the relative probabilities of post–RF ablation and post-NSS local recurrence, and to short-term RF ablation and NSS costs and post-NSS quality of life. By extrapolation, results may be considered sensitive to relative differences in short-term RF ablation and NSS costs and quality of life. To date, one study has shown RF ablation to have favorable postprocedural quality of life compared with surgery, although patient utilities, a key metric for cost-effectiveness analysis, were not elicited (45). Further primary investigation should target these parameters that are most influential to long-term cost-effectiveness outcomes.
This study focused on percutaneous RF ablation and open NSS. Laparoscopic NSS, used increasingly for small RCC in some centers, was not specifically evaluated because fewer long-term follow-up data are available (46,47). Lotan and Cadeddu (16) reported the cost of laparoscopic NSS to be only $754 less than that of open NSS. Assuming that the long-term effectiveness of laparoscopic and open NSS are similar, ICERs of laparoscopic NSS relative to RF ablation would similarly greatly exceed the $75 000 per QALY willingness-to-pay threshold level. Other promising ablative therapies for which further experience is needed, such as cryoablation and high-intensity focused ultrasonography, are likely to have cost and effectiveness profiles similar to those of RF ablation, although insufficient data were available for their full evaluation in this analysis (48,49).
This study represents one of the first analyses of RCC treatment outcomes from decision-analytic and cost-effectiveness perspectives. Ideally, cost-effectiveness analysis should precede diffusion of a new technology such as RF ablation to routine care settings. This practice serves to (a) determine whether a new technology is likely to result in favorable and affordable health outcomes relative to existing care paradigms and (b) identify efficacy and cost factors most critical to its future viability.
The results of this study support the future viability of RF ablation for small RCC from a cost-effectiveness perspective. Further model refinements are necessary prior to use of our analysis for policy-level decision-making. Primary data collection is necessary to inform long-term RF ablation effectiveness, and relevant short-term cost and quality of life parameters identified to be most critical to model results. RCC diagnostic uncertainty must be incorporated, including risks of false-positive and false-negative pre-RF ablation biopsy results. Nonetheless, our analysis establishes the potential for RF ablation as a routine alternative to NSS for small RCC from a cost-effectiveness perspective, and identifies future research priorities that will be most influential to defining its role in routine renal tumor therapy.
ADVANCES IN KNOWLEDGE
-
•.
When percutaneous radiofrequency (RF) ablation and nephron-sparing surgery (NSS) were compared for patients with small renal cell carcinoma (RCC) by using decision-analytic techniques, RF ablation was preferred from a cost-effectiveness perspective.
-
•.
Long-term outcomes following RF ablation remain unknown owing to its recent implementation, however, the annual probability of local RCC recurrence following RF ablation would need to be more than 48% higher relative to that of NSS for NSS to be preferred from a cost-effectiveness perspective.
IMPLICATION FOR PATIENT CARE
-
•.
This analysis establishes the potential for RF ablation as an alternative to NSS for small RCC treatment from a cost-effectiveness perspective, and identifies future research priorities that will be most influential to defining the role of RF ablation in renal tumor therapy.
The results of this study support the future viability of radiofrequency ablation for small renal cell carcinoma from a cost-effectiveness perspective.
Footnotes
Author contributions: Guarantor of integrity of entire study, P.V.P.; study concepts/study design or data acquisition or data analysis/interpretation, all authors; manuscript drafting or manuscript revision for important intellectual content, all authors; approval of final version of submitted manuscript, all authors; literature research, P.V.P.; experimental studies, all authors; statistical analysis, P.V.P., C.H., G.S.G.; and manuscript editing, all authors
References
- 1.American Cancer Society. Cancer facts and 2007 figures 2007. Atlanta, Ga: American Cancer Society, 2007.
- 2.Chow WH, Devesa SS, Warren JL, Fraumeni JF Jr. Rising incidence of renal cell cancer in the United States. JAMA 1999;281:1628–1631. [DOI] [PubMed] [Google Scholar]
- 3.Devesa SS, Silverman DT, McLaughlin JK, Brown CC, Connelly RR, Fraumeni JF Jr. Comparison of the descriptive epidemiology of urinary tract cancers. Cancer Causes Control 1990;1:133–141. [DOI] [PubMed] [Google Scholar]
- 4.Jayson M, Sanders H. Increased incidence of serendipitously discovered renal cell carcinoma. Urology 1998;51:203–205. [DOI] [PubMed] [Google Scholar]
- 5.Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst 2006;98:1331–1334. [DOI] [PubMed] [Google Scholar]
- 6.Frank I, Blute ML, Leibovich BC, Cheville JC, Lohse CM, Zincke H. Independent validation of the 2002 American Joint Committee on cancer primary tumor classification for renal cell carcinoma using a large, single institution cohort. J Urol 2005;173:1889–1892. [DOI] [PubMed] [Google Scholar]
- 7.Uzzo RG, Novick AC. Nephron sparing surgery for renal tumors: indications, techniques and outcomes. J Urol 2001;166:6–18. [PubMed] [Google Scholar]
- 8.Novick AC Nephron-sparing surgery for renal cell carcinoma. Annu Rev Med 2002;53:393–407. [DOI] [PubMed] [Google Scholar]
- 9.Gervais DA, Arellano RS, McGovern FJ, McDougal WS, Mueller PR. Radiofrequency ablation of renal cell carcinoma. II. Lessons learned with ablation of 100 tumors. AJR Am J Roentgenol 2005;185:72–80. [DOI] [PubMed] [Google Scholar]
- 10.Gervais DA, McGovern FJ, Arellano RS, McDougal WS, Mueller PR. Radiofrequency ablation of renal cell carcinoma. I. Indications, results, and role in patient management over a 6-year period and ablation of 100 tumors. AJR Am J Roentgenol 2005;185:64–71. [DOI] [PubMed] [Google Scholar]
- 11.McDougal WS, Gervais DA, McGovern FJ, Mueller PR. Long-term followup of patients with renal cell carcinoma treated with radio frequency ablation with curative intent. J Urol 2005;174:61–63. [DOI] [PubMed] [Google Scholar]
- 12.Schiller JD, Gervais DA, Mueller PR. Radiofrequency ablation of renal cell carcinoma. Abdom Imaging 2005;30:442–450. [DOI] [PubMed] [Google Scholar]
- 13.Zagoria RJ, Traver MA, Werle DM, Perini M, Hayasaka S, Clark PE. Oncologic efficacy of CT-guided percutaneous radiofrequency ablation of renal cell carcinomas. AJR Am J Roentgenol 2007;189:429–436. [DOI] [PubMed] [Google Scholar]
- 14.Mayo-Smith WW, Dupuy DE, Parikh PM, Pezzullo JA, Cronan JJ. Imaging-guided percutaneous radiofrequency ablation of solid renal masses: techniques and outcomes of 38 treatment sessions in 32 consecutive patients. AJR Am J Roentgenol 2003;180:1503–1508. [DOI] [PubMed] [Google Scholar]
- 15.Breen DJ, Rutherford EE, Stedman B, et al. Management of renal tumors by image-guided radiofrequency ablation: experience in 105 tumors. Cardiovasc Intervent Radiol 2007;30:936–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lotan Y, Cadeddu JA. A cost comparison of nephron-sparing surgical techniques for renal tumour. BJU Int 2005;95:1039–1042. [DOI] [PubMed] [Google Scholar]
- 17.Kletscher BA, Qian J, Bostwick DG, Andrews PE, Zincke H. Prospective analysis of multifocality in renal cell carcinoma: influence of histological pattern, grade, number, size, volume and deoxyribonucleic acid ploidy. J Urol 1995;153:904–906. [PubMed] [Google Scholar]
- 18.Butler BP, Novick AC, Miller DP, Campbell SA, Licht MR. Management of small unilateral renal cell carcinomas: radical versus nephron-sparing surgery. Urology 1995;45:34-40; discussion 40–41. [DOI] [PubMed] [Google Scholar]
- 19.Lerner SE, Hawkins CA, Blute ML, et al. Disease outcome in patients with low stage renal cell carcinoma treated with nephron sparing or radical surgery. J Urol 1996;155:1868–1873. [PubMed] [Google Scholar]
- 20.Manikandan R, Srinivasan V, Rane A. Which is the real gold standard for small-volume renal tumors? radical nephrectomy versus nephron-sparing surgery. J Endourol 2004;18:39–44. [DOI] [PubMed] [Google Scholar]
- 21.McAchran SE, Lesani OA, Resnick MI. Radiofrequency ablation of renal tumors: past, present, and future. Urology 2005;66:15–22. [DOI] [PubMed] [Google Scholar]
- 22.Winkelmayer WC, Weinstein MC, Mittleman MA, Glynn RJ, Pliskin JS. Health economic evaluations: the special case of end-stage renal disease treatment. Med Decis Making 2002;22:417–430. [DOI] [PubMed] [Google Scholar]
- 23.Goldman L Cost-effectiveness in a flat world: can ICDs help the United States get rhythm? N Engl J Med 2005;353:1513–1515. [DOI] [PubMed] [Google Scholar]
- 24.Neumann PJ, Rosen AB, Weinstein MC. Medicare and cost-effectiveness analysis. N Engl J Med 2005;353:1516–1522. [DOI] [PubMed] [Google Scholar]
- 25.Gillick MR Medicare coverage for technological innovations: time for new criteria? N Engl J Med 2004;350:2199–2203. [DOI] [PubMed] [Google Scholar]
- 26.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA 1996;276:1253–1258. [PubMed] [Google Scholar]
- 27.Fryback DG, Dasbach EJ, Klein R, et al. The Beaver Dam Health Outcomes Study: initial catalog of health-state quality factors. Med Decis Making 1993;13:89–102. [DOI] [PubMed] [Google Scholar]
- 28.Gazelle GS, McMahon PM, Beinfeld MT, Halpern EF, Weinstein MC. Metastatic colorectal carcinoma: cost-effectiveness of percutaneous radiofrequency ablation versus that of hepatic resection. Radiology 2004;233:729–739. [DOI] [PubMed] [Google Scholar]
- 29.Ness RM, Holmes AM, Klein R, Dittus R. Utility valuations for outcome states of colorectal cancer. Am J Gastroenterol 1999;94:1650–1657. [DOI] [PubMed] [Google Scholar]
- 30.Gyrd-Hansen D Willingness to pay for a QALY: theoretical and methodological issues. Pharmacoeconomics 2005;23:423–432. [DOI] [PubMed] [Google Scholar]
- 31.Hirth RA, Chernew ME, Miller E, Fendrick AM, Weissert WG. Willingness to pay for a quality-adjusted life year: in search of a standard. Med Decis Making 2000;20:332–342. [DOI] [PubMed] [Google Scholar]
- 32.King JT Jr, Tsevat J, Lave JR, Roberts MS. Willingness to pay for a quality-adjusted life year: implications for societal health care resource allocation. Med Decis Making 2005;25:667–677. [DOI] [PubMed] [Google Scholar]
- 33.Ries LAG, Harkins D, Krapcho M, et al, eds. SEER cancer statistics review, 1975. –2003. Bethesda, Md. National Cancer Institute. http://seer.cancer.gov/csr/1975_2003/. Published November 2005. Accessed April, 2006.
- 34.Beck JR, Pauker SG. The Markov process in medical prognosis. Med Decis Making 1983;3:419–458. [DOI] [PubMed] [Google Scholar]
- 35.Beck JR, Kassirer JP, Pauker SG. A convenient approximation of life expectancy (the “DEALE”). I. Validation of the method. Am J Med 1982;73:883–888. [DOI] [PubMed] [Google Scholar]
- 36.Beck JR, Pauker SG, Gottlieb JE, Klein K, Kassirer JP. A convenient approximation of life expectancy (the “DEALE”). II. Use in medical decision-making. Am J Med 1982;73:889–897. [DOI] [PubMed] [Google Scholar]
- 37.Itano NB, Blute ML, Spotts B, Zincke H. Outcome of isolated renal cell carcinoma fossa recurrence after nephrectomy. J Urol 2000;164:322–325. [PubMed] [Google Scholar]
- 38.Surveillance Epidemiology and End Results (SEER) Program Public-Use Data, 1973–2004. Bethesda, Md. National Cancer Institute. http://seer.cancer.gov/data/. Published April 2006. Accessed April, 2007.
- 39.Centers for Disease Control, National Center for Health Statistics. LEWK3 United States Life Tables 1999–2003. http://www.cdc.gov/nchs/datawh/statab/unpubd/mortabs/lewk3_10.htm. Accessed April, 2006.
- 40.Shekarriz B, Upadhyay J, Shekarriz H, et al. Comparison of costs and complications of radical and partial nephrectomy for treatment of localized renal cell carcinoma. Urology 2002;59:211–215. [DOI] [PubMed] [Google Scholar]
- 41.Taplin SH, Barlow W, Urban N, et al. Stage, age, comorbidity, and direct costs of colon, prostate, and breast cancer care. J Natl Cancer Inst 1995;87:417–426. [DOI] [PubMed] [Google Scholar]
- 42.Chang I, Mikityansky I, Wray-Cahen D, Pritchard WF, Karanian JW, Wood BJ. Effects of perfusion on radiofrequency ablation in swine kidneys. Radiology 2004;231:500–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamakado K, Nakatsuka A, Kobayashi S, et al. Radiofrequency ablation combined with renal arterial embolization for the treatment of unresectable renal cell carcinoma larger than 3.5 cm: initial experience. Cardiovasc Intervent Radiol 2006;29:389–394. [DOI] [PubMed] [Google Scholar]
- 44.Johnson DB, Solomon SB, Su LM, et al. Defining the complications of cryoablation and radio frequency ablation of small renal tumors: a multi-institutional review. J Urol 2004;172:874–877. [DOI] [PubMed] [Google Scholar]
- 45.Onishi T, Nishikawa K, Hasegawa Y, et al. Assessment of health-related quality of life after radiofrequency ablation or laparoscopic surgery for small renal cell carcinoma: a prospective study with medical outcomes Study 36-Item Health Survey (SF-36). Jpn J Clin Oncol 2007;37:750–754. [DOI] [PubMed] [Google Scholar]
- 46.Matin SF, Gill IS, Worley S, Novick AC. Outcome of laparoscopic radical and open partial nephrectomy for the sporadic 4 cm. or less renal tumor with a normal contralateral kidney. J Urol 2002;168:1356-1359. [DOI] [PubMed] [Google Scholar]
- 47.Weld KJ, Venkatesh R, Huang J, Landman J. Evolution of surgical technique and patient outcomes for laparoscopic partial nephrectomy. Urology 2006;67:502-506. [DOI] [PubMed] [Google Scholar]
- 48.Stein RJ, Kaouk JH. Renal cryotherapy: a detailed review including a 5-year follow-up. BJU Int 2007;99:1265–1270. [DOI] [PubMed] [Google Scholar]
- 49.Hafron J, Kaouk JH. Ablative techniques for the management of kidney cancer. Nat Clin Pract Urol 2007;4:261–269. [DOI] [PubMed] [Google Scholar]