This article clarifies the relationships among the related but distinct regulatory pathways needed for quantitative imaging biomarker development and dissemination, in addition to describing important modifications to facilitate their adoption.
Abstract
Quantitative imaging biomarkers could speed the development of new treatments for unmet medical needs and improve routine clinical care. However, it is not clear how the various regulatory and nonregulatory (eg, reimbursement) processes (often referred to as pathways) relate, nor is it clear which data need to be collected to support these different pathways most efficiently, given the time- and cost-intensive nature of doing so. The purpose of this article is to describe current thinking regarding these pathways emerging from diverse stakeholders interested and active in the definition, validation, and qualification of quantitative imaging biomarkers and to propose processes to facilitate the development and use of quantitative imaging biomarkers. A flexible framework is described that may be adapted for each imaging application, providing mechanisms that can be used to develop, assess, and evaluate relevant biomarkers. From this framework, processes can be mapped that would be applicable to both imaging product development and to quantitative imaging biomarker development aimed at increasing the effectiveness and availability of quantitative imaging.
© RSNA, 2011
Supplemental material: http://radiology.rsna.org/lookup/suppl/doi:10.1148/radiol.10100800/-/DC1
Introduction
The United States Food and Drug Administration (FDA) launched the Critical Path Initiative (1,2) to accelerate the adoption of new product development and clinical evaluation methods, such as biomarkers, for demonstrating safety and effectiveness. The development of biomarkers for safety and efficacy is also a major theme in the European Innovative Medicines Initiative (3,4). Quantitative imaging methods have the potential to serve as biomarkers, to improve patient care and speed the development of new treatments. Numerous efforts are underway to develop both specimen and in vivo biomarkers (5–7). However, a great deal of work is still needed to achieve efficient implementation, not just in individual research laboratories but ultimately in general use (8). Similar regulatory pathways are required whether devices or drugs are pursued separately, as has historically been the case, or are to be considered together in the concept of drug-diagnostic combinations (9) (sometimes referred to as companion diagnostics).
Potential Conflicts of Interest
Individual company interests are not addressed, but the health of the industry and the effectiveness of working relationships are to the advantage of all stakeholders. B.B. works for Siemens Healthcare; whereas there is no direct financial interest related to Siemens alone, there is industry-wide interest. M.L.G. is a stockholder in R2 Technology/Hologic and receives royalties from Hologic, GE Medical Systems, MEDIAN Technologies, Riverain Medical, Mitsubishi, and Toshiba. S.G. is an employee of GE Global Research. G.M. is a founder and part owner of a lung image analysis software company, VIDA Diagnostics. C.G.M. is employed by BioClinica, a commercial imaging core laboratory that conducts central assessments of radiologic images. B.A.S. is a stockholder in and is on the Medical Advisory Board of Radiology Corporation of America and is on the Senior Scientific Advisory Board of Siemens Molecular Imaging. A.G.S. receives research support from and is a consultant to pharmaceutical companies using imaging to monitor treatment effects and magnetic resonance (MR) imager manufacturers; details are available at www.biomarkers.org.
Definition of Biomarker
The currently accepted definition of a biomarker is “a characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention” (10). This definition carries practical implementation implications, as does the extended definition, “A biomarker is a validated disease characteristic which can be reliably measured in a cost-effective, repeatable and generalizable manner, and which acts as a meaningful surrogate for disease presence, absence, activity, or outcome in individuals or groups with the disease process” (11). These broad definitions include in vitro diagnostic measurements made from biologic fluids (eg, low-density lipoprotein cholesterol), measurements made from solid-tissue pathologic specimens (eg, tumor estrogen receptor status), physiologic and biophysical measurements (eg, electrocardiographic data, blood pressure), and measurements from images (eg, tumor size, tumor pharmacokinetics, rheumatoid erosions).
Biomarkers can be classified as follows:
1. Diagnostic or prognostic (biomarker measurement improves accuracy of patient diagnosis or prognosis)
2. Predictive (biomarker measurement predicts which treatment would be most beneficial or not)
3. Response (change in biomarker after treatment predicts whether treatment will lead to beneficial outcome)
4. Monitoring (regular measurement of quantitative imaging biomarker to detect relapse or emergence of toxicity)
Because biomarkers are useful only if they provide additional accuracy in predicting clinical outcome beyond that which is attained without them, ultimately, objective evidence regarding their relationships with health status must be established (12). Quantitative imaging biomarkers are usually used in concert with other types of biomarkers and with clinical end points (eg, patient-reported outcomes, survival). In addition, imaging and other biomarkers are often essential to the qualification of each other.
In 2009, the Toward Quantitative Imaging task force of the Radiological Society of North America (RSNA) developed a working definition of quantitative imaging (12):
Quantitative imaging is the extraction of quantifiable features from medical images for the assessment of normal or the severity, degree of change, or status of a disease, injury, or chronic condition relative to normal. Quantitative imaging includes the development, standardization, and optimization of anatomical, functional, and molecular imaging acquisition protocols, data analyses, display methods, and reporting structures. These features permit the validation of accurately and precisely obtained image-derived metrics with anatomically and physiologically relevant parameters, including treatment response and outcome, and the use of such metrics in research and patient care.
The FDA makes a distinction between approval (or clearance) of a diagnostic drug or device for commercialization purposes and qualification of a measurement itself for use as a biomarker in clinical trials. Approval is based on a demonstration of safety and effectiveness. The criteria for effectiveness are different for devices, which are approved by the FDA Center for Devices and Radiological Health (CDRH), versus drugs, which are approved by the FDA Center for Drug Evaluation and Research (CDER), because their relative risks are generally considered to be different. Furthermore, the criteria for effectiveness will depend on the labeling claim that the sponsor is proposing. (See Appendix E1 [online] for an example use of the pathways.)
Qualification is a measure of the use of a biomarker in a specific context (13):
That context may be selecting or deselecting people for a clinical trial, monitoring drug-induced toxicity, or some other purpose. The amount of evidence needed to qualify a biomarker for a given purpose is related to the consequences of using the result to make decisions, such as whether to pursue the development of a drug or whether to withhold a drug from individuals in a clinical trial. Qualification requires context-specific measurement of the performance of the biomarker in relation to an outcome or outcomes of interest.
The FDA uses the term validation to refer to the analytic or technical (design) performance of a test or device. Demonstrating technical performance requires a stable platform and the establishment of standards that facilitate the linking of results across performance sites. Validation also requires study of variability among users and among performance sites. Both approval and qualification require prior validation, but validation is not sufficient to achieve approval or qualification.
Because the purposes of approval and qualification are different, and because the pathways to achieve them within the FDA are different, it is possible for devices capable of elucidating a quantitative imaging biomarker to be approved but the quantitative imaging biomarker elucidated by them not to be qualified, or conversely for a quantitative imaging biomarker to be qualified but not supported by products approved for market. It may also not be evident what data are needed to support these different pathways most efficiently, given the time- and cost-intensive nature of data collection. It is also not always clear which clinical trial design is best for a given purpose (14), nor has a mechanism been in place to allow data from activities related to the use of medical imaging in clinical trials (ie, qualification) be used to directly support regulatory labeling changes for clinical care (ie, claims). If there were clearer processes and precedents to allow parties to reuse data from one pathway to another, it would substantially enhance the value proposition for individual stakeholders and very likely speed the time-to-market of qualified methodologies (15).
The need to clarify and streamline the regulatory pathways is a critical issue for the diagnostic device and drug industry because their financial return on investment is often not favorable in the current model of discovery, development, approval, and commercialization. Imaging test discovery and development, followed by technical validation and approval, are generally pursued by manufacturers, whereas quantitative imaging biomarker qualification is usually undertaken by technology users such as practicing physicians and medical researchers. If the groups and data involved in these two activities (approval and qualification) could be brought into closer alignment, the efficiencies could translate into better diagnostic products in the health care marketplace.
The regulatory organizations that approve imaging tests, those that qualify quantitative imaging biomarkers, and those that make payment decisions have different objectives. Interagency coordination is needed to provide clear and efficient pathways. Regulatory issues are even more complex when other jurisdictions are considered, an important consideration for device and contrast agent manufacturers, who may need to recoup their investments through global sales.
The purpose of this article is to suggest an approach to minimize these hurdles. Our descriptions of regulatory pathways and procedures are drawn from FDA Guidances and other published articles, from experiences of many of the authors, and from discussions with FDA staff members. However, the opinions expressed in this article do not represent official FDA policy. Terms and abbreviations are summarized in Appendix E2 (online).
Process Maps
In November 2006, the RSNA convened a group of stakeholders to advise it on what role it could most constructively play with regard to imaging biomarkers. The RSNA subsequently initiated, and continues to sponsor, multiple initiatives to promote the objective extraction of quantitative information from clinical images (16). It sees this approach as the appropriate one to stimulate the necessary groundwork to support use of imaging as a biomarker (6,7,16). The initial focus has been on imaging in clinical trials, with the intent to generalize to imaging in clinical care. The Quantitative Imaging Biomarkers Alliance arose from a public session at the 2007 RSNA Annual Meeting. It first met as a group in May 2008. The Imaging Biomarkers Roundtable has met once or twice annually for the past 2 years. Together, members have built on previous work to develop consensus positions on process maps for both imaging devices and contrast agents for application in clinical care and clinical trials. These maps provide a flexible framework that may be adapted for each biomarker, providing a mechanism to develop, assess, and evaluate them. We consider here only imaging biomarkers that require an element of quantitation and standardization to allow useful within-patient or between-patient comparison. The activities focused on in this discussion are subsequent to the initial discovery and development of imaging techniques, the steps of which are generally well described in the literature (17).
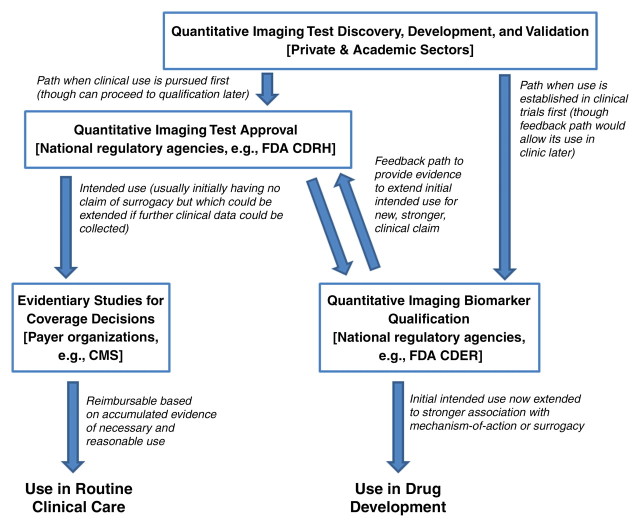
The proposed relationships among four related, but semi-independent, activities are shown in Figure 1.
Figure 1:
Flowchart shows interrelated activities, with feedback. From a regulatory point of view, the introduction of new technology solutions for medical purposes is generally considered as applying to either clinical research or clinical practice. In the United States, these activities generally follow different requirements and are administered by different departments within the agency. Quantitative imaging is relevant to both clinical research and clinical practice, but in the absence of an organized effort to do so it is generally difficult to utilize evidence across both of these uses owing to differences in expectations and requirements related to the collection and interpretation of evidence. To meet constraints imposed in both the use and the business models of sponsors, it may be possible to organize efforts that enable efficient and effective study designs to address multiple needs from collective activities. CMS = Centers for Medicare and Medicaid Services.
Quantitative imaging test approval is an extension of the general concept of “design validation” for the commercialization of a medical device intended to perform a quantitative imaging test. The FDA Quality System Regulation 820.3 indicates that “Design validation shall ensure that devices conform to defined user needs and intended uses and shall include testing of production units under actual or simulated use conditions” (18). Design validation is undertaken by medical physicists, biochemists, and engineers in collaboration with physicians.
Quantitative imaging biomarkers may require the use of any combination of the following: (a) an approved device used in a novel way, (b) an investigational device, (c) an approved imaging agent used in a novel way (ie, off label), and (d) an investigational imaging agent.
Hardware and software considerations usually dominate when an endogenous contrast mechanism is exploited or an approved exogenous imaging agent (eg, radionuclide tracers such as fluorine 18 [18F] fluorodeoxyglucose [FDG], iodinated x-ray or computed tomography [CT] contrast agents, gadolinium chelate–based contrast agents for MR imaging, microbubble contrast agents for ultrasonography) is used. If an investigational exogenous contrast agent is required, the potential toxicologic or radiation biologic risks of the tracer become primary considerations in developing a quantitative imaging biomarker.
To optimize a methodology, a thorough understanding of the physical and biochemical basis of the imaging measurement is essential. However, to design a quantitative imaging biomarker that is fit for a purpose, its intended purpose must be clearly specified. Specifically, exploratory imaging tests are developed, in part, through use of the Investigational Device Exemption mechanism of the FDA CDRH for imaging hardware and software components or through use of the Investigational New Drug mechanism of the FDA CDER for imaging agents.
If an existing device is already on the market (referred to as a predicate device) for the claimed intended use of an imaging test and the risk of the device is deemed moderate, clearance is pursued according to Section 510(k) of the Food, Drug, and Cosmetic Act (19). If, however, the risk of the device is not moderate or if there is no extant predicate device, then additional steps are necessary. When there is a predicate device, but the risk is considered more than moderate, a premarket approval (PMA) is required (20). When the risk is considered moderate but there is no predicate device, a de novo 510(k) is required, possibly following a 513(g) request for classification. Level of risk is based on intended use. If there is no predicate and the use is considered high risk, then a PMA is needed. Likewise, if a sponsor takes a product cleared for a general use to a specific use, CDRH can and may ask for more clinical data, as in the example of uterine artery embolization claims for general embolic agents. For the combination of a new agent (investigational new drug) with an established imaging test, there may still be a need for an investigational device exemption or PMA if the resulting application is a new claim for an existing imaging method.
The quantitative imaging biomarker qualification pathway starts with an imaging test that has undergone technical validation and has been approved for use under some initial claim. The initial intended use need not indicate a mechanism of action and generally does not make a “strong” claim of clinical relevance, pending the accumulation of clinical data.
Imaging tests are sometimes referred to as imaging assays to emphasize a similarity with their nonimaging counterparts and may be utilized in different ways. “Integral assays refer to tests that must be performed for the trial to proceed, whereas integrated assays include assays that will be performed on all samples or cases (for imaging studies) but are not required for the trial to proceed and will not inform treatment decisions or actions within the current trial” (21). Integral assays are typically associated with inclusion criteria or primary end points, while integrated assays are typically associated with secondary end points or exploratory analyses. The requirements for integrated assays are generally less restrictive (12), but they should also be handled as rigorously as practical, since they may, in fact, become integral at some future point.
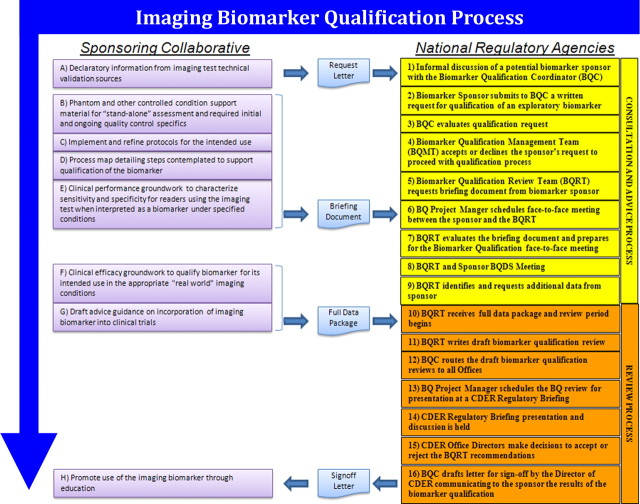
We utilize concepts and language from the current FDA process for the qualification of biomarkers (22–24) to make clear the specific steps necessary for a sponsoring collaborative to use it for qualification of putative quantitative imaging biomarkers. This is shown in Figure 2.
Figure 2:
Chart shows quantitative imaging biomarker qualification process. A detailed following of the FDA process is shown, where steps outlined on the right are steps within the regulatory agency and steps outlined on the left are steps that a collaborative activity of industrial sponsors, practicing clinicians, and academic stakeholders may take to pursue biomarker qualification. Processes in other geographic areas such as Europe and Asia differ somewhat, but cooperative activities across the agencies have worked to minimize differences and ideally allow parallel or near parallel pursuit in multiple countries at the same time. BQDS = Biomarker Qualification Data Submission.
In Figure 2, actions by the sponsor are indicated on the left. Actions by national regulatory agencies (eg, FDA, European Medicines Agency) are indicated on the right, and documents used to facilitate the communication are indicated in the center. It should be noted that the sponsor in this schematic could be a collaborative enterprise rather than a single commercial entity, to reflect the multi-stakeholder nature of the activity.
Evidentiary Studies for Coverage Decisions
Data from clinical trials are necessary for technical validation and approval of imaging tests and quantitative imaging biomarker qualification. Payer coverage decisions are often focused on comparative analysis of published clinical trial reports. The time delay inherent in large randomized clinical trials is often perceived to be an impediment to dissemination of useful medical products or procedures. Consequently, the research community has recently been exploiting observational studies that can generate large amounts of data at reasonable cost and that can be used to inform coverage decisions that support public policy (25). However, there are controversies about the conclusions that can be drawn from observational trials because of the effects of unknown and uncontrolled biases.
Large clinical studies and trials are expensive and lengthy. The use of quantitative imaging biomarkers, both to shorten drug development and to more effectively monitor treatment efficacy, would be in the financial interests of payers and thus be worthy of reimbursement (26). This in turn provides justification for sponsors to invest in their development.
Steps for Imaging Test Approval
When an imaging test reaches the point where its utility has been demonstrated in well-controlled settings, whether by an individual sponsor or by a collaboration, the following steps must be completed prior to commercialization (27,28):
1. Make available the following declaratory information from the discovery and initial development phases: (a) Statement of the claimed intended use of the imaging test, which must be clearly articulated before initiating technical validation studies so that appropriate data are generated to support that use. (b) Summary of current Good Manufacturing Practices issues as they relate to producing the imaging test. (c) Adherence to quality system requirements for the devices and software used to perform the imaging test (29). These should ideally be as generic as possible, and not be restricted to one particular brand of device or software. (d) Assurance that the device is reasonably safe—that is, that the benefits of the procedure (either to the individual patient or to medical knowledge as a whole) outweigh the risks to the patient. Safety includes the consequences of false-positive and false-negative results for the patient, as well as how diagnostic testing can harm a patient (either in the short term or the long term) (eg, ionizing radiation, adverse contrast agent reactions, complications arising from the imaging procedure). (e) Imaging type and volume and method of delivery of any imaging agents. Note that there is a difference between imaging modalities that require 100% agent or tracer use, such as nuclear medicine modalities, and those that use an enhancement agent only for a portion of studies. This may affect the choice of comparator and reference standard, thus complicating the design of clinical studies. The choice of imaging agents should ideally be as generic as possible (eg, several gadolinium chelates permitted) and not be restricted to one particular tracer unless this is scientifically justified.
2. Acquire or develop reference object(s) (phantoms) and other support material(s) for controlled experimentation and ongoing quality control (QC). This serves as the basis for “stand-alone” assessment (with the caveat that phantoms can never replicate in vivo imaging conditions, nor can they adequately cover the variety of imaging applications at reasonable cost). Well-controlled and defined reference objects, however, enable study design and allow planning for statistical analysis of the data. (a) For maximum applicability and impact, both healthy and disease states must be reflected in both objects and digital reference data sets across the range expected in the full target population. (b) Implementation of a comprehensive QC program, including the process for initial acceptance of an imaging device, as well as required ongoing QC procedures, data analysis, and reporting requirements.
3. Implement and refine protocols that include recommended operating points for acquisition, analysis, interpretation, and QC, according to the documented intended use, and develop and merge training and test data sets from various sources to support the technical performance of the imaging test. (a) Algorithms included in the imaging test for data and results interpretation must be prespecified before the study data are analyzed. Alterations of the algorithm to better fit the data are generally not acceptable and may invalidate a study. (b) High-level descriptions of the processing hardware and software, highlighting potential weaknesses, should be provided.
4. Define and iteratively refine the imaging test and its protocol, including assessment of intrinsic imaging unit variability, minimum detectable change, and other aspects of imaging test performance in controlled conditions. Include subject variability associated with the physiologic and pathophysiologic processes for which such imaging tests are being developed. (a) Sources of imaging test variability should be evaluated through studies that characterize reproducibility, limits of detection, and limits of quantification. (b) Prospective trials or collections of samples may sometimes be necessary for certain intended-use claims or to exclude biases that could arise from the use of existing images. (c) Data to support proposed cut points (ie, decision thresholds), if imaging results are not reported as a continuous variable, and performance characteristics (including sensitivity, specificity, and accuracy) are reported to complete this step.
5. Assess intra- and interreader (consistent with intended use) test-retest statistics in real operating conditions: To show the clinical performance of an imaging test, the sponsor generally needs to provide performance data for a properly-sized data set, acquired with a validated system that represents a true patient population in which the test will be used. For most novel devices or imaging agents, this is the pivotal clinical study that will establish whether performance is adequate.
Steps to Quantitative Imaging Biomarker Qualification
To facilitate the qualification, a sponsoring collaborative may be used to leverage efforts. The sponsoring collaborative seeks to:
1. Make available the following declaratory information from the Imaging Test Approval process described above: (a) a mechanistic understanding or “rationale” of the role of the feature(s) assessed with the imaging test in healthy and disease states. (b) A statement of value to stakeholders (eg, patients, manufacturers, biopharmaceutical companies) expressed in the context of the alternatives (eg, with explicit reference to methods that are presently used in lieu of the proposed biomarker) as opposed to in an “absolute” sense that may appear arbitrary. (c) Statements indicating whether the test is quantitative, semiquantitative, or qualitative (descriptive); what platform will be used; what is to be measured; controls; scoring procedures, including the values that will be used (eg, positive vs negative; 1+, 2+ 3+); interpretation; and so forth.
2. Describe phantom and other controlled condition support material for stand-alone assessment and required initial and ongoing QC specifics (as in the Steps for Imaging Test Approval process described above).
3. Implement and refine protocols for the intended use, and develop a process map detailing the steps in consideration to support qualification of the quantitative imaging biomarker. (a) Detailed descriptions of the procedures to be used for image acquisition and analysis and interpretation of the quantitative imaging biomarker as a clinical metric should be included. (b) Procedures to be used when results are not interpretable or are discrepant from other known test results must be described; this is especially important for imaging tests used for eligibility or assignment to treatment arms.
4. Develop a process map detailing steps intended to support qualification of the quantitative imaging biomarker. (a) Description of information on the statistical design used to establish the correlation with the clinical parameter of interest should be provided. (b) Steps to determine intrareader test-retest performance across vendors and centers in all relevant operating conditions should also be included.
5. Perform clinical performance groundwork to characterize sensitivity and specificity for readers using the imaging test when interpreted as a biomarker in specified conditions. This will include: (a) Performance of necessary studies to the extent that the literature does not already fully support the process map. These may be retrospective or prospective. (b) Development of a briefing document for the regulatory agency that describes all known evidence accumulated that pertains to the quantitative imaging biomarker’s qualification and that lays out a plan to complete steps to conclude the qualification process. (c) Pursuit of a face-to-face meeting with the regulatory agency Biomarker Qualification Review Team to elicit agency feedback on the clinical performance results, as well as the plan to complete the “full data package.”
6. Perform clinical efficacy groundwork to qualify the quantitative imaging biomarker for its intended use in the appropriate “real world” imaging conditions (30), to include the following: (a) Extension of prior results to interreader variability and test-retest reproducibility in multivendor, multisite settings. (b) Meeting with the Biomarker Qualification Review Team to elicit regulatory agency feedback on the clinical efficacy study results for the purpose of obtaining agency acceptance that the quantitative imaging biomarker becomes known as qualified.
7. Draft guidance on incorporation of the quantitative imaging biomarker into clinical trials.
8. Promote use of the quantitative imaging biomarker through education.
Example Process Maps for Quantitative Imaging Biomarkers Currently of Interest
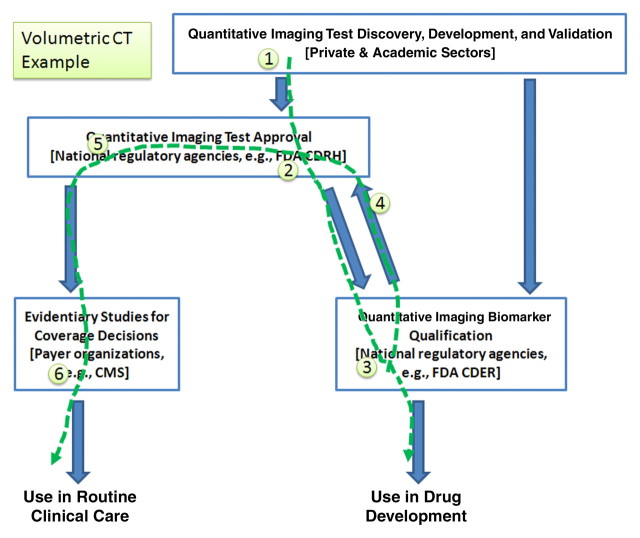
The flow of activity for volumetric image analysis for therapy response in cancer by using CT starts with the current state and extends to the future activities that would exploit the marker (Fig 3).
1. Vendors have developed, and are refining, volumetric CT applications.
2. Many of these solutions have been approved by CDRH, but with weak intended use (no explicit connection with biology or response).
3. A sponsoring collaborative would make a connection to response by qualifying the class of devices for clinical research in an indicated disease setting.
4. These “qualification data” would be available to be contributory as evidence for individual device sponsors as they reregister their products (if they are already a compliant implementation) or as they reengineer them (to become compliant).
5. Given the availability of these data, individual vendors can pursue approval for their volumetric CT products, but now with stronger claims as established in the qualification activity.
6. The qualification data collected would provide the scientific basis for reimbursement.
Figure 3:
Volumetric image analysis with CT serves as an example of how the interrelated activities outlined in Figure 1 may be pursued for this marker. The numbered sequence refers to the chronologic order in which steps may be accomplished, on the basis of the current state of the marker and projecting possible steps that may be undertaken by the community to advance the marker. CMS = Centers for Medicare and Medicaid Services.
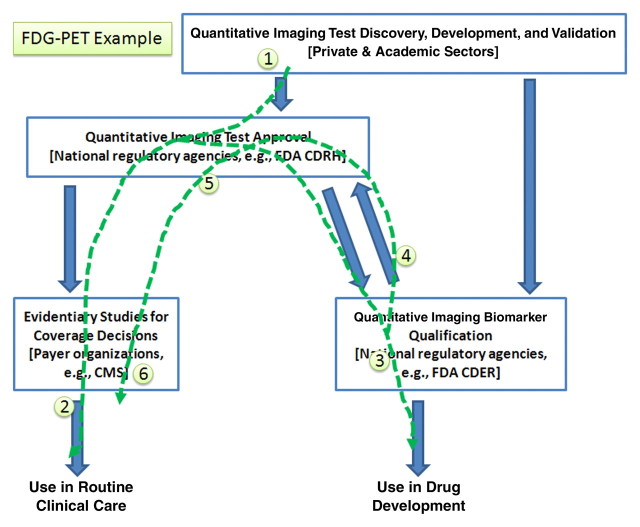
A different situation is represented by 18F-FDG positron emission tomography (PET) (Fig 4). In this case, the flow of activity, starting with the current status and extending to the future activities to exploit the marker, is as follows:
1. Vendors have developed and continue to refine FDG-related products (hardware, software, the agent).
2. Products have been approved by CDRH using the approval pathway, and—based partly on data from the National Oncologic PET Registry–they are reimbursed for clinical care, but only for disease stratification and diagnosis, not in quantitative applications for therapy monitoring.
3. A sponsoring collaborative would qualify the class of devices for clinical research applications by following the qualification pathway.
4. Data collected during the qualification activity, substantiating performance as a response measure, could be referenced by vendors to add therapy monitoring (and thereby expand their market) as a new indicated use (claim). This would be done by establishing compliance with the class by referencing data collected as part of the qualification pathway in the validation pathway.
5. These qualification data would be available to be contributory as evidence for individual device sponsors as they reregister their products (if they are already a compliant implementation) or as they reengineer them (to become compliant).
6. Payers could extend coverage decisions to include therapy monitoring as an additional code for reimbursement.
7. Subsequently, the intended use claims may be extended to additional settings (eg, tumor types or subtypes) and/or for different therapeutic approaches (eg, cytotoxic vs targeted therapy).
Figure 4:
Standardized uptake value in FDG PET serves as an example of how the interrelated activities outlined in Figure 1 may be pursued. This image may be read by itself, as well as compared with Figures 3 and 5 to highlight the differences in sequence that may be pursued by the community as appropriate for each marker. CMS = Centers for Medicare and Medicaid Services.
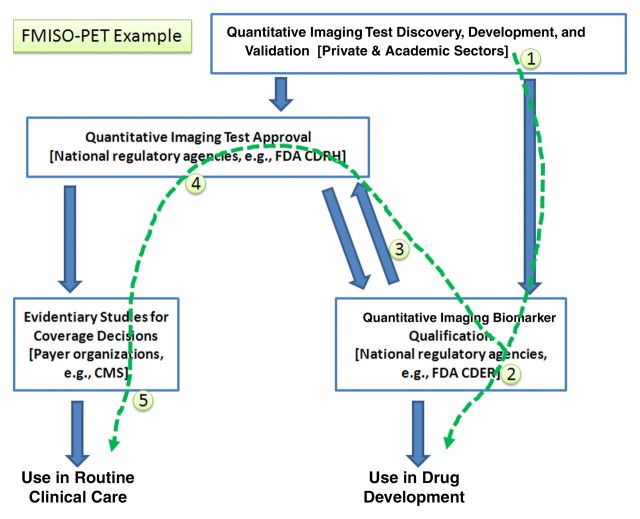
PET with 18F-fluoromisonidazole (FMISO) applied for purposes of measuring tissue hypoxia can be used to illustrate a different scenario (Fig 5). In this case, the complexity of taking the new tracer through qualification is dominant. While there are issues related to the instrumentation and the software and algorithms for analysis, there are certainly going to be issues around the agent and its reliable and reproducible manufacture according to current “good manufacturing process” requirements and validation at different sites, which may be more of an obstacle to disseminated use than the instrumentation aspects. With this understanding, the flow of activity, beginning with the current status and extending to future activities that would exploit the marker, includes:
1. Vendors develop and refine FMISO imaging methods for hypoxia.
2. The first application might be in clinical trials and not clinical care, so qualification would precede approval to market.
3. The qualification data may be used by vendors if they also intend to sell a product for clinical care to efficiently seek approval from the CDRH.
4. Ultimately, payers might make decisions on the basis of already collected qualification data, or with additional collection performed by using a model similar to that used by the National Oncologic PET Registry.
Figure 5:
The use of FMISO PET to evaluate hypoxia serves as a third example highlighting the different sequence of activities that may be pursued, in this case as applying to a marker that has been the subject of considerable interest but that may not be as mature as the examples given in Figures 3 and 4. CMS = Centers for Medicare and Medicaid Services.
Summary
Advances in technology have made imaging an essential component of health care. Many screening, diagnostic, and therapeutic decisions are based on imaging data. When used as biomarkers, imaging technologies have the potential to speed the development of new products to improve patient care. However, to fully realize the potential of medical imaging, quantitative imaging biomarkers ideally must be both approved and qualified.
Whether biomarkers are imaging based or specimen based (obtained from serum, plasma or tissue), we hope that guidance on clear, efficient, and robust processes will pave the way for an increased number of approved and qualified specific biomarkers. In this way, it will be possible to use a variety of important measures to assess how therapies in development might modify disease pathways. Many of these markers will subsequently aid in regulatory review of New Drug Applications and, ultimately, support commercialization of products for clinical practice as stand-alone diagnostics or as diagnostics associated with therapeutics.
By using the steps listed in this article, it is possible to create process maps for regulatory pathways that cover development and clinical evaluation of imaging tests as biomarkers to answer a variety of questions in therapy development such as efficacy; patient selection; pharmacokinetic, pharmacodynamic, and biologic effects; and so forth.
We propose improvements to the current mechanisms. Specifically, we clarify the relationships among the related but distinct pathways of imaging test and quantitative imaging biomarker development and propose two important modifications:
1. Document the steps needed by a collaborative sponsor to qualify quantitative imaging biomarkers under the national regulatory agencies (including the FDA), thereby accelerating the use of quantitative imaging for clinical research.
2. Suggest a feedback path to allow use of data collected to qualify a quantitative imaging biomarker (across a multiplicity of implementations) to be contributory as evidence for individual device sponsors to use in seeking market approval of individual implementations, thereby accelerating commercialization.
These clarifications and additions are needed to unlock the potential of the imaging device and contrast agent industries at this rapidly changing stage of health care evolution.
Next Steps
This article clarifies the relationships among the related but distinct regulatory pathways needed for quantitative imaging biomarker development and dissemination, in addition to describing important modifications to facilitate their adoption. Formal application of these processes and process maps requires both specific pursuit of the examples given here and proactive engagement with regulatory officials. The Quantitative Imaging Biomarker Alliance is presently engaged in these activities and will continue to provide updates to the community as we progress.
Advances in Knowledge.
-
•.
The process of imaging test approval and clearance is related to but distinct from biomarker qualification, but both of these are formal regulatory pathways.
-
•.
The term validation is used to describe multiple activities but is not in itself a regulatory pathway.
-
•.
Quantitative imaging offers considerable capabilities in both drug development and patient care but needs appropriate clinical evidence to be utilized.
-
•.
The biomarker qualification process may be applied to quantitative imaging biomarkers if it is interpreted to encompass terms and activities associated with imaging.
-
•.
Evidence accumulated in one pathway may be contributory to other pathways if accommodation for this is considered early enough to meet the requirements of both intended pathways.
Implications for Patient Care.
-
•.
The promise of quantitative imaging biomarkers for increasing the efficiency of drug development, as well as for creating new models for clinical trial designs, should speed the availability of promising novel therapeutics to patients.
-
•.
The ability to accommodate collection of clinical evidence for devices, software, and contrast agents into stakeholder business models would provide greater incentives for companies to produce and market them for improved patient care.
-
•.
Increasing use of quantitative imaging techniques should make patient care more objective, consistent, and personalized.
Acknowledgments
The complete list of authors is Hugo J. W. L. Aerts, MSc; Bernard Bendriem, PhD; Claus Bendtsen, PhD; Ronald Boellaard, PhD; John M. Boone, PhD; Linda Bresolin, PhD, MBA, CAE; Andrew J. Buckler, MS; Patricia E. Cole, PhD, MD; James J. Conklin, MD, MS; Gary S. Dorfman, MD; Pamela S. Douglas, MD; N. Reed Dunnick, MD; Willy Eidsaunet, BA, BM; Cathy Elsinger, PhD; Richard A. Frank, MD, PhD, FFPM; Constantine Gatsonis, MD; Maryellen L. Giger, PhD; Sandeep N. Gupta, PhD; David Gustafson, PhD; Otto S. Hoekstra, MD, PhD; Edward F. Jackson, PhD; Lisa Karam, PhD; Gary J. Kelloff, MD; Paul E. Kinahan, PhD; Geoffrey McLennan, MB, BS, FRACP, PhD; Colin G. Miller, CR, CSci; P. David Mozley, MD; Keith E. Muller, PhD; Rick Patt, MD; David Raunig, PhD; Mark Rosen, MD, PhD; Haren Rupani, MD; Lawrence H. Schwartz, MD; Barry A. Siegel, MD; A. Gregory Sorensen, MD; Daniel C. Sullivan, MD; Richard L. Wahl, MD; John C. Waterton, PhD; Walter Wolf, PhD; Gudrun Zahlmann, PhD; and Brian Zimmerman, PhD.
Author affiliations: Maastricht University, Maastricht, the Netherlands (H.J.W.L.A.); Siemens Healthcare, Knoxville, Tenn (B.B.); Merck Sharp and Dohme, Rome, Italy (C.B.); VU University Medical Center Amsterdam, the Netherlands (R.B.; O.S.H.); University of California–Davis, Sacramento, Calif (J.M.B.); Radiological Society of North America, Oak Brook, Ill (L.B.); Buckler Biomedical, Wenham, Mass (A.J.B.); Imagepace, Cincinnati, Ohio (P.E.C.); ICON Medical Imaging, Warrington, Pa (J.J.C.); Weill Cornell Medical College, New York, NY (G.S.D.); Duke University, Durham, NC (P.S.D., D.C.S.); University of Michigan, Ann Arbor, Mich (N.R.D.); IC Contrast, Oslo, Norway (W.E.); NordicNeuroLab, Milwaukee, Wis (C.E.); GE Healthcare, Princeton, NJ (R.A.F.); Brown University, Providence, RI (C.G.); University of Chicago, Chicago, Ill (M.L.G.); GE Healthcare, Niskayuna, NY (S.N.G.); Intio, Broomfield, Colo (D.G.); University of Texas M. D. Anderson Cancer Center, Houston, Tex (E.F.J.); National Institute of Standards and Technology, Gaithersburg, Md (L.K., B.Z.); National Cancer Institute, Colorado Springs, Colo (G.J.K.); University of Washington, Seattle, Wash (P.E.K.); University of Iowa, Iowa City, Iowa (G.M.); Bioclinica, Newtown, Pa (C.G.M.); Merck, West Point, Pa (P.D.M.); University of Florida, Gainesville, Fla (K.E.M.); RadMD, Doylestown, Pa (R.P.); Pfizer Global Research and Development, Groton, Conn (D.R.); University of Pennsylvania, Philadelphia, Pa (M.R.); Novartis Pharmaceuticals Corporation, Florham Park, NJ (H.R.); Columbia University, New York, NY (L.H.S.); Mallinckrodt Institute of Radiology, Washington University School of Medicine, St Louis, Mo (B.A.S.); Massachusetts General Hospital, Boston, Mass (A.G.S.); Johns Hopkins University, Baltimore, Md (R.L.W.); AstraZeneca, Cheshire, England (J.C.W.); University of Southern California, Los Angeles, Calif (W.W.); and Roche Pharmaceuticals, Basel, Switzerland (G.Z.).
Author contributions: Guarantors of integrity of entire study, A.J.B., G.S.D., H.R.; study concepts/study design or data acquisition or data analysis/interpretation, all authors; manuscript drafting or manuscript revision for important intellectual content, all authors; manuscript final version approval, all authors; literature research, A.J.B., H.J.W.L.A., B.B., R.B., P.E.C., J.J.C., P.S.D., N.R.D., W.E., C.E., D.G., E.F.J., L.K., P.E.K., G.M., P.D.M., H.R., L.H.S., A.G.S., R.L.W., J.C.W., G.Z., D.C.S.; statistical analysis, H.J.W.L.A., C.G., D.R.; and manuscript editing, A.J.B., H.J.W.L.A., R.B., J.M.B., L.B., P.E.C., J.J.C., G.S.D., P.S.D., N.R.D., W.E., C.E., R.A.F., M.L.G., D.G., S.N.G., O.S.H., E.F.J., L.K., G.J.K., P.E.K., G.M., C.G.M., P.D.M., R.P., D.R., M.R., H.R., L.H.S., B.A.S., A.G.S., R.L.W., J.C.W., W.W., G.Z., B.Z., D.C.S.
Received May 12, 2010; revision requested June 11; revision received June 29; accepted August 17; final version accepted August 23.
Abbreviations:
- CDER
- Center for Drug Evaluation and Research
- CDRH
- Center for Devices and Radiological Health
- FDA
- Food and Drug Administration
- FDG
- fluorodeoxyglucose
- FMISO
- fluoromisonidazole
- PMA
- premarket approval
- RSNA
- Radiological Society of North America
- QC
- quality control
References
- 1.Woodcock J, Woosley R. The FDA Critical Path Initiative and its influence on new drug development. Annu Rev Med 2008;59:1–12. [DOI] [PubMed] [Google Scholar]
- 2.Challenges and Opportunities Report—March 2004. Innovation or stagnation: challenge and opportunity on the critical path to new medical products. U.S. Food and Drug Administration. http://www.fda.gov/ScienceResearch/SpecialTopics/CriticalPathInitiative/CriticalPathOpportunitiesReports/ucm077262.htm. Last updated July 20, 2010. Accessed January 5, 2010.
- 3.The Innovative Medicines Initiative Web site. http://www.imi-europe.org/. Accessed March 17, 2010.
- 4.Hunter AJ. The Innovative Medicines Initiative: a pre-competitive initiative to enhance the biomedical science base of Europe to expedite the development of new medicines for patients. Drug Discov Today 2008;13(9-10):371–373. [DOI] [PubMed] [Google Scholar]
- 5.Biomarkers Consortium Web site. http://www.biomarkersconsortium.org/. Accessed March 17, 2010.
- 6.Buckler AJ, Mozley PD, Schwartz L, et al. Volumetric CT in lung cancer: an example for the qualification of imaging as a biomarker. Acad Radiol 2010;17(1):107–115. [DOI] [PubMed] [Google Scholar]
- 7.Buckler A. A procedural template for the qualification of imaging as a biomarker, using volumetric CT as an example. Presented at the IEEE Applied Imagery and Pattern Recognition Workshop, Washington, DC, October 14–16, 2009. [Google Scholar]
- 8.Sanhai WR. Working together to enhance the efficiency of medical product development. J Nucl Med 2008;49(6):43N–45N. [PubMed] [Google Scholar]
- 9.Drug-diagnostic co-development concept paper (draft, not for implementation), April 2005. Department of Health and Human Services, Food and Drug Administration. http://www.fda.gov/downloads/Drugs/ScienceResearch/ResearchAreas/Pharmacogenetics/UCM116689.pdf. Accessed January 7, 2010.
- 10.Biomarkers Definitions Working Group Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 2001;69(3):89–95. [DOI] [PubMed] [Google Scholar]
- 11.Meyer CR, Armato SG, Fenimore CP, et al. Quantitative imaging to assess tumor response to therapy: common themes of measurement, truth data, and error sources. Transl Oncol 2009;2(4):198–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toward Quantitative Imaging (TQI) Workshop RSNA Headquarters, Oak Brook, IL. July 10–11, 2008. Radiological Society of North America. http://www.rsna.org/Research/TQI/upload/Workshop-Summary-FINAL.pdf. Accessed March 17, 2010.
- 13.Olson S, Robinson S, Giffin R. Accelerating the development of biomarkers for drug safety: workshop summary Washington, DC: National Academies Press, 2009. [PubMed] [Google Scholar]
- 14.Levine MN, Julian JA. Registries that show efficacy: good, but not good enough. J Clin Oncol 2008;26(33):5316–5319. [DOI] [PubMed] [Google Scholar]
- 15.Buckler AJ, Bresolin L, Dunnick NR, et al. A collaborative enterprise for multi-stakeholder participation in the advancement of quantitative imaging. Radiology 2011;258(3):906–914. [DOI] [PubMed] [Google Scholar]
- 16.Quantitative Imaging Biomarkers Alliance. Radiological Society of North America. http://www.rsna.org/Research/QIBA/. Accessed June 19, 2010.
- 17.Dorfman GS, Sullivan DC, Schnall MD, Matrisian LM. Translational Research Working Group The Translational Research Working Group developmental pathway for image-based assessment modalities. Clin Cancer Res 2008;14(18):5678–5684. [DOI] [PubMed] [Google Scholar]
- 18.CFR–Code of Federal Regulations Title 21. Department of Health and Human Services, Food and Drug Administration. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=820&showFR=1. Accessed February 28, 2010.
- 19.510(k) Clearances Department of Health and Human Services, Food and Drug Administration. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/510kClearances/default.htm. Accessed January 5, 2010.
- 20.PMA Approvals Department of Health and Human Services, Food and Drug Administration. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/PMAApprovals/default.htm. Accessed January 5, 2010.
- 21.National Cancer Institute Division of Cancer Treatment and Diagnosis Major ongoing initiatives: program for the assessment of clinical cancer tests. http://dctd.cancer.gov/ProgramPages/cdp/major_initiatives_program_assessment.htm. Accessed November 15, 2010.
- 22.Goodsaid F, Frueh F. Process map proposal for the validation of genomic biomarkers. Pharmacogenomics 2006;7(5):773–782. [DOI] [PubMed] [Google Scholar]
- 23.Goodsaid FM, Frueh FW. Questions and answers about the pilot process for biomarker qualification at the FDA. Drug Discov Today Technol 2007;4(1):9–11. [DOI] [PubMed] [Google Scholar]
- 24.Goodsaid FM, Frueh FW, Mattes W. Strategic paths for biomarker qualification. Toxicology 2008;245(3):219–223. [DOI] [PubMed] [Google Scholar]
- 25.Dreyer NA, Garner S. Registries for robust evidence. JAMA 2009;302(7):790–791. [DOI] [PubMed] [Google Scholar]
- 26.Kramer BS. Comparative effectiveness research. Presented at the ACRIN 2009 Fall meeting, Arlington, Va, October 1, 2009. [Google Scholar]
- 27.Rodriguez H, Tezak Z, Mesri M, et al. Analytical validation of protein-based multiplex imaging assays: a workshop report by the NCI-FDa interagency oncology task force on molecular diagnostics. Clin Chem 2010;56(2):237–243. [DOI] [PubMed] [Google Scholar]
- 28.Regnier FE, Skates SJ, Mesri M, et al. Protein-based multiplex assays: mock presubmissions to the US Food and Drug Administration. Clin Chem 2010;56(2):165–171. [DOI] [PubMed] [Google Scholar]
- 29.Food and Drug Administration 21 CFR 820, Quality system regulation. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=820. Accessed January 5, 2010.
- 30.Sargent DJ, Rubinstein L, Schwartz L, et al. Validation of novel imaging methodologies for use as cancer clinical trial end-points. Eur J Cancer 2009;45(2):290–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.