Abstract
Glomerulonephritis (GN) affects patients of all ages and is an important cause of morbidity and mortality. Non-selective immunosuppressive drugs have been used in immune-mediated GN but often result in systemic side effects and occasionally fatal infective complications. There is increasing evidence from both preclinical and clinical studies that abnormal activation of receptor and non-receptor tyrosine kinase signalling pathways are implicated in the pathogenesis of immune-mediated GN. Activation of spleen tyrosine kinase (SYK), Bruton's tyrosine kinase (BTK), platelet-derived growth factor receptor (PDGFR), epidermal growth factor receptor (EGFR) and discoidin domain receptor 1 (DDR1) have been demonstrated in anti-GBM disease. SYK is implicated in the pathogenesis of ANCA-associated GN. SYK, BTK, PDGFR, EFGR, DDR1 and Janus kinase are implicated in the pathogenesis of lupus nephritis. A representative animal model of IgA nephropathy (IgAN) is lacking. Based on the results from in vitro and human renal biopsy study results, a phase II clinical trial is ongoing to evaluate the efficacy and safety of fostamatinib (an oral SYK inhibitor) in high-risk IgAN patient. Various tyrosine kinase inhibitors (TKIs) have been approved for cancer treatment. Clinical trials of TKIs in GN may be justified given their long-term safety data. In this review we will discuss the current unmet medical needs in GN treatment and research as well as the current stage of development of TKIs in GN treatment and propose an accelerated translational research approach to investigate whether selective inhibition of tyrosine kinase provides a safer and more efficacious option for GN treatment.
Keywords: crescentic glomerulonephritis, glomerulonephritis, IgA nephropathy, immunosuppression, lupus nephritis, tyrosine kinase
INTRODUCTION
Glomerulonephritis (GN) affects patients of all ages and is an important cause of morbidity and mortality. It is estimated that there were >100 million prevalent cases of chronic kidney disease (CKD) secondary to GN globally in 2013, the number of which had increased by >30% since 1990 [1]. Immune-mediated glomerular injury plays an important role in the pathogenesis of anti-glomerular basement membrane (anti-GBM) disease, anti-neutrophil cytoplasmic antibody (ANCA)–associated glomerulonephritis (AAGN), lupus nephritis (LN) and immunoglobulin A nephropathy (IgAN). In recent years, advances in understanding the immunopathogenesis of these entities have provided translational opportunities for the development of novel therapeutic interventions [2].
Protein tyrosine kinases (PTKs) catalyze phosphorylation of tyrosine residues on protein substrates. They play a crucial role in the modulation of enzymatic activity and recruitment of downstream signalling molecules, which in turn regulate cellular growth and transformation [3]. PTKs can be classified into receptor tyrosine kinases (RTKs) and non-receptor tyrosine kinases (NRTKs). RTKs are transmembrane receptors that have intrinsic tyrosine kinase activity, whereas NRTKs are involved in different intracellular signalling pathways [4]. RTKs typically have an extracellular domain (for binding of different ligands), a transmembrane domain (for anchorage) and an intracellular domain (for signal transduction). Upon ligand binding to an RTK, it triggers dimerization and autophosphorylation of the receptor, followed by activation of various downstream signalling pathways [5]. NRTKs are subdivided into nine main families based on their similarities in domain structure. They interact with RTKs and mediate important signalling pathways that regulate cellular proliferative, differentiation, survival and apoptosis [6]. Dysregulation of PTK activity (e.g. overexpression) has been implicated in tumourigenesis, and the development of tyrosine kinase inhibitors (TKIs) has been one of the most important recent advances in oncology [7–9].
Recently there is increasing evidence from both preclinical and clinical studies that targeting tyrosine kinase signalling pathways is a potential therapeutic strategy for immune-mediated GN [10–13]. In this review we will focus our discussion on anti-GBM disease, AAGN, LN and IgAN. The potential clinical applications of TKIs in these conditions, their stage of development and preliminary results from clinical studies will be emphasized.
CURRENT UNMET MEDICAL NEEDS
Rapidly progressive glomerulonephritis (RPGN) is an aggressive disease and the renal prognosis is often poor despite intensive treatment. A recent study from China showed that the 5-year the renal survival of anti-GBM disease and AAGN was 17.6 and 44.3%, respectively [14]. In another UK study of 43 patients (81% dialysis dependent at presentation), the 1-year renal survival of anti-GBM disease was just 16% [15]. AAGN usually affects elderly patients, and the use of non-selective immunosuppressive therapy can result in significant systemic side effects and sometimes fatal infectious complications. Rituximab (an anti-CD20 monoclonal antibody) is increasingly used in AAGN, but a recent study showed that there was no difference in clinical outcome of AAGN patients who were treated before and after the introduction of rituximab as an induction agent [16]. More importantly, the toxicity of rituximab was comparable to cyclophosphamide in the RAVE [17] and RITUXVAS [18] studies.
LN usually affects young female patients of child-bearing age. Some patients experience frequent relapses and require long-term immunosuppressive drugs. Corticosteroid-related systemic side effects and cyclophosphamide-related gonadal toxicity are important safety concerns. Multiple randomized controlled trials (RCTs) in ANCA-associated vasculitis (AAV) and LN have compared cyclophosphamide-based regimens with newer agents such as rituximab and mycophenolate mofetil. Disappointingly, their adverse event profiles were similar to those of cyclophosphamide-based protocols [19]. In high-risk IgAN patients with persistent proteinuria despite maximal supportive therapy and preserved renal function, the latest Kidney Disease: Improving Global Outcomes (KDIGO) guideline recommended immunosuppressive therapy using 6 months of corticosteroid [20]. However, the efficacy and safety of non-selective immunosuppressive treatment were recently challenged by the STOP-IgAN trial [21]. Compared with patients receiving supportive treatment alone, patients in the immunosuppression group had no significant improvement in the annual estimated glomerular filtration rate (eGFR) decline after 3 years but experienced significantly higher rates of severe infections, impaired glucose tolerance and weight gain.
With the current limitations of non-selective immunosuppressive therapy, a targeted approach using selective immunosuppressive drugs is more desirable and warrants further investigation.
CURRENT LIMITATIONS OF TRANSLATIONAL RESEARCH IN GN
The essence of translational research is to make use of biomedical advances in basic science to address unmet medical needs of patients so as to improve patient outcomes [22]. In GN research, although various useful animal (mostly rodent) models of anti-GBM disease (e.g. experimental autoimmune GN, nephrotoxic nephritis), AAGN (e.g. experimental autoimmune vasculitis) and LN (e.g. lupus-prone mice) have been developed, none of them is perfect (Table 1) [23–25]. Development of an animal model of IgAN has been attempted, but none was sufficiently representative of human IgAN, partly attributed to the complex pathophysiology of IgAN [26]. This underscores the uncertainty of the predictive value of data from animal studies in human diseases. In the absence of a perfect animal model, immunohistochemistry (IHC) study of human renal biopsy becomes a valuable tool to provide additional evidence on the pathogenic role of a certain therapeutic target, assuming that the target protein is expressed in the kidney and not in circulating cells that regulate the autoimmune response. Using combined results from in vitro studies and IHC study of human renal biopsy may be a reasonable approach to provide a scientific basis for future clinical studies [27]. Various TKIs have been approved for the treatment of malignancy and have long-term efficacy and safety data in oncology patients. As a result, targeting the tyrosine kinase signalling pathways provides an attractive opportunity for accelerated translation research in GN treatment.
Table 1.
Selected commonly used animal models of immune-mediated GN
| Model | Resemblance of human disease | Animal | Method of induction | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Experimental autoimmune GN (EAG) | Anti-GBM disease | Wistar Kyoto rat | Single intramuscular injection of collagenase-solubilized GBM (e.g. from Sprague-Dawley rat or sheep) in FCA or single intramuscular injection of recombinant rat α3(IV)NC1 in FCA |
Invariable progression to chronic phase of injury which resembles human disease | Technically more demanding Some strains (e.g. Lewis rats) are resistant to EAG More gradual onset of disease compared with nephrotoxic nephritis |
| NTN | Anti-GBM disease | rat | Single intravenous injection of rabbit anti-GBM antisera | Relatively simple Rapid onset of renal injury |
Rabbit antisera may contain antibodies towards other components apart from GBM |
| Accelerated nephrotoxic nephritis | Sprague-Dawley rat C57BL/6 mouse |
Subcutaneous injection of sheep IgG in FCA followed by intravenous injection of sheep anti-rat/mouse GBM serum 5–10 days later | Rapid onset of renal injury | Variable progression to chronic phase of injury | |
| Attenuated passive model of anti-GBM disease | Anti-GBM disease | C57BL/6 mouse | Intravenous injection of rabbit anti-mouse GBM antibody followed by intraperitoneal injection of purified mouse anti-rabbit IgG monoclonal antibody | Rapid onset of renal injury Degree of proteinuria is dependent on the amount of antibody used |
Attenuated form of anti-GBM disease Only ∼50% of wild-type mice progressed to chronic phase |
| Passive anti-MPO transfer | ANCA-associated vasculitis | C57BL/6 wild-type or RAG2-deficient mice, with or without LPS priming | Anti-MPO antibody induced in MPO-deficient mice and transferred into recipients | Pauci-immune GN resembling human disease | Technically demanding Mild disease severity (reported crescent fraction 5–15%) |
| Experimental autoimmune vasculitis | ANCA-associated vasculitis | Wistar Kyoto rat | Immunization with human MPO in CFA | Dose-dependent effect of MPO on disease severity | Technically demanding for MPO purification No urinary abnormalities were seen in the other rat strains (BN, Lewis, WF) |
| Spontaneous mouse models of lupus nephritis | Lupus nephritis | MRL/lpr mouse | Spontaneous disease | A broad spectrum of SLE features including arthritis, inflammatory skin lesions and GN are seen | Nephritis is independent of FcγRs so the relevance to human lupus nephritis may not be totally appropriate |
| NZB/NZW F1 mouse | Spontaneous disease | Closest approximation of human lupus nephritis in terms of characteristics of disease development and the underlying genetics driving autoimmunity | Slow onset of disease Progressive proteinuria beginning ∼5 months and azotemia ∼7 months onward | ||
| Anti-Thy 1.1 GN | Mesangial proliferative/IgAN | rat | Single intravenous injection of a mouse monoclonal anti-rat Thy 1.1 antibody | Mesangial cell proliferation and mesangial matrix expansion, histologically similar to human IgAN | No evidence of IgA deposition in glomeruli Lesions do not fully mimic the wide range of lesions seen in human IgAN |
| Spontaneous animal model for IgAN | IgAN | ddY strain mouse | Spontaneous disease | Elevated levels of circulating IgA and mouse IgA mesangial deposits, similar to human IgAN | Only a variable proportion of mice develop the disease model No haematuria and mild proteinuria |
| IgAN | IgA1-expressing mouse | sCD89 injection | Mouse expressing both human IgA1 and CD89 have circulating and mesangial deposition of IgA1-sCD89 complexes resulting in kidney inflammation, haematuria and proteinuria | Issues with reproducibility Human IgA1 may not be representative of the pathogenetic IgA1 in patients |
FCA, Freund's complete adjuvant; GBM, glomerular basement membrane; MPO, myeloperoxidase.
EVIDENCE FROM PRECLINICAL STUDIES TO JUSTIFY FURTHER CLINICAL TRIALS OF TKIs IN IMMUNE-MEDIATED GN
Anti-GBM disease
Compared with other types of immune-mediated GN, anti-GBM disease has been more extensively studied due to the availability of more robust animal models and it is considered a ‘prototypic’ autoimmune disease, such that findings may translate to other forms of GN.
Spleen tyrosine kinase (SYK) is an NRTK that plays a crucial role in a variety of biological functions, including intracellular signalling cascade for classic immunoreceptors like activatory Fc receptors (FcRs) and B-cell receptors (BCRs) [28]. IHC study showed increased SYK expression in both experimental [29–31] and human anti-GBM disease [32]. Increased SYK expression seemed to localize predominantly to areas of crescent formation and proliferating cells within the glomeruli [29, 32]. Administration of fostamatinib (an oral SYK inhibitor) completely aborted the development of nephritis when given before induction [29] and significantly reduced disease severity when given after established disease [29, 33]. In experimental autoimmune GN (EAG), fostamatinib treatment starting from Day 18 (where there were severe segmental necrotizing injury and crescent formation in ∼26% of glomeruli) to Day 36 led to a rapid and complete resolution of urinary abnormalities (100% reduction of both haematuria and proteinuria) that was sustained until Day 36 [29]. Fostamatinib-treated animals also had preserved levels of serum urea compared with a 103% increase in the vehicle group [29]. In nephrotoxic nephritis (NTN), high-dose fostamatinib treatment starting from Day 7 (where cellular crescents were present in ∼90% of glomeruli) to Day 14 significantly reduced proteinuria (23%), glomerular crescents (21%), infiltration of glomerular macrophages (93%) and CD8+ cells (74%) and serum creatinine (28%) [33]. SYK appeared to mediate glomerular injury by upregulation of pro-inflammatory cytokines, glomerular leukocyte recruitment and activation of c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPK) pathways [30]. JNK inhibitor (CC-401) suppressed glomerular and tubulointerstitial damage when given before induction of experimental anti-GBM disease [34]. When given from Day 7 (where there was significant proteinuria, focal glomerular lesions, marked glomerular macrophage and T-cell accumulation and upregulation of pro-inflammatory mediators) to Day 14, CC-401 prevented renal impairment, suppressed proteinuria and prevented the development of severe glomerular and tubulointerstitial lesions, including crescent formation [35]. Pharmacological inhibition of p38 MAPKα/β, both early (1 h before induction) and late (starting from Day 4), have also been shown to be effective in reducing GN severity in NTN [36].
Bruton's tyrosine kinase (BTK) is an NRTK that plays an important role in signal transduction pathways that regulate B-cell survival, activation, proliferation and differentiation [37]. Activated SYK can induce phosphorylation of BTK, which cooperatively activates phospholipase C (PLC)-γ. PLC-γ catalyzes the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) into diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3). IP3 induces calcium mobilization from the endoplasmic reticulum. DAG and calcium promote the activation of protein kinase C (PKC) and MAPK family downstream signalling cascades [38]. In experimental anti-GBM disease, administration of PF-06250112 (an oral BTK inhibitor) at the time of induction reduced proteinuria in a dose-dependent manner [39]. Interestingly, PF-06250112 inhibited disease development even in the presence of glomerular deposition of antibody and C3, indicating that the antiproteinuric effect was secondary to inhibition of the BTK signalling pathway instead of the effect on deposition or clearance of anti-GBM antibody. The effect of late treatment was not assessed in this study.
Platelet-derived growth factor receptors (PDGFRs) are RTKs that are expressed constitutively or inducibly in most renal cells. PDGFRs regulate cellular proliferation and migration, extracellular matrix accumulation, production of pro-inflammatory cytokines, tissue permeability and intrarenal haemodynamics [40]. PDGFR-β and PDGF-BB are overexpressed in the crescents of experimental and human anti-GBM disease [41]. An early study showed that intraperitoneal trapidil (a PDGFR antagonist) administration was associated with worse outcome in vivo [42]. However, recent studies using intraperitoneal imatinib (a multitargeted RTK inhibitor that can block PDGFR) showed significant renoprotective effects in vivo. In NTN, late imatinib treatment from Day 7 (where there was endocapillary proliferation, severe fibrinoid necrosis, cellular crescent formation and prominent glomerular fibrin deposition) to Day 20 led to less crescent formation and fibrinoid necrosis, reduced proteinuria and preserved renal function [43]. Using a similar NTN model, longer-term imatinib treatment from Day 7 to Day 49 significantly suppressed proteinuria, improved renal function and attenuated the development of glomerulosclerosis and tubulointerstitial injury [44]. In these in vivo studies, however, it was uncertain to what extent the beneficial effects were mediated specifically via inhibition of PDGFR signalling.
Epidermal growth factor receptor (EGFR) is an RTK that plays an important role in many cellular functions, including proliferation, migration and differentiation [45]. Heparin-binding epidermal growth factor-like growth factor (HB-EGF), a member of the EGFR family, is a potent inducer of cellular proliferation and migration (e.g. macrophages, T-lymphocytes). Upregulation of HB-EGF was found in both experimental and human anti-GBM disease [46]. HB-EGF deficiency status and pharmacological EGFR blockade (before induction) in vivo prevented renal leukocytic infiltration before the appearance of crescents and interstitial fibrosis, suggesting that the HB-EGF/EGFR pathway was involved in the very early stage of renal damage [46]. Pharmacological blockade of EGFR using erlotinib from Day 4 to Day 14 after induction of NTN was shown to reduce the expression of EGFR in the renal cortex, the proportion of crescentic glomeruli and blood urea nitrogen [46].
Discoidin domain receptor 1 (DDR1) is a collagen receptor with tyrosine kinase activity. As with most RTKs, MAPK and PI3 pathways are the downstream effectors of DDR1 [47]. DDR1 expression was increased in experimental and human anti-GBM disease [48]. DDR1-deficient mice had less severe renal disease and lower mortality than their wild-type littermates after induction of anti-GBM disease [49]. Administration of DDR1-specific antisense oligodeoxynucleotides at the time of induction decreased DDR1 expression and reduced disease severity. DDR1 antisense administration given on Day 4 (presence of proteinuria) and Day 8 both prevented progression of NTN, although the protective effect of the antisense treatment started at Day 8 was less efficient compared with antisense treatment started at Day 4 [49].
ANCA-associated GN
In vitro activation of neutrophil respiratory burst by ANCA from patients with systemic vasculitis required PTK and PKC activation. Blocking both kinases using pharmacological inhibitors abrogated ANCA-induced superoxide generation [50]. However, the specific tyrosine kinases involved were not investigated in this study. A previous study showed that p38 MAPK inhibition markedly reduced ANCA-induced neutrophil activation in vitro and partly reduced crescent formation in vivo [51].
SYK phosphorylation is induced during ANCA-triggered neutrophil activation [52]. In a study using the experimental autoimmune vasculitis model, where WKT rats developed haematuria and proteinuria at 4 weeks, fostamatinib treatment from Week 4 to Week 6 significantly reduced proteinuria, haematuria, glomerular histological abnormalities, glomerular macrophage infiltration, pulmonary haemorrhage severity and haemosiderin deposition in lung tissue [53]. Since SYK is involved in upstream signalling pathways of MAPK, the beneficial effect of SYK inhibition may be explained by its inhibitory effect on downstream MAPK signalling pathways. In patients with AAGN, glomerular SYK expression was increased and correlated with serum creatinine. SYK expression was highest in patients with crescentic GN (active disease) and minimal in those with sclerotic GN (chronic disease) [32].
In the kidney, vascular endothelial growth factor (VEGF) plays a crucial role in maintaining the integrity of the glomerular filtration barrier. Soluble fms-like tyrosine kinase 1 (sFlt-1) acts as an antagonist of VEGF. An imbalance of VEGF/sFlt-1 has been observed in many diseases with endothelial dysfunction, including diabetic nephropathy [54]. An in vitro study showed that ANCA antibodies increased sFlt1 during acute AAV, leading to an anti-angiogenic state that hinders endothelial repair [55].
Lupus nephritis
In prediseased lupus-prone NZB/NZW mice 6–7 months of age, fostamatinib treatment (up to Day 240) significantly delayed the onset of proteinuria and azotaemia, reduced renal inflammatory infiltrates and significantly prolonged animal survival [56]. In mice with established disease and proteinuria, fostamatinib treatment reduced proteinuria and preserved renal function in a dose-dependent manner and prolonged mice survival [56]. Up to 47% of mice with established disease demonstrated no microscopic evidence of renal changes after high-dose fostamatinib treatment, compared with only 10% in the vehicle group [56]. In MRL/lpr mice, fostamatinib treatment for 16 weeks starting from Week 4 (prediseased state) prevented the development of renal disease at Week 20, whereas fostamatinib for 8 weeks starting from Week 16 (established disease) significantly reduced proteinuria [57]. In a human renal biopsy study, patients with diffuse proliferative LN had the highest SYK expression, whereas those with membranous LN had minimal SYK expression [32]. Several BTK inhibitors have also been shown to reduce the severity of renal disease in experimental models of LN [13]. Ibrutinib treatment for 2 months in prediseased mice (starting from 4 months) alleviated renal damage and decreased circulating antinucleosome, antihistone and anti-ssDNA autoantibodies [58]. BTK inhibitors RN486 [59] and PF-06250112 [38] both reduced the severity of established GN in NZB/NZW mice.
In murine LN, imatinib treatment starting at 5 months of age (where focal glomerular hypercellularity and immune complex deposition were evident) significantly delayed the onset of proteinuria and renal impairment, protected against abnormal histological changes and prolonged animal survival, suggesting that inhibition of PDGFR might be a potential therapeutic strategy [60]. In another in vivo study using MRL/lpr mice, higher-dose imatinib treatment starting from Week 16 (advanced stage of GN) to Week 24 significantly reduced serum IgG and anti-dsDNA levels, ameliorated histological changes, reduced expression of PDGFR and transforming growth factor-β messenger RNA, reduced proteinuria, preserved renal function and prolonged survival [61]. An early IHC study showed increased EGFR expression in ∼35% of LN patients [62]. Autoantibodies to the extracellular domain of EGFR have been found in Fas-defective mice and in SLE patients [63]. A recent study showed that human epidermal growth factor receptor 2 (HER-2, an RTK) was overexpressed in lupus-prone NZM2410 mice and in patients with LN, but not in other mesangioproliferative GN [64]. DDR1 was found in podocytes and crescents in renal biopsies from patients with LN and genetic inhibition of DDR1 protected mice against development of crescentic GN [48].
Janus kinases (JAKs) are NRTKs that mediate the intracellular signalling initiated by interferons (IFNs), interleukins (ILs), colony-stimulating factors and hormones. Upon activation, JAKs phosphorylate the signal transducers and activators of transcription (STAT), which in turn regulate gene transcription. A series of JAK-STAT signalling cytokines, especially type I IFNs, IL-10 and IL-6, have been implicated in the pathogenesis of SLE [65]. Treatment of lupus-prone mice with JAK2 inhibitors led to prevention or improvement of established disease [66, 67]. In MRL/lpr mice, tyrphostin AG490 (a selective JAK2 inhibitor) treatment from Week 12 to Week 20 significantly inhibited renal expression of monocyte chemotactic protein (MCP)-1 and IFN-γ, reduced renal infiltration of T cells and macrophages, reduced proteinuria and improved renal function [66]. In an elegantly designed study, Lu et al. [67] tested the efficacy of CEP-33779 (a selective JAK2 inhibitor) in age-matched MRL/lpr or BWF1 mice with established SLE or LN, respectively. In this study, reference standard treatments including dexamethasone and cyclophosphamide were included. Treatment with CEP-33779 reduced serum pro-inflammatory cytokines and renal JAK2 activity, improved renal histopathology, decreased splenomegaly and lymphomegaly and prolonged animal survival. The therapeutic effect of CEP-33779 was comparable with that of cyclophosphamide and superior to dexamethasone alone. Tofacitinib, a JAK inhibitor, has been proven efficacious in rheumatoid arthritis. It is currently being investigated in a Phase I clinical trial of SLE patients (NCT02535689). Ruxolitinib, which inhibits JAK2, has been approved for the treatment of myelofibrosis. However, it has not been used in renal disease.
IgAN
Despite years of effort, a representative animal model of IgAN is still lacking. We [27] and others [68] have overcome this limitation by studying the effect of IgA1 purified from IgAN patients on human mesangial cells in vitro. In particular, we showed that IgA1 from patients with IgAN (but not IgA1 from the healthy volunteers) stimulated phosphorylation of SYK, production of inflammatory cytokines and growth factors and proliferation of mesangial cells in vitro [27]. These biological effects are similar to the pathological features of IgAN in patients. Inhibition of SYK by the active metabolite of fostamatinib or specific knockdown of SYK using siRNA reduced the synthesis of inflammatory cytokines and suppressed cell proliferation in IgA1-stimulated human mesangial cells [27]. In human IgAN, patients with endocapillary proliferation on renal biopsy had a higher level of SYK expression than those without [32].
Previous IHC study also showed that glomerular PDGFR-β expression significantly correlated with mesangial cell proliferation [69]. PDGFR inhibitor (in particular imatinib) and EGFR inhibitor reduced mesangial cell proliferation and matrix accumulation in rat acute anti-Thy 1.1 GN [40, 70]. In rat chronic anti-Thy 1.1 GN, PDGFR inhibition using B-specific oligonucleotide aptamer and neutralizing anti-PDGF-D IgG reduced proteinuria and improved renal function [40]. In acute anti-rat Thy-1.1, early erlotinib (an EGFR inhibitor) significantly prevented progression of mesangial cell proliferation and matrix accumulation and preserved renal function [41]. It should be noted, however, that the anti-rat Thy-1.1 GN model is not a representative model of human IgAN. In IgAN patients, elevated sFlt-1 (low VEGF:sFlt-1 ratio) correlated with the severity of proteinuria and hypertension [71]. Renal biopsy of IgAN patients also showed focal loss of VEGF in podocytes [72].
POTENTIAL APPLICATIONS AND SAFETY CONCERNS OF TKIs IN IMMUNE-MEDIATED GN
TKIs are widely used clinically for the treatment of malignancies such as chronic myeloid leukemia (CML), gastrointestinal stromal tumors (GISTs), non-small-cell lung cancer and renal cell carcinoma. There is now accumulating evidence to suggest that further clinical studies of TKIs may be justified in selected immune-mediated GN (Table 2). Multiple in vivo studies have demonstrated beneficial effects of pharmacological inhibition of tyrosine kinases in established renal disease. Some of these tyrosine kinases are also upregulated in human renal biopsies. It should be noted, however, that the pathogenesis of anti-GBM disease and AVV are complex. Although targeting tyrosine kinase signalling pathways is attractive, it is unlikely that a single selective TKI can replace traditional induction therapy. Nevertheless, it might be reasonable to consider TKIs as adjunctive induction agents such that the dosage and side effects of non-selective immunosuppressive drugs may be reduced. Using TKIs as a steroid-sparing maintenance therapy may be another possible treatment strategy. In murine LN, JAK2 inhibitor was equally effective compared with cyclophosphamide [67]. The use of TKIs as induction and maintenance therapy in human LN might be justified.
Table 2.
Summary of existing evidence of tyrosine kinase involvement in immunopathogenesis of immune-mediated GN
| Tyrosine kinase | Disease | In vitro study | In vivo study | Human renal biopsy study | Justifiable for further clinical study |
|---|---|---|---|---|---|
| Spleen tyrosine kinase | Anti-GBM disease | √ | √ | √ | √ |
| AAGN | √ | √ | √ | √ | |
| LN | √ | √ | √ | √ | |
| IgAN | √ | No representative animal model | √ | √ | |
| Bruton's tyrosine kinase | Anti-GBM disease | √ | √ | No data | Insufficient evidence |
| AAGN | No data | No data | No data | Insufficient evidence | |
| LN | √ | √ | No data | Insufficient evidence | |
| IgAN | No data | No representative animal model | No data | Insufficient evidence | |
| Platelet-derived growth factor receptor | Anti-GBM disease | √ | Conflicting data | No data | Insufficient evidence |
| AAGN | No data | No data | No data | Insufficient evidence | |
| LN | √ | √ | No data | Insufficient evidence | |
| IgAN | √ | √ (in anti-Thy 1.1 model) | √ | √ | |
| Epidermal growth factor receptor | Anti-GBM disease | √ | √ | √ | √ |
| AAGN | No data | No data | No data | Insufficient evidence | |
| LN | √ | √ | √ | √ | |
| IgAN | √ | √ (in anti-Thy 1.1 model) | No data | Insufficient evidence | |
| Discoidin domain receptor 1 | Anti-GBM disease | √ | √ | √ | √ |
| AAGN | No data | No data | No data | Insufficient evidence | |
| LN | √ | √ | √ | √ | |
| IgAN | No data | No representative animal model | No data | Insufficient evidence | |
| Janus kinase | LN | √ | √ | No data | Insufficient evidence |
| Vascular endothelial growth factor | IgAN | √ | No representative animal model | √ | √ |
Multiple TKIs have been approved for anti-cancer therapy. Imatinib was the first Bcr-Abl TKI approved by the US Food and Drug Administration for the treatment of CML. Imatinib also has inhibitory effects on other RTKs that make it a potent immunomodulatory agent. There have been promising results with the use of imatinib in murine models of kidney disease, including experimental anti-GBM disease, anti-Thy 1.1 GN and LN [73]. Besides, a number of case reports have described its successful (off-label) use in human membranoproliferative GN and cryoglobulinemia [74–76].
Although the clinical outcomes of these cases are encouraging, it should be noted that imatinib may have deleterious off-target effects on the kidney. In a recent long-term study of CML patients treated with different TKIs, imatinib was associated with a higher incidence of acute kidney injury (AKI) compared with dasatinib and nilotinib [77]. Imatinib-associated AKI has been reported previously [78]. It has also been associated with tubular dysfunction causing renal potassium and phosphate wasting [79] and thrombotic microangiopathy (TMA) [80]. Imatinib may also increase serum creatinine by inhibiting tubular secretion [81]. In another study of CML patients, patients with baseline renal dysfunction had a greater incidence of transient reversible AKI after dasatinib and nilotinib treatment [82]. Dasatinib has been reported to be associated with AKI [83, 84], thrombotic thrombocytopenia purpura [85] and nephrotic range proteinuria [86].
Fostamatinib has been evaluated in >3200 rheumatoid arthritis patients enrolled in three Phase 2, one Phase 2b and three Phase 3 trials [87, 88]. It is currently the only TKI that is being studied in a Phase 2, multicentre RCT in high-risk IgAN patients (NCT02112838). This clinical trial is testing a novel SYK-targeted approach for treating IgAN and will provide important information to guide further development of novel treatment strategies. Up to 35% of subjects on fostamatinib versus 11% on placebo developed hypertension or required adjustment to their antihypertensive regimen [89]. The effect of fostamatinib on blood pressure (mean elevation of ∼3 mmHg in both systolic and diastolic) appeared to be dose dependent and secondary to reduced VEGF-induced nitric oxide release from the endothelium [90]. This suggests that fostamatinib may also have off-target inhibitory effects on VEGF. Anti-VEGF therapy has been reported to be associated with hypertension, proteinuria and TMA [91]. However, previous trials of fostamatinib did not suggest an increased risk of nephrotoxic side effects. The current stages of development of TKIs in immune-mediated GN are summarized in Table 3.
Table 3.
Stage of development of selected TKIs in immune-mediated GN
| Drug | Target tyrosine kinase | Animal studies | Human studies |
|---|---|---|---|
| Fostamatinib | Spleen tyrosine kinase | Anti-GBM disease, ANCA-associated GN, lupus nephritis | Phase 2 clinical trial in IgAN |
| Ibrutinib | Bruton's tyrosine kinase | Lupus nephritis | No data |
| Imatinib | Platelet-derived growth factor receptor | Anti-GBM, lupus nephritis, anti-Thy 1.1 GN | Case reports of off-label use in membranoproliferative GN and cryoglobulinemia |
| Tofacitinib | Janus kinase | Lupus nephritis | Phase 1 clinical trial in SLE |
FUTURE DIRECTIONS
Clinical trials have been the Achilles' heel of translational nephrology. This is particularly true in the field of GN research. Some diseases (e.g. anti-GBM disease) are rare, and it is almost impossible to perform an RCT due to the long recruitment period and lack of statistical power of a study with a limited sample size. In this regard, establishing national or international patient registries in the field of rare diseases may be required. Despite numerous efforts, however, the development of novel treatment in these rare conditions remains difficult.
Another major difficulty in performing RCTs in nephrology is the definition of adequate surrogate end points. In many renal diseases (e.g. IgAN), the natural history is measured in terms of decades. While patients suffering from advanced cases should be recruited to get a sufficient number of events, patients with severe disease may be less responsive to therapy and experience more complications. In the recent STOP-IgAN trial, for instance, it has been challenging to give cyclophosphamide to patients with Stage 4 CKD, which may result in significant infective complications [21]. The relatively short duration of follow-up is another area of criticism, as a long-term renoprotective effect may not be apparent in the first few years, especially when the immunosuppression group has higher rate of proteinuria remission.
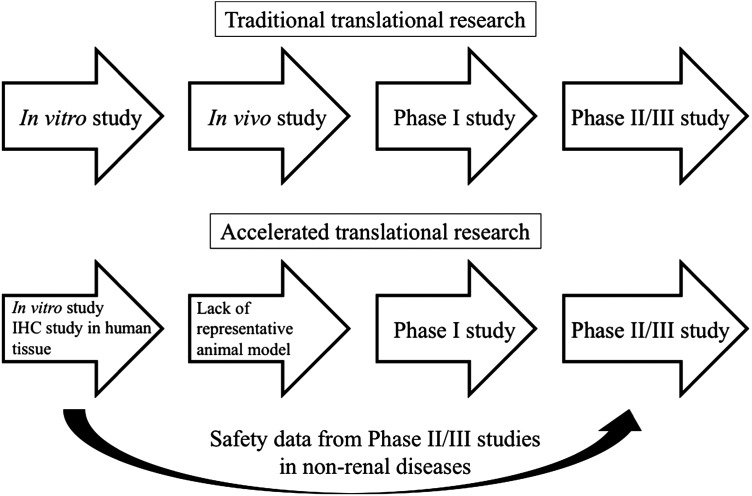
The use of TKIs in selected immune-mediated GN appears to be supported by animal models and human biopsy studies. Observational studies or case series, despite a lack of randomization, have much lower costs and are relatively easy to perform. Provided that the nephrologist in charge has an adequate understanding of the pharmacology and potential side effects, off-label use of TKIs may be justified on a case-by-case basis after adequate explanation to patients with close monitoring. In some diseases where a representative animal model is lacking (e.g. IgAN), the development of a clinical trial may still be justified based on valid in vitro models and human renal biopsy data. Therefore, we propose an accelerated pathway of translational research for the study of TKIs in GN research (Figure 1).
FIGURE 1:
Schematic diagram showing a proposed accelerated pathway of translational research for the study of tyrosine kinase inhibitors in GN research.
CONCLUSION
Targeting the tyrosine kinase signalling pathways represents a novel therapeutic target for the treatment of immune-mediated GN. Nonetheless, there is a persistent and even growing gap between advances in basic research and the development of clinical trials in GN research. Collaborations between scientists and clinicians are needed to address the current unmet medical needs and provide potential solutions to speed up translation into clinical practice and implementation of biomedical science advances.
ACKNOWLEDGEMENTS
T.K.W.M. received a scholarship for overseas training from the Hong Kong Kidney Foundation. S.P.M. received a Clinical Research Training Fellowship funded by the UK Medical Research Council (grant number G0901997/1). F.W.K.T. is supported by the Diamond Fund from the Imperial College Healthcare Charity and a ‘Making Every Kidney Count’ programme grant from Kidney Research UK. Part of this work was also supported by the UK Medical Research Council, the National Institute for Health Research Biomedical Research Centre based at Imperial College Healthcare NHS Trust and Imperial College London.
CONFLICT OF INTEREST STATEMENT
F.W.K.T. is the chief investigator of the randomized controlled trial of Syk inhibitor in IgAN. He has received research project grants from AstraZeneca, Baxter Biosciences, GlaxoSmithKline, MedImmune and Roche Palo Alto and has consultancy agreements with MedImmune and Rigel Pharmaceuticals.
REFERENCES
- 1.Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015; 386: 743–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Couser WG. Basic and translational concepts of immune-mediated glomerular diseases. J Am Soc Nephrol 2012; 23: 381–399 [DOI] [PubMed] [Google Scholar]
- 3.Hubbard SR, Till JH. Protein tyrosine kinase structure and function. Annu Rev Biochem 2000; 69: 373–398 [DOI] [PubMed] [Google Scholar]
- 4.Robinson DR, Wu YM, Lin SF. The protein tyrosine kinase family of the human genome. Oncogene 2000; 19: 5548–5557 [DOI] [PubMed] [Google Scholar]
- 5.Hubbard SR, Mohammadi M, Schlessinger J. Autoregulatory mechanisms in protein-tyrosine kinases. J Biol Chem 1998; 273: 11987–11990 [DOI] [PubMed] [Google Scholar]
- 6.Gocek E, Moulas AN, Studzinski GP. Non-receptor protein tyrosine kinases signaling pathways in normal and cancer cells. Crit Rev Clin Lab Sci 2014; 51: 125–137 [DOI] [PubMed] [Google Scholar]
- 7.Wu P, Nielsen TE, Clausen MH. FDA-approved small-molecule kinase inhibitors. Trends Pharmacol Sci 2015; 36: 422–439 [DOI] [PubMed] [Google Scholar]
- 8.Gaumann AK, Kiefer F, Alfer J, et al. Receptor tyrosine kinase inhibitors: are they real tumor killers? Int J Cancer 2016; 138: 540–554 [DOI] [PubMed] [Google Scholar]
- 9.Hojjat-Farsangi M. Targeting non-receptor tyrosine kinases using small molecule inhibitors: an overview of recent advances. J Drug Target 2016; 24: 192–211 [DOI] [PubMed] [Google Scholar]
- 10.Grimminger F, Schermuly RT, Ghofrani HA. Targeting non-malignant disorders with tyrosine kinase inhibitors. Nat Rev Drug Discov 2010; 9: 956–970 [DOI] [PubMed] [Google Scholar]
- 11.Flamant M, Bollée G, Hénique C, et al. Epidermal growth factor: a new therapeutic target in glomerular disease. Nephrol Dial Transplant 2012; 27: 1297–1304 [DOI] [PubMed] [Google Scholar]
- 12.Tang J, Liu N, Zhuang S. Role of epidermal growth factor receptor in acute and chronic kidney injury. Kidney Int 2013; 83: 804–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen JS, Chang LC, Huang SJ, et al. Targeting spleen tyrosine kinase-Bruton's tyrosine kinase axis for immunologically mediated glomerulonephritis. Biomed Res Int 2014; 2014: 814869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen S, Tang Z, Xiang H, et al. Etiology and outcome of crescentic glomerulonephritis from a single center in China: a 10-year review. Am J Kidney Dis 2016; 67: 376–383 [DOI] [PubMed] [Google Scholar]
- 15.Alchi B, Griffiths M, Sivalingam M, et al. Predictors of renal and patient outcomes in anti-GBM disease: clinicopathologic analysis of a two-centre cohort. Nephrol Dial Transplant 2015; 30: 814–821 [DOI] [PubMed] [Google Scholar]
- 16.Tanna A, Guarino L, Tam FW, et al. Long-term outcome of anti-neutrophil cytoplasm antibody-associated glomerulonephritis: evaluation of the international histological classification and other prognostic factors. Nephrol Dial Transplant 2015; 30: 1185–1192 [DOI] [PubMed] [Google Scholar]
- 17.Stone JH, Merkel PA, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 2010; 363: 221–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones RB, Tervaert JW, Hauser T, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med 2010; 363: 211–220 [DOI] [PubMed] [Google Scholar]
- 19.Hogan J, Avasare R, Radhakrishnan J. Is newer safer? Adverse events associated with first-line therapies for ANCA-associated vasculitis and lupus nephritis. Clin J Am Soc Nephrol 2014; 9: 1657–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. KDIGO clinical practice guideline for glomerulonephritis. Kidney Inter Suppl 2012; 2: 139–274 [Google Scholar]
- 21.Rauen T, Eitner F, Fitzner C, et al. Intensive supportive care plus immunosuppression in IgA nephropathy. N Engl J Med 2015; 373: 2225–2236 [DOI] [PubMed] [Google Scholar]
- 22.Ortiz A. Translational nephrology: what translational research is and a bird's-eye view on translational research in nephrology. Clin Kidney J 2015; 8: 14–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Becker GJ, Hewitson TD. Animal models of chronic kidney disease: useful but not perfect. Nephrol Dial Transplant 2013; 28: 2432–2438 [DOI] [PubMed] [Google Scholar]
- 24.Salama AD, Little MA. Animal models of ANCA associated vasculitis. Curr Opin Rheumatol 2012; 24: 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chalmers SA, Chitu V, Ramanujam M, et al. Therapeutic targeting of macrophages in lupus nephritis. Discov Med 2015; 20: 43–49 [PubMed] [Google Scholar]
- 26.Magistroni R, D'Agati VD, Appel GB, et al. New developments in the genetics, pathogenesis, and therapy of IgA nephropathy. Kidney Int 2015; 88: 974–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim MJ, McDaid JP, McAdoo SP, et al. Spleen tyrosine kinase is important in the production of proinflammatory cytokines and cell proliferation in human mesangial cells following stimulation with IgA1 isolated from IgA nephropathy patients. J Immunol 2012; 189: 3751–3758 [DOI] [PubMed] [Google Scholar]
- 28.Mócsai A, Ruland J, Tybulewicz VL. The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat Rev Immunol 2010; 10: 387–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McAdoo SP, Reynolds J, Bhangal G, et al. Spleen tyrosine kinase inhibition attenuates autoantibody production and reverses experimental autoimmune GN. J Am Soc Nephrol 2014; 25: 2291–2302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ryan J, Ma FY, Kanellis J, et al. Spleen tyrosine kinase promotes acute neutrophil-mediated glomerular injury via activation of JNK and p38 MAPK in rat nephrotoxic serum nephritis. Lab Invest 2011; 12: 1727–1738 [DOI] [PubMed] [Google Scholar]
- 31.Ryan J, Ma FY, Han Y, et al. Myeloid cell-mediated renal injury in rapidly progressive glomerulonephritis depends upon spleen tyrosine kinase. J Pathol 2016; 238: 10–20 [DOI] [PubMed] [Google Scholar]
- 32.McAdoo SP, Bhangal G, Page T, et al. Correlation of disease activity in proliferative glomerulonephritis with glomerular spleen tyrosine kinase expression. Kidney Int 2015; 88: 52–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith J, McDaid JP, Bhangal G, et al. A spleen tyrosine kinase inhibitor reduces the severity of established glomerulonephritis. J Am Soc Nephrol 2010; 21: 231–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flanc RS, Ma FY, Tesch GH, et al. A pathogenic role for JNK signaling in experimental anti-GBM glomerulonephritis. Kidney Int 2007; 72: 698–708 [DOI] [PubMed] [Google Scholar]
- 35.Ma FY, Flanc RS, Tesch GH, et al. Blockade of the c-Jun amino terminal kinase prevents crescent formation and halts established anti-GBM glomerulonephritis in the rat. Lab Invest 2009; 89: 470–484 [DOI] [PubMed] [Google Scholar]
- 36.Sheryanna A, Bhangal G, McDaid J, et al. Inhibition of p38 mitogen-activated protein kinase is effective in the treatment of experimental crescentic glomerulonephritis and suppresses monocyte chemoattractant protein-1 but not IL-1beta or IL-6. J Am Soc Nephrol 2007; 18: 1167–1179 [DOI] [PubMed] [Google Scholar]
- 37.López-Herrera G, Vargas-Hernández A, González-Serrano ME, et al. Bruton's tyrosine kinase—an integral protein of B cell development that also has an essential role in the innate immune system. J Leukoc Biol 2014; 95: 243–250 [DOI] [PubMed] [Google Scholar]
- 38.Mohamed AJ, Yu L, Bäckesjö CM, et al. Bruton's tyrosine kinase (Btk): function, regulation, and transformation with special emphasis on the PH domain. Immunol Rev 2009; 228: 58–73 [DOI] [PubMed] [Google Scholar]
- 39.Rankin AL, Seth N, Keegan S, et al. Selective inhibition of BTK prevents murine lupus and antibody-mediated glomerulonephritis. J Immunol 2013; 191: 4540–4550 [DOI] [PubMed] [Google Scholar]
- 40.Floege J, Eitner F, Alpers CE. A new look at platelet-derived growth factor in renal disease. J Am Soc Nephrol 2008; 19: 12–23 [DOI] [PubMed] [Google Scholar]
- 41.Boor P, Ostendorf T, Floege J. PDGF and the progression of renal disease. Nephrol Dial Transplant 2014; 29(Suppl 1): i45–i54 [DOI] [PubMed] [Google Scholar]
- 42.Shinkai Y, Cameron JS. Trial of platelet-derived growth factor antagonist, trapidil, in accelerated nephrotoxic nephritis in the rabbit. Br J Exp Pathol 1987; 68: 847–852 [PMC free article] [PubMed] [Google Scholar]
- 43.Iyoda M, Shibata T, Kawaguchi M, et al. Preventive and therapeutic effects of imatinib in Wistar-Kyoto rats with anti-glomerular basement membrane glomerulonephritis. Kidney Int 2009; 75: 1060–1070 [DOI] [PubMed] [Google Scholar]
- 44.Iyoda M, Shibata T, Wada Y, et al. Long- and short-term treatment with imatinib attenuates the development of chronic kidney disease in experimental anti-glomerular basement membrane nephritis. Nephrol Dial Transplant 2013; 28: 576–584 [DOI] [PubMed] [Google Scholar]
- 45.Kovacs E, Zorn JA, Huang Y, et al. A structural perspective on the regulation of the epidermal growth factor receptor. Annu Rev Biochem 2015; 84: 739–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bollée G, Flamant M, Schordan S, et al. Epidermal growth factor receptor promotes glomerular injury and renal failure in rapidly progressive crescentic glomerulonephritis. Nat Med 2011; 17: 1242–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alfieri C, Kavvadas P, Simonini P, et al. Discoidin domain receptor-1 and periostin: new players in chronic kidney disease. Nephrol Dial Transplant 2015; 30: 1965–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kerroch M, Guerrot D, Vandermeersch S, et al. Genetic inhibition of discoidin domain receptor 1 protects mice against crescentic glomerulonephritis. FASEB J 2012; 26: 4079–4091 [DOI] [PubMed] [Google Scholar]
- 49.Kerroch M, Alfieri C, Dorison A, et al. Protective effects of genetic inhibition of Discoidin Domain Receptor 1 in experimental renal disease. Sci Rep 2016; 6: 21262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Radford DJ, Lord JM, Savage COS. The activation of the neutrophil respiratory burst by anti-neutrophil cytoplasm autoantibody (ANCA) from patients with systemic vasculitis requires tyrosine kinases and protein kinase C activation. Clin Exp Immunol 1999; 118: 171–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van der Veen BS, Chen M, Müller R, et al. Effects of p38 mitogen-activated protein kinase inhibition on anti-neutrophil cytoplasmic autoantibody pathogenicity in vitro and in vivo. Ann Rheum Dis 2011; 70: 356–365 [DOI] [PubMed] [Google Scholar]
- 52.Hewins P, Williams JM, Wakelam MJ, et al. Activation of Syk in neutrophils by antineutrophil cytoplasm antibodies occurs via Fcgamma receptors and CD18. J Am Soc Nephrol 2004; 15: 796–808 [DOI] [PubMed] [Google Scholar]
- 53.McAdoo SP, Tanna A, McDaid J, et al. SYK inhibition in experimental autoimmune vasculitis and its glomerular expression in ANCA-associated vasculitis. Lancet 2014; 383: 72 [Google Scholar]
- 54.Kim NH, Oh JH, Seo JA, et al. Vascular endothelial growth factor (VEGF) and soluble VEGF receptor FLT-1 in diabetic nephropathy. Kidney Int 2005; 67: 167–177 [DOI] [PubMed] [Google Scholar]
- 55.Le Roux S, Pepper RJ, Dufay A, et al. Elevated soluble Flt1 inhibits endothelial repair in PR3-ANCA-associated vasculitis. J Am Soc Nephrol 2012; 23: 155–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bahjat FR, Pine PR, Reitsma A, et al. An orally bioavailable spleen tyrosine kinase inhibitor delays disease progression and prolongs survival in murine lupus. Arthritis Rheum 2008; 58: 1433–1444 [DOI] [PubMed] [Google Scholar]
- 57.Deng GM, Liu L, Bahjat FR, et al. Suppression of skin and kidney disease by inhibition of spleen tyrosine kinase in lupus-prone mice. Arthritis Rheum 2010; 62: 2086–2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hutcheson J, Vanarsa K, Bashmakov A, et al. Modulating proximal cell signaling by targeting Btk ameliorates humoral autoimmunity and end-organ disease in murine lupus. Arthritis Res Ther 2012; 14: R243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mina-Osorio P, LaStant J, Keirstead N, et al. Suppression of glomerulonephritis in lupus-prone NZB × NZW mice by RN486, a selective inhibitor of Bruton's tyrosine kinase. Arthritis Rheum 2013; 65: 2380–2391 [DOI] [PubMed] [Google Scholar]
- 60.Zoja C, Corna D, Rottoli D, et al. Imatinib ameliorates renal disease and survival in murine lupus autoimmune disease. Kidney Int 2006; 70: 97–103 [DOI] [PubMed] [Google Scholar]
- 61.Sadanaga A, Nakashima H, Masutani K, et al. Amelioration of autoimmune nephritis by imatinib in MRL/lpr mice. Arthritis Rheum 2005; 52: 3987–3996 [DOI] [PubMed] [Google Scholar]
- 62.Nakopoulou L, Stefanaki K, Boletis J, et al. Immunohistochemical study of epidermal growth factor receptor (EGFR) in various types of renal injury. Nephrol Dial Transplant 1994; 9: 764–769 [PubMed] [Google Scholar]
- 63.Planque S, Zhou YX, Nishiyama Y, et al. Autoantibodies to the epidermal growth factor receptor in systemic sclerosis, lupus, and autoimmune mice. FASEB J 2003; 17: 136–143 [DOI] [PubMed] [Google Scholar]
- 64.Costa-Reis P, Russo PA, Zhang Z, et al. The role of microRNAs and human epidermal growth factor receptor 2 in proliferative lupus nephritis. Arthritis Rheumatol 2015; 67: 2415–2426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dong J, Wang QX, Zhou CY, et al. Activation of the STAT1 signaling pathway in lupus nephritis in MRL/lpr mice. Lupus 2007; 16: 101–109 [DOI] [PubMed] [Google Scholar]
- 66.Wang S, Yang N, Zhang L, et al. Jak/STAT signaling is involved in the inflammatory infiltration of the kidneys in MRL/lpr mice. Lupus 2010; 19: 1171–1180 [DOI] [PubMed] [Google Scholar]
- 67.Lu LD, Stump KL, Wallace NH, et al. Depletion of autoreactive plasma cells and treatment of lupus nephritis in mice using CEP-33779, a novel, orally active, selective inhibitor of JAK2. J Immunol 2011; 187: 3840–3853 [DOI] [PubMed] [Google Scholar]
- 68.Leung JC, Tang SC, Chan LY, et al. Polymeric IgA increases the synthesis of macrophage migration inhibitory factor by human mesangial cells in IgA nephropathy. Nephrol Dial Transplant 2003; 18: 36–45 [DOI] [PubMed] [Google Scholar]
- 69.Ranieri E, Gesualdo L, Grandaliano G, et al. The role of alpha-smooth muscle actin and platelet-derived growth factor-beta receptor in the progression of renal damage in human IgA nephropathy. J Nephrol 2001; 14: 253–262 [PubMed] [Google Scholar]
- 70.Rintala JM, Savikko J, Rintala SE, et al. Epidermal growth factor receptor inhibition with erlotinib ameliorates anti-Thy 1.1-induced experimental glomerulonephritis. J Nephrol 2016; 29: 359–365. [DOI] [PubMed] [Google Scholar]
- 71.Zhai YL, Zhu L, Shi SF, et al. Elevated soluble VEGF receptor sFlt-1 correlates with endothelial injury in IgA nephropathy. PLoS One 2014; 9: e101779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hill GS, Karoui KE, Karras A, et al. Focal segmental glomerulosclerosis plays a major role in the progression of IgA nephropathy. I. Immunohistochemical studies. Kidney Int 2011; 79: 635–642 [DOI] [PubMed] [Google Scholar]
- 73.Wallace E, Gewin L. Imatinib: novel treatment of immune-mediated kidney injury. J Am Soc Nephrol 2013; 24: 694–701 [DOI] [PubMed] [Google Scholar]
- 74.Dwyer JP, Yates KM, Sumner EL, et al. Chronic myeloid leukemia-associated membranoproliferative glomerulonephritis that responded to imatinib mesylate therapy. Clin Nephrol 2007; 67: 176–181 [DOI] [PubMed] [Google Scholar]
- 75.Subramanian M, Kilara N, Manjunath R, et al. Membranoproliferative glomerulonephritis secondary to chronic myeloid leukemia. Saudi J Kidney Dis Transpl 2010; 21: 738–741 [PubMed] [Google Scholar]
- 76.Wallace E, Fogo AB, Schulman G. Imatinib therapy for non-infection-related type II cryoglobulinemia with membranoproliferative glomerulonephritis. Am J Kidney Dis 2012; 59: 122–125 [DOI] [PubMed] [Google Scholar]
- 77.Yilmaz M, Lahoti A, O'Brien S, et al. Estimated glomerular filtration rate changes in patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. Cancer 2015; 121: 3894–3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gafter-Gvili A, Ram R, Gafter U, et al. Renal failure associated with tyrosine kinase inhibitors—case report and review of the literature. Leuk Res 2010; 34: 123–127 [DOI] [PubMed] [Google Scholar]
- 79.de Oliveira RA, Marques ID, Seguro AC, et al. Electrolyte disturbances and acute kidney injury induced by imatinib therapy. NDT Plus 2009; 2: 27–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Al Aly Z, Philoctête Ashley JM, Gellens ME, et al. Thrombotic thrombocytopenic purpura in a patient treated with imatinib mesylate: true association or mere coincidence? Am J Kidney Dis 2005; 45: 762–768 [DOI] [PubMed] [Google Scholar]
- 81.Vidal-Petiot E, Rea D, Serrano F, et al. Imatinib increases serum creatinine by inhibiting its tubular secretion in a reversible fashion in chronic myeloid leukemia. Clin Lymphoma Myeloma Leuk 2016; 16: 169–174 [DOI] [PubMed] [Google Scholar]
- 82.Sasaki K, Lahoti A, Jabbour E, et al. Clinical safety and efficacy of nilotinib or dasatinib in patients with newly diagnosed chronic-phase chronic myelogenous leukemia and pre-existing liver and/or renal dysfunction. Clin Lymphoma Myeloma Leuk 2016; 16: 152–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ozkurt S, Temiz G, Acikalin MF, et al. Acute renal failure under dasatinib therapy. Ren Fail 2010; 32: 147–149 [DOI] [PubMed] [Google Scholar]
- 84.Holstein SA, Stokes JB, Hohl RJ. Renal failure and recovery associated with second-generation Bcr-Abl kinase inhibitors in imatinib-resistant chronic myelogenous leukemia. Leuk Res 2009; 33: 344–347 [DOI] [PubMed] [Google Scholar]
- 85.Martino S, Daguindau E, Ferrand C, et al. A successful renal transplantation for renal failure after dasatinib-induced thrombotic thrombocytopenic purpura in a patient with imatinib-resistant chronic myelogenous leukaemia on nilotinib. Leuk Res Rep 2013; 2: 29–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wallace E, Lyndon W, Chumley P, et al. Dasatinib-induced nephrotic-range proteinuria. Am J Kidney Dis 2013; 61: 1026–1031 [DOI] [PubMed] [Google Scholar]
- 87.Scott IC, Scott DL. Spleen tyrosine kinase inhibitors for rheumatoid arthritis: where are we now? Drugs 2014; 74: 415–422 [DOI] [PubMed] [Google Scholar]
- 88.Salgado E, Maneiro JR, Carmona L, et al. Safety profile of protein kinase inhibitors in rheumatoid arthritis: systematic review and meta-analysis. Ann Rheum Dis 2014; 73: 871–882 [DOI] [PubMed] [Google Scholar]
- 89.Weinblatt ME, Kavanaugh A, Genovese MC, et al. An oral spleen tyrosine kinase (Syk) inhibitor for rheumatoid arthritis. N Engl J Med 2010; 363: 1303–1312 [DOI] [PubMed] [Google Scholar]
- 90.Skinner M, Philp K, Lengel D, et al. The contribution of VEGF signalling to fostamatinib-induced blood pressure elevation. Br J Pharmacol 2014; 171: 2308–2320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Izzedine H. Anti-VEGF cancer therapy in nephrology practice. Int J Nephrol 2014; 2014: 143426. [DOI] [PMC free article] [PubMed] [Google Scholar]