Abstract
SLE is a chronic autoimmune disease caused by perturbations of the immune system. The clinical presentation is heterogeneous, largely because of the multiple genetic and environmental factors that contribute to disease initiation and progression. Over the last 60 years, there have been a number of significant leaps in our understanding of the immunological mechanisms driving disease processes. We now know that multiple leucocyte subsets, together with inflammatory cytokines, chemokines and regulatory mediators that are normally involved in host protection from invading pathogens, contribute to the inflammatory events leading to tissue destruction and organ failure. In this broad overview, we discuss the main pathways involved in SLE and highlight new findings. We describe the immunological changes that characterize this form of autoimmunity. The major leucocytes that are essential for disease progression are discussed, together with key mediators that propagate the immune response and drive the inflammatory response in SLE.
Keywords: systemic lupus erythematosus, SLE, SLE pathogenesis, autoantibodies, immunology, tolerance, inflammation, tissue destruction
Rheumatology key messages
SLE is a heterogeneous disease influenced by a variety of genetic and environmental factors.
Innate and adaptive immune responses contribute to the autoimmune dysfunction observed in SLE.
Autoantibodies form immune complexes that drive target organ inflammation in most individuals with SLE.
Introduction
Autoimmunity affects ∼8% of the global population. However, the incidence is increasing because of a number of factors, including awareness and improved clinical diagnoses [1]. Moreover, there is evidence that autoimmune diseases are a leading cause of death in young females within the USA [2]. SLE affects primarily women, with a gender bias of 9:1, which has been attributed in part to oestrogen receptor-1 and to undefined immunomodulatory genes on the X chromosome [3]. There is no prevention or cure, and the mainstay of treatment is immunosuppressive therapy. Disease aetiology involves genetic predisposition and environmental factors, with the influence of gender [4]. Over 60 genetic regions have been associated with the development or severity of human disease [5]. These genetic associations have directed research towards multiple pathways involved in innate and adaptive immunity.
Comprehensive discussions of the history of SLE have been described previously [6, 7]. An important step in determining that SLE is a disease of the immune system, or an immunological disease, was taken in 1948 by Dr Hargraves, who discovered the LE cell or LE body. The LE cell is a neutrophil or macrophage in the bone marrow that has a distinct morphology by haematoxylin staining as a result of the phagocytosis of nuclear debris. The presence of these cells is indicative of SLE or other connective tissues disorders. Later, the LE cell phenotype was defined as an ANA reaction because it could be reproduced from bone marrow preparations with the addition of SLE serum [7]. This observation and test provided the key to linking SLE to immunological dysfunction. The LE test was subsequently replaced by serum ANA assays [8]. Since these early discoveries, there has been a dramatic progression in our understanding of the immunological pathways driving disease.
The cellular roles of individual leucocytes, including B cells, T cells and myeloid cells, in the initiation and progression of SLE have been recently reviewed elsewhere [9–12]. There are several known factors that contribute to the initiation and progression of autoimmunity in SLE. These factors include the following: the initial break in tolerance and generation of autoantigen-specific effector B and T lymphocytes and the subsequent production of ANAs; defects in cell death or debris clearance pathways and the continued generation of self-antigens; and tissue inflammation and deficiencies in immune regulation combined with mechanisms that propagate chronicity to drive lupus immunopathology.
Breaking immune tolerance and the development of autoimmunity
The loss of tolerance to self and the subsequent elevation in serum ANA levels is proposed to be a crucial first step in the development of SLE [13–15]. This observation is supported by the finding that autoantibodies can be detected before clinical symptoms in most SLE patients [15]. The presence of autoantibodies in patients, including anti-dsDNA, anti-SSA (Ro), anti-SSB (La), anti-Sm and anti-RNPs, suggests that a common mechanism is involved in the peripheral expansion of autoreactive B cells that has yet to be delineated fully [15–17].
The majority of B cells generated are self-reactive and are usually removed by central tolerance mechanisms in the bone marrow, including receptor editing, deletion or the induction of anergy, reviewed by Meffre [13] and Goodnow et al. [18]. These are B-cell-intrinsic mechanisms and are known to be controlled by B-cell antigen receptor signalling thresholds and regulators of the phosphoinositide 3-kinase pathway [19–21]. Recently, the miR-17–92 family of miRNA has been shown to regulate central B-cell tolerance by targeting phosphatase and tensin homolog [20, 22]. Additional removal of autoreactive B cells occurs by selection mechanisms in the periphery, which are less clear but can involve impaired survival and anergy [23]. Elevated levels of B-cell activating factor (BAFF, also known as B lymphocyte stimulator (BLyS) or CD257), which are observed in SLE patients (see below) [24, 25], have been shown in mouse models to promote a breach in B-cell tolerance and enhance the survival of autoreactive B cells [26]. Evidence from SLE patients has shown that there is failure in both central B-cell checkpoints in the bone marrow and peripheral checkpoints at the transitional-naive B-cell stage [27]. Furthermore, SLE patients exhibit a defect in anergy of naive B cells [28].
Additionally, the generation of de novo autoreactive B cells occurs after maturation as a result of somatic hypermutation in germinal centres (GCs) [29]. It is believed that the majority of pathogenic autoantibodies are somatically hypermutated, class-switched IgGs. This class-switching from IgM to IgG occurs primarily, but not solely, in GCs, through interactions of the B cell with antigen and with CD4+ T follicular helper (Tfh) cells, which are identified by the markers CD4, inducible T-cell costimulator (ICOS), C-X-C chemokine receptor type 5 (CXCR5), CD57 and programmed cell death protein 1 (PD-1) [12, 30–33]. B cell activation in GCs is followed by expansion and differentiation into autoreactive plasmablasts and plasma cells that secrete high levels of antibodies to autoantigens. The targeted deletion of IFN-γ, Toll-like receptor 7 (TLR7) and signal transducer and activator of transcription 1 (STAT1) in mice results in the disruption of autoreactive GCs and impaired production of IgG autoantibodies [34–36] (Fig. 1). Importantly, it has been shown that the IFN-γ receptor (IFN-γR) requirement for the class-switch recombination of pathogenic autoantibodies and the subsequent development of systemic autoimmunity is B-cell intrinsic [35, 37]. A T-box transcription factor (T-bet) also contributes to pathogenic autoantibody production [35, 37]. TLR7 within the B cell is also required for spontaneous GC formation and antibody production [35, 36, 38, 39].
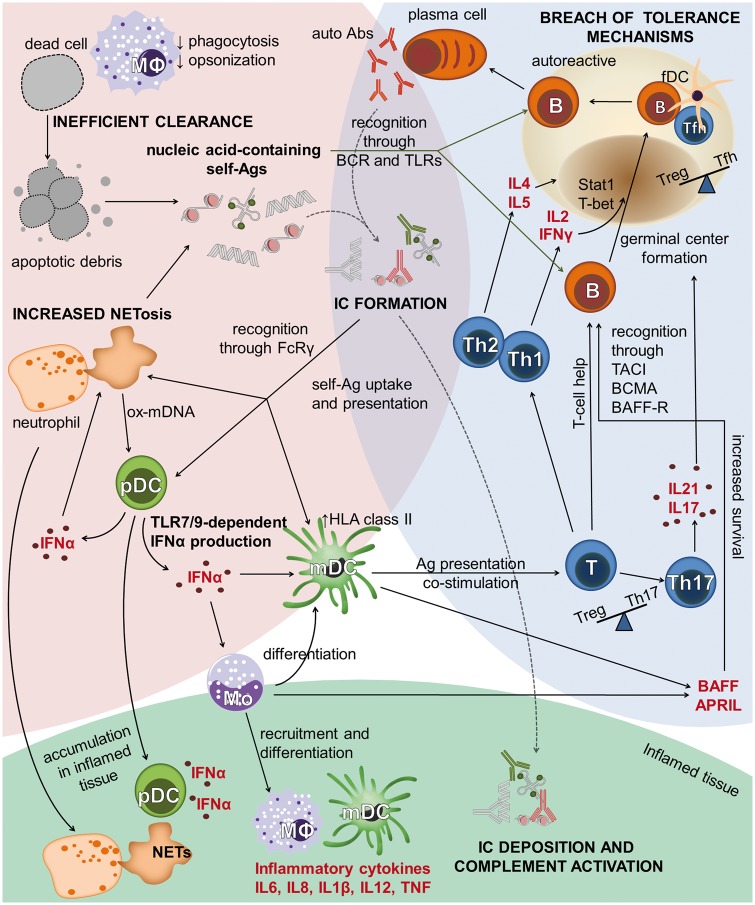
Fig. 1.
Overview of immunological pathways leading to SLE
The development of SLE occurs in three interconnected phases, illustrated by coloured backgrounds. Loss of adaptive immune tolerance (blue) leads to an increase in autoreactive B cells. Signals from self-antigens, TLR ligands, BAFF/APRIL and T-cell-derived cytokines promote the formation of germinal centres and the production of autoantibodies. Innate immune defects leading to increased availability of self-antigens (pink) include increased NETosis, impaired clearance of apoptotic debris and reduced phagocytosis. Self-antigens form ICs with autoantibodies, enabling FcRγ-mediated uptake and activation of several downstream pathways. Inflammation and tissue damage (green) is caused by mediators released by recruited inflammatory cells and IC-induced complement activation. Abs: antibodies; Ags: antigens; APRIL (CD256): a proliferation-inducing ligand; B: B cell; BAFF (CD257): B-cell-activating factor; BAFF-R: B-cell-activating factor receptor; BCMA: B-cell maturation antigen; BCR: B-cell antigen receptor; FcRγ: Fc receptor-γ; fDC: follicular dendritic cell; HLA class II: human leucocyte antigen class II; mDC: myeloid dendritic cell; MΦ: macrophage; Mo: monocyte; NET: neutrophil extracellular trap; ox-mDNA: oxidized mitochondrial DNA; pDC: plasmacytoid dendritic cell; Stat1: signal transducer and activator of transcription (a transcription factor); T: T cell; TACI (CD267): transmembrane activator, calcium modulator and cyclophilin ligand interactor; T-bet: a T-box transcription factor; Tfh: T follicular helper; TLR7/9: Toll-like receptors 7 and 9.
Other mechanisms that might contribute to ANA production include molecular mimicry; for example, autoantibodies can be induced during an infection as a result of activation of lymphocytes that recognize foreign antigens that cross-react with autoantigens [40]. Alternatively, injury to tissues during infection can induce epitope spreading from immune responses against pathogens to tissue antigens. Often these self-antigens have undergone chemical modifications as a result of the inflammatory response [41, 42]. Autoantibodies in target tissues form immune complexes (ICs), which, combined with inflammatory cytokines from infiltrating leucocytes, perpetuate organ inflammation and tissue injury.
Similar to B lymphocytes, T cells undergo tolerance mechanisms to restrict autoreactivity. Multiple autoimmune-prone strains have demonstrated a requirement for both B and CD4+ T cells for the production of IgG autoantibodies, indicating that loss of T-cell tolerance may play a role in lupus [43]. Tolerance mechanisms include deletion of autoreactive T cells in the thymus during development, and peripheral mechanisms such as apoptosis, anergy or inhibition by Treg [44]. In SLE, there are decreased numbers of recent thymic emigrants, suggesting that central T-cell tolerance mechanisms are dysregulated [45]. A contributing factor could be the upregulated gene expression of HLA-D region and antigen-presentation pathways in SLE patients’ dendritic cells (DCs), as was recently reported [46]. Moreover, elevated expression of HLA-DR on DCs could impact tolerance of autoreactive T cells in secondary lymphoid organs. Peripheral autoreactive T-cell tolerance can be disrupted by exposure to pathogens and by a variety of other mechanisms [47]. In SLE, abnormalities in proximal signalling pathways can contribute to altered T-cell tolerance and responsiveness [48]. Dysfunction in autoreactive T-cell anergy can be mediated by several factors, including genetic variants, epigenetic modifications or alterations in gene regulation. Such dysfunction can result in hyperactive T-cell responses, disruption of T-cell trafficking patterns and alter the balance of T-cell pro-inflammatory and anti-inflammatory cytokine production [48].
Treg cells appear to play a major role in the maintenance of peripheral autoreactive T-cell anergy. Although reports of altered Treg numbers and function in SLE have been contradictory, Treg defects are observed in some SLE patients [49]. Low-dose IL-2 therapy can correct these defects, reduce the relative frequency of Tfh and Th17 cells and decrease disease activity, suggesting that disruption of the balance of Treg to Tfh and/or Th17 cells may contribute to loss of tolerance in SLE [50, 51]. These observations are also supported by studies in murine models [52].
Tfh cells are important for the activation and selection of B cells within GCs [53]. Tfh cells are found at increased frequencies in the peripheral circulation of SLE patients undergoing flares, and in LN, Tfh cells can be found in the kidneys [12]. Elevated numbers of Tfh cells are associated with increased disease activity and decreased Treg numbers and/or function in SLE patients and autoimmune mouse models [30–33, 54–56]. IFN-γR signalling is also necessary for Tfh cell development, and consistent with this, excess of IFN-γR signalling leads to an accrual of Tfh cells, which is associated with elevated ANA titres, a higher frequency of circulating activated B cells (plasmablasts) and disease activity [35, 37, 57] . Interestingly, higher levels of serum IFN-γ, along with IL-5 and IL-6, have been detected >3 years before SLE diagnosis, suggesting their importance in the development of disease [58].
Generation of self-ligand and impaired clearance mechanisms
It is believed that pathogenic class-switched IgG autoantibodies play a significant role in the inflammatory processes that lead to tissue destruction. These antibodies are produced by B cells that have been activated by self-antigens [59]. Current lines of evidence suggest that increased nuclear debris in the periphery of SLE patients might provide a source of excess autoantigens that contribute to increased serum ANA levels [59–61]. Nuclear debris results from increased apoptotic or necrotic cells or impaired uptake of dying cells through phagocytosis by neutrophils and macrophages [60, 62, 63]. Phagocytosis is defective in macrophages derived from SLE patients [64, 65]. Studies in murine models suggest that deficiencies in cell surface proteins, such as receptor tyrosine kinases Mer and Axl or the combination of milk fat globule-EGF factor 8 and T-cell immunoglobulin- and mucin-domain-containing molecule, involved in the clearance of circulating cellular debris, may contribute to autoimmunity [66–68]. Opsonins, such as CRP, C1q, serum amyloid P, mannose-binding lectin, pentraxin 3 and other soluble proteins, bind to apoptotic cells and facilitate their clearance [64]. In SLE, reduced levels of CRP, which binds to nuclear proteins and phosphatidylethanolamine on damaged cell membranes, contribute to defects in cell debris clearance [69]. In addition, autoantibodies binding to opsonins on circulating necrotic cell debris can generate ICs and promote pro-inflammatory responses and tissue damage, as discussed below [69].
Neutrophils also undergo a unique process ultimately resulting in cell death, known as NETosis, the release of neutrophil extracellular traps (NETs), which is enhanced in paediatric SLE [70, 71]. This specific form of liberating nuclear contents is believed to contribute to the autoantigens necessary for the persistent autoreactive inflammatory response [70] (Fig. 1). In SLE, neutrophils can undergo NETosis after priming by type I IFNs and activation induced by cytokines (IL-1β, IL-8, IL-17 and TNF) and anti-RNP antibodies or autoantibodies to cell surface anti-microbial peptides (cathelicidin LL-37, human neutrophil peptide) [70, 71]. The nuclear contents (DNA, RNA, histones, etc.) are released in the form of spider web-like NETs [72]. The released DNA and RNA is coated with self-proteins, including anti-microbial peptides which stabilize the NETs in an immunogenic form [70, 71]. Serum samples from active SLE patients fail to degrade NETs efficiently because they contain autoantibodies and C1q, which inhibit DNase-I degradation of chromatin [73, 74]. Moreover, renal DNase-I is downregulated in late-stage LN [73, 75]. These findings are consistent with observations in murine models demonstrating that loss of DNase-I accelerates LN [76].
The nuclear material forms ICs with the autoantibodies, which are then taken up through either the B-cell antigen receptor on B cells or FcγR on DCs [77, 78] (Fig. 1). This can then activate specific intracellular innate receptors, including TLR7 and Toll-like receptor 9 (TLR9), which drive cellular activation and the production of pro-inflammatory cytokines, including IL-6, IL-8, IL-1β, IL-12 and TNF. Additionally, oxidized mitochondrial DNA released by SLE neutrophils can stimulate plasmacytoid DCs (pDCs) to produce IFN [79]. In SLE, neutrophils potentially represent a major reservoir of autoantigens to drive B-cell and DC activation and effector function, resulting in propagation of the inflammatory response.
The complement system is a major mechanism of innate immunity. It plays an important role in the lysis of invading bacteria, in the clearance of antibodies found in ICs and in the removal of cellular debris [80]. Activation of three possible pathways (classical, alternative or lectin) induces an enzymatic cascade of activated and cleaved complement proteins. However, genetic deficiencies in predominantly the classical components, including C1 (C1s-C1r, C1q), C2 and C4 have been associated with the development of SLE, reviewed by Sturfelt and Truedsson [81]. In most SLE patients, complement activity is reduced during episodes of inflammation.
The classical pathway is activated by the binding of C1q to clusters of IgG or IgM, CRP or apoptotic cell debris. This triggers the enzymatic cascade through a series of proteases to cleave C4, C3 and, ultimately, C5. The resulting products, C3a, C4a and C5a, are potent chemoattractive agents, which can recruit inflammatory cells. The C3b and C4b fragments bind ICs, which then activate complement receptors on macrophages that clear them from circulation in the spleen and liver.
The complement system also plays a role in peripheral tolerance. Genetic ablation of C4 in a murine model reduced negative selection during the progression from transitional to mature naive B cells [82]. These findings are consistent with the high frequency of patients with deficiencies in early complement components who have SLE or other autoimmune disorders [81, 83]. Taken together, these studies demonstrate that while complement activation can promote inflammation and tissue damage in SLE, defects in complement-dependent clearance of ICs and apoptotic debris results in more antigen availability. This, in turn, promotes loss of adaptive immune tolerance and promotes autoimmunity.
Immune-mediated perpetuation of inflammation
Positive feedback loops resulting from the loss of adaptive immune tolerance, the formation of ICs and the activation of the innate immune system perpetuate inflammatory responses that may result in target organ injury (Fig. 1) [84].
Below, we discuss key mediators involved in the immune-mediated perpetuation of the inflammatory response. A recent report has described elevations in a number of serum innate and adaptive cytokines before SLE disease onset [58]. These include the innate cytokines IL-23 and IL-12p70 and TNF superfamily members BAFF (CD257) and CD256 (a proliferation-inducing ligand, APRIL), the IFN-inducible chemokines IP-10 and CXCL9, the Th1 cytokines IFN-γ and IL-2, the Th2 cytokine IL-5 and the Th17-associated cytokine IL-21. In addition, soluble serum TNFRI and TNFRII levels were elevated, whereas regulatory TGFβ levels were decreased [58]. Importantly, the combination of certain mediators (CXCL9, IFN-γ, IL-5, IL-6) with ANA titres and the presence of anti-Ro and anti-SSA antibodies had a greater predictive power for the development of SLE than autoantibodies alone. These cytokines along with others demonstrated to be key in the autoimmune response, such as type I IFNs and IL-6, are discussed below. These studies further highlight the dysregulation of multiple innate and adaptive pathways required to perpetuate autoreactive immune responses by undergoing a series of amplification steps, each with increasing complexity, leading to the establishment of full-blown disease.
DCs and type I IFNs
DCs play an important role in the initial stages of lymphocyte activation, presenting antigen to drive the immune response. In SLE, debris from apoptotic cells can be presented as self-antigens to propagate B- and T-cell hyperreactivity [62, 85]. Furthermore, previous data have shown that monocytes cultured with serum from SLE patients mature into cells with myeloid DC-like morphology and function [86]. These studies demonstrated that differentiation of healthy control monocytes by SLE serum was dependent on IFN-α and correlated with SLE disease activity. This has important implications, as it has been proposed that lymph node myeloid dendritic cells (mDCs) participate in the maintenance of peripheral tolerance by regulating autoreactive T cells. IFN-α upregulates costimulatory expression and TLR7 mRNA expression in human mDCs, making them potentially potent antigen-presenting cells for foreign and self-antigens [87]. In BXSB and Y-autoimmune accelerator (Yaa)-associated murine models of lupus, a 2-fold increase in TLR7 expression is required for the development of severe disease [34, 88–91]. Moreover, the increase in DCs, and not B cells, is crucial for TLR7-induced severe pathology [87, 89].
In SLE, pDCs play an important role through the production of IFN-α. ICs activate immature pDCs via the innate TLRs, TLR7 and TLR9, to produce inflammatory cytokines, including type I IFNs [92]. Although pDCs are reduced in the periphery of SLE patients with active disease, they accumulate at inflammatory sites in tissues, including cutaneous lesions and kidneys [86, 93–95].
Type I IFNs serve to propagate autoimmune responses through activities including the maturation of monocytes into mDCs (see above) [86], the priming of neutrophils to undergo NETosis in the presence of anti-RNP autoantibodies [70] and the promotion of B-cell responses to TLR7 engagement [96]. Previous data have shown that an elevated serum IFN-α level is a heritable trait that can contribute to SLE disease susceptibility [97, 98]. The majority of SLE patients have a consistent upregulation in a broad array of type I IFN-responsive genes compared with controls [99–102]. This IFN signature could be induced by IFN and correlated with increased serum type I IFN levels [101, 103]. Mixed results were obtained in longitudinal studies correlating an IFN signature score with disease activity [104–106]. A specific IFN signature, represented by a select number (of the order of 5–30 mRNAs) of reproducibly affected expressed genes, has been successfully used as a diagnostic biomarker or in clinical trials as a pharmacodynamic biomarker [107, 108]. For example, in studies of sifalimumab and rontalizumab, mAbs targeting type I IFN, a dose-dependent suppression of the IFN signature was correlated with improvement in clinical symptoms in SLE patients [109, 110]. Thus, the IFN signature appears to be detectable in most, but perhaps not all, lupus patients’ peripheral blood cells, during increases in disease activity.
In addition to TLRs, type I IFN responses can be induced by stimulation of cytosolic DNA or RNA sensors. Mutations or deficiencies in related signalling pathway members have been shown to alter lupus-like disease significantly in humans and in murine models. For example, in humans a constitutively active mutant STING (stimulator of IFN genes) protein has been shown to be associated with elevated serum type I IFN and an IFN signature in peripheral blood mononuclear cells, resulting in a lupus-like syndrome [111]. Unexpectedly, the MRL/lpr mouse model of lupus intercrossed to mice deficient in the key downstream signalling component STING demonstrated accelerated lupus pathology, including increased autoantibody production and TLR-mediated cytokine production [112]. These studies suggest that in addition to mediating signalling by cytosolic sensors, STING might activate negative feedback mechanisms that control inflammatory responses. Further studies are warranted to gain a full understanding of the role of the cytosolic sensors and their signalling pathways in lupus.
IL-6
IL-6 is a potent cytokine produced by innate and adaptive cells. It stimulates B-cell growth and B- and T-cell differentiation, as observed by their diminished activity in mice deficient for IL-6 [113, 114]. IL-6 can also contribute to tissue damage independent of its role in B- and T-cell activation [115]. IL-6, like IL-1β, has a number of regulatory homeostatic functions beyond immune regulation and induces other mediators during the acute phase response to infections. Deficiency of IL-6 in numerous murine lupus models results in amelioration of autoantibody production, inflammation and glomerulonephritis [116–118]. Given the important role of IL-6 in the innate and adaptive immune responses, tocilizumab, an anti-IL-6 receptor antibody, was developed as a potential therapy for autoimmune diseases. Similar to murine models, blockade of IL-6 was shown to decrease B-cell hyperactivity evidenced by decreased serum anti-dsDNA levels and disease activity in SLE patients [116, 119–121].
IFN-γ
IFN-γ signalling in B cells promotes autoreactive GC formation and autoantibody production [35, 37]. Complementary to the findings of Rahman and Rawlings and colleagues discussed above for B-cell-intrinsic regulation of GC formation, genetic ablation of IFN-γ in the MRLlpr and B6.Sle1b murine models prevents lupus-like disease progression, including glomerulonephritis [37, 122]. IFN-γ was shown to amplify macrophage activation, enhance anti-dsDNA autoantibody production and upregulate the expression of MHC classes I and II on renal cells, which promoted the inflammatory response in the target tissue. IFN-γ-mediated production of BAFF by myeloid cells was also shown to contribute to disease in the Lyn-deficient lupus model [123]. Thus, IFN-γ contributes to the innate and adaptive arms of the immune response and augments inflammation in target tissues.
IL-21
IL-21 is produced primarily by Tfh cells and is important for B-cell expansion in the GC, class switch recombination and the generation of plasma cells [31, 33, 124, 125]. More recently, it has been shown that IL-21, together with IL-6, drives Tfh cell expansion in humans and mice [31, 126]. Furthermore, IL-21 also contributes to Th17 cell differentiation and the expansion of CD8+ suppressor T cells [127]. Despite its role in expansion of regulatory cells, IL-21 was found to play a crucial role in driving lupus-like disease in the BXSB.Yaa murine lupus model [127]. Therefore, IL-21 appears to have the potential to play both positive and negative roles in promotion of disease manifestations.
BAFF (CD257)
BAFF is primarily secreted by myeloid cells, but can also be produced by other types of cells. It stimulates the expansion, differentiation and antibody production of B cells [128, 129], and its overexpression promotes loss of B-cell tolerance [26]. It can be active in membrane or soluble form and it can bind to three receptors (transmembrane activator, calcium modulator, and cyclophilin ligand interactor (TACI), B cell maturation antigen (BCMA) and BAFF-R) on B cells, whose expression is modulated in SLE with increased disease activity [24, 25, 130–132]. BAFF is elevated in the circulation of SLE patients and was shown to be correlated with anti-dsDNA antibody titres. Mixed results were reported for correlations with disease activity, and BAFF levels were decreased in patients treated with CSs [24, 25, 132]. Of note, 17β-estradiol induced soluble BAFF, anti-dsDNA and anti-C1q in a murine lupus model [133]. A mAb to soluble BAFF, belimumab, has been approved by the US Food and Drug Administration for the treatment of SLE and has been found to reduce disease activity and the number of lupus flares [134, 135]. Although this is considered a breakthrough in lupus treatment, further studies are required to determine the best regimen incorporating this treatment for patient subgroups.
Adaptive immune pro-inflammatory mediators
Aside from being essential for ANA production, B cells and T cells play a crucial role in the inflammatory events that contribute to disease progression [136]. B cells can function as antigen-presenting cells for memory T-cell responses; they produce an array of cytokines and can function in a regulatory capacity [137]. Regulatory B-cell activity has been shown to be impaired in SLE patients [137, 138]. Multiple types of effector T cells have been implicated in disease development in both murine models and SLE patients. Depletion of CD4+ Th1 cells prevents disease progression in several murine models, and Th1 cells producing IL-2 and IFN-γ are required for antibody production by autoreactive B cells [9, 35, 37, 139]. IFN-γ, along with the Th2 cytokines IL4 and IL5, promotes the recruitment of lymphocytes to lymph nodes. Moreover, some studies have found that Th2 effector cells promote disease chronicity in target tissues [140, 141].
Immune-mediated end-organ damage
Inflammation of the skin or mucosal membranes or arthritis is seen in milder forms of SLE [142]. More commonly, severe disease manifests in neurological symptoms, renal disease, vasculitis, serositis involving the heart (pericarditis) or lungs (pleuritis), or haematological disorders, including leucopenia, lymphopenia, thrombocytopenia, thrombotic thrombocytopenic purpura, myelofibrosis and autoimmune haemolytic anaemia [142–144].
Ultimately, tissue inflammation can result from a variety of factors. Given the heterogeneous nature of the disease, antibodies can often, but not always, be involved in local inflammatory responses. Autoantibodies can form ICs, activate innate or resident cells and directly induce target organ injury. In addition to autoantibodies, defects in cell death, cell clearance or phagocytic machinery can initiate complement activation, IC formation with ensuing myeloid cell activation and cytokine production with resultant autoimmune tissue destruction [145]. Infiltrating and resident renal myeloid cells activated by ICs produce cytokines and chemokines, such as IL-6 and BAFF, that promote B-cell activation and differentiation. The chemokines CXCL13 and CCL21 direct lymphocyte migration to lymphoid follicles in lymph nodes and in target tissues. Specialized tissue-resident pDCs promote the maintenance of Treg populations, and this pDC population and Treg cell numbers are decreased during inflammation [146, 147].
As exemplified in LN, which affects up to 60% of SLE patients [148], mechanisms involving numerous inflammatory cytokines, leucocytes, responses by the resident tissue cells, along with alterations in vascular endothelial cell function contribute to tissue damage [149]. Each tissue’s response to immune-mediated injury is unique and has been reviewed extensively [84, 142–144, 149–153], but ultimately, chronic inflammation can result in tissue dysfunction caused by remodelling and fibrosis.
Summary and future directions
The presence of elevated levels of autoantibodies and numerous cytokines serve as markers of immune-mediated pathology in preclinical SLE disease. Most often, the deposition of ICs combined with inflammatory cytokines from infiltrating leucocytes perpetuate target organ inflammation. The unabated inflammatory response eventually results in autoimmune-mediated tissue destruction. However, no single mediator consistently serves as a diagnostic marker for patients with SLE. Although most patients at the time of diagnosis have elevated levels of autoantibodies, which appear to contribute to SLE pathology, similar disease manifestations can occur in individuals without elevated autoantibodies. Likewise, elevations in circulating type I IFNs have been found in a majority, but not all patients. Future strategies will incorporate new biomarkers to evaluate the patient’s disease and immunological signature based on our continually evolving understanding of relevant immunological pathways. The era of immunologics and biologics has only just begun.
Acknowledgements
O.Z.’s, T.C.’s and A.M.F.’s work is supported by core funding from A*STAR at the Singapore Immunology Network, Singapore. A.B.S. is funded by National Institutes of Health grants AR067625, AI122720.
Funding: A.B.S.'s work is funded by National Institutes of Health (NIH) grants AR067625, AI122720 and an Alliance for Lupus Research (ALR) Award 257549 (PI, Davis). L.S.D.'s work is funded by an ALR Award 257549 and NIH grants AR067625 (PI, Satterthwaite) and AI122720 (PI, Satterthwaite).
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1. Inês L, Silva C, Galindo M. et al. Classification of systemic lupus erythematosus: systemic lupus international collaborating clinics versus American College of Rheumatology Criteria. A comparative study of 2,055 patients from a real-life, international systemic lupus erythematosus cohort. Arthritis Care Res 2015;67:1180–5. [DOI] [PubMed] [Google Scholar]
- 2. Schmidt CW. Questions persist: environmental factors in autoimmune disease. Environ Health Perspect 2011;119:A249–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rubtsov AV, Rubtsova K, Kappler JW, Marrack P.. Genetic and hormonal factors in female-biased autoimmunity. Autoimmun Rev 2010;9:494–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kaul A, Gordon C, Crow MK. et al. Systemic lupus erythematosus. Nat Rev Dis Primers 2016;2:16039. [DOI] [PubMed] [Google Scholar]
- 5. Ramos PS, Shaftman SR, Ward RC, Langefeld CD.. Genes associated with SLE are targets of recent positive selection. Autoimmune Dis 2014;2014:203435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smith CD, Cyr M.. The history of lupus erythematosus. From Hippocrates to Osler. Rheum Dis Clin North Am 1988;14:1–14. [PubMed] [Google Scholar]
- 7. Mallavarapu RK, Grimsley EW.. The history of lupus erythematosus. South Med J 2007;100:896–8. [DOI] [PubMed] [Google Scholar]
- 8. Ippolito A, Wallace DJ, Gladman D. et al. Autoantibodies in systemic lupus erythematosus: comparison of historical and current assessment of seropositivity. Lupus 2011;20:250–5. [DOI] [PubMed] [Google Scholar]
- 9. Fairhurst AM, Wandstrat AE, Wakeland EK.. Systemic lupus erythematosus: multiple immunological phenotypes in a complex genetic disease. Adv Immunol 2006;92:1–69. [DOI] [PubMed] [Google Scholar]
- 10. Lino AC, Dorner T, Bar-Or A, Fillatreau S.. Cytokine-producing B cells: a translational view on their roles in human and mouse autoimmune diseases. Immunol Rev 2016;269:130–44. [DOI] [PubMed] [Google Scholar]
- 11. Son M, Kim SJ, Diamond B.. SLE-associated risk factors affect DC function. Immunol Rev 2016;269:100–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blanco P, Ueno H, Schmitt N.. T follicular helper (Tfh) cells in lupus: activation and involvement in SLE pathogenesis. Eur J Immunol 2016;46:281–90. [DOI] [PubMed] [Google Scholar]
- 13. Meffre E. The establishment of early B cell tolerance in humans: lessons from primary immunodeficiency diseases. Ann N Y Acad Sci 2011;1246:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Olsen NJ, Karp DR.. Autoantibodies and SLE: the threshold for disease. Nat Rev Rheumatol 2014;10:181–6. [DOI] [PubMed] [Google Scholar]
- 15. Arbuckle MR, McClain MT, Rubertone MV. et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med 2003;349:1526–33. [DOI] [PubMed] [Google Scholar]
- 16. Heinlen LD, McClain MT, Merrill J. et al. Clinical criteria for systemic lupus erythematosus precede diagnosis, and associated autoantibodies are present before clinical symptoms. Arthritis Rheum 2007;56:2344–51. [DOI] [PubMed] [Google Scholar]
- 17. Heinlen LD, Ritterhouse LL, McClain MT. et al. Ribosomal P autoantibodies are present before SLE onset and are directed against non-C-terminal peptides. J Mol Med 2010;88:719–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goodnow CC, Sprent J, Fazekas de St Groth B, Vinuesa CG.. Cellular and genetic mechanisms of self tolerance and autoimmunity. Nature 2005;435:590–7. [DOI] [PubMed] [Google Scholar]
- 19. Browne CD, Del Nagro CJ, Cato MH, Dengler HS, Rickert RC.. Suppression of phosphatidylinositol 3,4,5-trisphosphate production is a key determinant of B cell anergy. Immunity 2009;31:749–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lai M, Gonzalez-Martin A, Cooper AB. et al. Regulation of B-cell development and tolerance by different members of the miR-17∼92 family microRNAs. Nat Commun 2016;7:12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Verkoczy L, Duong B, Skog P. et al. Basal B cell receptor-directed phosphatidylinositol 3-kinase signaling turns off RAGs and promotes B cell-positive selection. J Immunol 2007;178:6332–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Benhamou D, Labi V, Novak R. et al. A c-Myc/miR17-92/Pten axis controls PI3K-mediated positive and negative selection in B cell development and reconstitutes CD19 deficiency. Cell Rep 2016;16:419–31. [DOI] [PubMed] [Google Scholar]
- 23. Meffre E, Casellas R, Nussenzweig MC.. Antibody regulation of B cell development. Nat Immunol 2000;1:379–85. [DOI] [PubMed] [Google Scholar]
- 24. Stohl W, Metyas S, Tan SM. et al. B lymphocyte stimulator overexpression in patients with systemic lupus erythematosus: longitudinal observations. Arthritis Rheum 2003;48:3475–86. [DOI] [PubMed] [Google Scholar]
- 25. Petri M, Stohl W, Chatham W. et al. Association of plasma B lymphocyte stimulator levels and disease activity in systemic lupus erythematosus. Arthritis Rheum 2008;58:2453–9. [DOI] [PubMed] [Google Scholar]
- 26. Brink R. Regulation of B cell self-tolerance by BAFF. Semin Immunol 2006;18:276–83. [DOI] [PubMed] [Google Scholar]
- 27. Yurasov S, Hammersen J, Tiller T, Tsuiji M, Wardemann H.. B-cell tolerance checkpoints in healthy humans and patients with systemic lupus erythematosus. Ann N Y Acad Sci 2005;1062:165–74. [DOI] [PubMed] [Google Scholar]
- 28. Malkiel S, Jeganathan V, Wolfson S. et al. Checkpoints for autoreactive B cells in the peripheral blood of lupus patients assessed by flow cytometry. Arthritis Rheumatol 2016;68:2210–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ray SK, Putterman C, Diamond B.. Pathogenic autoantibodies are routinely generated during the response to foreign antigen: a paradigm for autoimmune disease. Proc Natl Acad Sci USA 1996;93:2019–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Choi JY, Ho JH, Pasoto SG. et al. Circulating follicular helper-like T cells in systemic lupus erythematosus: association with disease activity. Arthritis Rheumatol 2015;67:988–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang L, Zhao P, Ma L. et al. Increased interleukin 21 and follicular helper T-like cells and reduced interleukin 10+ B cells in patients with new-onset systemic lupus erythematosus. J Rheumatol 2014;41:1781–92. [DOI] [PubMed] [Google Scholar]
- 32. Xu H, Liu J, Cui X. et al. Increased frequency of circulating follicular helper T cells in lupus patients is associated with autoantibody production in a CD40L-dependent manner. Cell Immunol 2015;295:46–51. [DOI] [PubMed] [Google Scholar]
- 33. Zhang X, Lindwall E, Gauthier C. et al. Circulating CXCR5+CD4+helper T cells in systemic lupus erythematosus patients share phenotypic properties with germinal center follicular helper T cells and promote antibody production. Lupus 2015;24:909–17. [DOI] [PubMed] [Google Scholar]
- 34. Deane JA, Pisitkun P, Barrett RS. et al. Control of Toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity 2007;27:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jackson SW, Jacobs HM, Arkatkar T. et al. B cell IFN-γ receptor signaling promotes autoimmune germinal centers via cell-intrinsic induction of BCL-6. J Exp Med 2016;213:733–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Soni C, Wong EB, Domeier PP. et al. B cell-intrinsic TLR7 signaling is essential for the development of spontaneous germinal centers. J Immunol 2014;193:4400–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Domeier PP, Chodisetti SB, Soni C. et al. IFN-γ receptor and STAT1 signaling in B cells are central to spontaneous germinal center formation and autoimmunity. J Exp Med 2016;213:715–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Berland R, Fernandez L, Kari E. et al. Toll-like receptor 7-dependent loss of B cell tolerance in pathogenic autoantibody knockin mice. Immunity 2006;25:429–40. [DOI] [PubMed] [Google Scholar]
- 39. Nickerson KM, Christensen SR, Shupe J. et al. TLR9 regulates TLR7- and MyD88-dependent autoantibody production and disease in a murine model of lupus. J Immunol 2010;184:1840–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. James JA, Robertson JM.. Lupus and Epstein-Barr. Curr Opin Rheumatol 2012;24:383–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Deshmukh US, Bagavant H, Sim D, Pidiyar V, Fu SM.. A SmD peptide induces better antibody responses to other proteins within the small nuclear ribonucleoprotein complex than to SmD protein via intermolecular epitope spreading. J Immunol 2007;178:2565–71. [DOI] [PubMed] [Google Scholar]
- 42. Kurien BT, Dorri Y, Bachmann M, Scofield RH.. Induction of anti-Ro60/anti-La by immunisation with spectrin and induction of anti-spectrin by immunisation with Ro60 and 4-hydroxy-2-nonenal-modified Ro60 immunisation. Clin Exp Rheumatol 2012;30:886–93. [PMC free article] [PubMed] [Google Scholar]
- 43. Pathak S, Mohan C.. Cellular and molecular pathogenesis of systemic lupus erythematosus: lessons from animal models. Arthritis Res Ther 2011;13:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Perry JS, Hsieh CS.. Development of T-cell tolerance utilizes both cell-autonomous and cooperative presentation of self-antigen. Immunol Rev 2016;271:141–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vieira QF, Kayser C, Kallas EG, Andrade LE.. Decreased recent thymus emigrant number is associated with disease activity in systemic lupus erythematosus. J Rheumatol 2008;35:1762–7. [PubMed] [Google Scholar]
- 46. Raj P, Rai E, Song R. et al. Regulatory polymorphisms modulate the expression of HLA class II molecules and promote autoimmunity. eLife 2016;5:e12089 DOI: http://dx.doi.org/10.7554/eLife.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rocken M, Urban JF, Shevach EM.. Infection breaks T-cell tolerance. Nature 1992;359:79–82. [DOI] [PubMed] [Google Scholar]
- 48. Moulton VR, Tsokos GC.. T cell signaling abnormalities contribute to aberrant immune cell function and autoimmunity. J Clin Invest 2015;125:2220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ohl K, Tenbrock K.. Regulatory T cells in systemic lupus erythematosus. Eur J Immunol 2015;45:344–55. [DOI] [PubMed] [Google Scholar]
- 50. von Spee-Mayer C, Siegert E, Abdirama D. et al. Low-dose interleukin-2 selectively corrects regulatory T cell defects in patients with systemic lupus erythematosus. Ann Rheum Dis 2016;75:1407–15. [DOI] [PubMed] [Google Scholar]
- 51. He J, Zhang X, Wei Y. et al. Low-dose interleukin-2 treatment selectively modulates CD4+ T cell subsets in patients with systemic lupus erythematosus. Nat Med 2016;22:991–3. [DOI] [PubMed] [Google Scholar]
- 52. Choi SC, Hutchinson TE, Titov AA. et al. The lupus susceptibility gene Pbx1 regulates the balance between follicular helper T cell and regulatory T cell differentiation. J Immunol 2016;197:458–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Woods M, Zou YR, Davidson A.. Defects in germinal center selection in SLE. Front Immunol 2015;6:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Simpson N, Gatenby PA, Wilson A. et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum 2010;62:234–44. [DOI] [PubMed] [Google Scholar]
- 55. Wu HY, Center EM, Tsokos GC, Weiner HL.. Suppression of murine SLE by oral anti-CD3: inducible CD4+CD25-LAP+ regulatory T cells control the expansion of IL-17+ follicular helper T cells. Lupus 2009;18:586–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mittereder N, Kuta E, Bhat G. et al. Loss of immune tolerance is controlled by ICOS in Sle1 mice. J Immunol 2016;197:491–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lee SK, Silva DG, Martin JL. et al. Interferon-γ excess leads to pathogenic accumulation of follicular helper T cells and germinal centers. Immunity 2012;37:880–92. [DOI] [PubMed] [Google Scholar]
- 58. Lu R, Munroe ME, Guthridge JM. et al. Dysregulation of innate and adaptive serum mediators precedes systemic lupus erythematosus classification and improves prognostic accuracy of autoantibodies. J Autoimmun 2016;74:182–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Liu Y, Anders HJ.. Lupus nephritis: from pathogenesis to targets for biologic treatment. Nephron Clin Pract 2014;128:224–31. [DOI] [PubMed] [Google Scholar]
- 60. Hakkim A, Fürnrohr BG, Amann K. et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci USA 2010;107:9813–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mistry P, Kaplan MJ.. Cell death in the pathogenesis of systemic lupus erythematosus and lupus nephritis. Clin Immunol 2016; http://dx.doi.org/10.1016/j.clim.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shao WH, Cohen PL.. Disturbances of apoptotic cell clearance in systemic lupus erythematosus. Arthritis Res Ther 2011;13:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Vaishnaw AK, Toubi E, Ohsako S. et al. The spectrum of apoptotic defects and clinical manifestations, including systemic lupus erythematosus, in humans with CD95 (Fas/APO-1) mutations. Arthritis Rheum 1999;42:1833–42. [DOI] [PubMed] [Google Scholar]
- 64. Mahajan A, Herrmann M, Muñoz LE.. Clearance deficiency and cell death pathways: a model for the pathogenesis of SLE. Front Immunol 2016;7:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Muñoz LE, Chaurio RA, Gaipl US, Schett G, Kern P.. MoMa from patients with systemic lupus erythematosus show altered adhesive activity. Autoimmunity 2009;42:269–71. [DOI] [PubMed] [Google Scholar]
- 66. Khan TN, Wong EB, Soni C, Rahman ZS.. Prolonged apoptotic cell accumulation in germinal centers of Mer-deficient mice causes elevated B cell and CD4+ Th cell responses leading to autoantibody production. J Immunol 2013;190:1433–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Miyanishi M, Segawa K, Nagata S.. Synergistic effect of Tim4 and MFG-E8 null mutations on the development of autoimmunity. Int Immunol 2012;24:551–9. [DOI] [PubMed] [Google Scholar]
- 68. Rahman ZS, Shao WH, Khan TN, Zhen Y, Cohen PL.. Impaired apoptotic cell clearance in the germinal center by Mer-deficient tingible body macrophages leads to enhanced antibody-forming cell and germinal center responses. J Immunol 2010;185:5859–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Janko C, Franz S, Munoz LE. et al. CRP/anti-CRP antibodies assembly on the surfaces of cell remnants switches their phagocytic clearance toward inflammation. Front Immunol 2011;2:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Garcia-Romo GS, Caielli S, Vega B. et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med 2011;3:73ra20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lande R, Ganguly D, Facchinetti V. et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med 2011;3:73ra19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Brinkmann V, Reichard U, Goosmann C. et al. Neutrophil extracellular traps kill bacteria. Science 2004;303:1532–5. [DOI] [PubMed] [Google Scholar]
- 73. Leffler J, Ciacma K, Gullstrand B. et al. A subset of patients with systemic lupus erythematosus fails to degrade DNA from multiple clinically relevant sources. Arthritis Res Ther 2015;17:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yeh TM, Chang HC, Liang CC, Wu JJ, Liu MF.. Deoxyribonuclease-inhibitory antibodies in systemic lupus erythematosus. J Biomed Sci 2003;10:544–51. [DOI] [PubMed] [Google Scholar]
- 75. Skiljevic D, Jeremic I, Nikolic M. et al. Serum DNase I activity in systemic lupus erythematosus: correlation with immunoserological markers, the disease activity and organ involvement. Clin Chem Lab Med 2013;51:1083–91. [DOI] [PubMed] [Google Scholar]
- 76. Fenton K, Fismen S, Hedberg A. et al. Anti-dsDNA antibodies promote initiation, and acquired loss of renal Dnase1 promotes progression of lupus nephritis in autoimmune (NZBxNZW)F1 mice. PLoS One 2009;4:e8474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Means TK, Latz E, Hayashi F. et al. Human lupus autoantibody–DNA complexes activate DCs through cooperation of CD32 and TLR9. J Clin Invest 2005;115:407–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Moody KL, Uccellini MB, Avalos AM, Marshak-Rothstein A, Viglianti GA.. Toll-like receptor-dependent immune complex activation of B cells and dendritic cells. Methods Mol Biol 2016;1390:249–72. [DOI] [PubMed] [Google Scholar]
- 79. Caielli S, Athale S, Domic B. et al. Oxidized mitochondrial nucleoids released by neutrophils drive type I interferon production in human lupus. J Exp Med 2016;213:697–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ricklin D, Hajishengallis G, Yang K, Lambris JD.. Complement: a key system for immune surveillance and homeostasis. Nat Immunol 2010;11:785–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sturfelt G, Truedsson L.. Complement in the immunopathogenesis of rheumatic disease. Nat Rev Rheumatol 2012;8:458–68. [DOI] [PubMed] [Google Scholar]
- 82. Chatterjee P, Agyemang AF, Alimzhanov MB. et al. Complement C4 maintains peripheral B-cell tolerance in a myeloid cell dependent manner. Eur J Immunol 2013;43:2441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Elkon KB, Santer DM.. Complement, interferon and lupus. Curr Opin Immunol 2012;24:665–70. [DOI] [PubMed] [Google Scholar]
- 84. Liu Z, Davidson A.. Taming lupus—a new understanding of pathogenesis is leading to clinical advances. Nat Med 2012;18:871–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Coutant F, Miossec P.. Altered dendritic cell functions in autoimmune diseases: distinct and overlapping profiles. Nat Rev Rheumatol 2016;12:703–15. [DOI] [PubMed] [Google Scholar]
- 86. Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J.. Induction of dendritic cell differentiation by IFN-α in systemic lupus erythematosus. Science 2001;294:1540–3. [DOI] [PubMed] [Google Scholar]
- 87. Celhar T, Hopkins R, Thornhill SI. et al. RNA sensing by conventional dendritic cells is central to the development of lupus nephritis. Proc Natl Acad Sci USA 2015;112:E6195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Fairhurst AM, Hwang SH, Wang A. et al. Yaa autoimmune phenotypes are conferred by overexpression of TLR7. Eur J Immunol 2008;38:1971–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hwang SH, Lee H, Yamamoto M. et al. B cell TLR7 expression drives anti-RNA autoantibody production and exacerbates disease in systemic lupus erythematosus-prone mice. J Immunol 2012;189:5786–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Pisitkun P, Deane JA, Difilippantonio MJ. et al. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science 2006;312:1669–72. [DOI] [PubMed] [Google Scholar]
- 91. Santiago-Raber ML, Kikuchi S, Borel P. et al. Evidence for genes in addition to Tlr7 in the Yaa translocation linked with acceleration of systemic lupus erythematosus. J Immunol 2008;181:1556–62. [DOI] [PubMed] [Google Scholar]
- 92. Blanco P, Palucka AK, Pascual V, Banchereau J.. Dendritic cells and cytokines in human inflammatory and autoimmune diseases. Cytokine Growth Factor Rev 2008;19:41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Blomberg S, Eloranta ML, Cederblad B. et al. Presence of cutaneous interferon-alpha producing cells in patients with systemic lupus erythematosus. Lupus 2001;10:484–90. [DOI] [PubMed] [Google Scholar]
- 94. Farkas L, Beiske K, Lund-Johansen F, Brandtzaeg P, Jahnsen FL.. Plasmacytoid dendritic cells (natural interferon-α/β-producing cells) accumulate in cutaneous lupus erythematosus lesions. Am J Pathol 2001;159:237–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Tucci M, Quatraro C, Lombardi L. et al. Glomerular accumulation of plasmacytoid dendritic cells in active lupus nephritis: role of interleukin-18. Arthritis Rheum 2008;58:251–62. [DOI] [PubMed] [Google Scholar]
- 96. Bekeredjian-Ding IB, Wagner M, Hornung V. et al. Plasmacytoid dendritic cells control TLR7 sensitivity of naive B cells via type I IFN. J Immunol 2005;174:4043–50. [DOI] [PubMed] [Google Scholar]
- 97. Kariuki SN, Ghodke-Puranik Y, Dorschner JM. et al. Genetic analysis of the pathogenic molecular sub-phenotype interferon-alpha identifies multiple novel loci involved in systemic lupus erythematosus. Genes Immun 2015;16:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Niewold TB, Hua J, Lehman TJ, Harley JB, Crow MK.. High serum IFN-α activity is a heritable risk factor for systemic lupus erythematosus. Genes Immun 2007;8:492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Baechler EC, Batliwalla FM, Karypis G. et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci USA 2003;100:2610–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Becker AM, Dao KH, Han BK. et al. SLE peripheral blood B cell, T cell and myeloid cell transcriptomes display unique profiles and each subset contributes to the interferon signature. PLoS One 2013;8:e67003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Bennett L, Palucka AK, Arce E. et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med 2003;197:711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Crow MK, Kirou KA, Wohlgemuth J.. Microarray analysis of interferon-regulated genes in SLE. Autoimmunity 2003;36:481–90. [DOI] [PubMed] [Google Scholar]
- 103. Palucka AK, Blanck JP, Bennett L, Pascual V, Banchereau J.. Cross-regulation of TNF and IFN-α in autoimmune diseases. Proc Natl Acad Sci USA 2005;102:3372–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Banchereau R, Hong S, Cantarel B. et al. Personalized immunomonitoring uncovers molecular networks that stratify lupus patients. Cell 2016;165:551–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Petri M, Singh S, Tesfasyone H. et al. Longitudinal expression of type I interferon responsive genes in systemic lupus erythematosus. Lupus 2009;18:980–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Landolt-Marticorena C, Bonventi G, Lubovich A. et al. Lack of association between the interferon-α signature and longitudinal changes in disease activity in systemic lupus erythematosus. Ann Rheum Dis 2009;68:1440–6. [DOI] [PubMed] [Google Scholar]
- 107. Yao Y, Higgs BW, Morehouse C. et al. Development of potential pharmacodynamic and diagnostic markers for anti-IFN-α monoclonal antibody trials in systemic lupus erythematosus. Hum Genomics Proteomics 2009;2009: 374312. doi: 10.4061/2009/374312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Feng X, Huang J, Liu Y. et al. Identification of interferon-inducible genes as diagnostic biomarker for systemic lupus erythematosus. Clin Rheumatol 2015;34:71–9. [DOI] [PubMed] [Google Scholar]
- 109. Petri M, Wallace DJ, Spindler A. et al. Sifalimumab, a human anti-interferon-α monoclonal antibody, in systemic lupus erythematosus: a phase I randomized, controlled, dose-escalation study. Arthritis Rheum 2013;65:1011–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Kalunian KC, Merrill JT, Maciuca R. et al. A Phase II study of the efficacy and safety of rontalizumab (rhuMAb interferon-α) in patients with systemic lupus erythematosus (ROSE). Ann Rheum Dis 2016;75:196–202. [DOI] [PubMed] [Google Scholar]
- 111. Jeremiah N, Neven B, Gentili M. et al. Inherited STING-activating mutation underlies a familial inflammatory syndrome with lupus-like manifestations. J Clin Invest 2014;124:5516–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Sharma S, Campbell AM, Chan J. et al. Suppression of systemic autoimmunity by the innate immune adaptor STING. Proc Natl Acad Sci USA 2015;112:E710–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Ataie-Kachoie P, Pourgholami MH, Richardson DR, Morris DL.. Gene of the month: Interleukin 6 (IL-6). J Clin Pathol 2014;67:932–7. [DOI] [PubMed] [Google Scholar]
- 114. Zhang C, Zhang X, Chen XH.. Inhibition of the interleukin-6 signaling pathway: a strategy to induce immune tolerance. Clin Rev Allergy Immunol 2014;47:163–73. [DOI] [PubMed] [Google Scholar]
- 115. Tsantikos E, Maxwell MJ, Putoczki T. et al. Interleukin-6 trans-signaling exacerbates inflammation and renal pathology in lupus-prone mice. Arthritis Rheum 2013;65:2691–702. [DOI] [PubMed] [Google Scholar]
- 116. Cash H, Relle M, Menke J. et al. Interleukin 6 (IL-6) deficiency delays lupus nephritis in MRL-Faslpr mice: the IL-6 pathway as a new therapeutic target in treatment of autoimmune kidney disease in systemic lupus erythematosus. J Rheumatol 2010;37:60–70. [DOI] [PubMed] [Google Scholar]
- 117. Gutierrez T, Halcomb KE, Coughran AJ, Li QZ, Satterthwaite AB.. Separate checkpoints regulate splenic plasma cell accumulation and IgG autoantibody production in Lyn-deficient mice. Eur J Immunol 2010;40:1897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Jain S, Park G, Sproule TJ. et al. Interleukin 6 accelerates mortality by promoting the progression of the systemic lupus erythematosus-like disease of BXSB.Yaa mice. PLoS One 2016;11:e0153059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Illei GG, Shirota Y, Yarboro CH. et al. Tocilizumab in systemic lupus erythematosus: data on safety, preliminary efficacy, and impact on circulating plasma cells from an open-label phase I dosage-escalation study. Arthritis Rheum 2010;62:542–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Tang B, Matsuda T, Akira S. et al. Age-associated increase in interleukin 6 in MRL/lpr mice. Int Immunol 1991;3:273–8. [DOI] [PubMed] [Google Scholar]
- 121. Mihara M, Takagi N, Takeda Y, Ohsugi Y.. IL-6 receptor blockage inhibits the onset of autoimmune kidney disease in NZB/W F1 mice. Clin Exp Immunol 1998;112:397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Balomenos D, Rumold R, Theofilopoulos AN.. Interferon-γ is required for lupus-like disease and lymphoaccumulation in MRL-lpr mice. J Clin Invest 1998;101:364–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Scapini P, Hu Y, Chu CL. et al. Myeloid cells, BAFF, and IFN-γ establish an inflammatory loop that exacerbates autoimmunity in Lyn-deficient mice. J Exp Med 2010;207:1757–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Ettinger R, Sims GP, Fairhurst AM. et al. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J Immunol 2005;175:7867–79. [DOI] [PubMed] [Google Scholar]
- 125. Deng XM, Yan SX, Wei W.. IL-21 acts as a promising therapeutic target in systemic lupus erythematosus by regulating plasma cell differentiation. Cell Mol Immunol 2015;12:31–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Choi YS, Eto D, Yang JA, Lao C, Crotty S.. Cutting edge: STAT1 is required for IL-6-mediated Bcl6 induction for early follicular helper cell differentiation. J Immunol 2013;190:3049–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. McPhee CG, Bubier JA, Sproule TJ. et al. IL-21 is a double-edged sword in the systemic lupus erythematosus-like disease of BXSB.Yaa mice. J Immunol 2013;191:4581–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Moore PA, Belvedere O, Orr A. et al. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science 1999;285:260–3. [DOI] [PubMed] [Google Scholar]
- 129. Scapini P, Nardelli B, Nadali G. et al. G-CSF-stimulated neutrophils are a prominent source of functional BLyS. J Exp Med 2003;197:297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Salazar-Camarena DC, Ortiz-Lazareno PC, Cruz A. et al. Association of BAFF, APRIL serum levels, BAFF-R, TACI and BCMA expression on peripheral B-cell subsets with clinical manifestations in systemic lupus erythematosus. Lupus 2016;25:582–92. [DOI] [PubMed] [Google Scholar]
- 131. Zhang J, Roschke V, Baker KP. et al. Cutting edge: a role for B lymphocyte stimulator in systemic lupus erythematosus. J Immunol 2001;166:6–10. [DOI] [PubMed] [Google Scholar]
- 132. Cheema GS, Roschke V, Hilbert DM, Stohl W.. Elevated serum B lymphocyte stimulator levels in patients with systemic immune-based rheumatic diseases. Arthritis Rheum 2001;44:1313–9. [DOI] [PubMed] [Google Scholar]
- 133. Bassi N, Luisetto R, Ghirardello A. et al. 17-β-estradiol affects BLyS serum levels and the nephritogenic autoantibody network accelerating glomerulonephritis in NZB/WF1 mice. Lupus 2015;24:382–91. [DOI] [PubMed] [Google Scholar]
- 134. Furie R, Petri M, Zamani O. et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum 2011;63:3918–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Navarra SV, Guzman RM, Gallacher AE. et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet 2011;377:721–31. [DOI] [PubMed] [Google Scholar]
- 136. Busser BW, Adair BS, Erikson J, Laufer TM.. Activation of diverse repertoires of autoreactive T cells enhances the loss of anti-dsDNA B cell tolerance. J Clin Invest 2003;112:1361–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Menon M, Blair PA, Isenberg DA, Mauri C.. A regulatory feedback between plasmacytoid dendritic cells and regulatory B cells is aberrant in systemic lupus erythematosus. Immunity 2016;44:683–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Blair PA, Noreña LY, Flores-Borja F. et al. CD19+CD24hiCD38hi B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity 2010;32:129–40. [DOI] [PubMed] [Google Scholar]
- 139. Koga T, Ichinose K, Tsokos GC.. T cells and IL-17 in lupus nephritis. Clin Immunol 2015; http://dx.doi.org/10.1016/j.clim.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Charles N, Hardwick D, Daugas E, Illei GG, Rivera J.. Basophils and the T helper 2 environment can promote the development of lupus nephritis. Nat Med 2010;16:701–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Shiroiwa W, Tsukamoto K, Ohtsuji M. et al. IL-4Rα polymorphism in regulation of IL-4 synthesis by T cells: implication in susceptibility to a subset of murine lupus. Int Immunol 2007;19:175–83. [DOI] [PubMed] [Google Scholar]
- 142. Yu C, Gershwin ME, Chang C.. Diagnostic criteria for systemic lupus erythematosus: a critical review. J Autoimmun 2014;48–49:10–3. [DOI] [PubMed] [Google Scholar]
- 143. Fayyaz A, Igoe A, Kurien BT. et al. Haematological manifestations of lupus. Lupus Sci Med 2015;2:e000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Velo-García A, Castro SG, Isenberg DA.. The diagnosis and management of the haematologic manifestations of lupus. J Autoimmun 2016;74:139–60. [DOI] [PubMed] [Google Scholar]
- 145. Pickering MC, Botto M.. Are anti-C1q antibodies different from other SLE autoantibodies? Nat Rev Rheumatol 2010;6:490–3. [DOI] [PubMed] [Google Scholar]
- 146. Zheng D, Cao Q, Lee VW. et al. Lipopolysaccharide-pretreated plasmacytoid dendritic cells ameliorate experimental chronic kidney disease. Kidney Int 2012;81:892–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Ghali JR, Wang YM, Holdsworth SR, Kitching AR.. Regulatory T cells in immune-mediated renal disease. Nephrology 2016;21:86–96. [DOI] [PubMed] [Google Scholar]
- 148. Isenberg D, Appel GB, Contreras G. et al. Influence of race/ethnicity on response to lupus nephritis treatment: the ALMS study. Rheumatology 2010;49:128–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Davidson A. What is damaging the kidney in lupus nephritis? Nat Rev Rheumatol 2016;12:143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Kuhn A, Landmann A, Wenzel J.. Advances in the treatment of cutaneous lupus erythematosus. Lupus 2016;25:830–7. [DOI] [PubMed] [Google Scholar]
- 151. Mackay M. Lupus brain fog: a biologic perspective on cognitive impairment, depression, and fatigue in systemic lupus erythematosus. Immunol Res 2015;63:26–37. [DOI] [PubMed] [Google Scholar]
- 152. Bhattacharyya S, Helfgott SM.. Neurologic complications of systemic lupus erythematosus, Sjögren syndrome, and rheumatoid arthritis. Semin Neurol 2014;34:425–36. [DOI] [PubMed] [Google Scholar]
- 153. Tunnicliffe DJ, Singh-Grewal D, Kim S, Craig JC, Tong A.. Diagnosis, monitoring, and treatment of systemic lupus erythematosus: a systematic review of clinical practice guidelines. Arthritis Care Res 2015;67:1440–52. [DOI] [PubMed] [Google Scholar]