Abstract
Microbial colonization of the gastrointestinal tract is an essential process that modulates host physiology and immunity. Recently, researchers have begun to understand how and when these microorganisms colonize the gut and the early-life factors that impact their natural ecological establishment. The vertical transmission of maternal microbes to the offspring is a critical factor for host immune and metabolic development. Increasing evidence also points to a role in the wiring of the gut-brain axis. This process may be altered by various factors such as mode of delivery, gestational age at birth, the use of antibiotics in early life, infant feeding, and hygiene practices. In fact, these early exposures that impact the intestinal microbiota have been associated with the development of diseases such as obesity, type 1 diabetes, asthma, allergies, and even neurodevelopmental disorders. The present review summarizes the impact of cesarean birth on the gut microbiome and the health status of the developing infant and discusses possible preventative and restorative strategies to compensate for early-life microbial perturbations.
Keywords: cesarean section, metabolism, microbiota, immunity, prebiotics, probiotics
INTRODUCTION
Humans share a mutualistic relationship with the complex community of microbes living in their bodies, collectively known as the microbiota. The microbiota is well known to play a critical role in the development and later function of the gastrointestinal,1 metabolic,2 and immune systems.3,4 There is now a growing body of evidence that suggests the gut microbiota also influences the brain and behavior.5–10 Robust preclinical and clinical findings indicate that a bidirectional route of communication exists between the brain and the gut microbiota and this is termed the microbiota-gut-brain axis (for detailed description of this axis in health and disease across the lifespan, see recent reviews5,11–15).
The exact mechanisms by which the gut microbiota communicates with the brain are not yet clear; however, they include immunological, endocrine, metabolic, and neural pathways (for detailed discussion of pathways of communications, see recent reviews7,9,16–18). Although immune signaling through the production of cytokines is an important route of communication between the gut and the brain, it is not involved in all conditions. For example, subclinical infection by pathogenic bacteria fails to induce a change in cytokine release, yet it alters behavior and central nervous system (CNS) neurochemistry.19 However, it is clear that mice raised without exposure to microorganisms (ie, germ-free mice[GF]) have underdeveloped adaptive and innate immune systems.20,21 The biochemical complexity of the gut is even greater than that of the brain.16,22 Indeed, many of the hormones produced by the gut microbiota also act as neurotransmitters within the CNS, including catecholamines, gamma-aminobutyric acid, serotonin, glutamate, histamine, acetylcholine, and tryptophan.20,23 These metabolic compounds have bioactive properties and can be transported throughout the entire body via the circulatory system.24 Of particular note is tryptophan, an essential amino acid and a precursor of many biologically active agents, including the neurotransmitter serotonin.25 A growing body of evidence points to dysregulation of the often-overlooked kynurenine arm of the tryptophan metabolic pathway in many disorders of both the brain and the gastrointestinal tract.25,26 Another important signaling aspect of microbiota-to-brain signaling is the hypothalamus-pituitary-adrenal axis, which regulates cortisol secretion. Cortisol can affect immune cells both locally in the gut and systemically. Cortisol can also alter gut permeability and barrier function and change gut microbiota composition.7 In addition, microbes can impact the responsivity of the hypothalamic-pituitary-adrenal axis, as seen in several studies demonstrating that neuroendocrine signaling is altered in response to microbial manipulation.27–30
Given that the human gut is heavily innervated,31,32 it is not surprising that neural pathways represent another important route connecting the brain and the gut. The involvement of the vagus nerve in bottom-up microbiota-gut-brain communication appears to be dependent on the bacterial strain under investigation: Some studies have found that the vagus nerve is necessary for communication to the brain,19,28,33 while others have documented vagus-independent effects.34,35 The enteric nervous system is also responsive to microbial interventions. Both potential probiotic treatment and the absence of bacteria can alter the excitability of enteric nervous system sensory neurons.36,37 Together, these findings indicate there are likely numerous systems simultaneously involved in the bidirectional transfer of information between the brain and the gut.
ALTERED MICROBIOTA COMPOSITION IN PERINATAL PERIOD, NEURODEVELOPMENT, AND HEALTH
A number of diverse factors can contribute to mammalian gut microbiota content and complexity, and the progression of bacterial colonization is not random.38 Over the last few centuries, especially in the industrialized world, increases in births by cesarean (C)-section, prematurity rates, and the use of antibiotics in pregnancy, in addition to changes in infant feeding, living conditions, diet, lifestyle, and general hygiene may have altered the ways in which enteric microbial communities are acquired.39,40,180 These microbial alterations have been suggested to correlate with a variety of immune (eg, asthma) and metabolic (eg, childhood obesity) disorders.41,42 However, the causal relationship between C-sections and such interactions is less studied.
One of the first and most important developmental windows is the post birth neonatal period. The exposure to bacteria during birth is a critical juncture in the establishment of a stable core gut microbiota.43,44 The effects of the intestinal microbiota on brain physiology include synaptogenesis, regulation of microglia development and maturation, and regulation of neurotransmitters and neurotrophic factors such as brain-derived neurotrophic factor.27,29,30,45–47
Recently, several preclinical studies using GF mice have highlighted the ability of early-life microbiota to influence neurodevelopment, with long-lasting effects on neural function.27,29,30,45,48 During development, the nervous system is assembled and sculpted by a series of temporally regulated developmental processes that shape the functional neural circuitry, which is critical for normal cognitive, motor, and emotional development. This is a complex process consisting of an orchestrated series of neurodevelopmental events including, but not limited to, neurogenesis (the birth of new neurons), axonal and dendritic growth, synaptogenesis, and refinement of these synaptic connections, which generate the required numbers of neurons and the appropriate synaptic density to match the requirements of the neural circuit they become part of, a process known as systems matching.49,50 These developmental processes begin in utero and are later refined and modified during early postnatal development. A large body of scientific evidence indicates that the first 1000 days – from conception until a child’s second birthday – is the most critical time for a positive impact on a child’s cognitive development.43,51 Maternally derived environmental disturbances (eg, infection, stress, drug and alcohol exposure, preterm birth) during this time period can have profound and enduring structural and functional consequences for brain development in affected offspring.52–55
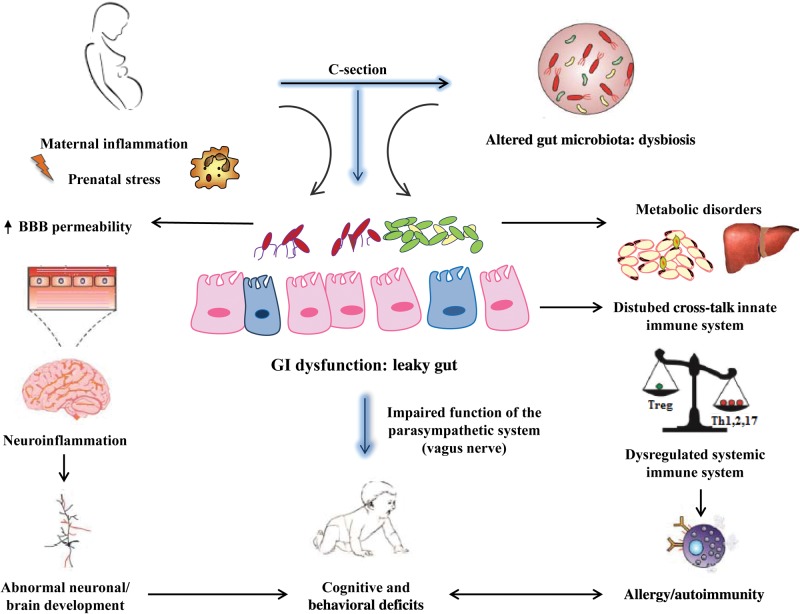
Despite the overwhelming evidence of the crucial role that the microbiota plays in the postnatal development and maturation of the CNS, the pathways as to how disturbances in microbiota development could lead to abnormal brain development, cognitive and behavioral deficits, allergy/autoimmunity, and metabolic disorders remain to be elucidated (Figure 1). Importantly, a recent study has provided one potential signaling mechanism involving the blood-brain barrier (BBB) through which the microbiota may modulate brain function during development.56 It is now becoming more apparent that intestinal microbiota-brain communication is initiated during gestation and propagated throughout life. The BBB ensures an optimal microenvironment for neuronal growth and development.57 An intact and regulated BBB is essential for protecting neonates during the critical periods of neurodevelopment.58,59 Increased BBB permeability has been associated with cytokine infiltration and neuroinflammation, resulting in abnormal neuronal development and disrupted immune priming, leading to neurodevelopmental, immune, and autoimmune disorders.60
Figure 1.
Cesarean section can alter colonization of the newborn intestine, which is a critical event influencing many developmental and physiological processes and, thereby, the functioning of the immune and neuroendocrine systems, with long-lasting effects on health. It is thought that an unhealthy microbiota can promote the increased translocation of pathogenic bacterial components from the intestinal mucosa to the systemic circulation, where they activate innate immunity characterized by production of proinflammatory cytokines, resulting in metabolic inflammation and abnormal gut function. Abbreviation: BBB, blood-brain barrier.
The mammalian lifespan can be arbitrarily divided into 5 stages – infancy, adolescence, adulthood, middle age, and old age – based on a variety of physiological and psychological parameters.61 Studies in GF mice have demonstrated that critical time windows exist during which certain deficits of the microbiota-gut-brain axis are amenable to microbial intervention. For example, the enhanced hypothalamic-pituitary-adrenal axis response of GF mice was partly corrected by reconstitution with specific pathogen-free microbiota at an early stage, but not by colonization exerted at a later stage.27 This indicates the existence of a critical window in early adolescence (in rodents, adolescence is generally thought to be the period between postnatal day 21 and postnatal day 60)61 during which the CNS is still sensitive to the microbial signals involved in normal hypothalamic-pituitary-adrenal axis development. Bacterial colonization at the post-weaning stage also normalizes alterations in anxiety-like behavior, microglial homeostasis, and BBB permeability in GF mice.30,46,56 Moreover, it has been shown that dietary administration of short-chain fatty acids, microbiota-derived metabolites, or the bacteria that produce them can reverse alterations in microglia homeostasis and the BBB permeability observed in GF mice.46,56 However, some microbial-based alterations appear to be permanent. For example, colonization of GF mice at the post-weaning stage does not normalize changes in hippocampal neurogenesis and central levels of serotonin.30,62 Colonization post weaning also only restores certain aspects of the social deficits in GF mice, ie, social preference is normalized, but social cognition is unaffected.48 These data suggest that the gut microbiota is capable of modulating brain development and behavior, but that critical time windows exist for intestinal microbes to exert this influence.
Alteration to the composition of the gut microbiota often occurs during the postnatal period. The use of antibiotics and feeding regime (whether breast fed or formula fed) can have a tremendous impact on the development and complexity of the microbiota,63 as well as influencing neural development.64 C-section birth has recently been suggested as another way in which this microbial alteration is occurring. While medically-necessary C-sections occur in 10% to 15% of pregnancies worldwide,65,66 the number of elective surgeries has increased over the last few decades. For example, in the United States approximately 1 in 3 babies was delivered via C-section in 2013, while in Northern European countries rates of C-section births are lower.67 In other regions, such as parts of Brazil and China, C-section rates are skyrocketing for cultural,68 cosmetic, and healthcare reasons rather than medical reasons.69 Moreover, it is worth noting that some extreme socioeconomic disparities exist, which limit the access to C-section delivery (especially in sub-Saharan Africa).70 However, despite these cultural differences, accumulating data suggests that in order to improve maternal and perinatal results, C-sections should only be performed when there is a medical indication.71
Disrupting the mother-to-newborn bacterial transmission by C-section delivery may increase the risk of disease in later life. For example, early-life microbiota perturbations, such as low levels of Bifidobacterium, have been reported to precede the development of certain disorders such as allergy and obesity.72–75 Indeed, C-section delivery has been associated with an increased risk of celiac disease,76 asthma,77,78 type 1 diabetes,79 and obesity.80,81 The available clinical association data for C-section delivery are summarized in Table 1. It should be noted that most of the evidence is association based, and clear evidence of causality of microbiome changes to functional outcomes have not been proven.
Table 1.
Summary of the impact of C-section on newborn health
| System | Effects | Specific changes | Specific changes in health condition/disorder/dysbiosis | Role of microbiota | Type of study | Reference |
|---|---|---|---|---|---|---|
| Immune system dysregulation | Allergy |
|
|
|
Clinical | Bisgaard et al. (2011)82 |
| Clinical | Watanabe et al. (2003)72 | |||||
|
Chronic inflammatory disease of the airways |
|
|
Clinical | Kero et al. (2002)78 | |
| Clinical | Roduit et al. (2009)83 | |||||
| Clinical and preclinical evidences | von Mutius (2007)84 | |||||
| Autoimmune diseases | Type 1 diabetes |
|
Probiotics that stimulate TLRs also protect from autoimmune diseases Microbiota development in infants is affected by mode of delivery and relates | Clinical | Algert et al. (2009)79 | |
| Clinical | Cardwell et al. (2008)85 | |||||
| Preclinical (mouse) | Aumeunier et al. (2010)86 | |||||
| Clinical | Mezoff et al. (2013)87 | |||||
| Systematic review and meta-analysis of observational studies | Akobeng et al. (2006)88 | |||||
| Clinical | Marild et al. (2012)76 | |||||
| Celiac disease | Clinical | Jakobsson et al. (2014)89 | ||||
|
differences in colonization patterns to the maturation of a balanced Th1/Th2 immune response | |||||
| Preclinical (mouse) | Hansen et al. (2014)90 | |||||
| Clinical Systematic review and meta-analysis | Sevelsted et al. (2015)91 Li et al. (2014)92 | |||||
| Metabolic dysregulation | Obesity |
|
|
Clinical (prospective prebirth cohort study) | Huh et al. (2012)80 | |
| Clinical (longitudinal birth cohort study) | Blustein et al. (2013)81 | |||||
| Clinical | Kalliomaki et al. (2008)74 | |||||
| Clinical | Pandey et al. (2012)75 |
Abbreviations: C-section, cesarean section; IL, interleukin; TLR, toll-like receptor.
Importantly, recent epidemiological findings suggest that C-section delivery is associated with a modest increase of some neuropsychiatric disorders such as bipolar disorder, autism spectrum disorders, and attention deficit hyperactivity disorder.93,94 However, other more definitive studies have not found an association between mode of delivery and autism or attention deficit/hyperactivity disorder or psychosis.95,96 More recently, an increased risk for obsessive compulsive disorder was associated with a variety of perinatal risk factors including birth by C-section, even after controlling for shared familial confounders and measured covariates (including sex, year of birth, maternal and paternal age at birth, and parity). Nevertheless, further studies are needed to determine whether C-section delivery is causally associated with autoimmune, metabolic, and neuropsychiatric diseases.97
The disruption of the normal maturation of the microbiota-brain-gut axis by C-section could, therefore, alter developmental trajectories and may lead to the onset of neurodevelopmental and other brain disorders later in life.5–10,43,98 The exposure to bacteria during birth is a critical event in the establishment of stable core gut microbiota, which is altered when infants are delivered via C-section. In fact, the microbiome of infants born vaginally most closely resembles that of the mother’s vagina and feces44 and is rich in beneficial bacteria such as Bifidobacterium longum subsp. infantis and Bacteroidetes.38,99 In contrast, the microbiome of infants born via C-section is more similar to the hospital environment and to the mother’s skin (eg, Staphylococcus, Corynebacterium, Propionibacterium spp.).44,100
STRATEGIES INVOLVED IN POSITIVELY SHAPING THE GUT MICROBIOTA AFTER C-SECTION
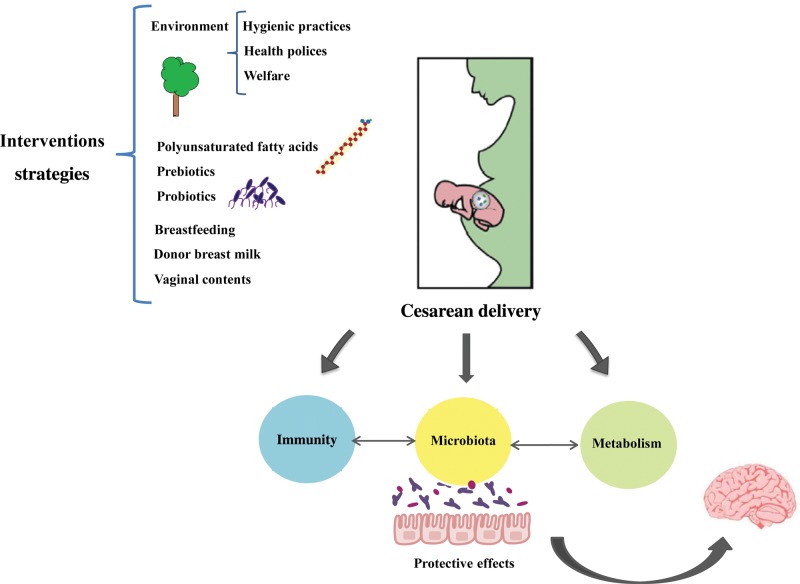
As mentioned above, the microbial composition of infants delivered by C-section differs from those born vaginally.44 The importance of the composition of the gastrointestinal microbiome in health (including brain health), particularly during early life, indicates that microbial-based interventions could represent effective strategies for targeting these potential negative health outcomes. Summarized here are the possible strategies that may be used to introduce a healthy balance of commensal gut microbiota. Many of these approaches have already been implicated in the improvement of microbial colonization of the gut in early childhood and may, thus, be associated with health benefits (Figure 2).
Figure 2.
Schematic representation of the main strategic points of intervention to reverse the effects of cesarean section delivery. This can be done by improving the environment through different hygienic habits and health practices. Alternatively, the intervention could be focused on the mother herself by using probiotics and/or prebiotics and/or polyunsaturated fatty acids during pregnancy. Finally, the intervention could focus on the newborn with “seeding” approaches: breastfeeding instead of formula feeding or the use of infant formulas enriched and improved with probiotics/prebiotics. This figure summarizes the current modulating therapies to improve the composition of the microbiota and neurodevelopmental health of the infant.
Vaginal seeding
The inoculation of a neonate with maternal vaginal microbiota immediately following C-section delivery is known as vaginal seeding. As previously mentioned, the composition of the microbiota in early life is strongly influenced by mode of delivery,44 which could account for the increased risk of certain diseases associated with C-section delivery. Therefore, vaginal seeding is a potential way to colonize C-section infants with the microbes they would have received had they been delivered vaginally.101
Recently, Dominguez-Bello et al.101 published results of the first pilot study demonstrating that vaginal seeding could successfully colonize C-section infants with vaginal bacteria. Two minutes after birth, C-section-delivered infants were swabbed with vaginal fluid over their entire bodies. Similar to vaginally delivered babies, the gut, oral cavity, and skin of seeded newborns were enriched with vaginal microbes for the first 30 days of life. Despite these findings, the study has some considerable drawbacks: most notably, only 4 babies were included in the study and the microbiome analysis was only conducted for 30 days following birth. The authors stress, however, that this was a proof-of-principle experiment and that reproduction in a larger cohort with a longer follow-up period is necessary.
Vaginal seeding has continued to garner increasing attention in the media, as well as curiosity from expectant mothers. Noticing this trend, Cunnington et al.102 published an editorial in the British Medical Journal focusing on the risks of the approach and cautioning against its unsupervised usage. The authors argue that there is not yet conclusive evidence proving that vaginal seeding is beneficial to the child. In fact, by performing this procedure, parents could unknowingly infect their child with pathogens present in vaginal fluid, which the mothers may carry asymptomatically. The authors are mostly concerned about accidental infection with group B. Streptococcus, which is the most common cause of neonatal sepsis and is carried by 20% to 30% of pregnant women.103,104 Finally, the authors caution that more studies are needed to demonstrate if the benefits are worth the potential risks and if parents do choose to perform vaginal seeding, they are advised to disclose this information to their healthcare providers.
In response to Cunnington et al.102 Knight and Gilbert105 published an editorial in support of vaginal seeding. Therein, they present the multitude of health-related dilemmas parents face on a daily basis for which there is no clear, evidence-based right or wrong decision. The authors argue that vaginal seeding does indeed modify the infant’s microbiome,101 while also admitting that the procedure may not ultimately improve clinical endpoints. As for the risk of infection, the authors recommend that mothers be screened for group B. Streptococcus and other pathogens before vaginal seeding is performed. It is worth noting that all pregnant women in Canada and the United States are offered group B. Streptococcus screening106,107 but this is not universally offered to pregnant women in the United Kingdom and Ireland.108 In conclusion, the authors stress that in both life and science it is necessary to make decisions before all the evidence is available.
Vaginal seeding is a promising, albeit controversial, approach to the colonization of C-section-delivered babies with vaginal microbes. However, it is clear that further research is necessary to determine the efficacy and safety of the procedure. Thus, targeted nutritional or environmental interventions may represent the best strategy to compensate for early-life microbial perturbations in the meantime, and these are described below.
Microbial environment
Epidemiological evidence indicates that exposure to “green spaces” rapidly induces positive changes to our psychological, physiological, and endocrine systems. These effects could be due to the bacteria found in green spaces as numerous studies now report that microbial exposure has beneficial outcomes.40,109 The biodiversity of the child’s environment, including family members who have contact with the baby and hygienic practices (eg, cleaning of baby’s soother through sucking or by other methods), can directly impact the diversity of microbes that are transferred to the infant. Indeed, the “old friends” hypothesis posits that a diverse community of symbiotic microorganisms is necessary to maintain optimal health.40,109 Diminished exposure to such microbes, which evolved together with the human organism both within the microbiota and in the environment, during the perinatal period may cause immunoregulatory and psychosocial deficits.109 Infants delivered by C-section have an altered microbial composition and reduced bacterial diversity, and this mode of delivery is associated with increased risk of developing some disorders, as outlined in Table 1. Therefore, health policies and clinical practice models should prioritize vaginal childbirth and re-evaluate when C-section is medically necessary.110 One further part of the puzzle is the recent postulation that the process of microbial gut colonization may be initiated prenatally by a distinct microbiota in the placenta and amniotic fluid.111 However, the relative contribution of this to postnatal colonization in either C-section or vaginally born infants is unclear, especially as only the presence of bacterial DNA, not live microbes, has been shown in the placenta.
Probiotic supplementation
Probiotics are live microorganisms that, when administered in adequate amounts, confer health benefits on the host.112 Probiotic administration confers a plethora of beneficial effects in a variety of disorders, such as inflammatory bowel disease, irritable bowel syndrome, obesity, diabetes, and asthma.112 The mechanisms by which probiotics exert these positive effects on the host are the focus of a number of preclinical and clinical investigative studies. For example, one study demonstrated that consumption of the commensal bacteria L. rhamnosus GG by the mother affects fecal Bifidobacterium transfer and composition during early infancy.113 In other words, supplementation with this bacteria appears to reinforce the development of a more complex and diverse Bifidobacterium microbiota.113 A separate study determined that treating expectant mothers with L. rhamnosus GG confers this strain to the newborn infant, with its presence documented for at least 6 months and, only in certain cases, persisting for as long as 24 months.114 In addition, infants whose mothers received L. rhamnosus GG during late pregnancy are more often colonized with species belonging to the most abundant group of Bifidobacterium microbiota present in the intestine of healthy infants and in human breast milk, B. longum, than infants whose mothers received placebo.114 However, it is worth noting that a separate clinical study reported that L. rhamnosus GG fails to modulate the microbial diversity of early infant gut microbiota despite promoting a beneficial Bifidobacterium profile.115 These results suggest that administration of potential probiotics to expectant mothers during late pregnancy can have beneficial effects on the development of the infant’s intestinal microbiota. Thus, probiotic treatment during pregnancy may represent an effective strategy to promote a healthy microbial composition in babies born via C-section. Moreover, systematic reviews of different studies suggest that the administration of certain probiotics to premature infants reduces the incidence of necrotizing enterocolitis, with resultant significant improvements in survival rates.116,117 To date, prophylactic administration of probiotics to premature infants (<32 weeks gestation) may be the only clinical circumstances in medicine where probiotic administration has been shown to save lives.
Prebiotic supplementation
In addition to probiotics, prebiotics have demonstrated promising effects in ameliorating immune and microbiota-derived health impairments.118 Prebiotics are “non-digestible substances that provide a beneficial physiological effect for the host by selectively stimulating the favorable growth or activity of a limited number of indigenous bacteria.”111,119–123 It is important to note that although all prebiotics are classified as dietary fiber, not all fiber is prebiotic: to be a prebiotic, the ingredient must not be digested in the upper gastrointestinal tract, must be fermented by the gut microbiota, and must stimulate the growth/activity or beneficial microbes, usually lactobaccilli and bifidobacteria.124 When prebiotics are fermented by these bacteria, short-chain fatty acids, lactic acids, and acetic acids are produced, which can have profound effects on host metabolism.122 Prebiotics occur naturally in foods such as vegetables, wheat, and soybeans and are typically oligosaccharides or more complex saccharides. So far, the most commonly studied compounds include inulin, fructo-oligosaccharides, and galacto-oligosaccharides.122,124
Preclinical studies using mice suggest that maternal consumption of fructo-oligosaccharide diminishes the severity of atopic dermatitis-like skin lesions in the offspring,125 suggesting that these compounds have a positive effect on the immune system. Another study in mice suggests that altering the fiber content of the maternal diet during both pregnancy and lactation enhances offspring growth through an effect on intestinal and muscle mass rather than fat mass accretion.126 In addition, results in piglets demonstrate that supplementation of prebiotics (short-chain fructo-oligosaccharides and polydextrose) modulates microbial colonization and alters signaling of short-chain fatty acids.127 Clinical studies have also reported that early prebiotic supplementation (such as with galacto-oligosaccharide and polydextrose) during the first 2 months of life may alleviate symptoms associated with crying and fussing in preterm infants.128 Moreover, prebiotic supplementation in the early neonatal period increases the prevalence of Bifidobacterium longum in the infant gut, in addition to promoting strain diversity.129 Although limited so far, these data suggest that prebiotic supplementation in infants exposed to early-life microbial perturbation may represent a viable strategy to benefit not only the gut microbiota, but also immunity,130–132 metabolism, and gastrointestinal function.122,124
Synbiotic supplementation
The combination of pre- and probiotics is thought to have synergistic beneficial effects on the immune and metabolic systems. Modification of the gut microbiota with a combination of specific prebiotics and probiotics (knows as synbiotics) might offer a novel and cost-effective strategy to reduce the risk of rhinovirus infections133 and to restore the delayed colonization of Bifidobacterium spp. in C-section-delivered babies.134 Indeed, evidence from clinical135 and preclinical136 studies indicates that some allergies can be prevented by using synbiotics. Synbiotics may increase the total antioxidant capacity levels in breast milk.137 Moreover, synbiotics may prevent weight loss in lactating mothers and increase the weight gain of infants.138
Human milk feeding
Natural selection has influenced the coevolution of hosts and microbes. This is clearly evidenced by mammalian mother-infant dyads, as the human microbiota is shaped by mothers and breast milk.139,140 Microbes are present in breast milk and may contribute to the composition of the infant microbiota,140,141 although this is, at present, an open question. Human breast milk consists of over 200 prebiotic oligosaccharide isomers, which influence the colonization and maturation of the infant gut microbiota.139 Oligosaccharides typically pass undigested from the infant stomach and are the major carbon source available to gut bacteria.142 Indeed, variation of the oligosaccharide profile in milk influences the microbial establishment in the infant gut.63 Importantly, the preponderance of the “breastfed-infant-type” of bacteria, ie, Bifidobacterium species, B. longum subsp. infantis – a species capable of utilizing the major oligosaccharides in human milk143 – is associated with better infant health and development.144,145 Therefore, the combination of probiotic and prebiotic components of human milk provides human milk–fed infants with a stable and uniform gut microbiota.146
It is consequently not surprising that the infant feeding regimen, ie, whether the infant is fed with formula or human milk, impacts the developing gut microbiota. Recent extended analysis of the Human Microbiome Project showed that it was possible to detect a microbial signature indicating whether an individual was ever or never breastfed as an infant.147 Evidence suggests that bacteria stimulated by human milk feeding can activate more immunoprotective genes in the host compared with formula feeding,148 and an extensive literature review has linked human breastfeeding with optimal infant health.149 Rhesus macaque infants that are breastfed by their mothers have a distinct microbiota profile and an expansion of Th17-based immune response in comparison with bottle-fed counterparts. In particular, human milk–fed infants develop robust populations of memory T cells as well as T helper 17 cells within the memory pool, whereas bottle-fed infants do not.150 In contrast, formula-fed infants have more diverse gut microbial communities typified by higher populations of Clostridium, Franulicatella, Citrobacter, Enterobacter, and Bilophila species compared with human milk–fed infants. Functionally, these formula-fed infants hosted higher proportions of antibiotic resistance genes, especially from ɣ-Proteobacteria.151 This suggests there may be previously unconsidered benefits from human milk, including that it reduces exposure to populations of microbes that contribute to antibiotic resistance.151 Given the numerous documented health advantages of breastfeeding and human milk, feeding infants human milk when possible represents a potential strategy to counteract early-life microbial perturbations including C-section delivery. However, it is important to acknowledge that since C-section delivery can impede early breastfeeding,15 this may not be a viable option in all cases.
Infant formula feeding with specific fatty acid supplementation
Human breast milk contains critical polyunsaturated fatty acids. Thus, another potential intervention strategy could be supplementation of infant milk formula with long-chain fatty acids such as docosahexaenoic and eicosapentaenoic acids and other n-3 polyunsaturated fatty acids, which have been extensively described in studies on allergic diseases, asthma, inflammatory bowel disease, and early-life stress.153–155 In fact, in clinical studies, n-3 polyunsaturated fatty acid consumption during pregnancy and infancy have been shown to prevent and/or improve onset and development of asthma;156 however, more research is warranted to determine the mechanism and the impact of dietary fatty acids on the intestinal microbiota composition of the host. It is possible that dietary supplementation with n-3 long-chain polyunsaturated fatty acids may exert indirect benefits in pregnancy through inhibition of placental inflammation.157 More specifically, some studies show the influence of dietary-specific docosahexaenoic acid and arachidonic acid on infant CNS with implications for neural development.158 It has recently been shown that a combination of docosahexaenoic acid and eicosapentaenoic acid could reverse the impact of early-life stress on the microbiota.155
Promoting breastfeeding and the use of human donor milk and breast milk fortifiers
Promoting exclusive breastfeeding for at least 6 months is the best approach to ensure the generation of a healthy microbiome in the infant. Mothers of preterm infants often cannot breastfeed. In these cases, human milk from human milk banks may be a possible alternative to improve neonatal health. However, strategies to boost donation rates should be identified to maintain donor human milk availability for preterm infant nutrition.159 Moreover, barriers remain for such availability including in terms of safety (eg, health status of mother, milk quality160) and variability in expressed milk composition (foremilk vs hind milk can vary in fatty acid composition),161 and the metabolome of preterm milk changes within 5 to 7 weeks postpartum to resemble that of term milk (eg, glutamate, caprylate, and caprate levels are increased in mature-term milk compared with colostrum).162 Logistical barriers such as monetary donations and shipping the milk over long distances also likely influence the availability of donor milk.163 Moreover, the impact of pasteurization on donor milk quality and the bioactivity of milk proteins/peptides should be minimized. All of these aspects indicate there should be more promotion and financial support of intrahospital human milk bank units to support the safe use of human milk in preterm infants. In the early weeks of their lives, premature infants are often fed maternally expressed breast milk enhanced with bovine-derived fortifiers to improve caloric intake and provide minerals, especially calcium and phosphate, to enhance bone health, and to prevent neonatal rickets. Human milk–derived fortifiers are currently being used in some neonatal centers as an alternative to bovine-derived fortifiers, albeit an expensive one. Whether using human fortifiers offers clinically measurable benefits and/or a more favorable microbiome composition compared with bovine-derived fortifiers remains to be seen.
Perspectives for the future
Thirty years ago, when experts from the World Health Organization met in Brazil to address the various issues surrounding childbirth, they agreed that there was “no justification for the rate of C-section exceeding 10-15% in any region of the world.” That percentage was then turned into a kind of universal dogma, valid for any hospital, anywhere in the world. However, while the C-section rates have continued to increase, the evidence collected in the past 3 decades has shown that this standard figure is not well adjusted to the complex and changing environment of labor. The World Health Organization itself revised its statement on the ideal C-section rate in April 2015, adding that “rates above 10% are not associated with a reduction in maternal and neonatal mortality.” The following phrase was also added, which was not in the 1985 document: “Every effort should be made to perform C-sections to all women who need it rather than trying to achieve a certain rate.”164
Like any surgery, C-section is not without risk. C-section surgery increases the risk of bleeding (and subsequent anemia), uterine ruptures, and problems with the placenta that can penetrate the wall of the uterus or complicate future pregnancies.165 Thus, the enduring risks associated with C-section delivery are now also beginning to be uncovered, and it is becoming clear that they are not limited to the mother. For example, compared with children born vaginally, children entering the world via C-section have an increased risk of asthma.77,166 The sensitivity of the newborn’s microbiome also should not be underestimated, as it can be affected by the location of birth, the type of birth, and the interventions, particularly maternal and infant antibiotic use, that may occur during or soon after birth. Most C-sections are accompanied by prophylactic maternal antibiotic administration that may affect the microbiota composition of the infant gut through subsequent breastfeeding.167 Therefore, the impact of C-section delivery on infant and maternal health as well as the microbiome should continue to be investigated.
Improving infant and maternal health
It should also be noted that maternal transfer of microbes may not always be beneficial. Indeed, studies in animals and humans show that maternal stress changes both the vaginal and offspring microbiota.168,169 Similarly, maternal pregestational weight correlates with offspring birth weight, and maternal obesity has been linked to fetal overgrowth, congenital defects, neural tube defects, stillbirth, preterm delivery, child morbidity, respiratory problems such as asthma, and neonatal mortality.170–172 The maternal gestational environment may create long-lasting and/or permanent modifications in fetal physiology, which can increase the risk of developing obesity, diabetes, and cardiovascular diseases in adulthood.173 Therefore, C-section with normative microbial interventions is a promising strategy warranting further investigation. Finally, health promotion strategies to lower the C-section rates and to educate against the potential risks are important strategies for the future, especially in jurisdictions where there are very high rates of C-sections.174
CONCLUSION
While research into the role of the gut microbiota on infant development and health is ongoing, a better understanding of the relevant communities of bacteria in the gut of healthy and compromised infants is needed. The relative contributions of biodiversity, mode of delivery, the introduction of pre- or probiotics in infant nutrition, feeding regime, and nutritional supplementation determine the diversity, abundance, and ratio of the gut microbiota. This bacterial community then becomes the fundamental core of commensal gut bacteria for one’s lifespan. As such, it is critical to unravel/decipher the links between gut microbiota composition and neurodevelopmental disorders and expand this important field of research. There is an excitement in the field about “seeding approaches” to reverse the effects of C-section delivery mode on the microbiome in early life, but there is an equal level of concern about the widespread utility and safety of this approach.102,105 Thus, targeted nutritional or environmental interventions and readjustments in obstetrical and newborn medicine practices may be the best strategies to compensate for early-life microbiota disturbances in the future (Table 2). Furthermore, there is a need to perform more in-depth studies on the role of the microbiota in neuropsychiatric disorders, such as autism, schizophrenia, and depression,14,175 in relation to mode of delivery and the possible consequences of this in later life. Future studies could also focus on the role of the microbiota in mediating fundamental brain processes ranging from prefrontal cortex myelination176 to amygdala function177 and hippocampal neurogenesis.62 Moreover, the potential of psychobiotics178,179 as novel nutritional strategies for brain disorders warrants further attention. Finally, the interventions and potential strategies detailed in this review are focused on microbiota-induced influences on the brain, but many will equally have implications for the impact of the microbiota on all systems in the body180 and, thus, should not be examined in isolation in future analyses.
Table 2.
Summary of different strategies involved in restoration of the gut microbiota after C-section
| Strategy | Featured effects | References |
|---|---|---|
| “Vaginal seeding” | Vaginal seeding could successfully colonize C-section infants with vaginal bacteria | Dominguez-Bello et al. (2016),101 Knight et al. (2016)105 |
| Microbial environment | Green spaces/natural environments rapidly induce positive changes to the psychological, physiological, and endocrine systems; diminished exposure to them in the perinatal period may cause immunoregulatory and psychosocial deficits | Rook (2013)109 |
| Probiotic supplementation | Supplementation with probiotics can confer a plethora of beneficial effects in variety of disorders, eg, consumption of the commensal bacteria L. rhamnosus GG affects fecal Bifidobacterium transfer and composition during early infancy | Hill et al. (2014),112 Gueimonde et al. (2006)113 |
| Prebiotic supplementation | Supplementation with prebiotics may represent a viable strategy to benefit the gut microbiota, immunity, metabolism, and gastrointestinal function of infants exposed to early-life microbial perturbation | Rastall et al. (2015),123 Slavin (2013),124 Barrett et al. (2015),129 Arslanoglu et al. (2008),130 Gruber et al. (2010)131 |
| Synbiotic supplementation | Supplementation with a combination of pre- and probiotics can have synergistic beneficial effects on the immune and metabolic system | Passeron et al. (2006),136 Nikniaz et al. (2013),137 Ostadrahimi et al. (2013)138 |
| Human milk feeding | Microbial establishment in the infant gut is influenced by the microbes present in breast milk | Diaz Heijtz (2016),64 Hinde et al. (2012)139 |
| Specific infant formula feeding | Supplementation of infant milk formula with long-chain fatty acids may prevent and/or improve development of asthma, inhibit placental inflammation, and have implications for neural development - even potentially reversing the impact of early-life stress on the microbiota | Miles et al. (2014),156 Melody et al. (2015),157 Hsieh et al. (2009),158 Pusceddu et al. (2015)155 |
| Human donor milk banks | Can be used as a possible alternative to maternal breastfeeding to improve neonatal health by supporting the safe use of human milk in preterm infants | Stevens et al. (2015),159 MacKenzie et al. (2013)163 |
Acknowledgments
Funding. JFC, TGD, and CS are funded by Science Foundation Ireland (SFI) through the Irish Government’s National Development Plan in the form of a center grant (APC Microbiome Institute Grant Number SFI/12/RC/2273) and through EU Grant 613979 (MYNEWGUT FP7-KBBE-2013-7). This work was partly funded by the Irish Department of Agriculture Food and Marine INFANTMET, SMARTFOOD, and TODDLERFOOD Projects.
Declaration of interest. The authors have conducted research in collaboration with several companies including Danone-Nutricia Research, Cremo, 4D Pharma, Mead Johnson, and Suntory Wellness. IR, SW, and JK are employees of Nutricia Research.
References
- 1. Hooper L V, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–1118. [DOI] [PubMed] [Google Scholar]
- 2. Nicholson J, Holmes E, Kinross J et al. . Host-gut microbiota metabolic interactions. Science. 2012;336:1262–1267. [DOI] [PubMed] [Google Scholar]
- 3. Bäckhed F, Ley RE, Sonnenburg JL et al. . Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. [DOI] [PubMed] [Google Scholar]
- 4. Hooper L V, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10:159–169. [DOI] [PubMed] [Google Scholar]
- 5. Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009;6:306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Micro. 2012;10:735–742. [DOI] [PubMed] [Google Scholar]
- 7. Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701–712. [DOI] [PubMed] [Google Scholar]
- 8. Dinan TG, Cryan JF. Regulation of the stress response by the gut microbiota: implications for psychoneuroendocrinology. Psychoneuroendocrinology. 2012;37:1369–1378. [DOI] [PubMed] [Google Scholar]
- 9. Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305–312. [DOI] [PubMed] [Google Scholar]
- 10. Sampson TR, Mazmanian SK. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe. 2015;17:565–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mayer EA. The neurobiology of stress and gastrointestinal disease. Gut. 2000;47:861–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bercik P. The microbiota-gut-brain axis: learning from intestinal bacteria? Gut. 2011;60:288–289. [DOI] [PubMed] [Google Scholar]
- 13. Cryan JF, O’Mahony SM. The microbiome-gut-brain axis: from bowel to behavior. Neurogastroenterol Motil. 2011;23:187–192. [DOI] [PubMed] [Google Scholar]
- 14. Sherwin E, Sandhu KV, Dinan TG et al. . May the force be with you: the light and dark sides of the microbiota-gut-brain axis in neuropsychiatry. CNS Drugs. 2016;30:1019–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dinan TG, Cryan JF. Gut instincts: microbiota as a key regulator of brain development, ageing and neurodegeneration. J Physiol. 2017;595:489–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Clarke G, Stilling RM, Kennedy PJ et al. . Minireview: gut microbiota: the neglected endocrine organ. Mol Endocrinol. 2014;28:1221–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. El Aidy S, Dinan TG, Cryan JF. Gut microbiota: the conductor in the orchestra of immune-neuroendocrine communication. Clin Ther. 2015;37:954–967. [DOI] [PubMed] [Google Scholar]
- 18. Mayer EA, Tillisch K, Gupta A. Gut/brain axis and the microbiota. J Clin Invest. 2015;125:926–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lyte M, Li W, Opitz N et al. . Induction of anxiety-like behavior in mice during the initial stages of infection with the agent of murine colonic hyperplasia Citrobacter rodentium. Physiol Behav. 2006;89:350–357. [DOI] [PubMed] [Google Scholar]
- 20. Shanahan F. The host-microbe interface within the gut. Best Pract Res Clin Gastroenterol. 2002;16:915–931. [DOI] [PubMed] [Google Scholar]
- 21. Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004;4:478–485. [DOI] [PubMed] [Google Scholar]
- 22. Lyte M. Microbial endocrinology in the microbiome-gut-brain axis: how bacterial production and utilization of neurochemicals influence behavior. PLoS Pathog. 2013;9:e1003726 doi: 10.1371/journal.ppat.1003726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barrett E, Ross RP, O'Toole PW et al. . γ-Aminobutyric acid production by culturable bacteria from the human intestine. J Appl Microbiol. 2012;113:411–417. [DOI] [PubMed] [Google Scholar]
- 24. Ross RP, Mills S, Hill C et al. . Specific metabolite production by gut microbiota as a basis for probiotic function. Int Dairy J. 2010;20:269–276. [Google Scholar]
- 25. O'Mahony SM, Clarke G, Borre YE et al. . Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res. 2015;277:32–48. [DOI] [PubMed] [Google Scholar]
- 26. Kennedy PJ, Cryan JF, Dinan TG et al. . Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology. 2017;112:399–412. [DOI] [PubMed] [Google Scholar]
- 27. Sudo N, Chida Y, Aiba Y et al. . Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004;558:263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bravo JA, Forsythe P, Chew MV et al. . Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108:16050–16055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Neufeld KM, Kang N, Bienenstock J et al. . Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil. 2011;23:255–265. [DOI] [PubMed] [Google Scholar]
- 30. Clarke G, Grenham S, Scully P et al. . The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. 2013;18:666–673. [DOI] [PubMed] [Google Scholar]
- 31. Furness JB. The organisation of the autonomic nervous system: peripheral connections. Auton Neurosci. 2006;130:1–5. [DOI] [PubMed] [Google Scholar]
- 32. Blackshaw LA, Brookes SJH, Grundy D et al. . Sensory transmission in the gastrointestinal tract. Neurogastroenterol Motil. 2007;19:1–19. [DOI] [PubMed] [Google Scholar]
- 33. Bercik P, Park AJ, Sinclair D et al. . The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol Motil. 2011;23:1132–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bercik P, Verdu EF, Foster JA et al. . Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology. 2010;139:2102–2112. [DOI] [PubMed] [Google Scholar]
- 35. Bercik P, Denou E, Collins J et al. . The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141:599–609, e3. [DOI] [PubMed] [Google Scholar]
- 36. Kunze WA, Mao Y, Wang B et al. . Lactobacillus reuteri enhances excitability of colonic AH neurons by inhibiting calcium-dependent potassium channel opening. J Cell Mol Med. 2009;13:2261–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mcvey Neufeld KA, Mao YK, Bienenstock J et al. . The microbiome is essential for normal gut intrinsic primary afferent neuron excitability in the mouse. Neurogastroenterol Motil. 2013;25:e183–e188. [DOI] [PubMed] [Google Scholar]
- 38. Dogra S, Sakwinska O, Soh SE et al. . Rate of establishing the gut microbiota in infancy has consequences for future health. Gut Microbes. 2015;6:321–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Goedert JJ, Hua X, Yu G et al. . Diversity and composition of the adult fecal microbiome associated with history of cesarean birth or appendectomy: analysis of the American Gut Project. EBioMedicine. 2014;1:167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lax S, Smith DP, Hampton-Marcell J et al. . Longitudinal analysis of microbial interaction between humans and the indoor environment. Science. 2014;345:1048–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mueller N T, Whyatt R, Hoepner L et al. . Prenatal exposure to antibiotics, cesarean section and risk of childhood obesity. Int J Obes (Lond). 2015;39:665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Metsälä J, Lundqvist A, Virta LJ et al. . Prenatal and post-natal exposure to antibiotics and risk of asthma in childhood. Clin Exp Allergy. 2015;45:137–145. [DOI] [PubMed] [Google Scholar]
- 43. Borre YE, O’Keeffe GW, Clarke G et al. . Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol Med. 2014;20:509–518. [DOI] [PubMed] [Google Scholar]
- 44. Dominguez-Bello MG, Costello EK, Contreras M et al. . Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107:11971–11975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Heijtz RD, Wang S, Anuar F et al. . Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A. 2011;108:3047–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Erny D, Hrabě de Angelis AL, Jaitin D et al. . Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18:965–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stilling RM, Ryan FJ, Hoban AE et al. . Microbes and neurodevelopment – absence of microbiota during early life increases activity-related transcriptional pathways in the amygdala. Brain Behav Immun. 2015;50:209–220. [DOI] [PubMed] [Google Scholar]
- 48. Desbonnet L, Clarke G, Shanahan F et al. . Microbiota is essential for social development in the mouse. Mol Psychiatry. 2014;19:146–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rager G. Systems-matching by degeneration. II. Interpretation of the generation and degeneration of retinal ganglion cells in the chicken by a mathematical model. Exp Brain Res. 1978;33:79–90. [DOI] [PubMed] [Google Scholar]
- 50. Herrup K, Sunter K. Numerical matching during cerebellar development: quantitative analysis of granule cell death in staggerer mouse chimeras. J Neurosci. 1987;7:829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wopereis H, Oozeer R, Knipping K et al. . The first thousand days–intestinal microbiology of early life: establishing a symbiosis. Pediatr Allergy Immunol. 2014;25:428–438. [DOI] [PubMed] [Google Scholar]
- 52. Ben-Ari Y. Neuropaediatric and neuroarchaeology: understanding development to correct brain disorders. Acta Paediatr. 2013;102:331–334. [DOI] [PubMed] [Google Scholar]
- 53. Rapoport JL, Giedd JN, Gogtay N. Neurodevelopmental model of schizophrenia: update 2012. Mol Psychiatry. 2012;17:1228–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Thompson BL, Levitt P, Stanwood GD. Prenatal exposure to drugs: effects on brain development and implications for policy and education. Nat Rev Neurosci. 2009;10:303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Workman AD, Charvet CJ, Clancy B et al. . Modeling transformations of neurodevelopmental sequences across mammalian species. J Neurosci. 2013;33:7368–7383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Braniste V, Al-Asmakh M, Kowal C et al. . The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6:263ra158 doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Engelhardt B. Development of the blood-brain barrier. Cell Tissue Res. 2003;314:119–129. [DOI] [PubMed] [Google Scholar]
- 58. Hensch TK. Critical period regulation. Annu Rev Neurosci. 2004;27:549–579. [DOI] [PubMed] [Google Scholar]
- 59. Knudsen EI. Sensitive periods in the development of the brain and behavior. J Cogn Neurosci. 2004;16:1412–1425. [DOI] [PubMed] [Google Scholar]
- 60. Stolp HB, Liddelow SA, Sá-Pereira I et al. . Immune responses at brain barriers and implications for brain development and neurological function in later life. Front Integr Neurosci. 2013;7:61 doi: 10.3389/fnint.2013.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Prenderville JA, Kennedy PJ, Dinan TG et al. . Adding fuel to the fire: the impact of stress on the ageing brain. Trends Neurosci. 2015;38:13–25. [DOI] [PubMed] [Google Scholar]
- 62. Ogbonnaya ES, Clarke G, Shanahan F et al. . Adult hippocampal neurogenesis is regulated by the microbiome. Biol Psychiatry. 2015;78:e7–e9. [DOI] [PubMed] [Google Scholar]
- 63. Knol J, Scholtens P, Kafka C et al. . Colon microflora in infants fed formula with galacto- and fructo-oligosaccharides: more like breast-fed infants. J Pediatr Gastroenterol Nutr. 2005;40:36–42. [DOI] [PubMed] [Google Scholar]
- 64. Diaz Heijtz R. Fetal, neonatal, and infant microbiome: perturbations and subsequent effects on brain development and behavior. Semin Fetal Neonatal Med. 2016;21:410–417. [DOI] [PubMed] [Google Scholar]
- 65. World Health Organization. WHO statement on caesarean section rates. Reprod Health Matters. 2015;23:149–150. [DOI] [PubMed] [Google Scholar]
- 66. World Health Organization. Appropriate technology for birth. Lancet. 1985;2:436–437. [PubMed] [Google Scholar]
- 67. OECD Health Data. Health at a Glance 2011: OECD Indicators. http://dx.doi.org/10.1787/888932524906. Published 2011:95–97. Accessed December 1, 2016. [Google Scholar]
- 68. Betrán AP, Merialdi M, Lauer JA et al. . Rates of caesarean section: analysis of global, regional and national estimates. Paediatr Perinat Epidemiol. 2007;21:98–113. [DOI] [PubMed] [Google Scholar]
- 69. Ye J, Betrán AP, Guerrero Vela M et al. . Searching for the optimal rate of medically necessary cesarean delivery. Birth. 2014;41:237–244. [DOI] [PubMed] [Google Scholar]
- 70. Ronsmans C, Holtz S, Stanton C. Socioeconomic differentials in caesarean rates in developing countries: a retrospective analysis. Lancet. 2006;368:1516–1523. [DOI] [PubMed] [Google Scholar]
- 71. Lumbiganon P, Laopaiboon M, Gülmezoglu AM et al. . Method of delivery and pregnancy outcomes in Asia: the WHO global survey on maternal and perinatal health 2007-08. Lancet. 2010;375:490–499. [DOI] [PubMed] [Google Scholar]
- 72. Watanabe S, Narisawa Y, Arase S et al. . Differences in fecal microflora between patients with atopic dermatitis and healthy control subjects. J Allergy Clin Immunol. 2003;111:587–591. [DOI] [PubMed] [Google Scholar]
- 73. Negele K, Heinrich J, Borte M et al. . Mode of delivery and development of atopic disease during the first 2 years of life. Pediatr Allergy Immunol. 2004;15:48–54. [DOI] [PubMed] [Google Scholar]
- 74. Kalliomaki M, Collado MC, Salminen S et al. . Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr. 2008;87:534–538. [DOI] [PubMed] [Google Scholar]
- 75. Pandey PK, Verma P, Kumar H et al. . Comparative analysis of fecal microflora of healthy full-term Indian infants born with different methods of delivery (vaginal vs cesarean): Acinetobacter sp. prevalence in vaginally born infants. J Biosci. 2012;37:989–998. [DOI] [PubMed] [Google Scholar]
- 76. Marild K, Stephansson O, Montgomery S et al. . Pregnancy outcome and risk of celiac disease in offspring: a nationwide case-control study. Gastroenterology. 2012;142:39–45, e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Xu B, Pekkanen J, Hartikainen AL et al. . Caesarean section and risk of asthma and allergy in adulthood. J Allergy Clin Immunol. 2001;107:732–733. [DOI] [PubMed] [Google Scholar]
- 78. Kero J, Gissler M, Gronlund MM et al. . Mode of delivery and asthma – is there a connection? Pediatr Res. 2002;52:6–11. [DOI] [PubMed] [Google Scholar]
- 79. Algert CS, McElduff A, Morris JM et al. . Perinatal risk factors for early onset of Type 1 diabetes in a 2000-2005 birth cohort. Diabet Med. 2009;26:1193–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Huh S Y, Rifas-Shiman S L, Zera CA et al. . Delivery by caesarean section and risk of obesity in preschool age children: a prospective cohort study. Arch Dis Child. 2012;97:610–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Blustein J, Attina T, Liu M et al. . Association of caesarean delivery with child adiposity from age 6 weeks to 15 years. Int J Obes (Lond). 2013;37:900–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bisgaard H, Li N, Bonnelykke K et al. . Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J Allergy Clin Immunol. 2011;128:646–652, e1–e5. [DOI] [PubMed] [Google Scholar]
- 83. Roduit C, Scholtens S, de Jongste JC et al. . Asthma at 8 years of age in children born by caesarean section. Thorax. 2009;64:107–113. [DOI] [PubMed] [Google Scholar]
- 84. von Mutius E. Allergies, infections and the hygiene hypothesis—the epidemiological evidence. Immunobiology. 2007;212:433–439. [DOI] [PubMed] [Google Scholar]
- 85. Cardwell CR, Stene LC, Joner G et al. . Caesarean section is associated with an increased risk of childhood-onset type 1 diabetes mellitus: a meta-analysis of observational studies. Diabetologia. 2008;51:726–735. [DOI] [PubMed] [Google Scholar]
- 86. Aumeunier A, Grela F, Ramadan A et al. . Systemic Toll-like receptor stimulation suppresses experimental allergic asthma and autoimmune diabetes in NOD mice. PLoS One. 2010;5:e11484 doi: 10.1371/journal.pone.0011484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mezoff EA, Aly H. The winding road to understanding the neonatal origins of inflammatory gastrointestinal disorders. J Pediatr Gastroenterol Nutr. 2013;57:543–549. [DOI] [PubMed] [Google Scholar]
- 88. Akobeng AK, Ramanan AV, Buchan I et al. . Effect of breast feeding on risk of celiac disease: a systematic review and meta-analysis of observational studies. Arch Dis Child. 2006;91:39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Jakobsson HE, Abrahamsson TR, Jenmalm MC et al. . Decreased gut microbiota diversity, delayed Bacteroidetes colonization and reduced Th1 responses in infants delivered by caesarean section. Gut. 2014;63:559–566. [DOI] [PubMed] [Google Scholar]
- 90. Hansen CH, Andersen LS, Krych L et al. . Mode of delivery shapes gut colonization pattern and modulates regulatory immunity in mice. J Immunol. 2014;193:1213–1222. [DOI] [PubMed] [Google Scholar]
- 91. Sevelsted A, Stokholm J, Bønnelykke K et al. . Cesarean section and chronic immune disorders. Pediatrics. 2015;135:e92–e98. [DOI] [PubMed] [Google Scholar]
- 92. Li Y, Tian Y, Zhu W et al. . Cesarean delivery and risk of inflammatory bowel disease: a systematic review and meta-analysis. Scand J Gastroenterol. 2014;49:834–844. [DOI] [PubMed] [Google Scholar]
- 93. Chudal R, Sourander A, Polo-Kantola P et al. . Perinatal factors and the risk of bipolar disorder in Finland. J Affect Disord. 2014;155:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Curran EA, O'Neill SM, Cryan JF et al. . Research review: birth by caesarean section and development of autism spectrum disorder and attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. J Child Psychol Psychiatry. 2015;56:500–508. [DOI] [PubMed] [Google Scholar]
- 95. Curran EA, Cryan JF, Kenny LC et al. . Obstetrical mode of delivery and childhood behavior and psychological development in a British cohort. J Autism Dev Disord. 2016;46:603–614. [DOI] [PubMed] [Google Scholar]
- 96. O'Neill SM, Curran EA, Dalman C et al. . Birth by caesarean section and the risk of adult psychosis: a population-based cohort study. Schizophr Bull. 2016;42:633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Brander G, Rydell M, Kuja-Halkola R et al. . Association of perinatal risk factors with obsessive-compulsive disorder: a population-based birth cohort, sibling control study. JAMA Psychiatry. 2016;73:1135–1144. [DOI] [PubMed] [Google Scholar]
- 98. Neufeld K-AM, Luczynski P, Dinan TG et al. . Reframing the teenage wasteland: adolescent microbiota-gut-brain axis. Can J Psychiatry. 2016;61:214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Jost T, Lacroix C, Braegger CP et al. . New insights in gut microbiota establishment in healthy breast fed neonates. PLoS One. 2012;7:e44595 doi: 10.1371/journal.pone.0044595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Azad MB, Konya T, Maughan H et al. . Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. CMAJ. 2013;185:385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Dominguez-Bello MG, De Jesus-Laboy KM, Shen N et al. . Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat Med. 2016;22:250–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Cunnington AJ, Sim K, Deierl A et al. . “Vaginal seeding” of infants born by caesarean section. BMJ. 2016;352:i227 doi: 10.1136/bmj.i227. [DOI] [PubMed] [Google Scholar]
- 103. Yancey MK, Schuchat A, Brown LK et al. . The accuracy of late antenatal screening cultures in predicting genital group B streptococcal colonization at delivery. Obstet Gynecol. 1996;88:811–815. [DOI] [PubMed] [Google Scholar]
- 104. Campbell JR, Hillier SL, Krohn MA et al. . Group B streptococcal colonization and serotype-specific immunity in pregnant women at delivery. Obstet Gynecol. 2000;96:498–503. [DOI] [PubMed] [Google Scholar]
- 105. Knight R, Gilbert JA. Opinion: a mother’s microbes. The Scientist. 2016. http://mobile.the-scientist.com/article/45505/opinion-a-mother-s-microbes. Accessed December 1, 2016. [Google Scholar]
- 106. Money DM, Dobson S. The prevention of early-onset neonatal group B streptococcal disease. J Obstet Gynaecol Can. 2004;26:826–840. [DOI] [PubMed] [Google Scholar]
- 107. Verani JR, McGee L, Schrag SJ. Prevention of perinatal group B streptococcal disease: revised guidelines from CDC, 2010. MMWR Recomm Rep. 2010;59:1–36. [PubMed] [Google Scholar]
- 108. UK National Screening Committee. The UK NSC policy on group B streptococcus screening in pregnancy. 2012. https://legacyscreening.phe.org.uk/groupbstreptococcus. Accessed December 1, 2016. [Google Scholar]
- 109. Rook GA. Regulation of the immune system by biodiversity from the natural environment: an ecosystem service essential to health. Proc Natl Acad Sci U S A. 2013;110:18360–18367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Renfrew MJ, McFadden A, Bastos MH et al. . Midwifery and quality care: findings from a new evidence-informed framework for maternal and newborn care. Lancet. 2014;384:1129–1145. [DOI] [PubMed] [Google Scholar]
- 111. Collado MC, Rautava S, Aakko J et al. . Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci Rep. 2016;6:doi:10.1038/srep23129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Hill C, Guarner F, Reid G et al. . Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–514. [DOI] [PubMed] [Google Scholar]
- 113. Gueimonde M, Sakata S, Kalliomaki M et al. . Effect of maternal consumption of lactobacillus GG on transfer and establishment of fecal bifidobacterial microbiota in neonates. J Pediatr Gastroenterol Nutr. 2006;42:166–170. [DOI] [PubMed] [Google Scholar]
- 114. Schultz M, Gottl C, Young RJ et al. . Administration of oral probiotic bacteria to pregnant women causes temporary infantile colonization. J Pediatr Gastroenterol Nutr. 2004;38:293–297. [DOI] [PubMed] [Google Scholar]
- 115. Ismail IH, Oppedisano F, Joseph SJ et al. . Prenatal administration of Lactobacillus rhamnosus has no effect on the diversity of the early infant gut microbiota. Pediatr Allergy Immunol. 2012;23:255–258. [DOI] [PubMed] [Google Scholar]
- 116. Anderson S. Probiotics for preterm infants: a premature or overdue necrotizing enterocolitis prevention strategy? Neonatal Netw. 2015;34:83–101. [DOI] [PubMed] [Google Scholar]
- 117. Abdulkadir B, Nelson A, Skeath T et al. . Routine use of probiotics in preterm infants: longitudinal impact on the microbiome and metabolome. Neonatology. 2016;109:239–247. [DOI] [PubMed] [Google Scholar]
- 118. Lin C S, Chang C J, Lu CC et al. . Impact of the gut microbiota, prebiotics, and probiotics on human health and disease. Biomed J. 2014;37:259–268. [DOI] [PubMed] [Google Scholar]
- 119. Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401–1412. [DOI] [PubMed] [Google Scholar]
- 120. Gibson GR, Probert HM, Van Loo J et al. . Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev. 2004;17:259–275. [DOI] [PubMed] [Google Scholar]
- 121. Delzenne NM, Neyrinck AM, Cani PD. Gut microbiota and metabolic disorders: how prebiotic can work? Br J Nutr. 2013;109:S81–S85. [DOI] [PubMed] [Google Scholar]
- 122. Marques TM, Cryan JF, Shanahan F et al. . Gut microbiota modulation and implications for host health: dietary strategies to influence the gut-brain axis. Innov Food Sci Emerg Technol. 2014;22:239–247. [Google Scholar]
- 123. Rastall RA, Gibson GR. Recent developments in prebiotics to selectively impact beneficial microbes and promote intestinal health. Curr Opin Biotechnol. 2015;32:42–46. [DOI] [PubMed] [Google Scholar]
- 124. Slavin J. Fiber and prebiotics: mechanisms and health benefits. Nutrients. 2013;5:1417–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Fujiwara R, Takemura N, Watanabe J et al. . Maternal consumption of fructo-oligosaccharide diminishes the severity of skin inflammation in offspring of NC/Nga mice. Br J Nutr. 2010;103:530–538. [DOI] [PubMed] [Google Scholar]
- 126. Desbuards N, Gourbeyre P, Haure-Mirande V et al. . Impact of perinatal prebiotic consumption on gestating mice and their offspring: a preliminary report. Br J Nutr. 2012;107:1245–1248. [DOI] [PubMed] [Google Scholar]
- 127. Wang M, Radlowski EC, Monaco MH et al. . Mode of delivery and early nutrition modulate microbial colonization and fermentation products in neonatal piglets. J Nutr. 2013;143:795–803. [DOI] [PubMed] [Google Scholar]
- 128. Partty A, Luoto R, Kalliomaki M et al. . Effects of early prebiotic and probiotic supplementation on development of gut microbiota and fussing and crying in preterm infants: a randomized, double-blind, placebo-controlled trial. J Pediatr. 2013;163:1272–1277, e1–e2. [DOI] [PubMed] [Google Scholar]
- 129. Barrett E, Deshpandey AK, Ryan CA et al. . The neonatal gut harbours distinct bifidobacterial strains. Arch Dis Child Fetal Neonatal Ed. 2015;100:F405–F410. [DOI] [PubMed] [Google Scholar]
- 130. Arslanoglu S, Moro GE, Schmitt J et al. . Early dietary intervention with a mixture of prebiotic oligosaccharides reduces the incidence of allergic manifestations and infections during the first two years of life. J Nutr. 2008;138:1091–1095. [DOI] [PubMed] [Google Scholar]
- 131. Grüber C, van Stuijvenberg M, Mosca F et al. . Reduced occurrence of early atopic dermatitis because of immunoactive prebiotics among low-atopy-risk infants. J Allergy Clin Immunol. 2010;126:791–797. [DOI] [PubMed] [Google Scholar]
- 132. Haarman M, Knol J. Quantitative real-time PCR assays to identify and quantify fecal Bifidobacterium species in infants receiving a prebiotic infant formula. Appl Environ Microbiol. 2005;71:2318–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Luoto R, Ruuskanen O, Waris M et al. . Prebiotic and probiotic supplementation prevents rhinovirus infections in preterm infants: a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2014;133:405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Chua MC, Chew C, Goh A et al. . A synbiotic mixture of scGOS/lcFOS and Bifidobacterium breve M-16V is able to restore the delayed colonization of Bifidobacteria ssp. in C-section delivered infants. J Pediatric Neonatal Individualized Med. 2015;4:e040210. [Google Scholar]
- 135. Schouten B, van Esch BC, Hofman GA et al. . Cow milk allergy symptoms are reduced in mice fed dietary synbiotics during oral sensitization with whey. J Nutr. 2009;139:1398–1403. [DOI] [PubMed] [Google Scholar]
- 136. Passeron T, Lacour JP, Fontas E et al. . Prebiotics and synbiotics: two promising approaches for the treatment of atopic dermatitis in children above 2 years. Allergy. 2006;61:431–437. [DOI] [PubMed] [Google Scholar]
- 137. Nikniaz L, Mahdavi R, Ostadrahimi A et al. . Effects of synbiotic supplementation on total antioxidant capacity of human breastmilk. Breastfeed Med. 2013;8:217–222. [DOI] [PubMed] [Google Scholar]
- 138. Ostadrahimi A, Nikniaz L, Mahdavi R et al. . Effects of synbiotic supplementation on lactating mothers' energy intake and BMI, and infants' growth. Int J Food Sci Nutr. 2013;64:711–714. [DOI] [PubMed] [Google Scholar]
- 139. Hinde K, German JB. Food in an evolutionary context: insights from mother’s milk. J Sci Food Agric. 2012;92:2219–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Hinde K, Lewis ZT. Mother’s littlest helpers. Science. 2015;348:1427–1428. [DOI] [PubMed] [Google Scholar]
- 141. Murphy K, Curley D, Callaghan T et al. . The composition of human milk and infant faecal microbiota over first three months of life. Sci Rep. 2017;7:40597. doi: 10.1038/srep40597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Bode L. Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology. 2012;22:1147–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Sela DA, Chapman J, Adeuya A et al. . The genome sequence of Bifidobacterium longum subsp. infantis reveals adaptations for milk utilization within the infant microbiome. Proc Natl Acad Sci U S A. 2008;105:18964–18969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Huda MN, Lewis Z, Kalanetra KM et al. . Stool microbiota and vaccine responses of infants. Pediatrics. 2014;134:e362–e372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Houghteling PD, Walker WA. Why is initial bacterial colonization of the intestine important to infants’ and children's health? J Pediatr Gastroenterol Nutr. 2015;60:294–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Bode L. Human milk oligosaccharides: prebiotics and beyond. Nutr Rev. 2009;67:S183–S191. [DOI] [PubMed] [Google Scholar]
- 147. Ding T, Schloss PD. Dynamics and associations of microbial community types across the human body. Nature. 2014;509:357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Schwartz S, Friedberg I, Ivanov IV et al. . A metagenomic study of diet-dependent interaction between gut microbiota and host in infants reveals differences in immune response. Genome Biol. 2012;13:doi: 10.1186/gb-2012-13-4-r32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. McGuire MK, McGuire MA. Human milk: mother nature's prototypical probiotic food? Adv Nutr. 2015;6:112–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Ardeshir A, Narayan NR, Mendez-Lagares G et al. . Breast-fed and bottle-fed infant rhesus macaques develop distinct gut microbiotas and immune systems. Sci Transl Med. 2014;6:doi: 10.1126/scitranslmed.3008791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Hu Y, Yang X, Qin J et al. . Metagenome-wide analysis of antibiotic resistance genes in a large cohort of human gut microbiota. Nat Commun. 2013;4:2151. [DOI] [PubMed] [Google Scholar]
- 152. Prior E, Santhakumaran S, Gale C et al. . Breastfeeding after cesarean delivery: a systematic review and meta-analysis of world literature. Am J Clin Nutr. 2012;95:1113–1135. [DOI] [PubMed] [Google Scholar]
- 153. Miyata J, Arita M. Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergol Int. 2015;64:27–34. [DOI] [PubMed] [Google Scholar]
- 154. Yates CM, Calder PC, Ed Rainger G. Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. Pharmacol Ther. 2014;141:272–282. [DOI] [PubMed] [Google Scholar]
- 155. Pusceddu MM, El Aidy S, Crispie F et al. . N-3 polyunsaturated fatty acids (PUFAs) reverse the impact of early-life stress on the gut microbiota. PLoS One. 2015;10:e0142228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Miles E.A, Calder PC. Omega-6 and omega-3 polyunsaturated fatty acids and allergic diseases in infancy and childhood. Curr Pharm Des. 2014;20:946–953. [DOI] [PubMed] [Google Scholar]
- 157. Melody M, Vincent R, Mori TA et al. . Effects of omega-3 and omega-6 fatty acids on human placental cytokine production. Placenta. 2015;36:34–40. [DOI] [PubMed] [Google Scholar]
- 158. Hsieh AT, Brenna JT. Dietary docosahexaenoic acid but not arachidonic acid influences central nervous system fatty acid status in baboon neonates. Prostaglandins Leukot Essent Fatty Acids. 2009;81:105–110. [DOI] [PubMed] [Google Scholar]
- 159. Stevens J, Keim SA. How research on charitable giving can inform strategies to promote human milk donations to milk banks. J Hum Lact. 2015;31:344–347. [DOI] [PubMed] [Google Scholar]
- 160. Cabrera-Rubio R, Mira-Pascual L, Mira A et al. . Impact of mode of delivery on the milk microbiota composition of healthy women. J Dev Orig Health Dis. 2016;7:54–60. [DOI] [PubMed] [Google Scholar]
- 161. Rodkiewicz B, Hardell LI, Pawlikowska-Rojewska B et al. . Fatty acid composition of human breast milk. Changes during the first week after delivery. Ups J Med Sci. 1981;86:279–289. [DOI] [PubMed] [Google Scholar]
- 162. Sundekilde UK, Downey E, O'Mahony JA et al. . The effect of gestational and lactational age on the human milk metabolome. Nutrients. 2016;8:doi: 10.3390/nu8050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. MacKenzie C, Javanparast S, Newman L. Mothers’ knowledge of and attitudes towards human milk banking in South Australia: a qualitative analysis. J Hum Lact. 2013;29:222–229. [DOI] [PubMed] [Google Scholar]
- 164. Betran AP, Torloni MR, Zhang JJ et al. . WHO Statement on caesarean section rates. BJOG. 2016;123:667–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Ahmed S, Tunçalp Ö. Burden of obstetric fistula: from measurement to action. Lancet Glob Health. 2015;3:e243–e244. [DOI] [PubMed] [Google Scholar]
- 166. Black M, Bhattacharya S, Philip S et al. . Planned cesarean delivery at term and adverse outcomes in childhood health. JAMA. 2015;314:2271–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Mazzola G, Murphy K, Ross RP et al. . Early gut microbiota perturbations following intrapartum antibiotic prophylaxis to prevent group B Streptococcal disease. PLoS One. 2016;11:doi: 10.1371/journal.pone.0157527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Jašarević E, Rodgers AB, Bale TL. A novel role for maternal stress and microbial transmission in early life programming and neurodevelopment. Neurobiol Stress. 2015;1:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Jašarević E, Howerton CL, Howard CD et al. . Alterations in the vaginal microbiome by maternal stress are associated with metabolic reprogramming of the offspring gut and brain. Endocrinology. 2015;156:3265–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Rasmussen SA, Chu SY, Kim SY et al. . Maternal obesity and risk of neural tube defects: a metaanalysis. Am J Obstet Gynecol. 2008;198:611–619. [DOI] [PubMed] [Google Scholar]
- 171. Papachatzi E, Dimitriou G, Dimitropoulos K et al. . Prepregnancy obesity: maternal, neonatal and childhood outcomes. J Neonatal Perinatal Med. 2013;6:203–216. [DOI] [PubMed] [Google Scholar]
- 172. Gaudet L, Ferraro ZM, Wen SW et al. . Maternal obesity and occurrence of fetal macrosomia: a systematic review and meta-analysis. Biomed Res Int. 2014;2014:doi: 10.1155/2014/640291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Lucas A. Programming by early nutrition: an experimental approach. J Nutr. 1998;128:401S–406S. [DOI] [PubMed] [Google Scholar]
- 174. Mueller NT, Bakacs E, Combellick J et al. . The infant microbiome development: mom matters. Trends Mol Med. 2015;21:109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Kelly JR, Borre Y, O'Brien C et al. . Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res. 2016;82:109–118. [DOI] [PubMed] [Google Scholar]
- 176. Hoban AE, Stilling RM, Ryan FJ et al. . Regulation of prefrontal cortex myelination by the microbiota. Transl Psychiatry. 2016;6:doi: 10.1038/tp.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Luczynski P, Whelan SO, O'Sullivan C et al. . Adult microbiota-deficient mice have distinct dendritic morphological changes: differential effects in the amygdala and hippocampus. Eur J Neurosci. 2016;44:2654–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiatry. 2013;74:720–726. [DOI] [PubMed] [Google Scholar]
- 179. Allen AP, Hutch W, Borre Y et al. . Bifidobacterium longum 1714 as a translational psychobiotic: modulation of stress, electrophysiology and neurocognition in healthy volunteers. Translational Psychiatry 2016;6:doi: 10.1038/tp.2016.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Hill CJ, Lynch DB, Murphy K et al. . Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome. 2017;5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]