Abstract
Background. Complement dysregulation occurs in thrombotic microangiopathies (TMAs) other than primary atypical haemolytic uraemic syndrome (aHUS). A few of these patients have been reported previously to be successfully treated with eculizumab.
Methods. We identified 29 patients with so-called secondary aHUS who had received eculizumab at 11 Spanish nephrology centres. Primary outcome was TMA resolution, defined by a normalization of platelet count (>150 × 109/L) and haemoglobin, disappearance of all the markers of microangiopathic haemolytic anaemia (MAHA), and improvement of renal function, with a ≥25% reduction of serum creatinine from the onset of eculizumab administration.
Results. Twenty-nine patients with secondary aHUS (15 drug-induced, 8 associated with systemic diseases, 2 with postpartum, 2 with cancer-related, 1 associated with acute humoral rejection and 1 with intestinal lymphangiectasia) were included in this study. The reason to initiate eculizumab treatment was worsening of renal function and persistence of TMA despite treatment of the TMA cause and plasmapheresis. All patients showed severe MAHA and renal function impairment (14 requiring dialysis) prior to eculizumab treatment and 11 presented severe extrarenal manifestations. A rapid resolution of the TMA was observed in 20 patients (68%), 15 of them showing a ≥50% serum creatinine reduction at the last follow-up. Comprehensive genetic and molecular studies in 22 patients identified complement pathogenic variants in only 2 patients. With these two exceptions, eculizumab was discontinued, after a median of 8 weeks of treatment, without the occurrence of aHUS relapses.
Conclusion. Short treatment with eculizumab can result in a rapid improvement of patients with secondary aHUS in whom TMA has persisted and renal function worsened despite treatment of the TMA-inducing condition.
Keywords: atypical haemolytic uraemic syndrome, complement activation, eculizumab, thrombotic microangiopathies
INTRODUCTION
Thrombotic microangiopathies (TMAs) is a group of devastating diseases with very different aetiologies that share common characteristic features at presentation: microangiopathic haemolytic anaemia (MAHA) [typified by anaemia, elevated lactate dehydrogenase (LDH), decreased serum haptoglobin and the presence of schistocytes in peripheral blood smear], thrombocytopenia and organ damage, mainly the kidneys and the nervous system. The pathogenesis of TMA is based on a diffuse and severe vascular endothelial injury inducing characteristic histological lesions in affected organs: platelet thrombus occluding vessels, swelling and detachment of endothelial cells, and thickening of arterioles and capillaries [1–5].
The most widely accepted classification establishes four categories of TMA: Shiga toxin-producing Escherichia coli haemolytic uraemic syndrome (STEC-HUS), thrombotic thrombocytopenic purpura (TTP), atypical haemolytic uraemic syndrome (aHUS) and secondary TMA [1–5]. This last group includes a long list of clinical entities in which the occurrence of TMA has been repeatedly reported: drug treatments [6, 7], systemic diseases [8–10], pregnancy/postpartum [11, 12], cancer [7, 13], haematopoietic stem cell and organ transplantation [14, 15], systemic infections [16, 17], glomerular diseases [18, 19], malignant hypertension [20] and some very rare conditions such as intestinal lymphangiectasia [21] or methylmalonicaciduria [22]. The pathogenesis of STEC-HUS, TTP and aHUS has been largely clarified in recent years [1–5], but the pathogenic pathways through which endothelial damage is induced in secondary TMA are not completely elucidated. It is generally accepted that in a significant number of patients within the group of secondary TMA there is a complement-related damage to the endothelium.
Eculizumab is a humanized monoclonal antibody that binds to complement C5 and prevents the formation of C5b-9, the membrane attack complex of the terminal complement pathway [23]. The efficacy and safety of eculizumab in aHUS has been demonstrated by observational studies and prospective multicentre trials [23, 24].
Complement is a tightly controlled part of our innate immunity, fundamental among other things for the elimination of pathogens and cellular debris generated by normal tissue homeostasis. aHUS caused by pathogenic variants in the genes coding for complement components and regulators, or by autoantibodies against these proteins [1–5, 25, 26], is referred here as primary aHUS, as suggested in the KDIGO Controversies Conference on aHUS and C3G [27]. The genetic and acquired factors associated with primary aHUS cause complement dysregulation resulting in damage to the endothelial cells in the microvasculature of the kidneys and other organs. While complement dysregulation is central to the pathogenesis of primary aHUS, cumulative evidence suggests that complement hyperactivation and dysregulation also occurs in other forms of TMA apart from primary aHUS, including some patients with STEC-HUS and TTP, and may be particularly relevant in an important number of secondary TMA cases (reviewed in [28]). We will refer to these cases of TMA as secondary aHUS. Further supporting the involvement of complement dysregulation in other forms of TMA apart from primary aHUS, individual case reports and small series of patients have been published in recent years showing a beneficial effect of eculizumab in cases of TMA associated with haematopoietic stem cell transplantation [29, 30], antiphospholipid syndrome [31, 32], systemic diseases [33, 34], pregnancy/postpartum [35] and drug treatments [36–38]. However, the small number of reported cases and the well-known tendency to preferentially publish those cases responding positively to new therapies prevents the generalization of such positive results.
To assess the potential benefit of eculizumab in this, so-called, secondary aHUS, we designed a collaborative retrospective study to identify these cases and revise those who had received eculizumab, independently of the aetiological factor causing the TMA and the final outcome of the eculizumab treatment.
MATERIALS AND METHODS
Study population and treatment
Patients with secondary aHUS treated with eculizumab were identified in 11 Spanish hospitals. Inclusion criteria were: (i) evidence of TMA, defined by the presence of low platelet count (<150 × 109/L), MAHA (low haemoglobin, LDH level above the upper limit of the normal range, decreased serum haptoglobin and presence of schistocytes in peripheral blood examination), negative Coombs test, normal activity of ADAMTS-13, negative Shiga toxin and impaired renal function fulfilling the criteria of acute kidney injury (AKI) [39]; (ii) diagnosis of secondary TMA, based on the presence of a precipitating cause listed among the recognized aetiologies of secondary TMA [5]; (iii) persistence of TMA and worsening of renal function despite the treatment of the TMA-precipitating trigger or disease; and (iv) treatment with eculizumab after previous treatments had failed. Exclusion criteria included previous episodes of TMA and, in renal transplant patients, the diagnosis of aHUS or C3 glomerulopathies in native kidneys. Patients fulfilling these inclusion and exclusion criteria were included in the study, independently of the TMA cause and patient’s outcome after eculizumab treatment.
Eculizumab was administered in all the patients intravenously at a dose of 900 mg/week for 4 weeks, and then 1200 mg every 2 weeks. Plasmapheresis was discontinued in all the patients once they started eculizumab. All the patients received anti-meningococcal vaccination, according to label instructions, and received antibiotic prophylaxis. The duration of eculizumab therapy was decided by the treating physician, based on the patient’s response and individual characteristics. Baseline data were considered to be the data coinciding with the detection of TMA. Follow-up was defined as the interval between the onset of eculizumab and the last visit or death.
Data collection
Data were compiled from the medical records of the participating centres using a uniform protocol and included age, gender, blood pressure and cause of TMA. Analytical variables included haemoglobin, platelet count, haptoglobin, LDH, presence of schistocytes in peripheral blood smear, Coombs test, ADAMTS-13 determination, Shiga toxin detection, serum creatinine (SCr), estimated glomerular filtration rate (eGFR), which was calculated using the four-variable Modification of Diet in Renal Disease equation, serum albumin and urinalysis. Results of the genetic analysis, performed in 22 patients were registered following a common protocol.
Treatments received before eculizumab, number of plasmapheresis sessions and the time interval between TMA onset and the initiation of eculizumab treatment were recorded, as well as the duration of eculizumab therapy and the cumulative dose administered to every patient. Complications occurring during plasmapheresis and eculizumab treatment, side effects, the occurrence of end-stage renal disease and deaths were also recorded.
Renal biopsies were performed in 18 patients and were reviewed for this study at every participating centre. Histological signs of TMA included glomerular and arteriolar thrombus, thickening of arteriolar walls, swelling and detachment of endothelial cells, widening of subendothelial space in glomerular capillaries and glomerular retraction.
Outcomes
The primary outcome was TMA resolution, defined by a normalization of platelet count (>150 × 109/L), normalization of haemoglobin, disappearance of all the markers of MAHA and recovery of renal function, with a ≥25% reduction in SCr in two consecutive measurements for ≥4 weeks from the onset of eculizumab administration. Secondary outcomes were the normalization of platelet count and haemoglobin with the disappearance of MAHA markers, the number of patients requiring dialysis, a ≥25% and a ≥50% reduction from the onset of eculizumab in SCr and the number of patients with an eGFR ≥60 mL/min/1.73 m2 at latest follow-up.
Statistical analysis
Continuous variables were expressed as median and interquartile (25th and 75th percentile) range. Categorical variables were expressed as frequencies and percentages. Categorical variables were compared with the Fisher’s exact probability test and the medians were compared using the Mann–Whitney U test. To compare more than two categorical variables, the chi-square (χ2) test was used. Statistical analyses were performed with STATA software for Windows, version 12.0 (Statacorp LP, College Station, TX, USA) with two-sided hypothesis testing and a P < 0.05 as the criteria for statistical significance.
Other methods
Complement genetic and molecular studies are described in detail in Supplementary Methods.
RESULTS
Clinical characteristics
Twenty-nine patients were included in the study. Their main clinical characteristics at the onset of eculizumab treatment are summarized in Tables 1–4. MAHA and AKI were evident in all patients. Normal ADAMTS-13 activity was assessed in all patients, with a median value of 68% (60–78%). Stool analysis for Shiga toxin detection and Coombs test, performed in 14 and 24 patients, respectively, were negative. Fourteen patients (48%) were undergoing dialysis at the onset of eculizumab due to the severity of renal failure and/or volume expansion. Severe extrarenal manifestations were observed in 11 patients (38%): five patients presented with seizures, three had decreased level of consciousness, four headache and blurred vision, and one had dilated cardiomyopathy.
Table 1.
Characteristics at the initiation of eculizumab treatment
| Patients (n = 29) | |
|---|---|
| Age (years)a | 51.8 (36.2–59.6) |
| Gender, no. (%), male | 16 (55.2) |
| Cause of aHUS, no. (%) | |
| Drug-induced | 15 (51.7) |
| Tacrolimus | 14 (93.3) |
| Everolimus | 4 (26.7) |
| Sirolimus | 1 (6.7) |
| Systemic disease | 8 (27.6) |
| SLE | 3 (37.5) |
| Scleroderma | 2 (25) |
| Vasculitis (EGPA) | 2 (25) |
| Antiphospholipid syndrome | 1 (12.5) |
| Other causes | 6 (20.7) |
| Pregnancy/postpartum | 2 (33.3) |
| Cancer related | 2 (33.3) |
| Acute humoral rejection renal transplant | 1 (16.7) |
| Primary intestinal lymphangiectasia | 1 (16.7) |
| Time from aHUS diagnosis to eculizumab treatment (days) | 13 (7–26) |
| Extrarenal manifestation, no. (%) | 11 (37.9) |
| Dialysis before eculizumab, no. (%) | 14 (48.3) |
| Laboratory findings | |
| SCr (mg/dL)a | 4 (3.4–5.6) |
| eGFR (mL/min/1.73 m2)a | 13 (7.7–19.0) |
| LDH (mg/dL)a | 960 (570–1950) |
| Haptoglobin (mg/dL)a | 5 (0–12) |
| Haemoglobin (g/dL)a | 8.8 (8.1–9.9) |
| Platelet count (×1000/µL)a | 73 (51–113) |
| Schystocites, no. (%) | 28 (96.6) |
| Follow-up (months)a | 5.2 (4.2–14.1) |
eGFR, estimated glomerular filtration rate; EGPA, eosinophilic granulomatosis with polyangiitis; SCr, serum creatinine; aHUS, atypical haemolytic uraemic syndrome; LDH, lactate dehydrogenase.
Median (25th–75th percentile).
Causes of aHUS and treatment before eculizumab
As shown in Table 1, aHUS was induced by drugs in 15 patients, by systemic diseases in 8, by postpartum in 2, by cancer in 2, by acute humoral rejection in 1 and by primary intestinal lymphangiectasia in 1. All drug-induced aHUS corresponded to transplanted patients who had received a kidney graft (seven patients), lung (four patients), haematopoietic stem cells (two patients), liver (one patient) or heart (one patient) (Table 2). In seven patients with a kidney graft (#1 to #7), median interval between transplantation and aHUS detection was 14 days (7–20). Induction treatment consisted of corticosteroids, tacrolimus and mycophenolate mofetil in all of them. Four patients (#1, #2, #3 and #6) also received antithymocyte globulin.
Table 2.
Drug-induced aHUS
| Patient | Age (years), gender | Offending drug | Treatment before eculizumab | PE no. of sessions | Eculizumab (duration, no. of doses) | Time from aHUS to eculizumab (days) | Highest SCr (mg/dL) HD (Yes/No) | Latest SCr (mg/dL) | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 43, female | Tacrolimusa | DW + PE | 3 | 2 weeks, 2 | 4 | 3.8 No | 1.1 | 16.2 |
| 2 | 63 female | Tacrolimusa | DW + PE | 10 | 18 weeks, 11 | 53 | 3.1 Yes | 2.0 | 5.9 |
| 3 | 34, male | Tacrolimusa | PE | 3 | 24 weeks, 14 | 10 | 9.8 Yes | 2.6 | 6.7 |
| 4 | 18, female | Tacrolimusa | DW + PE | 2 | 3 weeks, 3 | 14 | 2.1 No | 2 | 9.6 |
| 5 | 52, female | Tacrolimusa | DW | – | 8 weeks, 6 | 26 | 2.9 No | 1.2 | 8.3 |
| 6 | 43, male | Tacrolimusa | DW + PE | 6 | 30 weeks, 17 | 35 | 3.8 Yes | 1.1 | 16.2 |
| 7 | 36, female | Everolimusa | DW + PE | 6 | 6 weeks, 5 | 9 | 4.2 No | 2.1 | 17.5 |
| 8 | 60, male | Tacrolimusb | DW | – | 4 weeks, 4 | 53 | 3.4 Yes | 2.5 | 3.4 |
| 9 | 67, male | Tacrolimus, everolimusb | DW + PE | 6 | 6 weeks, 5 | 10 | 1.8 No | 2.0 | 1.7 |
| 10 | 59, male | Tacrolimus, everolimusb | DW + PE | 5 | 10 weeks, 7 | 7 | 3.5 No | 2.0 | 4.8 |
| 11 | 65, male | Tacrolimus, everolimusb | DW + PE | 6 | 2 weeks, 2 | 9 | 3.3 No | 2.4 | 4.5 |
| 12 | 51, male | Tacrolimusc | DW + PE | 2 | 4 weeks, 4 | 10 | 2.4 No | 0.6 | 2.9 |
| 13 | 54, female | Tacrolimus, sirolimusc | DW + PE | 7 | 3 weeks, 3 | 7 | 4.2 Yes | 1.2 | 1.5 |
| 14 | 55, male | Tacrolimusd | DW + PE | 12 | 18 weeks, 11 | 27 | 3.0 No | 2.4 | 14.1 |
| 15 | 42, female | Tacrolimuse | DW + PE | 3 | 3 weeks, 3 | 13 | 1.4 No | 0.5 | 17.0 |
DW, drug withdrawn; HD, haemodialysis (Yes/No); PE, plasma exchange; SCr, serum creatinine; aHUS, atypical haemolytic uraemic syndrome.
Kidney transplant.
Lung transplant.
Haematopoietic stem cell transplantation.
Liver transplant.
Heart transplant.
The offending drug was tacrolimus (combined with everolimus in patients #9, #10 and #11 and with sirolimus in patient #13, Table 2) in all but one patient (Table 2), in whom aHUS was attributed to everolimus (patient #7). Offending drugs were discontinued in all the patients but one (patient #3), without TMA improvement (Table 2). In this patient (hyperimmunized recipient of a second transplant), tacrolimus was maintained due to the high risk of rejection.
Eculizumab was initiated after only two to three sessions of plasmapheresis in patients #1, #3, #4 and #12 with drug-induced aHUS (Table 2) due to the progressive deterioration in these cases of renal function and the persistence of severe extrarenal manifestations (seizures, blurred vision, decreased level of consciousness), as well as the appearance of plasmapheresis-related complications, transfusion-related reaction and bleeding in patients #4 and #12.
Systemic disease-related aHUS occurred in eight patients: systemic lupus erythematosus (SLE) in three, systemic scleroderma in two, eosinophilic granulomatosis with polyangiitis (EGPA) in two and primary antiphospholipid syndrome in one (Table 3). All the patients with systemic diseases developed aHUS in their native kidneys, with the exception of the patient with primary antiphospholipid syndrome, who presented aHUS 7 days after renal transplantation. In four out of eight patients (50%), aHUS was the first manifestation of the disease (patients #16, #17, #19 and #20). Patients with systemic diseases received different types of immunosuppressive treatments before eculizumab (Table 3).
Table 3.
aHUS associated with systemic disease
| Patient | Age (years), gender | Systemic disease | Treatment before eculizumab | PE no. of sessions | Eculizumab (duration, no. of doses) | Time from aHUS to eculizumab (days) | Highest SCr (mg/dL) HD (Yes/No) | Latest SCr (mg/dL) | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|
| 16 | 51, female | SLEa | CS + Cyc + Rtx + PE | 10 | 4 weeks, 4 | 31 | 4.2 Yes | Dialysis | 0.9 |
| 17 | 16, female | SLEa | CS + MMF | – | 10 weeks, 7 | 1 | 7.1 Yes | Dialysis | 4.2 |
| 18 | 52, female | SLE | CS + Cyc + PE | 27 | 2 weeks, 2 | 38 | 6.4 Yes | 4.2 | 4.0 |
| 19 | 63, male | Scleroderma | CS + Cyc + PE | 10 | 6 weeks, 5 | 55 | 4.9 No | Dialysis | 12.9 |
| 20 | 56, male | Scleroderma | CS + Mtx + PE | 10 | 8 weeks, 6 | 25 | 3.7 No | 3.4 | 4.3 |
| 21 | 52, male | EGPA | CS + Cyc | – | 16 weeks, 10 | 5 | 3.5 No | 2.3 | 4.3 |
| 22 | 49, male | EGPA | CS + Cyc + PE | 2 | 130 weeks, ongoing | 15 | 6.5 Yes | Dialysis | 29.5 |
| 23 | 38, male | Primary APS | – | – | 14 weeks, 9 | 1 | 9.5 Yes | 3.4 | 4.4 |
aaHUS, atypical haemolytic uraemic syndrome; APS, Antiphospholip syndrome; CS, corticosteroids; Cyc, cyclophosphamide; EGPA, eosinophilic granulomatosis with polyangiitis; HD, Hemodialysis (Yes/No); Mtx, methotrexate; MMF, mycophenolate mofetil; ND, not done; PE, plasma exchange; Rtx, rituximab; SCr, serum creatinine; SLE, systemic lupus erythematosus.
Six patients presented a miscellany of secondary TMA causes: aHUS appearing after delivery in two patients, cancer-related aHUS in two (metastatic prostate cancer in both), aHUS associated with acute humoral rejection in a kidney transplant patient and with primary intestinal lymphangiectasia in another patient (Table 4). Antiandrogenic therapy, immunosuppressive anti-rejection treatments and antibiotics have been prescribed for prostate cancer, humoral rejection and intestinal lymphangiectasia, respectively.
Table 4.
Other causes of secondary aHUS
| Patient | Age (years), gender | TMA cause | Treatment before eculizumab | PE no. of sessions | Eculizumab (duration, no. of doses) | Time from aHUS to eculizumab (days) | Highest SCr (mg/dL) HD (Yes/No) | Latest SCr (mg/dL) | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|
| 24 | 27, female | Postpartum | PE | 15 | 46 weeks, 25 | 24 | 4.1 No | 1.5 | 13 |
| 25 | 35, female | Postpartum | PE | 8 | 16 weeks, 10 | 17 | 5.9 Yes | 1.2 | 4.2 |
| 26 | 61, male | Cancer-related aHUSa | Bilateral orchiectomy + PE | 2 | 10 weeks, 7 | 3 | 11.1 Yes | 0.9 | 5.2 |
| 27 | 73, male | Cancer-related aHUSa | Antiandrogentherapy + PE | 4 | 81 weeks, 43 | 4 | 8.7 Yes | 1.0 | 22.6 |
| 28 | 35, male | Acute humoral rejection | CS + IVIg + Rtx + PE | 5 | 3 weeks, 3 | 7 | 4.9 No | 1.6 | 10.7 |
| 29 | 13, female | Primary intestinal lymphangiectasia | PE | 4 | 64 weeks, ongoing | 21 | 5.4 Yes | 0.3 | 16.4 |
CS, corticosteroids; HD, haemodialysis; IVIg, intravenous immunoglobulin; PE, plasma exchange; Rtx, rituximab; SCr, serum creatinine; aHUS, atypical haemolytic uraemic syndrome.
Cancer-related aHUS:metastatic prostate cancer.
Plasmapheresis was performed before the onset of eculizumab in 24 patients (83%), 13 with drug-induced aHUS, 5 with systemic diseases, 2 with postpartum aHUS, 2 with cancer-related aHUS, 1 with acute humoral rejection and 1 with primary intestinal lymphangiectasia (Tables 2–4). Complications related to the performance of plasmapheresis were transfusion-related reactions (five), deep vein thrombosis (two), bleeding (one) and hypotension (one).
Renal biopsies
Renal biopsies were performed in 18 patients (62%). Characteristic lesions of TMA were found in all of them. A renal biopsy was performed in six out of seven kidney transplant patients with drug-induced aHUS (patients #2, #3, #4, #5, #6 and #7). No lesions of antibody-mediated rejection (AMR) were found and C4d staining was negative in all of them. C3 glomerulopathy was diagnosed in patient #11, on the basis of strong, isolated deposits of C3 in immunofluorescence studies. A membranoproliferative glomerulonephritis likely associated with hepatitis C virus infection was found in patient #14 (Table 2). Among patients with aHUS associated with systemic diseases, a class IV-S (C) lupus nephritis with advanced glomerular and interstitial sclerosing lesions was found in two patients with SLE (patients #16 and #17, Table 3) and a class IV-G (A) lupus nephritis in the remaining SLE patient (patient #18, Table 3). Patients with EGPA showed histological signs of vasculitis (fibrinoid necrosis in glomerular capillaries and arterioles with eosinophilic granulomas and interstitial infiltration by eosinophils) in addition to TMA. Renal biopsy in patient #24, with postpartum aHUS, showed characteristic lesions of TMA with no tubular necrosis. Renal biopsy in the patient with acute humoral rejection-induced aHUS showed histological signs of acute humoral rejection (tubulitis, arteritis, C4d-positive staining) in addition to TMA.
Eculizumab treatment
Eculizumab was started 13 (7–26) days after the onset of aHUS. In two patients (#22 and #29, Tables 2–4), investigators decided to maintain eculizumab after TMA resolution due to the identification of complement pathogenic variants in CFH and CFHR1 (see ‘Complement genetic studies’ section). In 27 remaining patients, eculizumab was discontinued after 8 (3–18) weeks. The average number of eculizumab doses in these 27 patients was 6 (3–11).
Outcomes
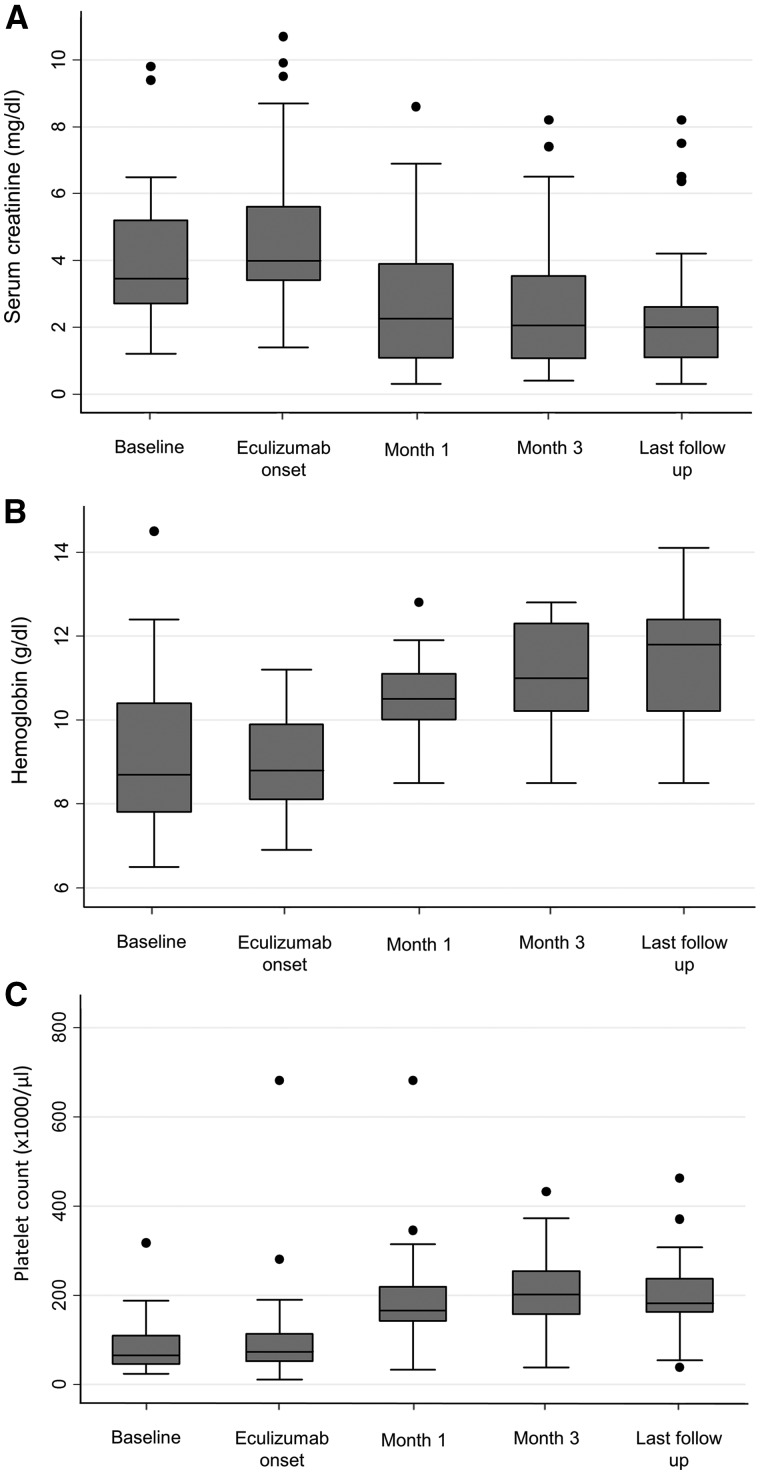
A rapid improvement in haematological and renal abnormalities after the onset of eculizumab was observed in 20 patients (69%) (Table 5 and Figure 1). The rate of response varied according to the aetiology of aHUS: 12/15 (80%) among drug-induced aHUS, 6/6 (100%) in postpartum, cancer-related, acute humoral rejection and intestinal lymphangiectasia, and 2/8 (25%) in aHUS associated with systemic diseases (Table 6). Among the latter, only one patient with EGPA and the patient with primary antiphospholipid syndrome showed TMA resolution after eculizumab treatment (Tables 2–4). The interval between eculizumab initiation and TMA resolution averaged 12 (7–17) days. All extrarenal manifestations showed a rapid and dramatic improvement.
Table 5.
Changes from baseline in haematological parameters and renal function
| Baseline | Eculizumab onset | 4 weeks | 12 weeks | Last follow-up | |
|---|---|---|---|---|---|
| Platelet count (×1000/µL)a | 65 (45–109) | 73 (51–113) | 166 (142–219) | 202 (157–254) | 182 (163–237) |
| Haemoglobin (g/dL)a | 8.7 (7.8–10.4) | 8.8 (8.1–9.9) | 10.5 (10–11.1) | 11 (10.2–12.3) | 11.8 (10.2–12.4) |
| Haptoglobin (mg/dL)a | 5 (0–5) | 5 (0–12) | 69 (32–115) | 118 (88–141) | 116 (86–135) |
| Serum creatinine (mg/dL)a | 3.5 (2.7–5.2) | 4.0 (3.4–5.6) | 2.3 (1.1–3.9) | 2.1 (1.1–3.5) | 2.0 (1.1–2.6) |
| eGFR (mL/min/1.73 m2)a | 18 (8.9–26) | 13 (7.7–19) | 27 (17–54) | 32 (26–71) | 34 (28–63) |
eGFR, glomerular filtrate rate.
Median (25th–75th percentile).
FIGURE 1.
Changes in (A) serum creatinine, (B) haemoglobin and (C) platelet count.
Table 6.
Outcomes
| Baseline | Eculizumab onset | 4 weeks | 12 weeks | Last follow-up | |
|---|---|---|---|---|---|
| TMA response, no. (%) | |||||
| Total | 0 (0) | 0 (0) | 11 (37.9) | 19 (65.5) | 20 (68.9) |
| Related to drugs | 0 (0) | 0 (0) | 6 (40) | 12 (80) | 12 (80) |
| Related to systemic disease | 0 (0) | 0 (0) | 1 (12.5) | 1 (12.5) | 2 (25) |
| Related to other causes | 0 (0) | 0 (0) | 4 (66.7) | 6 (100) | 6 (100) |
| Patients undergoing dialysis, no. (%) | 4 (13.8) | 14 (48.3) | 4 (13.8) | 4 (13.8) | 4 (13.8) |
| Patients with ≥50% decrease from baseline SCr, no. (%) | 0 (0) | 0 (0) | 10 (34.5) | 14 (48.3) | 15 (51.7) |
| Patients with eGFR ≥60 mL/min/1.73 m2 | 0 (0) | 0 (0) | 6 (20.7) | 10 (34.5) | 10 (34.5) |
TMA, thrombotic microangiopathy; eGFR, glomerular filtrate rate; SCr, serum creatinine.
Resolution of haematological abnormalities with no improvement in renal function was observed in six patients (patients #4, #9, #17, #18, #20 and #22). In three cases (patients #8, #16 and #19), both haematological abnormalities and renal function impairment persisted despite eculizumab treatment (Tables 2–4).
At the last follow-up, the number of patients requiring dialysis had decreased to 4 (13%), a ≥25% decrease in SCr was observed in 20 patients (69%), a ≥50% decrease in 15 (52%) and 10 patients (35%) had achieved an eGFR ≥60 mL/min/1.73 m2.
No differences in baseline characteristics were observed between responder and non-responder patients, but there was a significantly higher number of patients with aHUS associated with systemic diseases among non-responders. Interestingly, the duration of eculizumab treatment was significantly longer in responder patients and there was a non-significant trend towards a longer interval between aHUS diagnosis and onset of eculizumab in non-responder patients (Table 7).
Table 7.
Differences between responder and non-responder patients
| Responders (n = 20) | Non-responders (n = 9) | P | |
|---|---|---|---|
| Age (years)a | 47.4 (35.9–57.5) | 51.9 (49.9–60.1) | 0.57 |
| Gender, no. (%), male | 11 (55.0) | 5 (55.6) | 1 |
| Baseline SCr (mg/dL)a | 3.4 (2.8–4.5) | 3.4 (1.8–6.2) | 0.75 |
| SCr at the onset of Eculizumab (mg/dL)a,b | 3.8 (3.2–5.4) | 4.5 (3.4–6.7) | 0.82 |
| Dialysis, no. (%)b | 9 (45) | 5 (55.6) | 0.70 |
| Haemoglobin (g/dL)a,b | 9.0 (8.1–10) | 8.3 (7.7–104) | 0.65 |
| Platelet count (×1000/µL)a,b | 78 (51–138) | 61 (29–80) | 0.25 |
| Cause of aHUS, no. (%) | 0.004 | ||
| Systemic disease | 2 (25) | 6 (75) | |
| Drug induced | 12 (80) | 3 (20) | |
| Other causes | 6 (100) | 0 (0) | |
| Plasma exchange, no. (%) | 17 (85) | 7 (77.8) | 0.50 |
| Time between aHUS and eculizumab (days)a | 9.5 (6–22.5) | 25 (14–38) | 0.059 |
| Eculizumab duration (weeks)a | 11.6 (4–24.7) | 4.4 (3–8.4) | 0.003 |
SCr, serum creatinine; aHUS, atypical haemolytic uraemic syndrome.
Median (25th–75th percentile).
At the onset of eculizumab.
Complement genetic studies
Comprehensive complement studies were performed in 22/29 patients (Supplementary Table). These include western blot analyses and quantification of components, search for anti-Factor H (FH) autoantibodies and genetic screenings to identify pathogenic variants and copy number variations. Only two patients carry pathogenic variants. Patient #22, affected by EGPA, carries two pathogenic changes in CFHR1 (L290S and A296V) resulting in a mutant FHR-1 protein that competes with FH regulation on endothelial surfaces, and patient #29, affected by intestinal lymphangiectasia, who presents a pathogenic variant in CFH (C597*) that results in a partial FH deficiency. Patients #11 and #12 carry anti-FH autoantibodies, but presented normal C3 levels and do not have the CFHR3–CFHR1 deletion, suggesting that these anti-FH autoantibodies may not be functionally relevant in the context of aHUS. Finally, some patients carry genetic variants in complement genes with unknown functional significance that were considered non-pathogenic.
Safety
Eculizumab was well tolerated. Serious infections occurred in two transplanted patients during eculizumab administration (prostatic abscess in one, herpes zoster infection in the other) but they were likely related to immunosuppressive treatment and resolved with appropriate treatments.
DISCUSSION
Our study shows that eculizumab is an effective treatment for severe cases of secondary aHUS. In 20/29 eculizumab-treated patients (69%), there was a normalization of haemoglobin and platelet count together with a renal function improvement of at least a ≥25% reduction in SCr from the onset of eculizumab treatment. Renal function recovery was remarkable, allowing the discontinuation of dialysis in 10/14 patients. A decrease ≥50% in SCr was observed in 15 patients, and 10 patients (35%) showed an eGFR ≥60 mL/min/1.73 m2 at the last follow-up. Eculizumab has been reported to be beneficial in individual cases or small series of secondary TMA [29–38], haematopoietic stem cell transplantation-induced aHUS being the entity in which the effects of eculizumab treatment have been more extensively documented [29, 30]. Our study is the first to confirm these positive effects in a relatively large series of secondary aHUS patients.
TMA responses after eculizumab treatment were particularly remarkable in patients with drug-induced aHUS (80% of TMA resolution) and in a miscellaneous group of secondary aHUS that included postpartum, cancer, acute humoral rejection and intestinal lymphangiectasia (100%). Although many patients with drug-induced aHUS show a rapid resolution after withdrawal of the offending drug, TMA can persist in others, requiring additional therapeutic measures because of life-threatening organ involvement or progressive renal impairment [7, 40]. Plasmapheresis, immunosuppression and, in a few patients, eculizumab [36–38] have been tried in these resistant cases. Our results confirm that eculizumab is very effective to rapidly resolve TMA and improve renal function in this type of resistant drug-induced secondary aHUS.
Tacrolimus was the most common offending drug among our patients with drug-induced aHUS. Although tacrolimus discontinuation likely collaborated in TMA resolution, the rapid and dramatic improvement associated with eculizumab administration suggests a direct effect of complement blockade in TMA response in these patients. On the other hand, AMR in renal transplant patients can present features of TMA in renal biopsies and some reports have suggested a beneficial effect of eculizumab in AMR [41]. However, no lesions of AMR were observed in the renal biopsies of our kidney transplant patients with drug-induced aHUS and C4d staining was negative in all of them.
On the contrary, our results show limited benefit of eculizumab administration in patients with systemic diseases (25% of TMA responses). Only one patient with primary antiphospholipid syndrome and another with ANCA vasculitis responded to eculizumab, whereas patients with SLE (three), scleroderma (two) and ANCA vasculitis (one) did not. These data do not agree with the eculizumab benefits reported in anecdotic cases of scleroderma-like syndrome and lupus nephritis complicated with aHUS [33, 34], but it should be stressed that our patients with SLE, scleroderma and ANCA vasculitis presented severe lesions in their renal biopsies at the initiation of eculizumab treatment.
Patients included in the study were treated according with the condition triggering TMA (discontinuation of the offending drug in drug-induced cases, immunosuppressive therapy in systemic diseases and humoral rejection, antitumoral treatment in cancer-related cases), and plasmapheresis was performed in 24 patients (83%) before initiation of eculizumab treatment. Despite these measures, renal function continued to deteriorate in all the patients and, at the time of eculizumab initiation, 14 patients (48%) were requiring dialysis. Persistent severe anaemia and thrombocytopenia was present in all patients (Table 1). Eculizumab rapidly resulted in the responder patients (20/29) in an improvement of renal function and the disappearance of all haematological abnormalities (Table 5 and Figure 1). Among the non-responders, the haematological abnormalities disappeared with eculizumab in 6/9 patients, although none of them showed a substantial renal function recovery. Extrarenal manifestations showed a rapid resolution after eculizumab initiation. Particularly dramatic was the rapid disappearance of life-threatening neurological complications (seizures, coma) in patients #12, #13, #15, #19, #20 and #22.
Complement is activated in several types of TMA other than primary aHUS [28]. In STEC-HUS, Shiga toxin upregulates the membrane adhesion molecule P-selectin, which binds C3b and activates the complement alternative pathway (AP) [42, 43]. Low C3 and increased sC5b-9 have been reported in most severe cases of STEC-HUS [44, 45]. In TTP, levels of complement activation markers correlated with disease activity and deposition of C3 and C5b-9 have been demonstrated in endothelial cells [46, 47]. Interestingly, some patients with STEC-HUS and TTP not responding to plasmapheresis have been successfully treated with eculizumab [48, 49]. Regarding drug-induced aHUS, it has been shown that endothelial cells exposed to calcineurin inhibitors release microparticles that activate the AP [50]. Inflammatory conditions, platelet-derived microparticles and blood coagulation proteins induce complement activation, which in turn increments endothelial damage and microvascular thrombosis [51, 52].
Prospective studies with eculizumab in primary aHUS have shown a significant inverse correlation between the delay in the onset of the treatment and the degree of renal function recovery [24]. As shown in Table 7, an almost significant trend towards a longer delay in eculizumab therapy and a significantly shorter duration of treatment were observed among our non-responder patients. Further studies are needed to confirm whether an earlier treatment with eculizumab or a longer administration are associated with more positive responses and a greater recovery of renal function in patients with these secondary forms of aHUS.
Comprehensive complement molecular and genetic studies were performed in 22 patients, but pathogenic variants were found only in two patients (Supplementary Table). With the exception of these two patients (#22 and #29), eculizumab was discontinued after a median of 8 weeks, with no patients showing TMA relapses.
Tolerance to eculizumab was excellent with no important side effects recorded. Infectious complications coincidental with eculizumab administration in transplanted patients were attributable to immunosuppressive therapy.
Our study has the limitations inherent to its retrospective design, with a possible bias in the selection of patients and absence of a control group not treated with eculizumab. On the other hand, it has major strengths: the number of recruited patients is large and the work-up tests to exclude other types of TMA was complete, with ADAMTS-13 activity measured in all the patients and the presence of Shiga toxin ruled out in all the cases with gastrointestinal symptoms. Furthermore, renal biopsies and comprehensive genetic and molecular studies were performed in most patients. All the patients received the specific treatment for their aHUS aetiology and plasmapheresis was performed in most of them before eculizumab treatment.
Our findings are consistent with the observation that both primary aHUS, associated with genetic and acquired complement abnormalities, and secondary aHUS are characterized by complement dysregulation. In addition, they suggest that while complement dysregulation is constitutive in the first condition, it is likely temporary in the second and, therefore, both conditions should be considered differently regarding long-term treatment. Based on the results of this study, we suggest that eculizumab could be the first-line treatment in aggressive cases of secondary aHUS who do not respond to other therapies and that a rapid administration of eculizumab would enable an efficient recovery of renal function and complement normalization. Prospective studies are needed to corroborate these findings and to define the ideal duration of eculizumab treatment in secondary aHUS.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
Supplementary Material
ACKNOWLEDGEMENTS
Work in this report was funded by the Instituto de Salud Carlos III: REDinREN (RD 016/009 Feder Funds), the Fondo de Investigaciones Sanitarias (13/02502 and ICI14/00350), the Ministerio de Economia y Competitividad (SAF2015-66287R) and the Autonomous Region of Madrid (S2010/BMD-2316; Grupo de Investigación Complemento-CM). SRdeC is funded by the Seventh Framework Programme European Union Project EURenOmics (305608).
CONFLICT OF INTEREST STATEMENT
Conception, design, data collection and analysis, as well as writing of the study were performed by investigators with no support from pharmaceutical companies. C.R., E.R., A.A., M.B., M.M., M.E., P.A., E.M., M.C., S.R.d.C. and M.P. have received honoraria from Alexion Pharmaceuticals for giving lectures and participating in advisory boards. None of these activities has had any influence on the results or interpretations in this article. Other authors declare no conflicts of interest. The results presented in this article have not been published previously in whole or part, excepting an abstract that has been accepted for oral communication in the Renal Week, American Society of Nephrology 2016.
REFERENCES
- 1. George JN, Nester CM.. Syndromes of thrombotic microangiopathy. N Engl J Med 2014; 371: 654–666 [DOI] [PubMed] [Google Scholar]
- 2. Barbour T, Johnson S, Cohney S. et al. Thrombotic microangiopathy and associated renal disorders. Nephrol Dial Transplant 2012; 27: 2673–2685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Noris M, Remuzzi G.. Atypical hemolytic-uremic syndrome. N Engl J Med 2009; 361: 1676–1687 [DOI] [PubMed] [Google Scholar]
- 4. Besbas N, Karpman D, Landau D. et al. A classification of hemolytic uremic syndrome and thrombotic thrombocytopenic purpura and related disorders. Kidney Int 2006; 70: 423–431 [DOI] [PubMed] [Google Scholar]
- 5. Campistol JM, Arias M, Ariceta G. et al. An update for atypical haemolytic uraemic syndrome: diagnosis and treatment. A consensus document. Nefrologia 2015; 35: 421–447 [DOI] [PubMed] [Google Scholar]
- 6. Al-Nouri ZL, Reese JA, Terrell DR. et al. Drug induced thrombotic microangiopathy: a systemic review of published reports. Blood 2015; 125: 616–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Izzedine H, Perazella MA.. Thrombotic microangiopathy, cancer, and cancer drugs. Am J Kidney Dis 2015; 66: 857–866 [DOI] [PubMed] [Google Scholar]
- 8. Tektonidou MG, Sotsiou F, Nakopoulou L. et al. Antiphospholipid syndrome nephropathy in patients with systemic lupus erythematosus and antiphospholipid antibodies: prevalence, clinical associations, and long-term outcome. Arth Rheum 2004; 50: 2569–2579 [DOI] [PubMed] [Google Scholar]
- 9. Manadan AM, Harris C, Block JA.. Thrombotic thrombocytopenic purpura in the setting of systemic sclerosis. Semin Arthritis Rheum 2005; 34: 683–688 [DOI] [PubMed] [Google Scholar]
- 10. Gharbi C, Bourry E, Rouvier P. et al. Rapidly progressive lupus nephritis and concomitant thrombotic microangiopathy. Clin Exp Nephrol 2010; 14: 487–491 [DOI] [PubMed] [Google Scholar]
- 11. Fakhouri F, Vercel C, Frémeaux-Bacchi V.. Obstetric nephrology: AKI and thrombotic microangiopathies in pregnancy. Clin J Am Soc Nephrol 2012; 7: 2100–2106 [DOI] [PubMed] [Google Scholar]
- 12. Scully M, Thomas M, Underwood M. et al. Thrombotic thrombocytopenic purpura and pregnancy: presentation, management, and subsequent pregnancy outcomes. Blood 2014; 124: 211–219 [DOI] [PubMed] [Google Scholar]
- 13. Lechner K, Obermeier HL.. Cancer-related microangiopathic haemolytic anemia: clinical and laboratory features in 168 reported cases. Medicine 2012; 91: 195–205 [DOI] [PubMed] [Google Scholar]
- 14. Jodele S, Davies SM, Lane A. et al. Diagnostic and risk criteria for HSCT-associated thrombotic microangiopathy: a study in children and young adults. Blood 2014; 124: 645–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Laskin BL, Goebel J, Davies SM. et al. Small vessels, big trouble in the kidneys and beyond: hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Blood 2011; 118: 1452–1462 [DOI] [PubMed] [Google Scholar]
- 16. Allen U, Kicht C.. Pandemic H1N1 influenza A infection and (atypical) HUS – more than just another trigger. Pediatr Nephrol 2011; 26: 3–5 [DOI] [PubMed] [Google Scholar]
- 17. Lopes da Silva R. Viral-associated thrombotic microangiopathies. Hematol Oncol Stem Cell Ther 2011; 4: 51–59 [DOI] [PubMed] [Google Scholar]
- 18. Manenti L, Gnappi E, Vaglio A. et al. Atypical haemolytic uraemic syndrome with underlying glomerulopathies. A case series and a review of the literature. Nephrol Dial Transplant 2013; 28: 2246–2259 [DOI] [PubMed] [Google Scholar]
- 19. Sethi S, Fervenza FC.. Pathology of renal diseases associated with dysfunction of the alternative pathway of complement: C3 glomerulopathy and atypical hemolytic uremic syndrome (aHUS). Semin Thromb Hemost 2014; 40: 416–421 [DOI] [PubMed] [Google Scholar]
- 20. Mathew RO, Nayer A, Asif A.. The endothelium as the common denominator in malignant hypertension and thrombotic microangiopathy. J Am Soc Hypertens 2016; 10: 352–359 [DOI] [PubMed] [Google Scholar]
- 21. Kalman S, Bakkaloğlu S, Dalgiç B. et al. Recurrent hemolytic uremic syndrome associated with intestinal lymphangiectasia. J Nephrol 2007; 20: 246–249 [PubMed] [Google Scholar]
- 22. Cornec-Le Gall E, Delmas Y, De Parscau L. et al. Adult-onset eculizumab-resistant hemolytic uremic syndrome associated with cobalamin C deficiency. Am J Kidney Dis 2014; 63: 119–123 [DOI] [PubMed] [Google Scholar]
- 23. Zuber J, Fakhouri F, Roumenina LT. et al. Use of eculizumab for atypical haemolytic uraemic syndrome and C3 glomerulopathies. Nat Rev Nephrol 2012; 8: 643–657 [DOI] [PubMed] [Google Scholar]
- 24. Legendre CM, Licht C, Muus P. et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med 2013; 368: 2169–2181 [DOI] [PubMed] [Google Scholar]
- 25. Rodríguez de Córdoba S, Subías M, Pinto S. et al. Genetics of atypical hemolytic uremic syndrome (aHUS). Semin Thromb Hemost 2014; 40: 422–430 [DOI] [PubMed] [Google Scholar]
- 26. Nester CM, Barbour T, de Cordoba SR. et al. Atypical aHUS: state of the art. Mol Immunol 2015; 67: 31–42 [DOI] [PubMed] [Google Scholar]
- 27. Goodship THJ, Cook TH, Fakhouri F. et al. Atypical hemolytic uremic syndrome and C3 glomerulopathy: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 2016. (in press); doi: 10.1016/j.kint.2016.10.005 [DOI] [PubMed] [Google Scholar]
- 28. Noris M, Mescia F, Remuzzi G.. STEC-HUS, atypical HUS and TTP are all diseases of complement activation. Nat Rev Nephrol 2012; 8: 622–633 [DOI] [PubMed] [Google Scholar]
- 29. Jodele S, Fukuda T, Vinks A. et al. Eculizumab therapy in children with severe hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Biol Blood Marrow Transplant 2014; 20: 518–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. de Fontbrune FS, Galambrun C, Sirvent A. et al. Use of eculizumab in patients with allogeneic stem cell transplant-associated thrombotic microangiopathy: a study from the SFGM-TC. Transplantation 2015; 99: 1953–1959 [DOI] [PubMed] [Google Scholar]
- 31. Canaud G, Kamar N, Anglicheau D. et al. Eculizumab improves posttransplant thrombotic microangiopathy due to antiphospholipid syndrome recurrence but fails to prevent chronic vascular changes. Am J Transplant 2013; 113: 2179–2185 [DOI] [PubMed] [Google Scholar]
- 32. Strakhan M, Hurtado-Sbordoni M, Galeas N. et al. 36-year-old female with catastrophic antiphospholipid syndrome treated with eculizumab: a case report and review of literature. Case Rep Hematol 2014: 704371; doi: 10.1155/2014/704371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thomas CP, Nester CM, Phan AC. et al. Eculizumab for rescue of thrombotic microangiopathy in PM-Scl antibody-positive autoimmune overlap syndrome. Clin Kidney J 2015; 8: 698–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. El-Husseini A, Hannan S, Awad A. et al. Thrombotic microangiopathy in systemic lupus erythematosus: efficacy of eculizumab. Am J Kidney Dis 2015; 65: 127–130 [DOI] [PubMed] [Google Scholar]
- 35. Burwick RM, Feingerg BB.. Eculizumab or the treatment of preeclampsia/HELLP syndrome. Placenta 2013; 34: 201–203 [DOI] [PubMed] [Google Scholar]
- 36. Starck M, Wendtner CM.. Use of eculizumab in refractory gemcitabine-induced thrombotic microangiopathy. Br J Haematol 2014; 164: 888–902 [DOI] [PubMed] [Google Scholar]
- 37. Safa K, Logan MS, Batal I. et al. Eculizumab for drug-induced de novo posttransplantation thrombotic microangiopathy: a case report. Clin Nephrol 2015; 83: 125–129 [DOI] [PubMed] [Google Scholar]
- 38. Faguer S, Huart A, Frémeaux-Bacchi V. et al. Eculizumab and drug-induced haemolytic uremic syndrome. Clin Kidney J 2013; 6: 484–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kidney Disease: Improving Global Outcomes (KDIGO). AKI definition. Kidney Int Suppl 2012; 2: 19–36 [Google Scholar]
- 40. Zakarija A, Bennett C.. Drug-induced thrombotic microangiopathy. Semin Thromb Hemost 2005; 31: 681–690 [DOI] [PubMed] [Google Scholar]
- 41. Frémeaux-Bacchi V, Legendre CM.. The emerging role of complement inhibitors in transplantation. Kidney Int 2015; 88: 967–973 [DOI] [PubMed] [Google Scholar]
- 42. Morigi M, Galbusera M, Gastoldi S. et al. Alternative pathway activation of complement by Shiga toxin promotes exuberant C3a formation that triggers microvascular thrombosis. J Immunol 2011; 187: 172–180 [DOI] [PubMed] [Google Scholar]
- 43. Orth D, Khan AB, Naim A. et al. Shiga toxin activates complement and binds factor H: evidence for an active role of complement in hemolytic uremic syndrome. J Immunol 2009; 182: 6394–6400 [DOI] [PubMed] [Google Scholar]
- 44. Robson WL, Leung AK, Fick GH. et al. Hypocomplementemia and leukocytosis in diarrhea-associated hemolytic uremic syndrome. Nephron 1992; 62: 296–299 [DOI] [PubMed] [Google Scholar]
- 45. Thurman JM, Marians R, Emlen W. et al. Alternative pathway of complement in children with diarrhea-associated hemolytic uremic syndrome. Clin J Am Soc Nephrol 2009; 4: 1920–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Réti M, Farkas P, Csuka D. et al. Complement activation in thrombotic thrombocytopenic purpura. J Thromb Haemost 2012; 10: 791–798 [DOI] [PubMed] [Google Scholar]
- 47. Ruiz-Torres MP, Casiraghi F, Galbusera M. et al. Complement activation: the missing link between ADAMTS-13 deficiency and microvascular thrombosis of thrombotic microangiopathies. Thromb Haemost 2005; 93: 443–452 [DOI] [PubMed] [Google Scholar]
- 48. Lapeyraque AL, Malina M, Fremeaux-Bacchi V. et al. Eculizumab in severe Shiga-toxin-associated HUS. N Engl J Med 2011; 364: 2561–2563 [DOI] [PubMed] [Google Scholar]
- 49. Pecoraro C, Ferretti AV, Rurali E. et al. Treatment of congenital thrombotic thrombocytopenic purpura with eculizumab. Am J Kidney Dis 2015; 66: 1067–1070 [DOI] [PubMed] [Google Scholar]
- 50. Renner B, Klawitter J, Goldberg R. et al. Cyclosporine induces endothelial cell release of complement-activating microparticles. J Am Soc Nephrol 2013; 24: 1849–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Peerschke EI, Yin W, Ghebrehiwet B.. Complement activation on platelets: implications for vascular inflammation and thrombosis. Mol Immunol 2010; 47: 2170–2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Amara U, Flierl MA, Rittirsch D. et al. Molecular intercommunication between the complement and coagulation systems. J Immunol 2010; 185: 5628–5636 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.