Abstract
Background
Maternal vitamin D deficiency during pregnancy may impact fetal outcomes.
Objective
To examine whether maternal 25-hydroxyvitamin D (25(OH)D) concentrations in pregnancy affect fetal growth patterns and birth outcomes.
Design
Population-based prospective cohort in Rotterdam, the Netherlands among 7098 mothers and their offspring. We measured 25(OH)D concentrations at a median gestational age of 20.3 weeks (range 18.5-23.3 weeks). Vitamin D concentrations were analyzed continuously and in quartiles. Fetal head circumference, length and weight estimated by repeated ultrasounds, preterm birth (gestational age <37 weeks) and small-size for gestational age (<5th percentile).
Results
Adjusted multivariate regression analyses showed that as compared to mothers with second trimester 25(OH)D concentrations in the highest quartile, those with 25(OH)D concentrations in the lower quartiles had offspring with third trimester fetal growth restriction leading to a smaller birth head circumference, shorter birth length and lower weight at birth (all P values <0.05). Mothers who had 25(OH)D concentrations in the lowest quartile had increased risks of preterm delivery (Odds Ratio (OR) 1.72, 95% Confidence Interval (CI) 1.14, 2.60) and small-size for gestational age children (OR 2.07, 95% CI 1.33, 3.22). The estimated population attributable risks of 25(OH)D concentrations <50 nmol/L for preterm birth or small-size for gestational age were 17.3% and 22.6%, respectively. The observed associations were not based on extreme 25(OH)D deficiency, but present within the common ranges.
Conclusions
Low maternal 25(OH)D concentrations are associated with proportional fetal growth restriction and with increased risks of preterm birth and small-size for gestational age at birth. Further studies are needed to investigate the causality of these associations and the potential for public health interventions.
Introduction
Vitamin D deficiency is common and related to various non-communicable disease (1). An accumulating body of evidence suggest that vitamin D is also crucial for fetal development because of its important role during cell proliferation, differentiation and maturation processes (2, 3). Suboptimal vitamin D concentrations may affect early organogenesis and subsequently affect later health and disease (4). Also, vitamin D is important for placental function, calcium homeostasis, and bone mineralisation, which are all important determinants for fetal growth and development (5),(6). Thus far, most published studies focused on the associations of maternal vitamin D status during pregnancy with fetal development were mainly based on birth weight and showed inconsistent results (7–9). However, birth weight is just a proxy for fetal growth and development. Different fetal growth patterns and body proportions may lead to the same birth weight. Not much is known about the direct effects of maternal vitamin D status on fetal growth and development patterns in healthy populations (8, 10–13). The inconsistent results from previous studies may be explained by differences between study populations. Because ethnic differences in both vitamin D concentrations and birth outcomes have been reported, the effects of maternal vitamin D status on fetal outcomes may differ between specific populations (14, 15). Based on previous studies suggesting that low vitamin D concentrations, may lead to suboptimal placentation and fetal skeletal growth, we hypothesized that low vitamin D concentrations may lead to fetal growth restriction and increased risks of adverse birth outcomes.
Therefore, we examined in a multi-ethnic population-based prospective cohort study among 7098 mother-offspring pairs, the associations of circulating 25-hydroxyvitamin D (25(OH)D) concentrations with repeatedly measured fetal growth characteristics during second and third trimester and the risks of adverse birth outcomes. Additionally, we explored whether adverse birth outcomes were associated with cord blood 25(OH)D concentrations.
Methods
Design and Study Population
This study was embedded in the Generation R Study, a population-based prospective cohort study from fetal life onwards in Rotterdam, the Netherlands (16). All children were born between April 2002 and January 2006. Enrolment in the study was aimed at early pregnancy, but was allowed until the birth of the child. The study protocol was approved by the local Medical Ethical Committee. Written consent was obtained from all participating mothers. Second trimester 25(OH)D concentrations were measured in 7176 mothers. For the present study, we excluded pregnancies leading to twin births (n = 77) and loss to follow-up at birth (n = 1). Thus, the cohort for analysis comprised 7098 mothers with any fetal or birth outcome available. (Supplementary Figure 1. Flowchart).
Maternal and Cord 25(OH)D Blood Concentrations
Maternal venous blood samples were collected in second trimester (median 20.3 weeks of gestation, range 18.5-23.3 weeks), whereas umbilical cord blood samples were collected immediately after delivery (median 40.1 weeks of gestation, range 35.9-42.3 weeks). Cord blood vitamin D concentrations represent neonatal vitamin D status at birth. Measurements of 25(OH)D concentrations were conducted at the Eyles Laboratory at the Queensland Brain Institute, University of Queensland, Australia, in 2014.
Total 25(OH)D was calculated as the sum of 25(OH)D2 and 25(OH)D3 measured in plasma as previously described (17). Samples were quantified using isotope dilution liquid chromatography-tandem mass spectrometry. Linearity of 25(OH)D concentration was assessed using matrix-matched calibration standards, with R2 values of >0.99 across the calibration range (10 – 125 nmol/L). Inter-assay inaccuracy and imprecision were assessed at four concentration levels for 25(OH)D3 (48.3, 49.4, 76.4, 139.2 nmol/L) and a single level (32.3 nmol/L) for 25(OH)D2 using certified reference materials and were excellent at all concentration levels tested. Inter-assay inaccuracy and imprecision were both <10% for 25(OH)D3 and <17% for 25(OH)D2, respectively. We categorized vitamin D status into quartiles (quartile 1: median ( full range) 14.7 nmol/L (1.5 to 24.1); quartile 2: 35.1 nmol/L (24.2 to 46.6); quartile 3: 59.0 nmol/L (46.7 to 73.7); quartile 4: 91.6 nmol/L (73.8 to 193.2)). Since optimal vitamin D concentrations remain a subject of debate (18, 19), we performed a sensitivity analysis by using cut-offs concentrations according to previously used cut-offs and recommendations (severely deficient: <25.0 nmol/L; deficient: 25.0 to 49.9 nmol/L; sufficient: 50.0 to 74.9 nmol/L; optimal ≥75.0 nmol/L) (18, 20–24).
Fetal Growth Measurements
Fetal ultrasound examinations were carried out in two dedicated research centers in second (median 20.5 weeks of gestation, 95% range 18.6–24.3) and third trimester (median 30.3 weeks of gestation, 95% range 28.4–32.8) (25). The first trimester ultrasound was primarily used for establishing gestational age (26). In second and third trimester, we measured fetal head circumference, abdominal circumference and femur length to the nearest millimeter using standardized ultrasound procedures (27). Estimated fetal weight was subsequently calculated using the formula of Hadlock et al (28). Longitudinal growth curves and gestational-age-adjusted standard deviation scores (SDS) were constructed for all fetal growth measurements (26). These gestational-age-adjusted SDS were based on reference growth curves from the whole study population, and represent the equivalent of z-scores (26).
Birth Outcomes
We obtained information about offspring sex, gestational age, head circumference, length and weight at birth from medical records (29). Because head circumference and length were not routinely measured at birth in each delivery center, fewer measurements were available (n = 3681 and n = 4533 for head circumference and length at birth, respectively), as compared to birth weight. Gestational-age-adjusted SDS for head circumference, length and weight were constructed using North European growth standards (30). We defined preterm birth as a gestational age of <37 weeks at birth, low birth weight as a birthweight <2500 grams, and small-size for gestational age at birth as a gestational-age-adjusted birthweight below the 5th percentile (-1.79 SD).
Covariates
We used questionnaires at enrollment in the study to collect information about maternal age, ethnicity, educational level, parity, the presence of anorexia, smoking, alcohol usage, folic acid and vitamins supplementation (29). Maternal energy, iron, zinc and calcium dietary intake during pregnancy was measured at enrollment with a validated semi-quantitative food frequency questionnaire (FFQ) (31). Ethnicity and educational level were defined according to the demographic classification of Statistics Netherlands (32, 33). Ethnicity was categorized in the following groups: European, Cape Verdean, Dutch Antillean, Moroccan, Surinamese, Turkish and Others. We measured maternal height and weight at enrollment and calculated body mass index (kg/m2). Information about gestational hypertensive disorders (gestational hypertension, preeclampsia) and gestational diabetes was available from medical records (34). The date of blood sampling was categorized into summer, fall, winter, and spring, based on the European seasons.
Statistical Analysis
First, we performed regression analyses to relate second trimester maternal 25(OH)D concentrations with fetal growth characteristics in second, and third trimester separately. Second, we assessed the associations of maternal 25(OH)D concentrations with longitudinally measured of fetal head circumference, fetal femur length and body length at birth, and fetal weight, expressed as SDS, using unbalanced repeated measurement regression analyses. These analyses enable optimal use of available data, taking into account correlations within subjects and assessing both time dependent and independent associations (35). Since body length can not be estimated during fetal life, we used femur length in second and third trimester and body length at birth to assess length growth. We measured femur length to the nearest millimeter using standardized ultrasound procedures (27). For weight we used estimated fetal weight in second and third trimester and birth weight. Third, we used logistic regression models to assess the associations of maternal 25(OH)D concentrations with the risks of preterm birth, low birth weight, and small-size for gestational age. For all the analyses, 25(OH)D concentrations were analysed both continuously and by using quartiles. As sensitivity analysis, we also analysed 25(OH)D concentrations in groups of previously used and recommended cut-offs instead of quartiles (18, 20–24). We calculated the population attributable risk (PAR) for these outcomes as: PAR% = [(Ratetotal population – Rateunexposed)/(Ratetotal population)]*100%. Finally, we explored the associations of birth characteristics with cord blood 25(OH)D concentrations using linear regression models. All regression models were first adjusted for season when blood samples were drawn and maternal ethnicity (Basic models), and subsequently additionally for maternal age, education, parity, body mass index at enrolment, smoking, alcohol use, the presence of anorexia, folic acid and vitamins supplement use and energy, iron, zinc and calcium dietary intake during pregnancy, gestational hypertensive disorders and gestational diabetes (Adjusted models). These covariates were included in the models based on their associations on fetal and birth outcomes in previous studies, or a change in effect estimates of >10%. Adding maternal nutritional data (iron, zinc and calcium dietary intake and vitamin supplements) did not materially change the effect estimates but slightly improved the model fit (R2, -2log Likelihood and the Nagelkerk R2 values). Because of the strong associations of ethnicity with both 25(OH)D concentrations with and fetal outcomes, we first adjusted the regression models for maternal ethnicity and second, we restricted the analyses to Europeans only, the largest ethnic subgroup in our cohort (36, 37). A P value of <0.05 was considered as statistically significant. To adjust for multiple testing in the analyses of adverse birth outcomes with cord blood 25(OH)D concentrations, we applied Bonferroni correction considering a P value <0.025 (0.05/2) as significant. To diminish potential bias associated with attrition, missing values of covariates, were multiple imputed by generating 5 independent datasets using the Markov Chain Monte Carlo (MCMC) method. The multiple imputation procedure was based on the correlation between each variable with missing values and the other subject characteristics (38, 39). Statistical analyses were performed using SPSS version 21.0 (IBM SPSS Statistics for Windows, Armonk, NY: IBM Corp). Subject characteristics before and after imputation, including the percentage of missing values are given in the Supplementary Table 1. The unbalanced repeated-measurements analysis, including the Prox Mixed module, was performed with the Statistical Analysis System (version 9.3; SAS Institute Inc, Cary NC).
Results
Subject Characteristics
Table 1 shows that the median value and 95% range of maternal 25(OH)D is 46.7 (7.0, 119.4) nmol/L. Maternal and cord blood 25(OH)D concentrations were correlated (r=0.62, P value <0.01). Growth characteristics during second and third trimester and at birth are shown in Table 2. Supplementary Table 2 gives the subject characteristics for the each quartile of 25(OH)D concentrations. We observed that mothers with a Turkish or Moroccan ethnicity had the lowest 25(OH)D concentrations.
Table 1. Subject Characteristics (N = 7098)1.
| Maternal characteristics | |
| Age, mean (SD), y | 29.7 (5.2) |
| Body mass index at enrolment, median (95% range), kg/m2 | 23.7 (18.7, 36.3) |
| Gestational age at enrollment, median (95% range), wk | 13.9 (9.9, 22.9) |
| Nulliparous, No. (%) | 3987 (56.2) |
| Education level, No. (%) | |
| - No higher education | 4204 (59.2) |
| - Higher education | 2894 (40.8) |
| Ethnicity, No. (%) | |
| - European | 4069 (57.3) |
| - Cape Verdean | 311 (4.4) |
| - Dutch Antillean | 253 (3.5) |
| - Moroccan | 471 (6.6) |
| - Surinamese | 643 (9.1) |
| - Turkish | 651 (9.2) |
| - Other | 700 (9.9) |
| Presence of anorexia, No. (%) | |
| - No | 6352 (89.5) |
| - Yes | 503 (7.1) |
| - Maybe | 243 (3.4) |
| Smoking during pregnancy, No. (%) | |
| - Never | 5131 (72.3) |
| - Until pregnancy was known | 665 (9.4) |
| - Continued | 1302 (18.3) |
| Alcohol consumption during pregnancy, No. (%) | |
| - Never | 3493 (49.2) |
| - Until pregnancy was known | 974 (13.7) |
| - Continued | 2631 (37.1) |
| Folic acid supplement use, No. (%) | |
| - No | 2204 (31.0) |
| - Start in the first 10 weeks | 2219 (31.3) |
| - Start periconceptional | 2675 (37.7) |
| Vitamin supplement use, No. (%) | |
| - Yes | 5049 (71.1) |
| - No | 2049 (28.9) |
| Maternal energy intake (kcal) | 2039 (490) |
| Maternal zink dietary intake (mg) | 9.6 (1.6) |
| Maternal iron dietary intake (mg) | 11.1 (2.1) |
| Maternal calcium dietary intake (mg) | 1087 (418) |
| Maternal 25(OH)D concentrations, median (95% range), nmol/L | 46.7 (7.0, 119.4) |
| - Severely deficient (< 25.0 nmol/L), No. (%) | 1855 (26.1) |
| - Deficient (25.0 to 49.9 nmol/L), No. (%) | 1919 (27.1) |
| - Sufficient (50.0 to 74.9 nmol/L), No. (%) | 1619 (22.8) |
| - Optimal (≥ 75.0 nmol/L), No. (%) | 1705 (24.0) |
| Season when maternal blood sample was take, No. (%) | |
| - Spring | 2097 (29.5) |
| - Summer | 1622 (22.9) |
| - Autumn | 1702 (24.0) |
| - Winter | 1677 (23.6) |
| Pregnancy complications, No. (%) | |
| - Gestational Hypertensive disorders | 421 (5.9) |
| - Gestational Diabetes | 67 (0.9) |
| Birth characteristics | |
| Female sex, No. (%) | 3529 (49.7) |
| Preterm birth (<37 wk of gestation), No. (%) | 370 (5.2) |
| Low birth weight (<2500 g), No. (%) | 342 (4.8) |
| Small-size for gestational age at birth (<5th percentile), No. (%) | 355 (5.0) |
| 25(OH)D concentration in cord blood at birth, median (95% range), nmol/L | 27.4 (4.7, 81.4) |
| Season when cord blood sample was taken, No. (%) | 4.264 |
| - Spring | 1130 (26.5) |
| - Summer | 1164 (27.2) |
| - Autumn | 996 (23.4) |
| - Winter | 977 (22.9) |
Values are percentages for categorical variables, means (SD) for continuous variables with a normal distribution, or medians (95% range) for continuous variables with a skewed distribution.
Table 2. Fetal Growth Characteristics (N = 7098)1.
| Second trimester (N = 7098) | |
| - Gestational age, median (95% range), wk | 20.5 (18.6, 23.4) |
| - Head circumference, mean (SD), cm | 17.9 (1.5) |
| - Femur length, mean (SD), mm | 33.5 (3.6) |
| - Estimated fetal weight, mean (SD), g | 382 (94) |
| Third trimester (N = 7018) | |
| - Gestational age at birth, median (95% range), wk | 30.3 (28.4, 32.8) |
| - Head circumference, mean (SD), cm | 28.5 (1.2) |
| - Femur length, mean (SD), mm | 57.4 (3.0) |
| - Estimated fetal weight, mean (SD), g | 1614 (253) |
| Birth (N = 7046) | |
| - Gestational age, median (95% range), wk | 40.1 (35.6, 42.3) |
| - Head circumference, mean (SD), cm | 33.8 (1.7) |
| - Body length, mean (SD), cm | 50.2 (2.4) |
| - Weight, mean (SD), g | 3414 (561) |
Values are means (SD) for continuous variables with a normal distribution, or medians (95% range) for continuous variables with a skewed distribution.
Maternal 25(OH)D Concentrations and Fetal Growth Characteristics
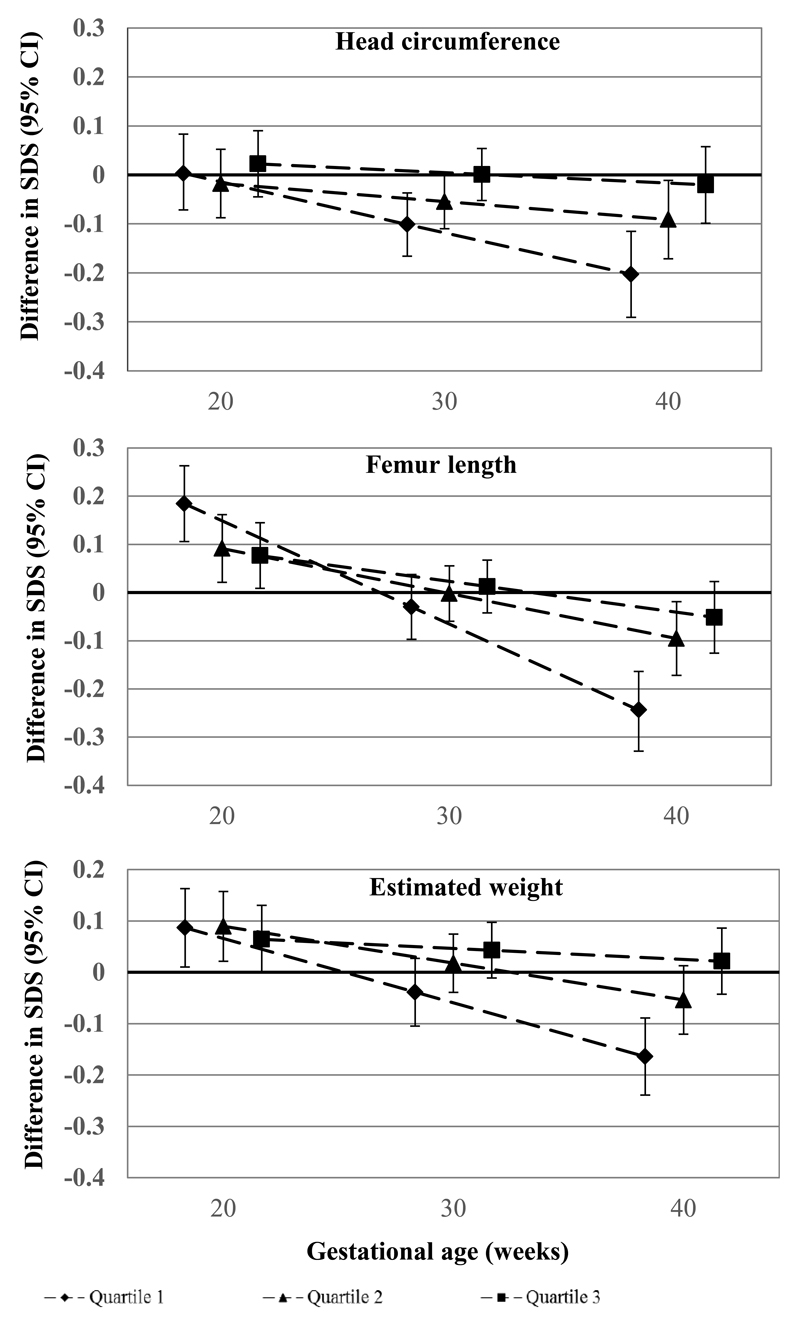
Figure 1 shows the associations of maternal 25(OH)D concentrations during pregnancy with longitudinally measured fetal head circumference, length and weight. As compared to mothers with 25(OH)D concentrations in the highest quartile (range 73.8 to 193.2 nmol/L), those with 25(OH)D concentrations in the first (range 1.5 to 24.1 nmol/L) and second (range 24.2 to 46.6 nmol/L) quartiles had offspring with restricted fetal head circumference growth from second trimester onwards, leading to a smaller head circumference at birth (differences: -0.20 SDS 95% Confidence Interval (CI) (-0.29, -0.12) and -0.09 SDS 95% CI (-0.17, -0.01) for first and second quartile of 25(OH)D concentrations, respectively). The associations of second trimester maternal 25(OH)D concentrations with fetal length growth and weight growth tended to be similar as those observed for head circumference. Results from the regression models focused on the associations of 25(OH)D concentrations with one fetal growth measure at each time point showed similar results (Supplementary Table 3). We observed similar associations when using vitamin D cut-offs according to previously used clinical cut-offs and recommendations (Supplementary Table 4). Also, although we observed tendencies for similar effect estimates when we restricted the analyses to Europeans only (N = 4069), not all associations reached statistical significance due to smaller sample sizes (Supplementary Table 5).
Figure 1. Associations of Maternal Second Trimester 25(OH)D Concentrations with Fetal Growth Patterns (N = 7098).
Values are estimates based on repeated linear regression models and reflect the standard deviation score for each growth characteristic in offspring of mothers whose 25(OH)D concentrations during pregnancy were in the first, second and third quartile compared to offspring from mothers who had 25(OH)D concentrations in the fourth quartile. Length at birth represents the full body length and weight at birth is the measured birth weight.
Maternal 25(OH)D Concentrations and Risks of Adverse Birth Outcomes
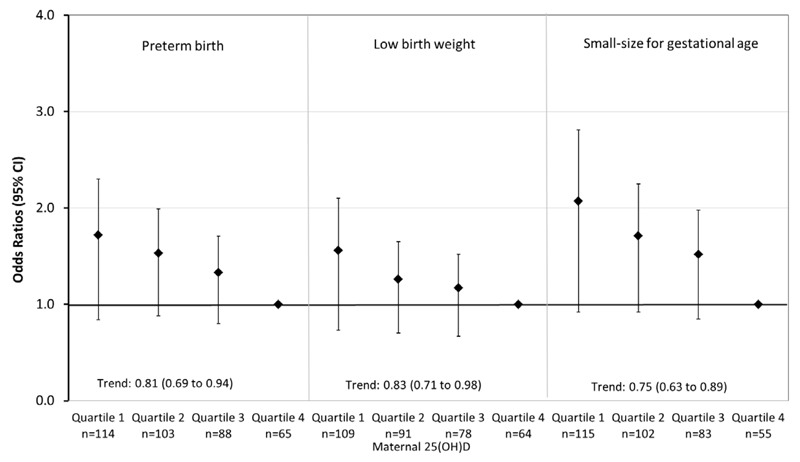
Figure 2 shows that as compared to mothers who had 25(OH)D concentrations in the highest quartile (range 73.8 to 193.2 nmol/L), those who were in the lowest quartile of 25(OH)D concentrations (range 1.5 to 24.1 nmol/L) had increased risks of children born preterm birth (Odds Ratio (OR) 1.72 (95% CI 1.14, 2.60)), with a low birth weight (OR 1.56 (95% CI 1.02, 2.39) and with a small-size for gestational age at birth (OR 2.07 (95% CI 1.33, 3.22)). We observed dose-response associations suggesting that higher maternal 25(OH)D concentrations across the full range were associated with lower risks of preterm birth, low birth weight and small-size for gestational age at birth (all P values <0.05). We observed tendencies for similar associations when using 25(OH)D cut-offs according to previously used clinical cut-offs and recommendations instead of quartiles (Supplementary Figure 2). Similar effect estimates were observed when we restricted our analyses to European subjects only, but not all associations were significant (Supplementary Figure 3). The estimated population attributable risks of vitamin D concentrations <50 nmol/L for preterm birth or low birth weight or for small-size for gestational age in our population were 17.3%, 18.4% and 22.6%, respectively.
Figure 2. Associations of Maternal Second Trimester 25(OH)D Concentrations with the Risks of Adverse Birth Outcomes (N= 7098).
Values are logistic regression coefficients (95% confidence interval) and reflect the risk of adverse birth outcomes compared to the reference group. Continuous analyses reflect the risks of being preterm, having a low birth weight or being small-size for gestational age at birth per 1 SDS increase in maternal 25(OH)D. Multivariable model is adjusted for maternal characteristics (age, body mass index at intake, alcohol consumption, smoking during pregnancy, folic acid and vitamin supplements, energy, iron, zinc, and calcium dietary intake during pregnancy, education, ethnicity, gestational hypertensive disorders, gestational diabetes, parity, season when blood samples were drawn and the presence of anorexia).
Adverse Birth Outcomes and Cord Blood 25(OH)D Concentrations
Table 3 shows that higher weight at birth and gestational age adjusted birth weight were associated with higher cord blood 25(OH)D concentrations (differences 0.04 SDS (95% CI 0.01, 0.07)) and 0.03 SDS (95% CI 0.01, 0.06) per 1 SD increase in birth weight and gestational age adjusted birth weight, respectively). The association of higher gestational age at birth with cord blood 25(OH)D concentrations was of borderline significance (difference -0.01 SDS (95% CI -0.03, 0) per 1SD increase in gestational age. Tendencies for similar effect estimates were observed when we restricted our analyses to European subjects only (Supplementary Table 6).
Table 3. Associations of Adverse Birth Outcomes with Cord Blood 25(OH)D Concentrations (N = 4262) 1.
| Birth characteristics | N | Cord blood 25(OH)D (nmol/L) |
|---|---|---|
| Gestational age | 4262 | |
| <37.0 weeks | 130 | 0.02 (-0.13, 0.17) |
| 37.0-41.9 weeks | 3849 | Reference |
| ≥42 weeks | 283 | -0.06 (-0.16, 0.05) |
| Trend | -0.01 (-0.03, 0) | |
| Birth weight | 4258 | |
| <2000 grams | 15 | -0.49 (-0.91, -0.06)2 |
| 2000 - 2499 g | 94 | -0.01 (-0.18, 0.17) |
| 2500 - 2999 g | 607 | -0.02 (-0.11, 0.06) |
| 3000 - 3499 g | 1535 | Reference |
| 3500 - 3999 g | 1412 | 0.01 (-0.05, 0.07) |
| 4000 - 4499 g | 502 | -0.01 (-0.10, 0.08) |
| ≥4500 grams | 93 | 0.06 (-0.12, 0.23) |
| Trend | 0.04 (0.01, 0.07)2,3 | |
| Birth weight for gestational age | 4257 | |
| Small | 164 | -0.09 (-0.22, 0.05) |
| Normal | 3881 | Reference |
| Large | 212 | 0.06 (-0.06, 0.18) |
| Trend | 0.03 (0.01, 0.06)2 |
Values are linear regression coefficients (95% confidence interval) and reflect the change in standard deviation (SDS) of cord blood 25(OH)D concentrations for each birth weight or gestational age group, compared to the reference group. Trend estimates represent the effect estimates for the continuous associations per SDS change in birth characteristics. Multivariable model is adjusted for fetal sex, maternal characteristics (age, body mass index at intake, alcohol consumption, smoking during pregnancy, folic acid and vitamin supplements, energy, iron, zinc and calcium dietary intake during pregnancy, education, ethnicity, gestational hypertensive disorders, gestational diabetes, parity and the presence of anorexia).
P value < 0.05.
Also significant after applying Bonferroni correction (P value <0.025).
Discussion
Results from this large population-based prospective cohort study suggest that second trimester lower maternal 25(OH)D concentrations are associated with third trimester fetal head, length, and weight growth restriction and with increased risks of preterm birth, low birth weight and small-size for gestational age at birth. These associations were not restricted to the extremes, but tended to be present across the full spectrum of maternal 25(OH)D concentrations. Also, a smaller size at birth was associated with lower cord blood 25(OH)D concentrations.
We hypothesized that suboptimal maternal 25(OH)D concentrations affect fetal development leading to an increased risk for adverse birth outcomes. Previous studies reported inconsistent results on the associations of maternal 25(OH)D concentrations with birth outcomes (2, 7–9, 40). A previous study among 2473 mother-children pairs, in a multi-center cohort in the United States reported that low concentrations of maternal 25(OH)D are associated with low weight at birth (9). In line with these findings, a study among 2146 mother-children pairs in the United States observed that maternal 25(OH)D concentrations were positively associated with weight at birth (40). Results from another population-based cohort study in the Netherlands among 3730 mother-children pairs, also suggested that lower maternal 25(OH)D concentrations are associated with lower birth weight (7). A recent meta-analysis of randomized controlled trials suggested that vitamin D supplementation during pregnancy was associated with increased circulating 25(OH)D concentrations and a higher birth weight (41). Thus, altogether previous studies suggest that higher vitamin D concentrations in pregnancy lead to higher birth weight.
Using birth weight as main fetal outcome has strong limitations. Birth weight does not give information about longitudinal fetal growth and development patterns and fetal body proportions. We examined the impact of maternal 25(OH)D concentrations on head circumference, length and estimated fetal weight during second and third trimester of pregnancy and at birth. We used repeatedly and directly measured fetal growth characteristics and observed that lower maternal 25(OH)D concentrations were associated with restricted fetal head circumference growth from second trimester onwards, correlating with a small head circumference at birth. Similar associations were observed for fetal length growth and fetal weight growth. In line with our findings on head circumference at birth, a multi-center cohort in the United States suggested that lower maternal 25(OH)D concentrations during mid-pregnancy are associated with a smaller head circumference at birth (40). In contrast, a study among 2382 mother-child pairs in Spain, showed that higher maternal 25(OH)D concentrations in early pregnancy are associated with a smaller head circumference at birth (8). Regarding birth length, results from a recent meta-analysis of randomized controlled trials suggested that neonates in the maternal vitamin D supplemented group had a higher birth length compared with the control group (41). In contrast to these findings, a study among 559 mother-children pairs in India, did not observe any association of maternal 25(OH)D concentrations and length at birth (42). These differences may be due to differences in study populations. Results from the longitudinal analyses of our large study, suggest that low maternal 25(OH)D concentrations are associated with a proportional growth restriction, though the largest effect may be present on fetal length growth.
We observed that lower maternal 25(OH)D concentrations during mid-pregnancy are associated with a higher risk of preterm birth. A previous study among 2382 mother-child pairs in Spain did not observe any association of maternal 25(OH)D concentrations during early pregnancy with the risk of preterm birth (8). These results are not in line with what we observe, this may be due to specific study population, and smaller sample size to detect these associations. Our results regarding the risk of low birth weight and of small-size for gestational age are in line with findings from another cohort study in the Netherlands, which observed that lower maternal 25(OH)D concentrations during early pregnancy are associated with an increased risk of low birth weight and small-size for gestational age (7). Also, a previous study among 3658 Chinese mothers, whose 25(OH)D concentrations were assessed at different time points during pregnancy, observed that lower maternal 25(OH)D concentrations throughout pregnancy elevate the risk of small-size for gestational age at birth (43). The observations linking low maternal vitamin D to both preterm birth, low birth weight and small-size for gestational age at birth are important. All three outcomes are associated with perinatal mortality and later-life chronic disease (44). We also observed that children born preterm or with a small-size at birth have lower cord blood 25(OH)D concentrations. Results from a study in Boston among 471 infants, suggested that preterm born infants have a higher risk of having lower 25(OH)D cord blood concentrations (45). Thus, preterm birth and small size at birth may be both a consequence and a risk factor of vitamin D deficiency. Low vitamin D concentrations in early life may affect health in later life (46, 47).
Form our observational study, it is not possible to establish the causality for the observed associations. However, several biological mechanisms have been suggested linking maternal 25(OH)D concentrations to fetal development. Maternal vitamin D may affect placental vascularisation (48). It has been suggested that vitamin D receptors and 1,25(OH)2D regulate placental secretion of human placental lactogen and other hormones that affect maternal glucose and fatty acid metabolism, which provide energy for fetal needs (45, 48). Further studies are needed focusing on the causality and mechanisms explaining the observed associations are needed.
Our findings are important from a population-based perspective. The upper limit of first 2 quartiles of maternal 25(OH)D concentrations correspond to the value of <50 nmol/L, which is defined as 25(OH)D deficiency (19, 20). In our study, 53% of all mothers had 25(OH)D deficient concentrations, leading to high estimated population attributable risks. In the Netherlands, pregnant women are advised to use folic acid supplements (400 µg/day) prior to and up to the 10-12th week of pregnancy and vitamin D supplements (10 µg/day). Therefore, results from our study support population-strategies to improve vitamin D concentrations in pregnant women.
Some methodological considerations need to be considered. To our knowledge, this is the largest multi-ethnic population-based prospective cohort study focused on the associations of maternal 25(OH)D concentrations with directly measured fetal growth characteristics in different periods of pregnancy. Among mothers with 25(OH)D concentrations available, we had a limited loss to follow-up, therefore we do not expect biased results due to selective follow-up. We used 25(OH)D concentrations, which are the best and most widely used indicator of vitamin D status (49). We analyzed vitamin D concnetrations as continuous data, quartiles and previously used clinical cut-offs (18, 20–24). The cut offs remain subject of debate (18, 19). In line with recommendations of the Endocrine Society and based on previous results from our and other cohort studies, we created four vitamin D groups, including severely deficient (<25.0 nmol/L), deficient (25.0 to 49.9 nmol/L), sufficient (50.0 to 74.9 nmol/L) and optimal (≥75.0 nmol/L) (18, 20–24). The Institute of Medicine (IOM) defines vitamin D as deficient (<50nmol/L), and sufficient (≥50nmol/L), which would lead to categorizing our severely deficient and deficient groups as deficient, and categorizing the sufficient and optimal groups as sufficient or adequate. We consider an advantage of our categories as compared to IOM categories that we have more groups and can compare our results with previous pregnancy studies. Also, our findings suggest that the effects of vitamin D on fetal outcomes are not restricted to IOM deficient groups, but present across the full common range. 25(OH)D concentrations were assessed during second trimester and in cord blood at birth. The correlation coefficient between maternal serum 25(OH)D concentrations during second trimester and in cord blood was r=0.62 (P value <0.01). This correlation varies between studies. Some studies report a higher degree of correlation, but still report a difference between maternal 25(OH)D concentrations during third trimester of pregnancy and 25(OH)D concentrations in cord blood at birth (50). A limitation of our study may be that we do not have maternal 25(OH)D concentrations measured during first trimester. We measured fetal development by direct second- and third trimester fetal ultrasound examinations. Since the main outcomes were correlated, we did not adjust the main analyses for multiple comparisons. Another limitation of our study is the lacking of detailed information on vitamin D supplementation and on conditions that may influence vitamin D status, such as bariatric surgery or different gastrointestinal diseases. However, other nutritional factors may influence the observed associations. Since previous studies suggested that iron, zinc, calcium and other vitamin supplements influence fetal growth and birth outcomes, we included these nutritional factors in our regression models. Adjustment for these factors did not materially change the effect estimates. Several trials suggested that supplementation of these micronutrients lower the risks of pregnancy complications in women at risk of deficiencies or adverse outcomes (51–56). Because, the intake of these micronutrients was estimated from a FFQ, we may not have the precision in these levels as compared to studies with levels. Maternal ethnicity is related to both vitamin D concentrations and fetal growth patterns (36, 37). The study cohort was a multi-ethnic sample in the city of Rotterdam, the Netherlands. As compared to the population distribution in the city of Rotterdam, the percentage of Europeans was higher whereas the percentage of Moroccans was lower (36). We used two approaches to explore the role of maternal ethnicity. All main analyses were first adjusted for maternal ethnicity. Second, we observed tendencies for similar associations when we restricted the analyses to Europeans only. However, not all effect estimates were significant, probably due to smaller numbers. Unfortunately, we did not have enough numbers in the other ethnic subgroups, to perform ethnic specific analyses. The results of these additional analyses suggest that the associations of vitamin D concentrations with fetal outcomes are not explained by maternal ethnicity. Further studies are needed to explore differences in effect estimates between ethnic subgroups. Finally, although we performed adjustment for a large number of potential maternal confounders, residual confounding by other lifestyle factors, might still be present, as in any observational study. The causality for the associations of vitamin D with fetal developmental outcomes cannot be established from observational studies only. Therefore, future studies are needed to establish causal relationships.
Conclusion
Our findings suggest that second trimester low maternal 25(OH)D concentrations are associated with third trimester fetal grow restriction and with increased risks of preterm birth, low birth weight, and small-size for gestational age at birth. These associations were not restricted to the extremes, but tended to be present across the full spectrum of maternal 25(OH)D concentrations. Although the causality of the observed associations need to be further established, these findings support current strategies to increase 25(OH)D concentrations in pregnant women.
Supplementary Material
Acknowledgments
The Generation R Study is conducted by the Erasmus University Medical Center in close collaboration with the School of Law and Faculty of Social Sciences of the Erasmus University Rotterdam, the Municipal Health Service Rotterdam area, Rotterdam, the Rotterdam Homecare Foundation, Rotterdam and the Stichting Trombosedienst and Artsenlaboratorium Rijnmond (STAR), Rotterdam. We gratefully acknowledge the contribution of participating mothers, general practitioners, hospitals, midwives and pharmacies in Rotterdam.
Financial support: The general design of the Generation R Study was made possible by financial support from Erasmus Medical Center, Rotterdam; Erasmus University, Rotterdam; the Dutch Ministry of Health, Welfare and Sport; and the Netherlands Organization for Health Research and Development (ZonMw). K. Miliku has been financially supported through Erasmus Mundus Western Balkans (ERAWEB), a project funded by the European Commission. The vitamin D assay was supported by the Australian National Health and Medical Research Council (NHMRC APP1062846). Dr J. McGrath received a NHMRC John Cade Fellowship (APP1056929). V.W.V. Jaddoe received an additional grant from the Netherlands Organization for Health Research and Development (VIDI 016.136.361) and a European Research Council Consolidator Grant (ERC-2014-CoG-648916).The funders had no role in the design of the study; the data collection and analyses; the interpretation of data; the preparation and review of the manuscript; or the decision to submit the manuscript.
Abbreviations
- CI
confidence interval
Footnotes
Author Contributions
Dr Miliku had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Jaddoe and Hofman.
Acquisition of data: Miliku and Jaddoe.
Analysis and interpretation of data: Miliku, Gaillard, Jaddoe.
Drafting of the manuscript: Miliku and Jaddoe.
Critical revision of the manuscript for important intellectual content: Miliku, Vinkhuyzen, Blanken, McGrath, Eyles, Burne, Hofman, Tiemeier, Steegers, Gaillard and Jaddoe.
Statistical analysis: Miliku and Gaillard.
Obtained funding: Miliku, McGrath and Jaddoe.
Study supervision: Jaddoe.
Financial Disclosures:
None reported
Potential competing interests: None
References
- 1.Holick MF. Vitamin D for health and in chronic kidney disease. Semin Dial. 2005;18(4):266–75. doi: 10.1111/j.1525-139X.2005.18402.x. [DOI] [PubMed] [Google Scholar]
- 2.Gale CR, Robinson SM, Harvey NC, Javaid MK, Jiang B, Martyn CN, Godfrey KM, Cooper C, Princess Anne Hospital Study G Maternal vitamin D status during pregnancy and child outcomes. Eur J Clin Nutr. 2008;62(1):68–77. doi: 10.1038/sj.ejcn.1602680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown AJ, Dusso A, Slatopolsky E. Vitamin D. Am J Physiol. 1999;277(2 Pt 2):F157–75. doi: 10.1152/ajprenal.1999.277.2.F157. [DOI] [PubMed] [Google Scholar]
- 4.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359(1):61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hollis BW, Wagner CL. Nutritional vitamin D status during pregnancy: reasons for concern. Cmaj. 2006;174(9):1287–90. doi: 10.1503/cmaj.060149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thorne-Lyman A, Fawzi WW. Vitamin D during pregnancy and maternal, neonatal and infant health outcomes: a systematic review and meta-analysis. Paediatr Perinat Epidemiol. 2012;26(Suppl 1):75–90. doi: 10.1111/j.1365-3016.2012.01283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leffelaar ER, Vrijkotte TG, van Eijsden M. Maternal early pregnancy vitamin D status in relation to fetal and neonatal growth: results of the multi-ethnic Amsterdam Born Children and their Development cohort. Br J Nutr. 2010;104(1):108–17. doi: 10.1017/S000711451000022X. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez A, Garcia-Esteban R, Basterretxea M, Lertxundi A, Rodriguez-Bernal C, Iniguez C, Rodriguez-Dehli C, Tardon A, Espada M, Sunyer J, et al. Associations of maternal circulating 25-hydroxyvitamin D3 concentration with pregnancy and birth outcomes. Bjog. 2014 doi: 10.1111/1471-0528.13074. [DOI] [PubMed] [Google Scholar]
- 9.Gernand AD, Klebanoff MA, Simhan HN, Bodnar LM. Maternal vitamin D status, prolonged labor, cesarean delivery and instrumental delivery in an era with a low cesarean rate. J Perinatol. 2015;35(1):23–8. doi: 10.1038/jp.2014.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Godfrey KM, Barker DJ. Fetal programming and adult health. Public Health Nutr. 2001;4(2B):611–24. doi: 10.1079/phn2001145. [DOI] [PubMed] [Google Scholar]
- 11.Mahon P, Harvey N, Crozier S, Inskip H, Robinson S, Arden N, Swaminathan R, Cooper C, Godfrey K, Group SWSS Low maternal vitamin D status and fetal bone development: cohort study. J Bone Miner Res. 2010;25(1):14–9. doi: 10.1359/jbmr.090701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prefumo F, Canini S, Crovo A, Pastorino D, Venturini PL, De Biasio P. Correlation between first trimester fetal bone length and maternal serum pregnancy-associated plasma protein-A (PAPP-A) Hum Reprod. 2006;21(11):3019–21. doi: 10.1093/humrep/del058. [DOI] [PubMed] [Google Scholar]
- 13.Smith GC. First trimester origins of fetal growth impairment. Semin Perinatol. 2004;28(1):41–50. doi: 10.1053/j.semperi.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Bodnar LM, Catov JM, Zmuda JM, Cooper ME, Parrott MS, Roberts JM, Marazita ML, Simhan HN. Maternal serum 25-hydroxyvitamin D concentrations are associated with small-for-gestational age births in white women. J Nutr. 2010;140(5):999–1006. doi: 10.3945/jn.109.119636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bodnar LM, Simhan HN. Vitamin D may be a link to black-white disparities in adverse birth outcomes. Obstet Gynecol Surv. 2010;65(4):273–84. doi: 10.1097/OGX.0b013e3181dbc55b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kruithof CJ, Kooijman MN, van Duijn CM, Franco OH, de Jongste JC, Klaver CC, Mackenbach JP, Moll HA, Raat H, Rings EH, et al. The Generation R Study: Biobank update 2015. Eur J Epidemiol. 2014;29(12):911–27. doi: 10.1007/s10654-014-9980-6. [DOI] [PubMed] [Google Scholar]
- 17.Eyles D, Anderson C, Ko P, Jones A, Thomas A, Burne T, Mortensen PB, Norgaard-Pedersen B, Hougaard DM, McGrath J. A sensitive LC/MS/MS assay of 25OH vitamin D3 and 25OH vitamin D2 in dried blood spots. Clin Chim Acta. 2009;403(1–2):145–51. doi: 10.1016/j.cca.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 19.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. 2011;96:53–8. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miliku K, Voortman T, Franco OH, McGrath JJ, Eyles DW, Burne TH, Hofman A, Tiemeier H, Jaddoe VW. Vitamin D status during fetal life and childhood kidney outcomes. Eur J Clin Nutr. 2015 doi: 10.1038/ejcn.2015.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voortman T, van den Hooven EH, Heijboer AC, Hofman A, Jaddoe VW, Franco OH. Vitamin D Deficiency in School-Age Children Is Associated with Sociodemographic and Lifestyle Factors. J Nutr. 2015 doi: 10.3945/jn.114.208280. [DOI] [PubMed] [Google Scholar]
- 22.Sioen I, Mouratidou T, Kaufman JM, Bammann K, Michels N, Pigeot I, Vanaelst B, Vyncke K, De Henauw S, consortium I Determinants of vitamin D status in young children: results from the Belgian arm of the IDEFICS (Identification and Prevention of Dietary- and Lifestyle-Induced Health Effects in Children and Infants) Study. Public Health Nutr. 2012;15(6):1093–9. doi: 10.1017/S1368980011002989. [DOI] [PubMed] [Google Scholar]
- 23.Hypponen E, Cavadino A, Williams D, Fraser A, Vereczkey A, Fraser WD, Banhidy F, Lawlor D, Czeizel AE. Vitamin D and pre-eclampsia: original data, systematic review and meta-analysis. Ann Nutr Metab. 2013;63(4):331–40. doi: 10.1159/000358338. [DOI] [PubMed] [Google Scholar]
- 24.Lips P. Worldwide status of vitamin D nutrition. J Steroid Biochem Mol Biol. 2010;121(1–2):297–300. doi: 10.1016/j.jsbmb.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 25.Gaillard R, Steegers EA, de Jongste JC, Hofman A, Jaddoe VW. Tracking of fetal growth characteristics during different trimesters and the risks of adverse birth outcomes. Int J Epidemiol. 2014;43(4):1140–53. doi: 10.1093/ije/dyu036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verburg BO, Steegers EA, De Ridder M, Snijders RJ, Smith E, Hofman A, Moll HA, Jaddoe VW, Witteman JC. New charts for ultrasound dating of pregnancy and assessment of fetal growth: longitudinal data from a population-based cohort study. Ultrasound Obstet Gynecol. 2008;31(4):388–96. doi: 10.1002/uog.5225. [DOI] [PubMed] [Google Scholar]
- 27.Royal College of Obstetricians and Gynecologists. Routine ultrasound screening in pregnancy: protocol. London, United Kingdom: RCOG Press; 2000. [Google Scholar]
- 28.Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK. Estimation of fetal weight with the use of head, body, and femur measurements--a prospective study. Am J Obstet Gynecol. 1985;151(3):333–7. doi: 10.1016/0002-9378(85)90298-4. [DOI] [PubMed] [Google Scholar]
- 29.Jaddoe VWV, van Duijn CM, Franco OH, van der Heijden AJ, van Iizendoorn MH, de Jongste JC, van der Lugt A, Mackenbach JP, Moll HA, Raat H, et al. The Generation R Study: design and cohort update 2012. Eur J Epidemiol. 2012;27(9):739–56. doi: 10.1007/s10654-012-9735-1. [DOI] [PubMed] [Google Scholar]
- 30.Niklasson A, Ericson A, Fryer JG, Karlberg J, Lawrence C, Karlberg P. An update of the Swedish reference standards for weight, length and head circumference at birth for given gestational age (1977-1981) Acta Paediatr Scand. 1991;80(8–9):756–62. doi: 10.1111/j.1651-2227.1991.tb11945.x. [DOI] [PubMed] [Google Scholar]
- 31.Klipstein-Grobusch K, den Breeijen JH, Goldbohm RA, Geleijnse JM, Hofman A, Grobbee DE, Witteman JC. Dietary assessment in the elderly: validation of a semiquantitative food frequency questionnaire. Eur J Clin Nutr. 1998;52(8):588–96. doi: 10.1038/sj.ejcn.1600611. [DOI] [PubMed] [Google Scholar]
- 32.Centre for Research and Statistics. Internet: http://www.rotterdam.nl/onderzoek.
- 33.Statistiek CBvd. Immigrants in the Netherlands 2004. Netherland: Statistics Netherlands; 2004. [Google Scholar]
- 34.Coolman M, de Groot CJ, Jaddoe VW, Hofman A, Raat H, Steegers EA. Medical record validation of maternally reported history of preeclampsia. J Clin Epidemiol. 2010;63(8):932–7. doi: 10.1016/j.jclinepi.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 35.Goldstein H. Multilevel Statistical Methods. 2nd edn. London: Edward Arnold; 1995. [Google Scholar]
- 36.Vinkhuyzen AA, Eyles DW, Burne TH, Blanken LM, Kruithof CJ, Verhulst F, Jaddoe VW, Tiemeier H, McGrath JJ. Prevalence and predictors of vitamin D deficiency based on maternal mid-gestation and neonatal cord bloods: The Generation R Study. J Steroid Biochem Mol Biol. 2015 doi: 10.1016/j.jsbmb.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 37.Troe EJ, Raat H, Jaddoe VW, Hofman A, Looman CW, Moll HA, Steegers EA, Verhulst FC, Witteman JC, Mackenbach JP. Explaining differences in birthweight between ethnic populations. The Generation R Study. Bjog. 2007;114(12):1557–65. doi: 10.1111/j.1471-0528.2007.01508.x. [DOI] [PubMed] [Google Scholar]
- 38.Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, Wood AM, Carpenter JR. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rubin DB, Schenker N. Multiple imputation in health-care databases: an overview and some applications. Stat Med. 1991;10(4):585–98. doi: 10.1002/sim.4780100410. [DOI] [PubMed] [Google Scholar]
- 40.Gernand AD, Simhan HN, Klebanoff MA, Bodnar LM. Maternal serum 25-hydroxyvitamin D and measures of newborn and placental weight in a U.S. multicenter cohort study. J Clin Endocrinol Metab. 2013;98(1):398–404. doi: 10.1210/jc.2012-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perez-Lopez FR, Pasupuleti V, Mezones-Holguin E, Benites-Zapata VA, Thota P, Deshpande A, Hernandez AV. Effect of vitamin D supplementation during pregnancy on maternal and neonatal outcomes: a systematic review and meta-analysis of randomized controlled trials. Fertil Steril. 2015;103(5):1278–88 e4. doi: 10.1016/j.fertnstert.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 42.Farrant HJ, Krishnaveni GV, Hill JC, Boucher BJ, Fisher DJ, Noonan K, Osmond C, Veena SR, Fall CH. Vitamin D insufficiency is common in Indian mothers but is not associated with gestational diabetes or variation in newborn size. Eur J Clin Nutr. 2009;63(5):646–52. doi: 10.1038/ejcn.2008.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen YH, Fu L, Hao JH, Yu Z, Zhu P, Wang H, Xu YY, Zhang C, Tao FB, Xu DX. Maternal vitamin d deficiency during pregnancy elevates the risks of small for gestational age and low birth weight infants in chinese population. J Clin Endocrinol Metab. 2015;100(5):1912–9. doi: 10.1210/jc.2014-4407. [DOI] [PubMed] [Google Scholar]
- 44.MK S, Gardosi J. Perinatal mortality and fetal growth restriction. Best Pract Res Clin Obstet Gynaecol. 2004;18(3):397–410. doi: 10.1016/j.bpobgyn.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 45.Lapillonne A. Vitamin D deficiency during pregnancy may impair maternal and fetal outcomes. Med Hypotheses. 2010;74(1):71–5. doi: 10.1016/j.mehy.2009.07.054. [DOI] [PubMed] [Google Scholar]
- 46.Crozier SR, Harvey NC, Inskip HM, Godfrey KM, Cooper C, Robinson SM, Group SWSS Maternal vitamin D status in pregnancy is associated with adiposity in the offspring: findings from the Southampton Women's Survey. Am J Clin Nutr. 2012;96(1):57–63. doi: 10.3945/ajcn.112.037473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harvey NC, Moon RJ, Sayer AA, Ntani G, Davies JH, Javaid MK, Robinson SM, Godfrey KM, Inskip HM, Cooper C, et al. Maternal antenatal vitamin D status and offspring muscle development: findings from the Southampton Women's Survey. J Clin Endocrinol Metab. 2014;99(1):330–7. doi: 10.1210/jc.2013-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shin JS, Choi MY, Longtine MS, Nelson DM. Vitamin D effects on pregnancy and the placenta. Placenta. 2010;31(12):1027–34. doi: 10.1016/j.placenta.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harvey NC, Holroyd C, Ntani G, Javaid K, Cooper P, Moon R, Cole Z, Tinati T, Godfrey K, Dennison E, et al. Vitamin D supplementation in pregnancy: a systematic review. Health Technol Assess. 2014;18(45):1–190. doi: 10.3310/hta18450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weisse K, Winkler S, Hirche F, Herberth G, Hinz D, Bauer M, Roder S, Rolle-Kampczyk U, von Bergen M, Olek S, et al. Maternal and newborn vitamin D status and its impact on food allergy development in the German LINA cohort study. Allergy. 2013;68(2):220–8. doi: 10.1111/all.12081. [DOI] [PubMed] [Google Scholar]
- 51.Castillo-Duran C, Weisstaub G. Zinc supplementation and growth of the fetus and low birth weight infant. J Nutr. 2003;133(5 Suppl 1):1494S–7S. doi: 10.1093/jn/133.5.1494S. [DOI] [PubMed] [Google Scholar]
- 52.Terrin G, Berni Canani R, Di Chiara M, Pietravalle A, Aleandri V, Conte F, De Curtis M. Zinc in Early Life: A Key Element in the Fetus and Preterm Neonate. Nutrients. 2015;7(12):10427–46. doi: 10.3390/nu7125542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hovdenak N, Haram K. Influence of mineral and vitamin supplements on pregnancy outcome. Eur J Obstet Gynecol Reprod Biol. 2012;164(2):127–32. doi: 10.1016/j.ejogrb.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 54.Imdad A, Bhutta ZA. Effects of calcium supplementation during pregnancy on maternal, fetal and birth outcomes. Paediatr Perinat Epidemiol. 2012;26(Suppl 1):138–52. doi: 10.1111/j.1365-3016.2012.01274.x. [DOI] [PubMed] [Google Scholar]
- 55.Hofmeyr GJ, Atallah AN, Duley L. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database Syst Rev. 2006;(3):CD001059. doi: 10.1002/14651858.CD001059.pub2. [DOI] [PubMed] [Google Scholar]
- 56.Belizan JM, Villar J, Gonzalez L, Campodonico L, Bergel E. Calcium supplementation to prevent hypertensive disorders of pregnancy. N Engl J Med. 1991;325(20):1399–405. doi: 10.1056/NEJM199111143252002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.