Abstract
PURPOSE
We aimed to evaluate whether morphologic magnetic resonance imaging (MRI) features could help to predict the maternal outcome after uterine artery embolization (UAE)-assisted cesarean section (CS) in patients with invasive placenta previa.
METHODS
We retrospectively reviewed the MRI data of 40 pregnant women who have undergone UAE-assisted cesarean section due to suspected high risk of massive hemorrhage caused by invasive placenta previa. Patients were divided into two groups based on the maternal outcome (good-outcome group: minor hemorrhage and uterus preserved; poor-outcome group: significant hemorrhage or emergency hysterectomy). Morphologic MRI features were compared between the two groups. Multivariate logistic regression analysis was used to identify the most valuable variables, and predictive value of the identified risk factor was determined.
RESULTS
Low signal intensity bands on T2-weighted imaging (P < 0.001), placenta percreta (P = 0.011), and placental cervical protrusion sign (P = 0.002) were more frequently observed in patients with poor outcome. Low signal intensity bands on T2-weighted imaging was the only significant predictor of poor maternal outcome in multivariate analysis (P = 0.020; odds ratio, 14.79), with 81.3% sensitivity and 84.3% specificity.
CONCLUSION
Low signal intensity bands on T2-weighted imaging might be a predictor of poor maternal outcome after UAE-assisted cesarean section in patients with invasive placenta previa.
Placenta previa is often associated with abnormal placentation (placenta accreta, increta, or percreta) when overlapping the previous cesarean section (CS) scar, leading to severe intrapartum hemorrhage during placenta removal and further increasing the rates of hysterectomy and maternal mortality (1, 2). To overcome this problem, many doctors have tried to use the uterine artery embolization (UAE) procedure to control intractable intrapartum bleeding during scheduled CS (3, 4). However, the volume of blood loss and the rate of hysterectomy have been variable at different clinical centers, and the treatment efficacy has not been uniform (3–6). A common and simple method that can help to predict the maternal clinical outcome after UAE-assisted CS is urgently needed, particularly for presurgical preparation and doctor-patient communication.
Previously, ultrasonography was the most commonly used imaging method in presurgical evaluation of abnormal placentation in patients with placenta previa (7). There are several reports on sonographic evaluation to predict the risk of massive bleeding in patients with placenta previa and previous CS (8–12). However, ultrasonography is an extremely subjective evaluation modality and mainly depends on the experience of the operator. In recent years, with the advance of improved soft tissue resolution, larger fields of interest, and ultra-fast scanning sequences, MRI has been increasingly used in the evaluation of placental invasiveness and to classify the degree of placenta invasion, particularly in cases of diagnostic doubt by ultrasonography, maternal obesity, and posterior placentation (13, 14). MRI can provide topographic and morphologic information regarding the placenta, and clarify the degree of invasion for optimal diagnosis and planning of surgical management. Abnormal uterine bulging sign, heterogeneous placental signal intensity, dark intraplacental bands on T2-weighted images, focal disruption of placental-myometrial interface, and placenta protrusion sign were listed as distinctive findings of placental invasion on MRI (15–18). However, to the best of our knowledge, there has been no study correlating MRI features with maternal clinical outcome after UAE-assisted CS delivery until now.
Therefore, our study aimed to evaluate whether morphologic features on conventional MRI could be used to predict the maternal clinical outcome after UAE-assisted CS in patients with invasive placenta previa.
Methods
Patients
The protocol of this study was approved by the ethics committee of our hospital, and written informed consent was waived due to the retrospective nature of the study.
From February 2012 and March 2015, a total of 53 consecutive pregnant women underwent abdominal MRI in our department due to placenta previa overlying the previous CS scar. All of these patients were suspected to experience significant hemorrhage due to placenta accreta, increta, or percreta on the surface of a previous CS scar. The inclusion criteria included: 1) an exact history of CS; 2) diagnosis of placenta previa overlying the previous CS scar based on both ultrasonography and MRI; and 3) invasive placenta confirmed during the CS delivery and pathologic exam result. The final diagnostic criteria used in this study included the following: 1) clinical diagnosis during the CS based on a difficult manual piecemeal removal of the placenta if there is no separation after 20 min despite active management of the third stage of labor; 2) heavy continuous bleeding from the implantation site of a well-contracted uterus after placenta removal during CS delivery. Patients who underwent hysterectomy had a final pathologic diagnosis of placenta accreta, increta, or percreta, classified according to the depth of myometrial infiltration. Placenta accreta means the attachment of the chorionic villi to the myometrium without invasion, unlike placenta increta that is defined by partial myometrial invasion. In placenta percreta, the chorionic villi penetrate the uterine serosa and may extend into adjacent pelvic organs.
Thirteen patients were excluded from the study due to motion artifact caused by fetal movement (n=4), patients who did not labor in our hospital (n=7), noninvasive placenta previa confirmed during the CS (n=2). At last, a total of 40 pregnant women (age, 31.95 years; range, 20–39 years; duration of gestation 34 weeks, ranging between 29 weeks 6 days and 37 weeks) were enrolled in the present study, including 16 cases with placenta accreta, 20 cases with placenta increta, and four cases with placenta percreta.
All patients received prophylactic UAE-assisted CS in our hospital. The UAE procedure was similar to the previous study (4). Following anesthesia, temporary bilateral ureteral stents were placed under cystoscopy and vesical catheter was inserted preoperatively. A 5 F sheath was placed in the right common femoral artery using Seldinger technique. Selective catheterization of the left uterine artery was performed with a 5 F catheter under fluoroscopic guidance, followed by a traditional CS delivery procedure. After the infant had been delivered, the obstetrician packed the vagina and uterus with the placenta still in situ. Under the fluoroscopic guidance, the left uterine artery was embolized first, following which, the right uterine artery was selectively catheterized and embolized with Gelform sponge pledgets. After confirming that the blood flow has slowed using angiography, the placenta was totally removed manually from the uterine wall in patients with placenta accreta, and the placenta was left in situ in patients with placenta increta and percreta. Specimens were taken from densely adherent placental areas for histopathology. If bleeding continued to be substantial even after bilateral UAE and internal iliac artery embolization and led to cardiovascular instability, emergency hysterectomy was performed. After surgery, when no more vaginal bleeding was observed, the 5 F sheath was removed. If a hysterectomy was performed, the blood lost during the hysterectomy was recorded. The total amount of blood lost during surgery was measured from the time of skin incision to closure of the abdominal wall. The Healthcare and Commission defined “significant” blood lost as >1000 mL in its recent review of maternity services in England and Wales (19). All patients involved in this study received prophylactic UAE procedure to reduce the blood lost during CS, so the blood loss of >1000 mL during surgery was defined as a significant hemorrhage in the present study. Blood loss of <1000 mL was defined as minor hemorrhage. Good maternal outcome was defined as parturient with minor hemorrhage and uterus preserved, while poor maternal outcome was defined as parturient with significant hemorrhage or emergency hysterectomy.
Scanning protocol
MRI examinations were performed using a 1.5 T MRI scanner (General Electric, HDxt) with a phase-array body coil. The MRI examination ranged from the diaphragm to the pubic symphysis using the following parameters. The imaging protocol included: 1) Axial, sagittal and coronal two-dimensional single-shot free-breathing fast-imaging employing steady state (SS FIESTA; TR 3.5 ms; TE 1.5 ms; 6 mm thick sections; 0.5 mm gap; field of view [FOV], 36×40 cm; matrix, 256×256). 2) Axial, sagittal and coronal breath-holding single-shot fast spin-echo T2-weighted imaging (BH SSFSE, TR 1800 ms; TE 80 ms; FOV, 42×40 cm; 6 mm thick sections; 2 mm gap; matrix, 256×324). 3) Axial and sagittal T1-weighted imaging (TR 400 ms; TE 8 ms; FOV, 40 mm; 4 mm thick sections; 1 mm gap; matrix, 356×220). All sequences were performed without intravenous contrast medium. The total imaging acquisition time was 30 minutes.
Imaging analysis
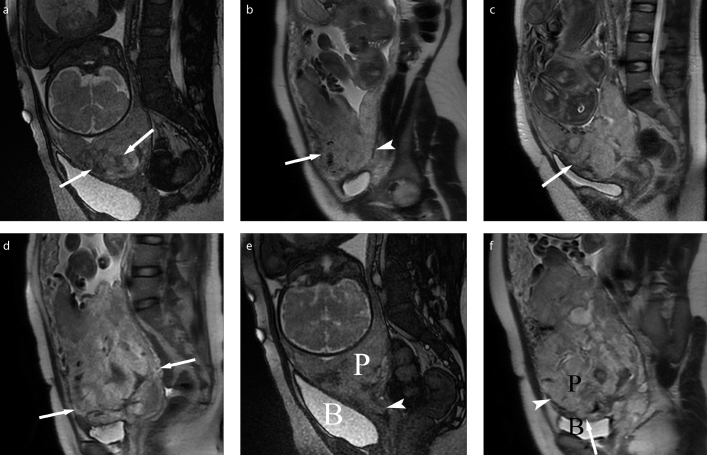
Conventional morphologic MRI features were analyzed as follows: 1) Placenta previa subtype classified as complete or partial depending on whether the placenta completely or partially overlapped the internal cervical ostium (8); 2) Attachment site of the placenta to the uterine wall classified as anterior previa or posterior previa: if the distance from the internal cervical ostium to the placental edge was longer in the anterior uterine wall than in the posterior wall, we defined it as anterior previa, if it was shorter in the anterior wall than the posterior wall, we defined it as posterior previa (9). 3) Signs of placental invasion. The MRI features indicating invasive placentation were low signal intensity bands on T2-weighted imaging that appear as areas with nodular or linear disposition, extending from the uterine-myometrial surface to placenta (Fig. 1a); a focally disrupted interface between myometrium and placenta at the site of placenta accreta, increta, or percreta (Fig. 1b); heterogeneous intraplacental signal intensity owing to repeated hemorrhage or lacunae (Fig. 1c); bulging of the lower segment of the uterus owing to the mass effect of placenta in the lower segment of uterus (Fig. 1d) (15); the placental cervical protrusion sign, which indicates placenta tissue protrusion into the cervical canal (Fig. 1e) (16); the tenting of the bladder and/or infiltration of the pelvic organ (Fig. 1f) (17).
Figure 1. a–f.
Schematic diagram for the assessed imaging features. Panel (a) shows low signal intensity bands of placenta on T2-weighted imaging (white arrows). Panel (b) shows focally interrupted myometrial interface on the anterior wall of uterus (white arrow); the myometrial/placental interface were intact on the posterior wall of uterus (white arrowhead). Panel (c) shows heterogeneous signal intensity of placenta located on anterior uterine wall (white arrow). Panel (d) shows the bulging of the lower segment of the uterus (white arrow). Panel (e) shows placenta extending into the cervix indicating the placental protrusion sign (white arrowhead) (e). Panel (f) shows placenta infiltration into the wall of the bladder indicating the percreta (white arrow); the myometrial/placental interface was disrupted on the anterior wall of the uterus (white arrowhead). P, placenta; B, bladder.
The images were independently and retrospectively reviewed by two radiologists (reader 1 and reader 2 with 10 and 7 years of experience in gynecology and obstetrics imaging, respectively), who were blinded to clinical and pathologic outcome. The imaging assessment results of the two radiologists were used to calculate interobserver agreement. In case of disagreement, a third senior radiologist with 20 years of experience in gynecology and obstetrics MRI was consulted. Reader 1 reviewed the images twice in one month for intraobserver agreement calculation.
Statistical analysis
Numeric data was averaged over all patients, reported as mean ± standard deviation (SD), and the Kolmogorov-Smirnov’s test was used to determine whether the quantitative parameters were normally distributed. Demographic data, including age, average gestational age, gravidity and parity, were compared between good outcome and poor outcome groups using unpaired t-test. The frequency distribution of qualitative MRI features between two groups was compared with chi-square test. Fisher exact test was performed if the sample size in the subgroup was too small. Multivariate logistic regression analysis was performed to determine the most significant risk factor that was predictive for poor outcome during UAE-assisted CS. Receiver operating characteristic curve analysis was used to determine the predictive value of the identified risk factor.
Interobserver agreement for the assessment of placental MRI between two readers was assed using Kappa analysis. Intraobserver agreement of reader 1 was assessed using Kappa analysis. The Kappa value ranged between 0 and 1.00, and values closer to 1.00 meant better reproducibility. They were interpreted as follows: (slight, 0.00–0.20; fair, 0.21–0.40; moderate, 0.41–0.60; substantial, 0.61–0.80; perfect, 0.81–1.00). Statistical analysis was performed using SPSS software (SPSS version 19.0, IBM Inc.). A two-sided P value less than 0.05 was considered statistically significant.
Results
Forty parturient women (average gestation age, 34 weeks) underwent prophylactic UAE-assisted CS, and no fetal or maternal mortality occurred. The average blood loss during CS was 1832 mL (range, 200–6500 mL). The poor outcome group contained 16 patients who had a significant hemorrhage of more than 1000 mL. Among these 16 patients, seven patients suffered an emergency hysterectomy due to refractory hemorrhage, including four patients who were diagnosed with placenta percreta preoperatively (Table 1). Meanwhile the good outcome group included 24 patients who had minor bleeding of less than 1000 mL, and the uterus was preserved in all 24 patients. The average blood loss of poor outcome group was significantly more than that of the good outcome group (3543 mL vs. 691 mL, P < 0.001). The maternal age, prior CS, dilation and curettage, prothrombin time, activated partial thromboplastin time, and international normalized ratio did not differ significantly between the two groups (P > 0.05). Detailed comparison of general obstetrical characteristics between the two groups is shown in Table 2.
Table 1.
The clinical and final pathologic diagnosis of patients who underwent hysterectomy
| Patient No. | Age (y) | Gravidity | Parity | Hemorrhage (mL) | Final diagnosis |
|---|---|---|---|---|---|
| 8 | 28 | 2 | 1 | 2500 | Increta and cervical involvement |
| 15 | 20 | 2 | 2 | 4000 | Percreta |
| 23 | 35 | 4 | 1 | 5000 | Increta |
| 26 | 22 | 2 | 1 | 6500 | Percreta and cervical involvement |
| 30 | 39 | 2 | 2 | 4000 | Increta |
| 37 | 35 | 3 | 2 | 6000 | Percreta and cervical involvement |
| 40 | 24 | 2 | 1 | 5000 | Percreta |
Table 2.
Clinical files of patients with placenta previa overlying the previous cesarean scar
| Demographic profile | Poor outcome (n=16) | Good outcome (n=24) | P |
|---|---|---|---|
| Age (years) | 32.54±4.03 (20–39) | 31.06±6 (24–40) | 0.356 |
| Gestational age (weeks±days) | 34±3 (32–37) | 34±1 (26–38) | 0.9770 |
| Gravidity | 1 (0–6) | 1 (0–4) | 0.741 |
| Parity | 0 (0–3) | 0 (0–2) | 0.322 |
| Prior cesarean section | 1 (1–2) | 1 (1–2) | 0.869 |
| Prior dilation and curettage | 1.92 (0–4) | 1.64 (0–5) | 0.60 |
| Hemorrhage (mL) | 3543±1656 (1000–6500) | 691±246 (80–800) | <0.001 |
| PT (s) | 11.07±1.47 (8.4–13.7) | 10.89±1.65 (9.1–14.8) | 0.728 |
| INR | 0.97±0.12 (0.78–1.52) | 0.89±0.17 (0.74–1.46) | 0.136 |
| APTT (s) | 23.52±4.59 (22.3–34.6) | 25.7±4.29 (20–32.8) | 0.162 |
Data are presented as mean ± standard deviation (range) or mean (range).
PT, prothrombin time; INR, international normalized ratio; APTT, activated partial thromboplastin time.
Perfect interobserver agreement was achieved during the evaluation of complete placenta previa and anterior placentation (k=0.818 and k=0.865, respectively), while substantial agreement was achieved in the evaluation of low signal intensity bands on T2-weighted imaging, heterogeneous intraplacental signal intensity, placental cervical protrusion sign, placenta percreta (k=0.624~0.767). Moderate agreement was achieved in the evaluation of focally interrupted myometrial border and bulging of the lower segment of uterus (k=0.578 and k=0.532, respectively). Detailed kappa values for interobserver agreement of MRI findings are shown in Table 3. The intraobserver agreement was prefect with a k value of 0.816 for reader 1.
Table 3.
Difference in MRI findings between patients with good and poor outcome
| MRI findings | Poor outcome | P | k | |
|---|---|---|---|---|
| Yes (n=16) | No (n=24) | |||
| Complete placenta previa | 14 | 17 | 0.2168 | 0.818 |
| Placenta on anterior wall | 13 | 20 | 1.000 | 0.865 |
| Low SI bands on T2WI | 13 | 4 | <0.001 | 0.748 |
| Focally interrupted myometrial border | 15 | 19 | 0.373 | 0.578 |
| Heterogeneous intraplacental SI | 14 | 13 | 0.063 | 0.674 |
| Bulging of the lower segment of uterus | 8 | 6 | 0.1040 | 0.532 |
| Placental cervical protrusion sign | 7 | 1 | 0.002 | 0.767 |
| The tenting of the bladder and/or infiltration of the pelvic organ | 4 | 0 | 0.011 | 0.624 |
SI, signal intensity; T2WI, T2-weighted imaging.
Low signal intensity bands on T2-weighted imaging (P < 0.001), the placental cervical protrusion sign (P = 0.002), the placenta percreta (P = 0.011) were more frequently observed in patients with poor outcome compared with those with good outcome (Table 3), while no significant difference was observed between the two groups on the following factors: complete placenta previa, placenta located on the anterior wall, presence of a focally interrupted myometrial border, heterogeneous intraplacental signal intensity, and bulging of the lower uterine segment (P > 0.05). Representative cases are shown in Figs. 2 and 3.
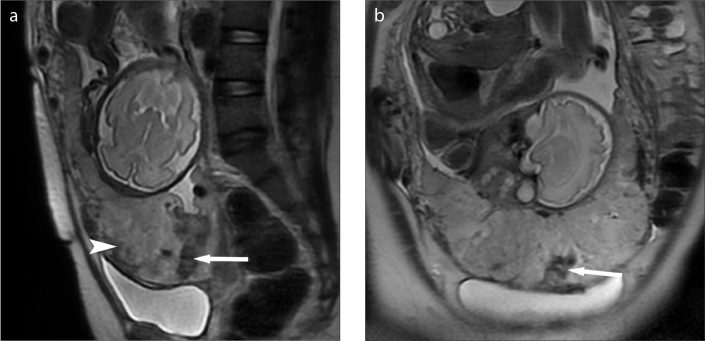
Figure 2. a, b.
Placenta previa associated with increta in a 35-year-old woman. Sagittal (a) and coronal (b) T2-weighted images showing the low signal intensity bands (white arrow) associated with heterogeneous signal intensity inside the placenta (white arrowhead), indicating high risk of significant hemorrhage during UAE-assisted CS. The patient had a significant hemorrhage of 5000 mL and experienced emergency hysterectomy due to refractory bleeding.
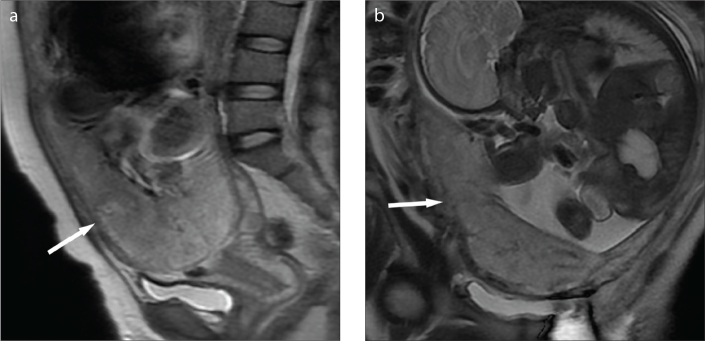
Figure 3. a, b.
A 20-year-old woman with complete placenta previa. Sagittal (a) and coronal (b) T2-weighted images showing the focally interrupted placenta/myometrial interface (arrow) in the lower anterior segment of the uterus without appearance of low signal intensity bands on T2-weighted imaging. The patient had minor hemorrhage of 800 mL, and thus uterus was preserved.
Multivariate logistic regression analysis indicated that low signal intensity bands on T2-weighted imaging was the only risk factor for a poor outcome after UAE-assisted CS (OR 14.79, P = 0.02), while placental cervical protrusion sign and placenta percreta were not significant risk factors (Table 4). Low signal intensity bands on T2-weighted imaging showed 81.3% sensitivity and 84.3% specificity in predicting a poor outcome.
Table 4.
Multiple logistic regression analysis results of potential predictors for poor outcome after UAE-assisted cesarean section
| Variable | P | Odds ratio (95% CI) |
|---|---|---|
| Low signal intensity bands on T2WI | 0.020 | 14.79 (1.03–185.84) |
| Placental cervical protrusion sign | 0.212 | 17.48 (0.21–1453.19) |
| The tenting of the bladder and/or infiltration of the pelvic organ | 0.999 | – |
UAE, uterine-artery embolization; CI, confidence interval; T2WI, T2-weighted imaging.
Discussion
Patients with invasive placenta previa are at high risk of massive hemorrhage during CS delivery (8–10). Previously, ultrasonography was the most frequently used imaging modality to identify abnormal placentation (7). There are several reports on sonographic evaluation for predicting the risk of massive bleeding in patients with invasive placenta previa (8–12). Hasegawa et al. (8) determined advanced maternal age (≥35 years old), previous CS, and sponge-like sonographic findings in the cervix as risk factors for massive bleeding during CS in cases of placenta previa. Baba et al. (9) reported anterior placenta position as a risk factor of massive hemorrhage during CS for placenta previa. Choi et al. (10) reported complete placenta previa as one of the risk factors for massive hemorrhage and peripartum hysterectomy. Recently, MRI has gained popularity in the evaluation of abnormal placentation. In the last two decades, UAE has become the first-line alternative treatment in controlling intractable peripartum hemorrhage, with a success rate of >90% (4–6, 20). This study focused on the preoperative evaluation of placental MRI features that can potentially predict high risk for significant hemorrhage associated with UAE-assisted CS in patients with invasive placenta previa. Our study demonstrated that the low signal intensity bands on T2-weighted imaging might be a high-risk factor that potentially correlates with poor maternal outcome after UAE-assisted CS in patients with invasive placenta previa. Low signal intensity bands on T2-weighted imaging is a promising predictor of greater blood loss or hysterectomy after UAE-assisted CS. The cervical protrusion sign and placenta percreta were potential risk factors for poor maternal outcome in univariate analysis; however, their significance was lost in the multivariate analysis. To the best of our knowledge, our study was the first to use the MRI features to predict the maternal outcome after UAE-assisted CS in patients with invasive placenta previa.
In the present study, we found the low signal intensity bands on T2-weighted imaging to be a high risk factor of significant intraoperative bleeding or hysterectomy during the UAE-assisted CS. Low signal intensity bands on T2-weighted imaging appear as areas with nodular or linear disposition, extending from the uterine-myometrial surface to placenta (16, 17). They represent abnormally thickened fibrous tissue or areas of fibrin deposition because of repetitive intraplacental hemorrhage. Alamo et al. (18) found that the presence of low signal intensity bands on T2-weighted imaging was the best single MRI feature indicating invasive placentation. These investigators also demonstrated higher sensitivity, specificity, and interobserver agreement compared with other MRI manifestations of placental invasion. In a review of MRI features of the gravid uterus, Azour et al. (21) indicated that the intraplacental thick T2 dark bands have been shown to be a significant feature of morbidly adherent placenta, with sensitivity and specificity of 87.9% and 71.9%, respectively; increasing volume of intraplacental dark bands on T2-weighted imaging has been shown to correspond to increasing degree of placental invasion. In a study by Bour et al. (22), the intraplacental thick T2 dark bands and thinning or focal defect of the uteroplacental interface were two MRI criteria that showed both moderate interobserver agreement and significant association with invasive placenta on univariate analysis. Placenta invasion has been reported as the main factor for significant hemorrhage in patients with placenta previa during the UAE-assisted CS (3, 4, 23, 24), which is consistent with our findings (P = 0.001).
Placental cervical protrusion sign was also regarded as a useful novel MRI finding for predicting placenta invasion by Ueno et al. (16), which is a known risk factor for the poor outcome of patients with placenta previa after CS. The potential reason for this might be related to the blood supply of the cervix. It is well known that not only the uterine artery but also internal pudendal arteries supply the cervix, which is different from the blood supply of the uterus (25). Therefore, just uterine artery embolization would not be sufficient to control the intractable bleeding from cervix-placenta adherent interface. Another possible reason for this is that the cervical stroma is mainly composed of connective tissue, resulting in weakness of contraction and intractable hemorrhage on the adherent surface of placenta accreta or increta. In our study, placental cervical protrusion sign was associated with poor maternal outcome in the univariate analysis. However, the significant association was lost during multivariate analysis. We speculate that this might be due to the limited number of patients. Further studies with larger sample sizes would be needed to clarify the value of cervical protrusion sign for predicting the poor outcome.
Placenta percreta is the most severe form of abnormal placental adherence, and the incidence is the lowest, accounting for only 7% of placenta invasion (26). The patient always experiences a significant hemorrhage during delivery when placenta removal is attempted. Highly aggressive and specialized surgical treatment is often required in patients with placenta percreta (27). Clausen et al. (28) reviewed 119 patients with placenta percreta, 66 of whom were managed by hysterectomy as the initial procedure. Yu et al. (3) mentioned that emergency hysterectomy was performed in two of four patients with placenta percreta due to massive hemorrhage in spite of the prophylactic uterine artery embolization. Grace Tan et al. (29) mentioned that in patients with placenta percreta, emergency hysterectomy must be considered if refractory hemorrhage is encountered. In our cohort of patients, we identified four patients with placenta percreta on MRI preoperatively, all of whom underwent hysterectomies in spite of the utility of prophylactic UAE procedure. In multivariate analysis, the sample size of patients with placenta percreta was too small to reach a statistically association with treatment outcome due to the rare incidence.
The treatment efficacy of prophylactic catheter placement is not uniform. Some authors advocate placement of arterial catheters before delivery because of the possibility of intractable hemorrhage (3–6); however, this is not a systematic procedure and only restricted to specific situations because of the risk of catheter displacement and because less than 50% of women with invasive placenta actually require transcatheter arterial embolization (30). In our department, the prophylactic UAE procedure was selectively performed on those patients who were suspected to experience significant hemorrhage due to invasive placenta previa. National guidelines do not recommended prophylactic balloon catheter placement because this approach conveys a risk of permanent sciatic nerve ischemia, arterial dissection, arterial rupture, and inferior leg ischemia (31). Should the prophylactic balloon catheter be used, it should be placed in the common iliac arteries instead of internal iliac arteries (32).
Previous studies revealed anterior placentation and complete placenta previa as independent risk factors for massive hemorrhage during CS (9, 10, 26); however, in the present study, they were not associated with significant hemorrhage possibly due to the added value of occlusion of the blood supply to the uterus and placenta by the UAE procedure.
In addition to its retrospective nature, there are some limitations to our study. First, the small number of the enrolled patients limited the power of statistical analysis. Given the rare incidence of patients with placenta percreta, it is difficult to gather large numbers of patients in short term. In our opinion, our study could serve as a preliminary finding for further studies with larger sample sizes. Second, our study focused only on the MRI findings of placenta abnormalities; MRI manifestations were not compared with pathologic findings to determine whether there is an underlying pathologic foundation for the low signal intensity bands on T2-weighted imaging.
In conclusion, our study demonstrates that the low signal intensity bands on T2-weighted imaging might reflect a high risk of significant hemorrhage after UAE-assisted CS in patients with invasive placenta previa. When this finding is identified preoperatively on MRI, effective management strategies should be prepared for potential significant intraoperative bleeding or hysterectomy.
Main points.
MRI was useful in the presurgical evaluation of abnormal placentation in cases with invasive placenta.
Low signal intensity bands on T2-weighted imaging was a significant predictor of poor maternal outcome in patients with invasive placenta.
Placental cervical protrusion sign and placenta percreta sign were more frequently observed in patients with poor outcome.
Footnotes
Conflict of interest disclosure
The authors declare no conflicts of interest.
References
- 1.Higgins MF, Monteith C, Foley M, O’Herlihy C. Real increasing incidence of hysterectomy for placenta accreta following previous caesarean section. Eur J Obstet Gynecol Reprod Biol. 2013;171:54–56. doi: 10.1016/j.ejogrb.2013.08.030. https://doi.org/10.1016/j.ejogrb.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 2.Chattopadhya SK, Kharihl-I, Sherbeeni MM. Placenta praevia and accreta after previous caesarean section. Euro J Obstet Gynecol Reprod Biol. 1993;53:151–156. doi: 10.1016/0028-2243(93)90064-j. https://doi.org/10.1016/0028-2243(93)90064-J. [DOI] [PubMed] [Google Scholar]
- 3.Yu PC, Ou HY, Tsang LL, Kung FT, Hsu TY, Cheng YF. Prophylactic intraoperative uterine artery embolization to control hemorrhage in abnormal placentation during late gestation. Fertil Steril. 2009;91:1951–1955. doi: 10.1016/j.fertnstert.2008.02.170. https://doi.org/10.1016/j.fertnstert.2008.02.170. [DOI] [PubMed] [Google Scholar]
- 4.Li Q, Yang ZQ, Mohammed W, Feng YL, Shi HB, Zhou X. Prophylactic uterine artery embolization assisted cesarean section for the prevention of intrapartum hemorrhage in high-risk patients. Cardiovasc Intervent Radiol. 2014;37:1458–1463. doi: 10.1007/s00270-014-0855-8. https://doi.org/10.1007/s00270-014-0855-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shrivastava V, Nageotte M, Major C, Haydon M, Wing D. Case-control comparison of caesarean hysterectomy with and without prophylactic placement of intravascular balloon catheters for placenta accreta. Am J Obstet Gynecol. 2007;197:401–402. doi: 10.1016/j.ajog.2007.08.001. https://doi.org/10.1016/j.ajog.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Ferrazzani S, Iadarola R, Perrelle A, et al. Use of an intrauterine inflated catheter balloon in massive post-partum hemorrhage: A series of 52 cases. J Obstet Gynaecol Res. 2014;40:1603–1610. doi: 10.1111/jog.12404. https://doi.org/10.1111/jog.12404. [DOI] [PubMed] [Google Scholar]
- 7.D’Antonio F, Iacovella C, Bhide A. Prenatal identification of invasive placentation using ultrasound: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2013;42:509–517. doi: 10.1002/uog.13194. https://doi.org/10.1002/uog.13194. [DOI] [PubMed] [Google Scholar]
- 8.Hasegawa J, Matsuoka R, Ichizuka K, et al. Predisposing factors for massive hemorrhage during Cesarean section in patients with placenta previa. Ultrasound Obstet Gynecol. 2009;34:80–84. doi: 10.1002/uog.6426. https://doi.org/10.1002/uog.6426. [DOI] [PubMed] [Google Scholar]
- 9.Baba Y, Matsubara S, Ohkuchi A, et al. Anterior placentation as a risk factor for massive hemorrhage during cesarean section in patients with placenta previa. J Obstet Gynaecol Res. 2014;40:1243–1248. doi: 10.1111/jog.12340. https://doi.org/10.1111/jog.12340. [DOI] [PubMed] [Google Scholar]
- 10.Choi SJ, Song SE, Jung KL, Oh SY, Kim JH, Roh CR. Antepartum risk factors associated with peripartum casarean hysterectomy in women with placenta previa. Am J Perinatol. 2008;25:37–41. doi: 10.1055/s-2007-1004834. https://doi.org/10.1055/s-2007-1004834. [DOI] [PubMed] [Google Scholar]
- 11.Saitoh M, Ishihara K, Sekiya T, Araki T. Anticipation of uterine bleeding in placenta previa based on vaginal sonographic evaluation. Gynecol Obstet Invest. 2002;54:37–42. doi: 10.1159/000064695. https://doi.org/10.1159/000064695. [DOI] [PubMed] [Google Scholar]
- 12.Jang DG, We JS, Shin JU, et al. Maternal outcomes according to placental position in placental previa. Int J Med Sci. 2011;8:439–444. doi: 10.7150/ijms.8.439. https://doi.org/10.7150/ijms.8.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Antonio F, Iacovella C, Palacios-Jaraquemada J, Bruno CH, Manzoli L, Bhide A. Prenatal identification of invasive placentation using magnetic resonance imaging (MRI): a systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2014;44:8–16. doi: 10.1002/uog.13327. https://doi.org/10.1002/uog.13327. [DOI] [PubMed] [Google Scholar]
- 14.Sivrioğlu AK, Ozcan UA, Türk A, et al. Evaluation of the placenta with relative apparent diffusion coefficient and T2 signal intensity analysis. Diagn Interv Radiol. 2013;19:495–500. doi: 10.5152/dir.2013.13106. https://doi.org/10.5152/dir.2013.13106. [DOI] [PubMed] [Google Scholar]
- 15.Allen BC, Levendecker JR. Placental evaluation with magnetic resonance. Radiol Clin North Am. 2013;51:955–966. doi: 10.1016/j.rcl.2013.07.009. https://doi.org/10.1016/j.rcl.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 16.Ueno Y, Kitajima K, Kawakami F, et al. Novel MRI finding for diagnosis of invasive placenta praevia: evaluation of findings for 65 patients using clinical and histopathological correlations. Eur Radiol. 2014;24:881–888. doi: 10.1007/s00330-013-3076-7. https://doi.org/10.1007/s00330-013-3076-7. [DOI] [PubMed] [Google Scholar]
- 17.Teo TH, Law YM, Tay KH, Tan BS, Cheah FK. Use of magnetic resonance imaging in evaluation of placental invasion. Clin Radiol. 2009;64:511–516. doi: 10.1016/j.crad.2009.02.003. https://doi.org/10.1016/j.crad.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Alamo L, Anaye A, Rey J, et al. Detection of suspected placental invasion by MRI: do the results depend on observer’ experience. Eur J Radiol. 2013;82:51–57. doi: 10.1016/j.ejrad.2012.08.022. https://doi.org/10.1016/j.ejrad.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 19.Wise A, Clark V. Challenges of major obstetric hemorrhage. Best Pract Res Obstet Gynaecol. 2010;24:353–365. doi: 10.1016/j.bpobgyn.2009.11.011. https://doi.org/10.1016/j.bpobgyn.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 20.Gonsalves M, Belli A. The role of interventional radiology in obstetric haemorrhage. Cardiovasc Intervent Radiol. 2010;33:887–895. doi: 10.1007/s00270-010-9864-4. https://doi.org/10.1007/s00270-010-9864-4. [DOI] [PubMed] [Google Scholar]
- 21.Azour L, Besa C, Lewis S, Kamath A, Oliver ER, Taouli B. The gravid uterus: MR imaging and reporting of abdominal placentation. Abdom Radiol. 2016;41:2411–2423. doi: 10.1007/s00261-016-0752-5. https://doi.org/10.1007/s00261-016-0752-5. [DOI] [PubMed] [Google Scholar]
- 22.Bour L, Placé V, Bendavid S, et al. Suspected invasive placenta: evaluation with magnetic resonance imaging. Eur Radiol. 2014;24:3150–3160. doi: 10.1007/s00330-014-3354-z. https://doi.org/10.1007/s00330-014-3354-z. [DOI] [PubMed] [Google Scholar]
- 23.Maassen MS, Lambers MD, Tutein Nolthenius RP, Van der Valk PH, Elgersma OE. Complications and failure of uterine artery embolisation for intractable postpartum haemorrhage. BJOG. 2009;116:55–61. doi: 10.1111/j.1471-0528.2008.01939.x. https://doi.org/10.1111/j.1471-0528.2008.01939.x. [DOI] [PubMed] [Google Scholar]
- 24.Descargues G, Douvrin F, Degré S, Lemoine JP, Marpeau L, Clavier E. Abnormal placentation and selective embolization of the uterine arteries. Eur J Obstet Gynecol Reprod Biol. 2001;99:47–52. doi: 10.1016/s0301-2115(01)00355-4. https://doi.org/10.1016/S0301-2115(01)00355-4. [DOI] [PubMed] [Google Scholar]
- 25.Soyer P, Morel O, Farqeaudou Y, et al. Value of pelvic embolization in the management of severe postpartum hemorrhage due to placenta accreta, increta or percreta. Eur J Radiol. 2011;80:729–735. doi: 10.1016/j.ejrad.2010.07.018. https://doi.org/10.1016/j.ejrad.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 26.Miller DA, Chollet JA, Goodwin TM. Clinical risk factors for placenta previa-placenta accreta. Am J Obstet Gynecol. 1997;177:210–214. doi: 10.1016/s0002-9378(97)70463-0. https://doi.org/10.1016/S0002-9378(97)70463-0. [DOI] [PubMed] [Google Scholar]
- 27.Usta IM, Hobeika EM, Musa AA, Gabriel GE, Nassar AH. Placenta previa-accreta: risk factors and complications. Am J Obstet Gynecol. 2005;193:1045–1049. doi: 10.1016/j.ajog.2005.06.037. https://doi.org/10.1016/j.ajog.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 28.Clausen C, Lönn L, Langhoff-Roos J. Management of placenta percreta: a review of published cases. Acta Obstet Gynecol Scand. 2014;93:138–143. doi: 10.1111/aogs.12295. https://doi.org/10.1111/aogs.12295. [DOI] [PubMed] [Google Scholar]
- 29.Grace Tan SE, Jobling TW, Wallace EM, McNeilage LJ, Manolitsas T, Hodges RJ. Surgical management of placenta accreta: a 10-year experience. Acta Obstet Gynecol Scand. 2013;92:445–450. doi: 10.1111/aogs.12075. https://doi.org/10.1111/aogs.12075. [DOI] [PubMed] [Google Scholar]
- 30.Soyer P, Dohan A, Dautry R, et al. Transcatheter arterial embolization for postpartum hemorrhage: indications, technique, results, and complications. Cardiovasc Intervent Radiol. 2015;38:1068–1081. doi: 10.1007/s00270-015-1054-y. https://doi.org/10.1007/s00270-015-1054-y. [DOI] [PubMed] [Google Scholar]
- 31.Teare J, Evans E, Belli A, Wendler R. Sciatic never ischemia after iliac artery occlusion balloon catheter placement for placenta percreta. Int J Obstet Anesth. 2014;23:178–181. doi: 10.1016/j.ijoa.2013.11.002. https://doi.org/10.1016/j.ijoa.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 32.Clausen C, Stensballe J, Albrechtsen CK, Hansen MA, Lönn L, Langhoff-Roos J. Balloon occlusion of the internal iliac arteries in the multidisciplinary management of placenta percreta. Acta Obstet Gynecol Scand. 2013;92:386–391. doi: 10.1111/j.1600-0412.2012.01451.x. https://doi.org/10.1111/j.1600-0412.2012.01451.x. [DOI] [PubMed] [Google Scholar]