Abstract
PURPOSE
We aimed to evaluate the safety and effectiveness of fluoroscopy-guided gastroduodenal metallic stent placement with different approaches in malignant obstruction.
METHODS
We retrospectively assessed 53 patients (33 men and 20 women; mean age, 58.7±15 years) who underwent stent placement between February 2004 and April 2014. All patients had unresectable tumors. The most common causes of obstruction were gastric (38%) and pancreatic cancers (36%). Uncovered self-expandable metallic stents (SEMS) were placed under fluoroscopic guidance. In addition to transoral approach in 46 patients (86.7%), transgastric and transhepatic approaches were used in six patients (11.3%) and one patient (1.8%), respectively. Gastric outlet obstruction scoring system (GOOSS) was used to evaluate oral intake before and after stenting. Patients were followed until death or the end of the study.
RESULTS
Technical and clinical success rates were 100% and 92%, respectively. The median stent patency was 76 days (range, 4–985 days). Mean preprocedural GOOSS score of 0.1 increased to postprocedural GOOSS score of 2.42 (P < 0.001). Afferent loop decompression was achieved in one symptomatic patient. Neither mortality nor major complications occurred due to stenting. Stent migration occurred in one patient (2%) and stent obstruction occurred in two patients (4%). Combined biliary and duodenal stenting were performed in 21 patients (40%). Post-stenting GOOSS scores were predictive of survival (P = 0.003).
CONCLUSION
Fluoroscopic metallic stent placement for palliation of malignant gastroduodenal obstruction is safe and effective with high technical and clinical success rates and minimal complications. High technical success rates can be achieved using different approaches.
Malignant gastroduodenal obstruction (GDO) is a common and debilitating complication of advanced gastric, duodenal, and pancreatobiliary cancers. It can also be seen due to lymphoma and metastatic spread of other malignancies (1, 2). Patients classically present with abdominal pain, nausea, and vomiting with resulting malnutrition and weight loss (1–3). The majority of patients have a median survival of only 3–6 months (4, 5).
Curative surgery is often not possible and palliative surgical procedures might have high complication rates with delayed postoperative gastric emptying and prolonged hospitalization (1, 5–8).
Fluoroscopic or endoscopic placement of covered and uncovered metallic stents has been commonly performed in the palliation of malignant GDO as an alternative to gastrojejunostomy (GJ) with high technical and clinical success and low complication rates (5). However, recurrent obstruction due to tumor ingrowth and stent migration is a drawback of uncovered and covered metallic stents.
The purpose of this study is to evaluate safety and effectiveness of fluoroscopy-guided gastroduodenal metallic stent placement using different approaches such as transoral, transgastric, and transhepatic in 53 patients with malignant obstruction. Patients who underwent combined biliary and duodenal stenting were also assessed.
Methods
Patients
We retrospectively studied 53 patients (33 men, 20 women) with unresectable malignant GDO who underwent uncovered self-expandable metallic stent (SEMS) placement in our department between February 2004 and April 2014. The characteristics of patient population are shown in the Table. All but one patient had unresectable malignancy due to metastasis and/or peritoneal carcinomatosis (79%) or locally advanced disease (19%). One patient (2%) with gastric cancer was accepted as inoperable due to low cardiac function. Thirty-eight patients (72%) received at least one chemotherapy protocol during disease period; 15 patients (28%) received chemotherapy after stent placement. Written informed consent was obtained from each patient prior to the procedure. Our study was approved by the local institutional review board (HEK 09/54–60). The gastric outlet obstruction score system (GOOSS; 0: no oral intake; 1: liquids; 2: soft solids; 3: full diet), was used for assessing oral intake before and after stenting.
Table.
Characteristics of the patient population
| Characteristic | Value |
|---|---|
| Age (years), mean±SD (range) | 58.7±15.07 (7–87) |
|
| |
| Sex, M/F, n (%) | 33/20 (62/38) |
|
| |
| Site of obstruction, n (%) | |
| Gastroduodenal | 18 (34) |
| Duodenal | 27 (51) |
| Gastrojejunal anastomosis | 6 (11) |
| Duodenojejunal | 1 (2) |
| Afferent loop | 1 (2) |
|
| |
| Cause of obstruction, n (%) | |
| Gastric cancer | 20 (38) |
| Pancreatic cancer | 19 (36) |
| Cholangiocarcinoma | 4 (7) |
| Duodenal cancer | 3 (6) |
| Periampullary tumor | 1 (2) |
| Metastasis | 5 (9) |
| External compression (neuroblastoma) | 1 (2) |
SD, standard deviation; F, female; M, male.
The patients were followed for a mean of 112.6±152 days from the procedure, until death or October 2014, which was the end-point of our study. Unfortunately two patients were lost to follow-up after stenting. Information was collected regarding patient demographics, diagnosis, number and type of stents deployed, complications, pre- and postprocedural oral intake, history of biliary stent placement, and survival time. Data were obtained from the medical records and phone calls.
Stent placement
Preprocedural computed tomography was performed to assess the site of obstruction and the presence of additional obstructions. Prior to the procedure, decompression of the stomach was obtained by nasogastric tube insertion to prevent aspiration and to facilitate access to stenosis. Uncovered SEMS 20–25 mm in diameter and 6–12 cm in length (Wallstent and Wallflex, Boston Scientific; Niti-S, Taewoong Medical) were placed using transoral approach in 46 patients (86.7%), transgastric approach in six patients (11.3%), and transhepatic approach in one patient (1.8%). One day after stenting, position and expansion of the stent were assessed by radiography. In case of no abdominal symptoms, oral intake was started with liquids, followed by semisolids and solids.
Transoral approach
Xylocaine spray was used for topical pharyngeal anesthesia. A 5 F angiographic catheter (Glidecath, Terumo) with a 0.035-inch hydrophilic guidewire (Radiofocus Guidewire, Terumo) was advanced fluoroscopically. Contrast material was injected to depict the stenotic segment. The stricture was traversed with hydrophilic guidewire and catheter manipulations. Hydrophilic guidewire was exchanged with an exchange length 0.035-inch guidewire (Amplatz Superstiff 260 cm or Back-up Meier 300 cm, Boston Scientific). In case of difficulty with crossing the obstruction or advancing the stent, a vascular sheath (Super Arrow-Flex, 10 F, 80 cm, Arrow) was used to prevent buckling of guidewire or stent in stomach. Without predilatation, the stent delivery system was inserted over the guidewire and deployed across the stricture or obstruction.
Transgastric approach
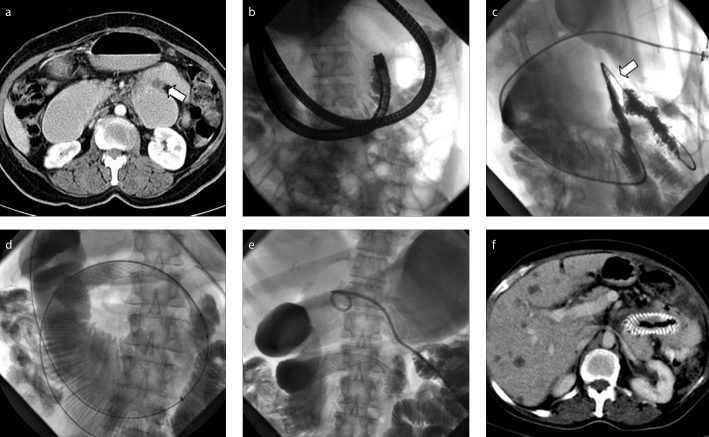
In six patients, obstructed segment could not be traversed transorally with fluoroscopic and/or endoscopic approach, and stenting was performed using percutaneous transgastric approach (Fig. 1). Under intravenous sedation, a nasogastric tube was passed to inflate the stomach with air. After local anesthetic administration and gastropexy, gastric body was punctured under fluoroscopic guidance. With the guidewire and catheter manipulations, metallic stent was deployed across the obstructed segment. Percutaneous gastrostomy catheters (10 F) were left for two weeks for tract maturation after procedure.
Figure 1. a–f.
A 59-year-old patient with metastatic duodenal cancer. Preprocedural axial abdominal computed tomography (CT) (a) shows malignant stricture at the distal duodenum (arrow). Fluoroscopic image (b) demonstrates failure of endoscopic and fluoroscopic transoral route to traverse the stricture. Fluoroscopic images (c–e) obtained during transgastric metallic stenting show traversing malignant stricture (arrow) with a catheter (c) and deployment of metallic stent over an exchanged wire (d). A gastrostomy catheter was left at the end of the procedure (e). Axial abdominal CT (f) performed 35 weeks after procedure shows patent stent without GDO.
Transhepatic approach
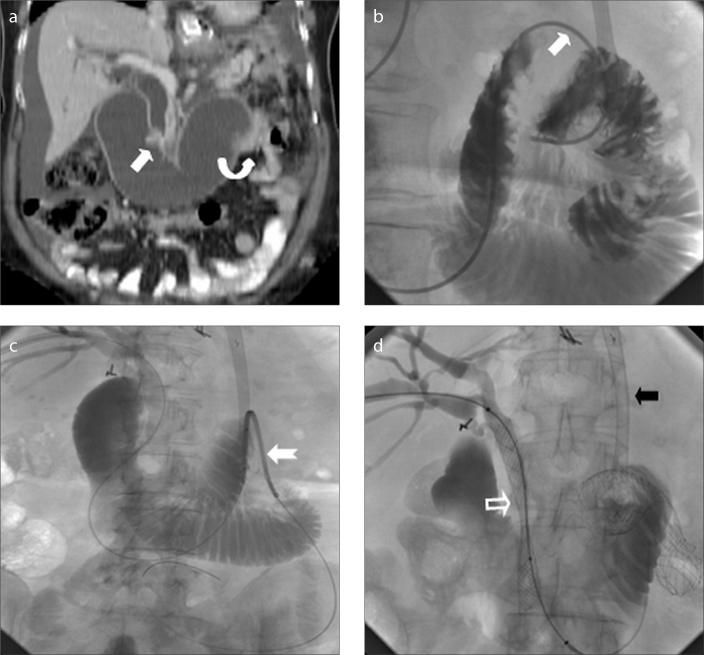
Afferent loop obstruction in a patient with recurrent gastric cancer after total gastrectomy and Roux-en-Y esophagojejunostomy was treated with stent placement using transhepatic route (Fig. 2). Under fluoroscopic guidance, through the previously placed external biliary drainage catheter, obstruction at papilla was traversed and contents in the afferent loop were drained with drainage catheter. After drainage of the dilated loop, a metallic stent was placed at the obstructed distal segment of the afferent loop. Then, a metallic biliary stent was deployed over the same guidewire for distal bile duct obstruction.
Figure 2. a–d.
A 53-year-old female with gastrectomy and Roux-en-Y anastomosis. Coronal reformatted abdominopelvic CT image (a) demonstrates biliary and afferent loop dilatation due to obstructive recurrent tumors at ampulla (arrow) and distal end of afferent loop (curved arrow). Fluoroscopic images (b–d) obtained during transhepatic metallic stenting show traversing obstructed afferent loop segment (arrow) with a catheter, placement of metallic stent (notched arrow) over an exchange wire and metallic biliary stenting (hollow arrow), respectively. Note previously placed esophagojejunal metallic stent (black arrow).
Combined metallic biliary and duodenal stenting
Twenty-one patients underwent both biliary and duodenal stenting. Biliary stenting was performed percutaneously under fluoroscopic guidance. Metallic biliary stents (Biliary Wallstent, Boston Scientific) of 10 mm diameter and 7–9 cm length were used.
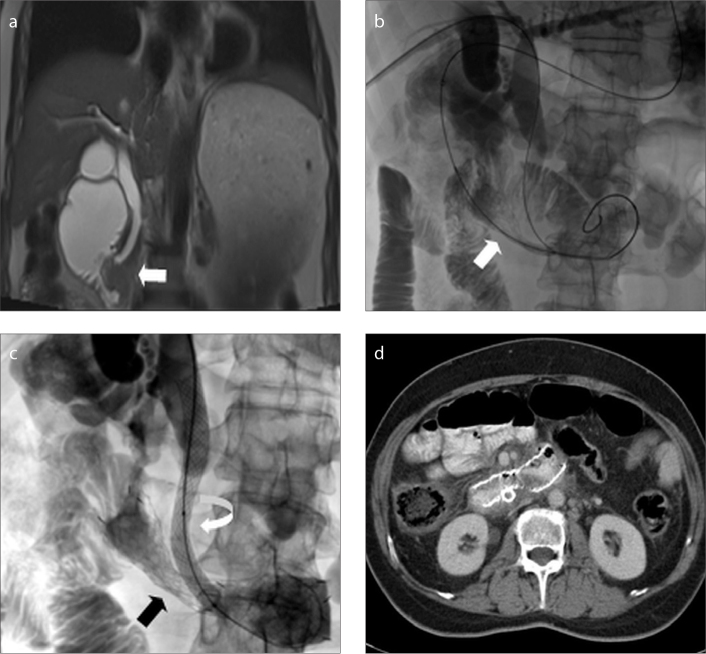
Biliary and duodenal stents were placed simultaneously in seven patients (Fig. 3), whereas duodenal stents were placed after biliary stenting in 14 patients. A patient who developed biliary and gastric obstruction symptoms after whipple surgery was treated with stenting of afferent and efferent loop obstructions 26 days after biliary stenting. Stenting of afferent loop was performed using transgastric approach, whereas efferent loop was stented transorally.
Figure 3. a–d.
A 55-year-old patient with pancreatic cancer. Coronal magnetic resonance imaging (a) shows an infiltrative mass causing both biliary and duodenal obstruction (arrow). Fluoroscopic images (b–c) obtained during simultaneous biliary and duodenal stenting demonstrate transoral placement of duodenal stent (arrow) and transhepatic placement of metallic biliary stent (curved arrow), respectively. Expanded duodenal stent is also shown (black arrow). Axial CT image (d) obtained 56 weeks after stenting shows patent stent at duodenum.
Statistical analysis
Statistical analysis was performed using STATA/MP (StataCorp® LP). Descriptive statistics were demonstrated as mean ± standard deviation or median (minimum–maximum) for enumerable variables, and nominal variables were shown as the number of cases and percentage. The Wilcoxon signed-rank test was performed to evaluate improvement in the GOOSS scores after stenting. Two-tailed P values of <0.05 were considered significant. Survival time was defined as the time from the date of stent insertion until death or the end of the study. Survival curves were drawn using Kaplan-Meier analysis and compared with the log-rank test.
Results
Technical success was 100%. Clinical success was defined as improvement of symptoms and oral intake after the procedure. Mean preprocedural GOOSS score of 0.1 increased to mean postprocedural GOOSS score of 2.42 and median preprocedural GOOSS score of 0 (0–1) increased to median postprocedural GOOSS score of 3 (0–3) (P < 0.001). After metallic stenting, 32 patients (61.5%) could resume solid, 14 patients (26.9%) could resume semisolid, and two patients (3.8%) could resume fluid foods. Oral intake of four patients did not improve. The symptoms of a patient with afferent loop obstruction improved. Our clinical success rate was 92%.
In three patients with no improvement of oral intake, stent patency was shown by fluoroscopic study. Lack of clinical improvement in these patients could be explained by failing of peristalsis due to peritoneal carcinomatosis and poor general condition. In another patient with no improvement of oral intake after stenting revealed unsatisfactory expansion of the stent due to external compression of the pancreatic cancer.
A patient with post-stenting increase of GOOSS score from 0 to 2 lost the ability of food ingestion four weeks after the procedure. Stent patency was shown by endoscopic study. Inability of oral intake in this patient with pancreas and ovarian cancer was considered to be due to gastroparesis and peritoneal carcinomatosis.
Balloon dilatation was performed in one patient due to inadequate expansion of the stent five days after deployment. Patient had improvement of oral intake with post-dilatation GOOSS score of 2.
Mortality and complications like bleeding or ulceration due to stenting did not occur. Stent migration was detected one week after stent placement in one patient and the symptoms of obstruction improved after second stent placement. The median stent patency was 76 days (4–985 days). Stent obstruction due to tumor ingrowth occurred in two patients, 13 and 20 weeks after stent placement. Restenting was not considered for these patients because of poor general condition; patients died shortly (3 and 7 days) after detection of reobstruction.
Twenty-one patients with both gastroduodenal and biliary obstruction were treated with combined stenting. Our technical success in combined stenting was %100.
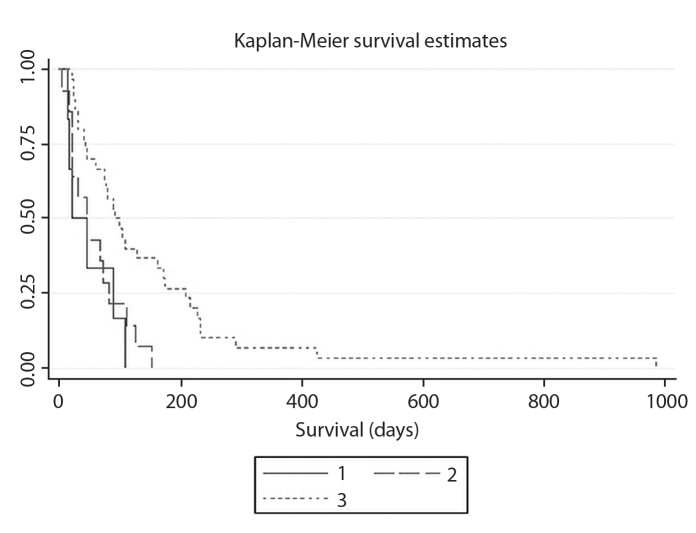
Mean survival time following stent insertion was 112.6 days (95% confidence interval [CI], 69.4–115.8) days. Post-stenting GOOSS scores were predictive of survival (P = 0.003). Mean survival was 49 days (95% CI, 5.6–92.3 days) for patients with GOOSS score of 0–1, 58.1 days (95% CI, 31.8–84.4 days) for those with GOOSS score of 2, 150.8 days (95% CI, 81.8–219.7 days) for those with GOOSS score of 3. Kaplan-Meier survival curves for the patients with different GOOSS scores is shown in Fig. 4.
Figure 4.
Kaplan-Meier survival cure for the patients with different gastric outlet obstruction score system (GOOSS) scores. Solid line (1) represents patients with GOOSS scores of 0-1, long dash line (2) represents patients with GOOSS score of 2, short dash line (3) represents patients with GOOSS score of 3.
Discussion
Our single center study confirmed the safety and effectiveness of fluoroscopic metallic stent placement in palliation of malignant gastroduodenal obstruction. Technical (100%) and clinical success (92%) rates are consistent with previously reported rates of endoscopic and fluoroscopic stenting. Technical success rates ranging from 92% to 100% and clinical success rates ranging from 76% to 94% have been previously reported (4, 9–12). Stenting with different approaches including transoral, transgastric, and transhepatic was also proven to be safe with no mortality or major complications. Migration occurred only in one patient (2%) and reobstruction due to tumor ingrowth occurred only in two patients (4%). Our reintervention rate was 4% with one balloon dilation and one restenting.
Malignant GDO develops in up to 20% of patients in advanced carcinoma of pancreas, stomach and the duodenum (1, 13). It severely affects quality of life with intractable vomiting and inability to eat. A minimally invasive method to palliate symptoms and restore oral intake is crucial in these patients with limited life expectancy. Surgical palliation of GDO is associated with high mortality (up to 7%) and morbidity rates (10%–16%) even with the recent advances in surgical techniques (5). Metallic stent placement has been increasingly used as a minimally invasive method with lower mortality and morbidity rates than surgical GJ (3, 7, 8, 12, 14, 15). It has high technical success rates similar to surgical palliation (8). It also results in earlier oral intake and shorter hospital stay than surgical GJ with lower costs (6, 7, 14, 15). Nevertheless, the need for reintervention seems to be more common after stent placement due to recurrent obstructive symptoms because of tumor ingrowth and stent migration (7, 8).
Gastroduodenal stents may be placed either endoscopically with fluoroscopic assistance or by fluoroscopy alone. Similar technical and clinical results have been reported with both techniques (3, 4, 11, 12, 16). Endoscopy supports wire-delivery system and prevents buckling due to redundant stomach or tortuous anatomy (11, 17). However, fluoroscopic difficulty of manipulating wire-delivery system can be overcome by advancing a sheath until the obstructed segment or using a transgastric approach (16, 17). In this study, transgastric approach was used in six patients in whom transoral approach has failed. Duodenal stent was placed using transhepatic approach in one patient with roux-en-Y operation. The use of percutaneous techniques including transgastric and transhepatic approaches as alternative to the transoral approach has contributed to our high technical success rate.
Dysfunction of uncovered SEMS after successful placement mainly occurs because of tumor growth through the stent mesh (11, 18, 19). Covered stents reduce the risk of tumor ingrowth; however, they have higher risks of stent migration (17, 18, 20). Recent studies comparing uncovered and covered SEMS in malignant GDO showed that the rates of reintervention due to stent dysfunction were similar between two stent groups (18, 21, 22). In case of stent dysfunction, additional coaxial stenting has been shown to enable oral intake (9, 10). High technical and clinical success rates of additional coaxial stenting have been reported as 100% and 95%, respectively (9, 10).
Large series involving fluoroscopically placed uncovered SEMS are limited. Bessoud et al. (4) inserted 108 uncovered SEMS in 72 patients under fluoroscopic guidance. They reported 97% technical and 90% clinical success. Their mortality and complication rates were 1% and 17%, respectively. Stent migration occurred in eight patients (11%) and restenosis due to tumor over- and ingrowth occurred in seven patients (10%). Their mean stent patency was 113 days (4–513 days). Our technical and clinical success rates and mean duration of stent patency were similar; however, unlike their study we did not experience mortality or complications like bleeding, ulceration, or perforation.
Miller et al. (3) compared the outcome of transoral and transgastric gastroduodenal stenting in 100 patients. They used the transoral route in 66 patients with more proximal obstructions and the transgastric route in 44 patients with duodenal obstructions. Technical rates for transoral and transgastric stenting were 83.3% and 90.9%, respectively. Although they immediately removed the gastrostomy tube after transgastric stent placement, they did not detect any complications related to peritoneal contamination or leakages probably due to complete aspiration of air and gastric fluid before gastrostomy tube removal. They also found that patients with successful stenting and return to soft or normal diet had significantly better survival, which is consistent with our results.
Concomitant obstruction is seen particularly in patients with pancreatic cancer, which causes biliary obstruction in 70%–90% and duodenal obstruction in 15%–20% of patients (16). Combined metallic stenting has been reported as a safe and effective method for palliation of both biliary and gastroduodenal obstruction (16, 23–25). We previously reported our initial experience in nine patients with combined stenting (16). Combined stenting can be performed at the same session or at different sessions under fluoroscopic or endoscopic guidance. However, because of difficulty in endoscopic access of the papilla, percutaneous approach might be necessary (26). Balloon dilation may be required to pass the endoscope through duodenal stricture and postdilation bleeding can complicate papillary cannulation (26). We performed combined gastroduodenal and biliary stenting under fluoroscopic guidance in 21 patients. Seven of these patients underwent simultaneous stenting. Our technical success in combined stenting was 100%.
This study has some limitations. First, it has a nonrandomized retrospective design. The second limitation is the relatively small number of patients. On the other hand, gastroduodenal stenting using different approaches and combined biliary and duodenal stenting are the advantages of this study.
In conclusion, our study shows the safety and effectiveness of fluoroscopy-guided gastroduodenal and combined biliary metallic stent placement with different approaches such as transoral, transgastric, and transhepatic in patients with symptomatic malignant obstruction.
Main points.
Fluoroscopic placement of metallic stents is a good alternative to gastrojejunostomy in the palliation of malignant gastroduodenal obstruction with high technical and clinical success and low complication rates.
We achieved a technical success rate of 100% using transgastric and transhepatic approaches in addition to oral route.
Our clinical success rate was 92% with mean GOOSS score increasing from 0.1 preprocedure to 2.42 postprocedure (P < 0.001).
Post-stenting GOOSS scores were predictive of survival (P = 0.003).
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Lopera JE, Brazzini A, Gonzales A, et al. Gastroduodenal stent placement: current status. Radiographics. 2004;24:1561–1573. doi: 10.1148/rg.246045033. https://doi.org/10.1148/rg.246045033. [DOI] [PubMed] [Google Scholar]
- 2.Mauro MA, Koehler RE, Baron TH. Advances in gastrointestinal intervention: the treatment of gastroduodenal and colorectal obstructions with metallic stents. Radiology. 2000;215:659–669. doi: 10.1148/radiology.215.3.r00jn30659. https://doi.org/10.1148/radiology.215.3.r00jn30659. [DOI] [PubMed] [Google Scholar]
- 3.Miller BH, Griffiths EA, Pursnani KG, et al. An assessment of radiologically inserted transoral and transgastric gastroduodenal stents to treat malignant gastric outlet obstruction. Cardiovasc Intervent Radiol. 2013;36:1591–1601. doi: 10.1007/s00270-013-0584-4. https://doi.org/10.1007/s00270-013-0584-4. [DOI] [PubMed] [Google Scholar]
- 4.Bessoud B, de Baere T, Denys A, et al. Malignant gastroduodenal obstruction: palliation with self-expanding metallic stents. J Vasc Interv Radiol. 2005;16:247–253. doi: 10.1097/01.RVI.0000145227.90754.76. https://doi.org/10.1097/01.RVI.0000145227.90754.76. [DOI] [PubMed] [Google Scholar]
- 5.Nagaraja V, Eslick GD, Cox MR. Endoscopic stenting versus operative gastrojejunostomy for malignant gastric outlet obstruction-a systematic review and meta-analysis of randomized and non-randomized trials. J Gastrointest Oncol. 2014;5:92–98. doi: 10.3978/j.issn.2078-6891.2014.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maetani I, Tada T, Ukita T, et al. Comparison of duodenal stent placement with surgical gastrojejunostomy for palliation in patients with duodenal obstructions caused by pancreaticobiliary malignancies. Endoscopy. 2004;36:73–78. doi: 10.1055/s-2004-814123. https://doi.org/10.1055/s-2004-814123. [DOI] [PubMed] [Google Scholar]
- 7.Siddiqui A, Spechler SJ, Huerta S. Surgical bypass versus endoscopic stenting for malignant gastroduodenal obstruction: a decision analysis. Dig Dis Sci. 2007;52:276–281. doi: 10.1007/s10620-006-9536-z. https://doi.org/10.1007/s10620-006-9536-z. [DOI] [PubMed] [Google Scholar]
- 8.Jeurnink SM, van Eijck CH, Steyerberg EW, et al. Stent versus gastrojejunostomy for the palliation of gastric outlet obstruction: a systematic review. BMC Gastroenterol. 2007;7:18. doi: 10.1186/1471-230X-7-18. https://doi.org/10.1186/1471-230X-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sato T, Hara K, Mizuno N, et al. Gastroduodenal stenting with Niti-S stent: long-term benefits and additional stent intervention. Dig Endosc. 2015;27:121–129. doi: 10.1111/den.12300. https://doi.org/10.1111/den.12300. [DOI] [PubMed] [Google Scholar]
- 10.Jang JK, Song HY, Kim JH, et al. Tumor overgrowth after expandable metallic stent placement: experience in 583 patients with malignant gastroduodenal obstruction. AJR Am J Roentgenol. 2011;196:W831–836. doi: 10.2214/AJR.10.5861. https://doi.org/10.2214/AJR.10.5861. [DOI] [PubMed] [Google Scholar]
- 11.Kim JH, Song HY, Shin JH, et al. Metallic stent placement in the palliative treatment of malignant gastroduodenal obstructions: prospective evaluation of results and factors influencing outcome in 213 patients. Gastrointest Endosc. 2007;66:256–264. doi: 10.1016/j.gie.2006.12.017. https://doi.org/10.1016/j.gie.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 12.Diamantopoulos A, Sabharwal T, Katsanos K, et al. Fluoroscopic-guided insertion of self-expanding metal stents for malignant gastroduodenal outlet obstruction: immediate results and clinical outcomes. Acta Radiol. 2015;56:1373–1379. doi: 10.1177/0284185114556491. https://doi.org/10.1177/0284185114556491. [DOI] [PubMed] [Google Scholar]
- 13.Ding NS, Alexander S, Swan MP, et al. Gastroduodenal outlet obstruction and palliative self-expandable metal stenting: a dual-centre experience. J Oncol. 2013;2013:167851. doi: 10.1155/2013/167851. https://doi.org/10.1155/2013/167851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehta S, Hindmarsh A, Cheong E, et al. Prospective randomized trial of laparoscopic gastrojejunostomy versus duodenal stenting for malignant gastric outflow obstruction. Surg Endosc. 2006;20:239–242. doi: 10.1007/s00464-005-0130-9. https://doi.org/10.1007/s00464-005-0130-9. [DOI] [PubMed] [Google Scholar]
- 15.Hosono S, Ohtani H, Arimoto Y, et al. Endoscopic stenting versus surgical gastroenterostomy for palliation of malignant gastroduodenal obstruction: a meta-analysis. J Gastroenterol. 2007;42:283–290. doi: 10.1007/s00535-006-2003-y. https://doi.org/10.1007/s00535-006-2003-y. [DOI] [PubMed] [Google Scholar]
- 16.Akinci D, Akhan O, Ozkan F, et al. Palliation of malignant biliary and duodenal obstruction with combined metallic stenting. Cardiovasc Intervent Radiol. 2007;30:1173–1177. doi: 10.1007/s00270-007-9045-2. https://doi.org/10.1007/s00270-007-9045-2. [DOI] [PubMed] [Google Scholar]
- 17.Park KB, Do YS, Kang WK, et al. Malignant obstruction of gastric outlet and duodenum: palliation with flexible covered metallic stents. Radiology. 2001;219:679–683. doi: 10.1148/radiology.219.3.r01jn21679. https://doi.org/10.1148/radiology.219.3.r01jn21679. [DOI] [PubMed] [Google Scholar]
- 18.Lee KM, Choi SJ, Shin SJ, et al. Palliative treatment of malignant gastroduodenal obstruction with metallic stent: prospective comparison of covered and uncovered stents. Scand J Gastroenterol. 2009;44:846–852. doi: 10.1080/00365520902929849. https://doi.org/10.1080/00365520902929849. [DOI] [PubMed] [Google Scholar]
- 19.Yang Z, Wu Q, Wang F, et al. A systematic review and meta-analysis of randomized trials and prospective studies comparing covered and bare self-expandable metal stents for the treatment of malignant obstruction in the digestive tract. Int J Med Sci. 2013;10:825–835. doi: 10.7150/ijms.5969. https://doi.org/10.7150/ijms.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung GS, Song HY, Kang SG, et al. Malignant gastroduodenal obstructions: treatment by means of a covered expandable metallic stent-initial experience. Radiology. 2000;216:758–763. doi: 10.1148/radiology.216.3.r00au05758. https://doi.org/10.1148/radiology.216.3.r00au05758. [DOI] [PubMed] [Google Scholar]
- 21.Lim SG, Kim JH, Lee KM, et al. Conformable covered versus uncovered self-expandable metallic stents for palliation of malignant gastroduodenal obstruction: a randomized prospective study. Dig Liver Dis. 2014;46:603–608. doi: 10.1016/j.dld.2014.02.024. https://doi.org/10.1016/j.dld.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 22.Park CI, Kim JH, Lee YC, et al. What is the ideal stent as initial intervention for malignant gastric outlet obstruction? Dig Liver Dis. 2013;45:33–37. doi: 10.1016/j.dld.2012.08.021. https://doi.org/10.1016/j.dld.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 23.Maire F, Hammel P, Ponsot P, et al. Long-term outcome of biliary and duodenal stents in palliative treatment of patients with unresectable adenocarcinoma of the head of pancreas. Am J Gastroenterol. 2006;101:735–742. doi: 10.1111/j.1572-0241.2006.00559.x. https://doi.org/10.1111/j.1572-0241.2006.00559.x. [DOI] [PubMed] [Google Scholar]
- 24.Kaw M, Singh S, Gagneja H. Clinical outcome of simultaneous self-expandable metal stents for palliation of malignant biliary and duodenal obstruction. Surg Endosc. 2003;17:457–461. doi: 10.1007/s00464-002-8541-3. https://doi.org/10.1007/s00464-002-8527-1. [DOI] [PubMed] [Google Scholar]
- 25.Profili S, Feo CF, Meloni GB, et al. Combined biliary and duodenal stenting for palliation of pancreatic cancer. Scand J Gastroenterol. 2003;38:1099–1102. doi: 10.1080/00365520310005532. https://doi.org/10.1080/00365520310005532. [DOI] [PubMed] [Google Scholar]
- 26.Mutignani M, Tringali A, Shah SG, et al. Combined endoscopic stent insertion in malignant biliary and duodenal obstruction. Endoscopy. 2007;39:440–447. doi: 10.1055/s-2007-966327. https://doi.org/10.1055/s-2007-966327. [DOI] [PubMed] [Google Scholar]