ABSTRACT
This review focuses on the role of trans-kingdom movement of small RNA (sRNA) molecules between parasites, particularly Plasmodium falciparum, and their respective host cells. While the intercellular transfer of sRNAs within organisms is well recognized, recent studies illustrate many examples of trans-kingdom sRNA exchange within the context of host-parasite interactions. These interactions are predominantly found in the transfer of host sRNAs between erythrocytes and the invading P. falciparum, as well as other host cell types. In addition, parasite-encoded sRNAs can also be transferred to host cells to evade the immune system. The transport of these parasite sRNAs in the body fluids of the host may also offer means to detect and monitor the parasite infection. These isolated examples may only represent the tip of the iceberg in which the transfer of sRNA between host and parasites is a critical aspect of host-pathogen interactions. In addition, the levels of these sRNAs and their speed of transfer may vary dramatically under different contexts to push the biologic equilibrium toward the benefit of hosts vs. parasites. Therefore, these sRNA transfers may offer potential strategies to detect, prevent or treat parasite infections. Here, we review a brief history of the discovery of host erythrocyte sRNAs, their transfers and interactions in the context of P. falciparum infection. We also provide examples and discuss the functional significance of the reciprocal transfer of parasite-encoded sRNAs into hosts. These understandings of sRNA exchanges are put in the context of their implications for parasite pathogenesis, host defenses and the evolution of host polymorphisms driven by host interactions with these parasites.
KEYWORDS: Erythrocyte, malaria, microRNA, microvescicles, plasmodium, sickle
Intraerythrocytic developmental cycle of the human malaria parasite Plasmodium falciparum
Malaria continues to be a global health burden worldwide, with an estimated 429,000 deaths and 212 million new cases in 2015 alone.1 The vast majority of these deaths are caused by Plasmodium falciparum, considered to be the most virulent of the malaria-causing species. The P. falciparum life cycle is characterized by multiple developmental stages in the human host and the female Anopheles mosquito vector, including the invasion of human hepatocytes followed by asexual replication in human red blood cells (RBCs), also known as erythrocytes. In human erythrocytes, P. falciparum parasites undergo a 48-hour intraerythrocytic developmental cycle (IDC) that corresponds to the 2-day fever periodicity characteristic of the most prominent symptoms of malaria. During the 48-hour IDC, merozoites invade erythrocytes, where they grow and undergo cell division (schizogony) (Fig. 1). This cell division results in 16–32 new merozoites that burst out of host erythrocytes and invade new ones. Most of these merozoites propagate asexually and maintain infection in the host erythrocytes, but approximately 5 percent commit to a sexual fate.2 These sexual parasites, also called gametocytes, are non-dividing male and female cells. Once mature, these gametocytes are the only form of the parasite transmissible from the human to the mosquito, where sexual reproduction takes place.
Figure 1.
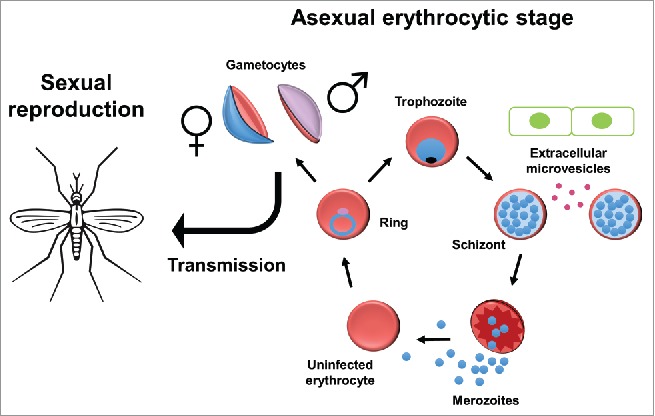
P. falciparum parasites replicate asexually in human erythrocytes in a 48-hour life cycle. Merozoites invade erythrocytes where they grow into rings and eventually larger trophozoites. During the schizont stage, cell-to-cell communication increases as extracellular microvesicles from infected cells relay messages to neighboring cells, including cells of the innate immune system and endothelial cells. The parasite also divides at this stage to form 16–32 new merozoites, most of which continue to reproduce asexually. A small proportion of merozoites is sexually committed and upon invasion of new erythrocytes will transition to a gametocyte fate. Mature male and female gametocytes can then be transmitted to the mosquito, where mating takes place.
While the signal for gametocyte induction is unknown, the cue for such development occurs in the previous schizogony cycle, as all merozoites from a single schizont are committed to either a sexual fate or an asexual fate.3 Environmental stress, such as high parasitemia or the addition of certain drugs4 or spent medium to the culture,5 can lead to an increase in gametocytogenesis. Interestingly, it has been noted that gametocytogenesis is significantly increased in P. falciparum grown in erythrocytes from patients of sickle cell disease and other anemia disorders.6 This suggests that these parasites can sense their environment and alter their IDC accordingly. However, the underlying environmental factors and involved molecular mechanisms are still poorly defined.
Reciprocally, many studies have also suggested that different Plasmodium species also shape the host environments to facilitate their propagation and invasion. One important tool used by parasites to remodel host environments is that circulating extracellular vesicles from various cellular sources. Several studies have shown that these extracellular vesicles are elevated during parasite infection and are associated with the severity of disease.7,8 These vesicles contain proteins, DNA, and RNA, including miRNAs,9,10,11 which can modulate gene expression and phenotypes of the recipient cells. These observations further strengthen the importance of extensive cell-to-cell communication during the infection of P. falciparum and other parasites.12 This review will further explore how the cross-kingdom transfer of these miRNAs, either in host erythrocytes or extracellular vesicles, affects P. falciparum growth and development as well as the their putative roles in the selection of human variants by human malaria.
Discovery of microRNAs in human erythrocytes
Human red cell disorders, including various types of anemia and malaria, affect millions of people and present huge medical and economic challenges. Although the basis for many red cell disorders is known, even for sickle cell disease (SCD) – the first “monogenetic molecular disease” – there are still significant unknowns about their complex phenotypes, clinical heterogeneity13 and malaria resistance. These gaps in our knowledge highlight a great need for a better understanding of all the biologic components of human erythrocytes and a conceptual framework for capturing this information on a genomic scale. Although advances in various genomic technologies have led to an explosion of quantitative knowledge in human diseases, the application of these genomic tools to erythrocyte diseases has been limited by the long-held belief that mature erythrocytes lack RNA expression. These terminally differentiated cells transport oxygen by a large number of hemoglobin proteins. Since mature erythrocytes have lost their nuclei during differentiation, a “textbook” conventional belief has been that erythrocytes do not contain any nucleic acids. In fact, one criterion to separate mature erythrocytes from reticulocytes is based on the absence of signals by RNA-staining dyes. Contrary to this belief, we and other groups have discovered that human erythrocytes actually contain abundant and diverse species of microRNAs and other transcripts that persist beyond terminal differentiation.14,15,16-20 While the initial focus was on microRNAs in mature erythrocytes,14,20 RNA-Seq has further revealed that mature erythrocytes have a large number of large-sized RNAs, including mRNAs and non-coding RNAs.21 The functional relevance of these erythrocyte transcripts has been summarized in a recent review.22
Elevated erythrocyte miR-451 clusters seen in various types of hemolytic anemia
The discovery of the erythrocyte transcriptome allows the use of genomics tools and advanced bioinformatics to analyze these genetic materials as a unique window into the developmental history and various physiologic/pathological adaptations of erythropoiesis. Since circulating erythrocytes are easily accessible and available for repeated sampling, the erythrocyte transcriptome may provide valuable biomarkers for various factors shaping erythropoiesis in bone marrow and other locations. For example, erythrocyte microRNA profiling of HbAA (non-sickle normal) and HbSS (homozygous sickle) showed that erythrocyte microRNA expression was dramatically altered in HbSS erythrocytes.14 Furthermore, the reduced expression of miR-320 contributes to the defective terminal differentiation of HbSS erythrocytes.14 When all the HbSS erythrocyte microRNA profiling was analyzed together with the clinical phenotypes, the high erythrocyte miR-144 level was found to associate with more severe anemia.23 Further investigation revealed that higher levels of miR-144 targeted NRF2, the master regulator of anti-stress capacity, and reduced oxidative tolerance, thus resulting in more severe anemia.23 Therefore, the analysis of the erythrocyte transcriptome may offer important insights into the clinical heterogeneity and pathogenesis of erythroid cells with therapeutic implications.
Both miR-451 and miR-144 reside in a locus that is driven by GATA1 and whose transcription is induced by erythropoietin.24 To put the microRNA dysregulation in HBSS erythrocytes in context, we extended the erythrocyte microRNA profiling to include individuals with Paroxysmal nocturnal hemoglobinuria (PNH) before and after treatment with eculizumab. PNH is characterized by episodic destruction of red blood cells (hemolytic anemia) due to the lack of protective mechanisms against complement-mediated hemolysis. Eculizumab is a humanized antibody that targets the hemolysis-inducing complement protein C5, thus preventing hemolysis and improving the anemia of PNH.25 The clustering analysis of all erythrocyte samples separated the samples into 2 groups based on the differential expression of a subset of microRNAs, including miR-451 and miR-320 (Fig. 2, Chi Lab unpublished). The samples with elevated miR-451 all exhibited prominent hemolytic anemia, including untreated PNH patients with an SS-like pattern (SS-like PNH). Importantly, eculizumab treatments significantly reduced levels of miR-451 in the PNH erythrocytes and converted the pattern to normal-like (NL-like PNH) (Fig. 2). Therefore, the elevated miR-451 in erythrocytes is likely to reflect erythrocyte hemolysis. At least 2 factors may contribute to the distinct erythrocyte expression pattern and elevated miR-451 associated with hemolysis. First, acute anemia induces a physiologic response that includes the rapid development of new erythrocytes. This process, distinct from steady-state erythropoiesis, is referred to as “stress erythropoiesis.”.26 Increased GATA-1 activities have been found during stress erythropoiesis27 and it is reasonable to expect an increased expression of GATA-1 driven miR-451/144. Another contributing factor is that the erythrocyte populations tend to be younger in most hemolytic anemia. Therefore, these 2 factors are likely to play important roles in the elevated miR-451/144 levels seen in erythrocytes from individuals with hemolytic anemia.
Figure 2.
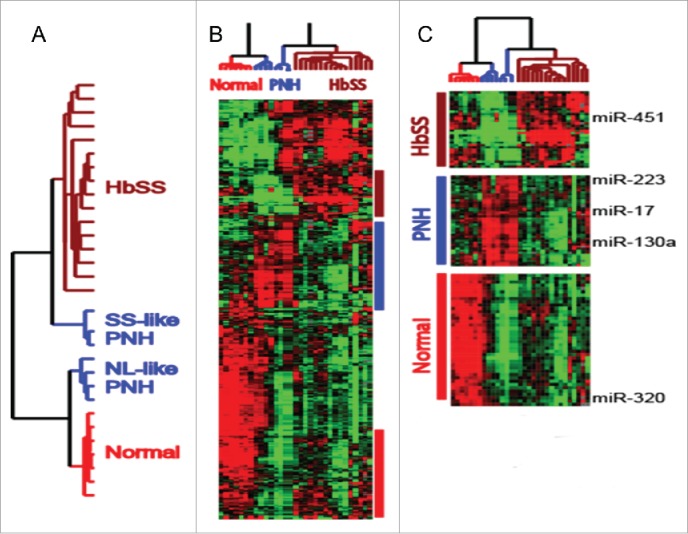
(A) Dendrogram showing the separation of all erythrocyte microRNA profiles into 2 large groups. While HbSS and normal (HbAA, NL) are clearly separated, the samples of PNH are separated into 2 distinct clusters termed HbSS-like PNH or NL-like PNH. The HbSS-like and NL-like PNH correspond to before and after eculizumab treatment. (B) Heatmap of the expression of erythrocyte microRNAs that separate the samples with microRNA clustering corresponding to NL-like, HbSS-like and PNH, which are shown and further expanded in (C)
The search for small non-coding RNAs in Plasmodium falciparum
While microRNAs were first discovered by genetic approaches in C. elegans, their broad presence and functional relevance in various organisms was not appreciated until several groups developed biochemical means to isolate microRNAs for sequencing.28-30 These approaches have led to the discovery of a large number of microRNAs in a wide variety of organisms, including metazoans and viruses.31 Similar methods have been used to identify parasite-encoded microRNAs from Trypanosoma brucei,32,33 Trichomonas vaginalis,32-34 Entamoeba histolytica35 and Toxoplasma gondii.36 However, similar approaches have not been as successful for other parasites. For example, the only identified small RNA species in P. falciparum was human microRNAs, especially miR-451.17,37 The initial interpretation was that these human microRNAs came from contamination by host cells used to propagate P. falciparum.17,37 The absence of P. falciparum-encoded microRNAs was also consistent with studies that had ruled out the presence of the canonical RNAi pathways in P. falciparum.38 However, it is not clear whether the host microRNAs found in Plasmodium have any functional roles.
The transfer of erythrocyte microRNAs into P. falciparum
During the blood stage of malaria infection, extensive material exchange occurs between parasites and the host erythrocytes. Many erythrocyte contents, including hemoglobin, are consumed by the parasites to support their growth, expansion, and differentiation.39 Conversely, many malaria proteins, including rifin, stevor and var, are exported from the parasites to erythrocytes and function to remodel host erythrocytes, supporting nutrient import and adhesion with endothelial cells.40 Therefore, it is reasonable to speculate that erythrocyte microRNAs and associated proteins may also be a part of such host-pathogen exchanges.
Consistent with this concept, human miRNAs, such as miR-451, were found within P. falciparum.17,37 To systematically profile the transported microRNAs, LaMonte et al. used multiplex RT-PCR and identified > 100 human microRNAs present within P. falciparum, including miR-451, miR-223, and miR-19b.41 As sickle erythrocytes are known to exhibit resistance to P. falciparum,42 it is interesting to note that several transported microRNAs, including miR-451 and miR-223, were also highly expressed in sickle erythrocytes.14 Based on the hypothesis that the transported sickle-enriched microRNAs may contribute to the resistant phenotypes, the authors transfected HbAA cells with SCD-enriched miR-451 and found that the increased erythrocyte miR-451 significantly reduced parasite growth. In addition, the inhibition of miR-451 in HbAS and HbSS erythrocytes increased parasite growth.41 Collectively, these data indicated a functional role of the translocated microRNAs in the malaria resistance of the sickle erythrocytes with highly elevated miR-451. However, it is important to point out that the role of miR-451 in the HbAA erythrocytes remains unclear.
Erythrocyte microRNAs in the malaria resistance of sickle cell erythrocytes
The next step was to determine how these translocated human microRNAs affected the gene expression and phenotypes of P. falciparum. Translocated miR-451 was found to be in the parasitophorous vacuolar membrane (PVM).41 Since P. falciparum lacks the orthologs of Dicer/Ago38,43, the mechanism by which these microRNAs could affect gene expression was unclear. Interestingly, 35 P. falciparum expressed sequence tags (ESTs) deposited into PlasmoDB contained several human microRNAs fused to the 5′ end of the P. falciparum transcripts. Among these human microRNAs, miR-451 occurred the most often, consistent with its high levels in parasites. Using northern blots, 5′ RACE-PCR, Illumina deep sequencing, and ribonuclease protection assays, LaMonte et al. confirmed that human microRNAs modified parasite mRNAs at a low rate in parasites grown in HbAA erythrocytes. This modification was dramatically increased in parasites grown in HbSS erythrocytes, with elevated miR-451, to exhibit functional relevance. These uncapped fusion transcripts were further shown to impair ribosomal loading of miR-451 target gene cAMP-dependent protein kinase (PKA-R).41,44 Consistently, parasites in HbSS and HbAS cells contained significantly less PKA-R protein,41 illustrating the effects of miR-451 in repressing translation. Regulation of PKA-R levels is crucial for parasite survival,45 and suppression may lead to increased cAMP-dependent catalytic (PKA-C) activity, which may cause an increase in gametocyte induction.46 Indeed, LaMonte et al. showed that miR-451 transfected erythrocytes have a 2.5-fold increase in the number of parasites that transition to a gametocyte fate.
The formation of chimeric fusions between human miRNAs and P. falciparum mRNAs may reflect a form of trans-splicing.41 Trans-splicing in parasites was first described in Trypanosomes, as all polycistronic mRNAs in T. brucei must be trans-spliced with a spliced leader (SL) RNA, which is a part of an RNP, to form mature processed mRNAs.47-49 With recent genome wide-studies, it has been shown that this trans-splicing increases mRNA diversity and the repertoire of proteins expressed.50-52 Trans-splicing has also since been found in metazoans such as Nematodes and Platyhelminthes.53,54 Such modification of parasite mRNAs by host transcripts may use similar trans-splicing machineries in other species to allow the modification of parasite gene expression by signals from host cells.
Erythrocyte microRNAs as intercellular communication tools of infected erythrocytes
Besides regulating the gene expression of P. falciparum, erythrocyte microRNAs also play a crucial role in the communication between infected erythrocytes and other cells via extracellular vesicles.11,55 Such intercellular communication is coordinated by infected erythrocytes to affect other host cells and facilitate malaria pathogenesis and parasite infection. The release of these extracellular microvesicles peaks during schizogony. These vesicles contain both human and P. falciparum proteins as well as host microRNAs that can be transferred to endothelial cells and immune cells.11
These extracellular vesicles strongly stimulate an immune response, including the activation of pro-inflammatory cytokines.11 This cytokine activation can increase the expression of cellular receptors and enhance the ability of infected erythrocytes to bind to endothelial cells. This can also result in increased vascular permeability and apoptosis of endothelial cells. Mantel et al. show that endothelial cells internalize extracellular vesicles via endocytosis and that these vesicles contain functional host microRNA-Ago2 complexes derived from the host erythrocytes.56 Interestingly, miR-451 is also the prominent microRNA in this setting, increased 50-fold upon vesicle internalization.56 The RISC complex was able to exert canonical microRNA-like regulatory function on its target genes, CAV-1 and ATF2.56 These changes, in turn, affect the barrier function of endothelial cells as manifested by decreased cortical actin, increased stress fiber formation, discontinuous junctional VE-Cadherin and the presence of gaps.56 These results show that extracellular vesicles and their cargo, particularly human miRNAs, are important in disease pathology as they lead to an increased immune response. In this context, Ago2 is an essential part of the activities of the erythrocyte miRNAs in extracellular vesicles that affect gene expression in the recipient cells via canonical microRNA function.56 The involvement of host Ago2 protein is especially essential for the formation of the RISC complex to target and cleave target mRNAs since P. falciparum lacks RNAi machinery and Ago proteins. Because severe malaria is associated with an increase in extracellular vesicles in the plasma,57 the microRNAs in the infected erythrocyte may play an important role in disease pathology.
Parasite small RNAs transported into host cells as mediators of host-pathogen interactions
While the previous sections outline how host-encoded small RNAs affect parasite growth and differentiation, several papers also report on the reciprocal ability of parasite-encoded small RNAs to be transferred to host cells during infection. For example, the rodent helminth nematode Heligmosomoides polygyrus secretes microvesicles that contain parasite-encoded microRNAs and Y-RNAs.58 Mouse cells can internalize these sRNAs, which suppress host immunity by targeting host mRNAs, including Il33r and Dusp158. While the extracellular vesicles contain a nematode AGO protein, it is still unclear whether the host or nematode AGO acts with the transported microRNAs to silence host genes.58
In addition, parasite-encoded small RNAs from T. cruzi,59 Schistosoma japonicum,60 and Litomosoides sigmodontis61 can be found in the bodily fluids of infected organisms. During T. cruzi infections, the parasites generate tRNA-derived small RNAs (tsRNAs) in T. cruzi-derived small vesicles that can be transferred to other parasites as well as susceptible host cells.59 This opens up studies to pursue the mechanisms that the parasite uses to specifically manipulate its target host cells during infection.
A similar occurrence has been shown between the mosquito and its endosymbiont Wolbachia.62 Two Wolbachia snRNAs, WsnRNA-46–5p and WsnRNA-59–5p, are highly expressed in infected Wol+ mosquitoes. Wsn-RNA-46 is exported out of Wolbachia and into the mosquito where it leads to an upregulation of the target gene Dynein heavy chain, which could be important for localization of the bacteria within the mosquito.62 Therefore, the transport of Wolbachia sRNAs modifies the shape of the host cells to facilitate the parasite infection. Collectively, these examples show the variability and prevalence of trans-kingdom cross-talk and demonstrate that such communication can be beneficial or detrimental to the host organism.
Different erythrocyte miR-451 levels dictates distinct modes of host-pathogen interactions
It is interesting to note that both the host and parasite can utilize erythrocyte microRNAs to their advantage in different contexts (Fig. 3). In HbAA cells with physiologic levels of miR-451, the low rate of miR-451 modification of parasite transcripts does not seem to affect the gene regulation and phenotypes of P. falciparum. Instead, as we previously predicted,41 erythrocyte microRNAs are used by parasites to benefit their pathogenesis by delivering signals to other cells via microvesicles to facilitate endothelial cell attachments and parasite sequestration. In such cases, erythrocyte microRNAs play an important part in the life cycle and pathogenesis of P. falciparum. In the pathological conditions of HbSS erythrocytes, the high level of miR-451 suppresses parasite growth by exaggerated transfer and affects the expression of PKA-R and other proteins of P. falciparum (Fig. 3). Thus, the aberrant miRNA profile and host environment in SCD erythrocytes short circuits the normal adaptation to the host-cell environment and contributes to an insurmountable challenge for the parasites. Therefore, erythrocyte microRNAs act like an innate immune system that suppresses the proliferation of P. falciparum. One variable that tips the interaction between hosts and pathogens is the level of miR-451 and the dominant modes of parasite suppression vs. microvesicle-mediated host cell remodeling.
Figure 3.
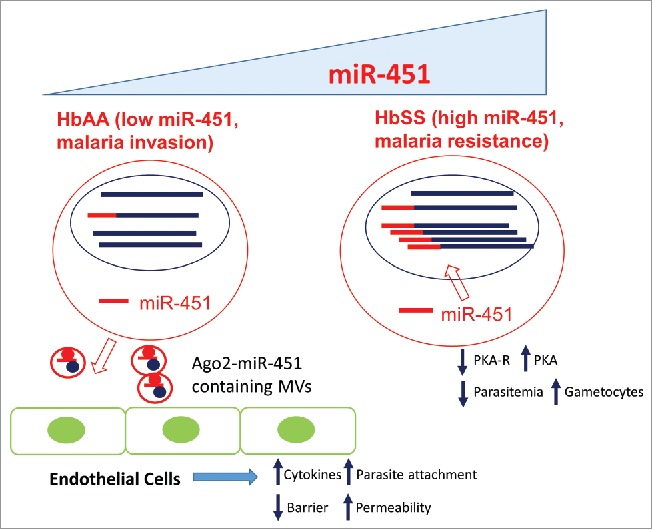
Distinct modes of host-parasite (blue circles) interactions in the HbAA (low miR-451, left) and HbSS (high miR-451, right) erythrocytes (red circles). The parasite transcripts (blue line) which are unmodified or modified by human microRNAs (red line) are indicated by respective color. In the erythrocytes with low miR-451, infected erythrocytes secrete miR-451-Ago2 containing microvesicles (MVs) to remodel endothelial cells to increase cytokine expression, facilitate erythrocyte attachment and to increase vascular permeability. These changes can benefit the parasites by facilitating their invasion and pathogenesis. In contrast, the erythrocytes with high miR-451 benefit host cells by exhibiting malaria resistance due to the increased import of miR-451 and enhanced modifications of parasite transcripts that reduce the protein of PKAR. This leads to the increased activities of PKA, reduced parasitemia and increased gametocytogenesis.
miR-451 as a common determinant of malaria-resistant hemoglobinopathies?
Many intra-cellular pathogens have complicated interactions with host cells that determine the outcome of infection. As a result of the high mortality and widespread impact of malaria, it is thought to be the strongest evolutionary selective force in recent human history.63,64 In fact, genes that confer resistance to malaria provide some of the best-known case studies of positive selection in modern humans. These hemoglobin variants include HbS, HbC, G6PD deficiency and thalassemia. Importantly, these malaria-resistant hemoglobinopathies also share the feature of hemolysis disease. Since several sickle-cell enriched microRNAs, especially miR-451, play significant roles as genetic determinants of growth for P. falciparum, it is possible that the elevated miR-451 associated with hemolysis contributes to the malaria resistance seen in many erythrocyte polymorphisms associated with malaria resistance. Consistently, miR-451 was found to also be elevated in β-thalassemia.65 This hypothesis can be tested in various malaria-resistant hemoglobinopathies similarly to what has been performed for sickle erythrocytes.41
The implications of sRNA transfer for hosts and pathogens during their interactions
We speculate that for the human host, the suppression of parasite growth through the modification of transferred host sRNAs may represent a novel mechanism of innate immunity. This concept is also supported by the increased sensitivity of the mosquito Anopheles gambiae to Plasmodium infection when its microRNA-producing proteins, Dicer1 and Ago1, are deleted.66 For P. falciparum, this use of host microRNAs to modify its transcripts may allow the parasite to tune its gene expression profile and adjust its development as it traverses different host cell types with distinct microRNA compositions. Furthermore, the parasites may also use exosomes and their contents to regulate the adhesion properties of endothelial cells to modulate the microcirculation. Therefore, the study of how host microRNAs participate in parasite gene regulation may offer important insights into the complex life cycle of P. falciparum, similar to what studies of viral pathogens have offered for mammalian host cells. From the Plasmodium perspective, the process may present an opportunity for the parasite to sample the host environment to adjust its gene expression. microRNAs are known to show very strong tissue-specific expression that drives and maintains the differentiated status of the cells. For example, miR-451 is highly expressed in erythrocytes. Similarly, miR-122 is highly expressed in the liver and plays a crucial role in the replication of HCV in hepatocytes.67 In the natural life cycle of Plasmodium, the parasites pass through many different cell types, including hepatocytes before red blood cells. Therefore, the host microRNAs may provide the relevant environmental cues. It is likely that different tissue-specific microRNAs may affect parasite gene regulation to adjust the gene expression to the host environments of humans or mosquitoes. Second, the elevated miR-451 indicates the shortened erythrocyte life span in hemolytic anemia. Therefore, the elevated miR-451 also indicates that the host cells are relatively undesirable and are short-lived host cells for P. falciparum.
Finally, it is interesting to note that the study of viral DNA, RNA and proteins provides a simple system to probe the gene regulation of their host mammalian cells. Similarly, we expect that the use of host or parasite small RNAs as molecular probes will provide a unique window into the import, processing and function of these transferred RNAs into the parasites and hosts, respectively. Since various modified microRNAs can be incorporated into the protein machineries, the inclusion of additional tags in the transfected microRNAs may be used to probe the interacting proteins and machineries involved in the transport, processing and activities of these small RNAs.
The examples we have covered here may only represent the tip of the iceberg in which the transfer of small RNAs serves as an important arsenal during the continuing arms race between host and pathogen. Therefore, the cross-kingdom transfer of small RNA may offer a unique opportunity to uncover novel aspects of host-pathogen interactions that have not been previously appreciated. Such understandings may have the potential to uncover novel means of detecting and treating parasite infections.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This research was funded by the Duke Chancellor's Pilot Project fund and the Burroughs Wellcome Fund. K.A.W. was supported by the NSF Graduate Research Fellowship Program.
References
- 1.Organization WH. World Malaria Report 2016. [Google Scholar]
- 2.Eksi S, Haile Y, Furuya T, Ma L, Su X, Williamson KC. Identification of a subtelomeric gene family expressed during the asexual-sexual stage transition in Plasmodium falciparum. Mol Biochem Parasitol 2005; 143:90-9; PMID:15996767; http://dx.doi.org/ 10.1016/j.molbiopara.2005.05.010 [DOI] [PubMed] [Google Scholar]
- 3.Bruce MC, Alano P, Duthie S, Carter R. Commitment of the malaria parasite Plasmodium falciparum to sexual and asexual development. Parasitology 1990; 100 Pt 2:191-200; PMID:2189114; http://dx.doi.org/19848586 10.1017/S0031182000061199 [DOI] [PubMed] [Google Scholar]
- 4.Peatey CL, Skinner-Adams TS, Dixon MW, McCarthy JS, Gardiner DL, Trenholme KR. Effect of antimalarial drugs on Plasmodium falciparum gametocytes. J Infect Dis 2009; 200:1518-21; PMID:19848586; http://dx.doi.org/ 10.1086/644645 [DOI] [PubMed] [Google Scholar]
- 5.Williams JL. Stimulation of Plasmodium falciparum gametocytogenesis by conditioned medium from parasite cultures. Am J Trop Med Hyg 1999; 60:7-13; PMID:9988315 [DOI] [PubMed] [Google Scholar]
- 6.Trager W, Gill GS, Lawrence C, Nagel RL. Plasmodium falciparum: enhanced gametocyte formation in vitro in reticulocyte-rich blood. Exp Parasitol 1999; 91:115-8; PMID:9990338; http://dx.doi.org/ 10.1006/expr.1998.4347 [DOI] [PubMed] [Google Scholar]
- 7.Pankoui Mfonkeu JB, Gouado I, Fotso Kuate H, Zambou O, Amvam Zollo PH, Grau GE, Combes V. Elevated cell-specific microparticles are a biological marker for cerebral dysfunctions in human severe malaria. PLoS One 2010; 5:e13415; PMID:20976232; http://dx.doi.org/ 10.1371/journal.pone.0013415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campos FM, Franklin BS, Teixeira-Carvalho A, Filho AL, de Paula SC, Fontes CJ, Brito CF, Carvalho LH. Augmented plasma microparticles during acute Plasmodium vivax infection. Malar J 2010; 9:327; PMID:21080932; http://dx.doi.org/ 10.1186/1475-2875-9-327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunter MP, Ismail N, Zhang X, Aguda BD, Lee EJ, Yu L, Xiao T, Schafer J, Lee ML, Schmittgen TD, et al.. Detection of microRNA expression in human peripheral blood microvesicles. PLoS One 2008; 3:e3694; PMID:19002258; http://dx.doi.org/ 10.1371/journal.pone.0003694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Regev-Rudzki N, Wilson Danny W, Carvalho Teresa G, Sisquella X, Coleman Bradley M, Rug M, Bursac D, Angrisano F, Gee M, Hill Andrew F, et al.. Cell-Cell Communication between Malaria-Infected Red Blood Cells via Exosome-like Vesicles. Cell 153:1120-33; PMID:23683579; http://dx.doi.org/ 10.1016/j.cell.2013.04.029 [DOI] [PubMed] [Google Scholar]
- 11.Mantel PY, Hoang AN, Goldowitz I, Potashnikova D, Hamza B, Vorobjev I, Ghiran I, Toner M, Irimia D, Ivanov AR, et al.. Malaria-infected erythrocyte-derived microvesicles mediate cellular communication within the parasite population and with the host immune system. Cell Host Microbe 2013; 13:521-34; PMID:23684304; http://dx.doi.org/ 10.1016/j.chom.2013.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mantel PY, Marti M. The role of extracellular vesicles in Plasmodium and other protozoan parasites. Cell Microbiol 2014; 16:344-54; PMID:24406102; http://dx.doi.org/ 10.1111/cmi.12259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pauling L, Itano HA, et al.. Sickle cell anemia a molecular disease. Science 1949; 110:543-8; PMID:15395398; http://dx.doi.org/ 10.1126/science.110.2865.543 [DOI] [PubMed] [Google Scholar]
- 14.Chen SY, Wang Y, Telen MJ, Chi JT. The genomic analysis of erythrocyte microRNA expression in sickle cell diseases. PLoS One 2008; 3:e2360; PMID:18523662; http://dx.doi.org/ 10.1371/journal.pone.0002360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neuman R, Hayek S, Rahman A, Poole JC, Menon V, Sher S, Newman JL, Karatela S, Polhemus D, Lefer DJ, et al.. Effects of storage-aged red blood cell transfusions on endothelial function in hospitalized patients. Transfusion 2014; PMID:25393772; http://dx.doi.org/ 10.1111/trf.12919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kannan M, Atreya C. Differential profiling of human red blood cells during storage for 52 selected microRNAs. Transfusion 2010; 50:1581-8; PMID:20158686; http://dx.doi.org/ 10.1111/j.1537-2995.2010.02585.x [DOI] [PubMed] [Google Scholar]
- 17.Rathjen T, Nicol C, McConkey G, Dalmay T. Analysis of short RNAs in the malaria parasite and its red blood cell host. FEBS Lett 2006; 580:5185-8; PMID:16963026; http://dx.doi.org/ 10.1016/j.febslet.2006.08.063 [DOI] [PubMed] [Google Scholar]
- 18.Hamilton AJ. MicroRNA in erythrocytes. Biochem Soc Trans 2010; 38:229-31; PMID:20074065; http://dx.doi.org/ 10.1042/BST0380229 [DOI] [PubMed] [Google Scholar]
- 19.Umemura T, Tanaka Y, Fujisaki M, Masaki S, Shiotsu H, Srinoun K, Fucharoen S, Abe Y, Ichihara H, Toh H, et al.. MicroRNA-Ago2 Complex in Mature Human Red Blood Cells. American Society of Hematology, 2009. [Google Scholar]
- 20.Azzouzi I, Moest H, Wollscheid B, Schmugge M, Eekels JJ, Speer O. Deep sequencing and proteomic analysis of the microRNA-induced silencing complex in human red blood cells. Exp Hematol 2015; 43:382-92; PMID:25681748; http://dx.doi.org/ 10.1016/j.exphem.2015.01.007 [DOI] [PubMed] [Google Scholar]
- 21.Doss J, Corcoran D, Jima D, Telen M, Dave S, Chi J-T. A comprehensive joint analysis of the long and short RNA transcriptomes of human erythrocytes. BMC Genomics 2015; 16:952; PMID:26573221; http://dx.doi.org/20709907 10.1186/s12864-015-2156-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen P-H, Hong J, Chi J-T. Discovery, Genomic Analysis, and Functional Role of the Erythrocyte RNAs. Current Pathobiology Reports 2017:1-6; http://dx.doi.org/ 10.1007/s40139-017-0124-z [DOI] [Google Scholar]
- 23.Sangokoya C, Telen MJ, Chi JT. microRNA miR-144 modulates oxidative stress tolerance and associates with anemia severity in sickle cell disease. Blood 2010; 116:4338-48; PMID:20709907; http://dx.doi.org/ 10.1182/blood-2009-04-214817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dore L, Amigo J, Dos Santos C, Zhang Z, Gai X, Tobias J, Yu D, Klein A, Dorman C, Wu W. A GATA-1-regulated microRNA locus essential for erythropoiesis. Proc Natl Acad Sci U S A 2008; 105:3333-8; PMID:18303114; http://dx.doi.org/ 10.1073/pnas.0712312105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kathula SK. Eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med 2006; 355:2786; author reply 7–8; PMID:17192547; http://dx.doi.org/ 10.1056/NEJMc062885 [DOI] [PubMed] [Google Scholar]
- 26.Paulson RF, Shi L, Wu DC. Stress erythropoiesis: new signals and new stress progenitor cells. Curr Opin Hematol 2011; 18:139-45; PMID:21372709; http://dx.doi.org/ 10.1097/MOH.0b013e32834521c8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki M, Ohneda K, Hosoya-Ohmura S, Tsukamoto S, Ohneda O, Philipsen S, Yamamoto M. Real-time monitoring of stress erythropoiesis in vivo using <em>Gata1</em>and β-globin <em>LCR</em>luciferase transgenic mice. Blood 2006; 108:726-33; PMID:16537808; http://dx.doi.org/ 10.1182/blood-2005-10-4064 [DOI] [PubMed] [Google Scholar]
- 28.Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science 2001; 294:853-8; PMID:11679670; http://dx.doi.org/ 10.1126/science.1064921 [DOI] [PubMed] [Google Scholar]
- 29.Lau NC, Lim LP, Weinstein EG, Bartel DP. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science 2001; 294:858-62; PMID:11679671; http://dx.doi.org/ 10.1126/science.1065062 [DOI] [PubMed] [Google Scholar]
- 30.Lee RC, Ambros V. An extensive class of small RNAs in Caenorhabditis elegans. Science 2001; 294:862-4; PMID:11679672; http://dx.doi.org/ 10.1126/science.1065329 [DOI] [PubMed] [Google Scholar]
- 31.Skalsky RL, Cullen BR. Viruses, microRNAs, and host interactions. Annu Rev Microbiol 2010; 64:123-41; PMID:20477536; http://dx.doi.org/ 10.1146/annurev.micro.112408.134243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mallick B, Ghosh Z, Chakrabarti J. MicroRNA switches in Trypanosoma brucei. Biochem Biophys Res Commun 2008; 372:459-63; PMID: 18510949; http://dx.doi.org/ 10.1016/j.bbrc.2008.05.084 [DOI] [PubMed] [Google Scholar]
- 33.Militello KT, Refour P, Comeaux CA, Duraisingh MT. Antisense RNA and RNAi in protozoan parasites: working hard or hardly working? Mol Biochem Parasitol 2008; 157:117-26; PMID:18053590; http://dx.doi.org/ 10.1016/j.molbiopara.2007.10.004 [DOI] [PubMed] [Google Scholar]
- 34.Chen XS, Collins LJ, Biggs PJ, Penny D. High throughput genome-wide survey of small RNAs from the parasitic protists Giardia intestinalis and Trichomonas vaginalis. Genome Biol Evol 2009; 1:165-75; PMID:20333187; http://dx.doi.org/ 10.1093/gbe/evp017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De S, Pal D, Ghosh SK. Entamoeba histolytica: computational identification of putative microRNA candidates. Exp Parasitol 2006; 113:239-43; PMID:16515787; http://dx.doi.org/ 10.1016/j.exppara.2006.01.009 [DOI] [PubMed] [Google Scholar]
- 36.Braun L, Cannella D, Ortet P, Barakat M, Sautel CF, Kieffer S, Garin J, Bastien O, Voinnet O, Hakimi MA. A complex small RNA repertoire is generated by a plant/fungal-like machinery and effected by a metazoan-like Argonaute in the single-cell human parasite Toxoplasma gondii. PLoS Pathog 2010; 6:e1000920; PMID:20523899; http://dx.doi.org/ 10.1371/journal.ppat.1000920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xue X, Zhang Q, Huang Y, Feng L, Pan W. No miRNA were found in Plasmodium and the ones identified in erythrocytes could not be correlated with infection. Malar J 2008; 7:47; PMID:18328111; http://dx.doi.org/ 10.1186/1475-2875-7-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baum J, Papenfuss AT, Mair GR, Janse CJ, Vlachou D, Waters AP, Cowman AF, Crabb BS, de Koning-Ward TF. Molecular genetics and comparative genomics reveal RNAi is not functional in malaria parasites. Nucleic acids research 2009; 37:3788-98; PMID:19380379; http://dx.doi.org/ 10.1093/nar/gkp239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deitsch K, Driskill C, Wellems T. Transformation of malaria parasites by the spontaneous uptake and expression of DNA from human erythrocytes. Nucleic Acids Res 2001; 29:850-3; PMID:11160909; http://dx.doi.org/ 10.1093/nar/29.3.850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maier AG, Cooke BM, Cowman AF, Tilley L. Malaria parasite proteins that remodel the host erythrocyte. Nat Rev Micro 2009; 7:341-54; PMID:19369950; http://dx.doi.org/22901539 10.1038/nrmicro2110 [DOI] [PubMed] [Google Scholar]
- 41.LaMonte G, Philip N, Reardon J, Lacsina JR, Majoros W, Chapman L, Thornburg CD, Telen MJ, Ohler U, Nicchitta CV, et al.. Translocation of sickle cell erythrocyte microRNAs into Plasmodium falciparum inhibits parasite translation and contributes to malaria resistance. Cell Host Microbe 2012; 12:187-99; PMID:22901539; http://dx.doi.org/ 10.1016/j.chom.2012.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Friedman MJ. Erythrocytic mechanism of sickle cell resistance to malaria. Proc Natl Acad Sci U S A 1978; 75:1994-7; PMID:347452; http://dx.doi.org/ 10.1073/pnas.75.4.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hall N, Karras M, Raine JD, Carlton JM, Kooij TW, Berriman M, Florens L, Janssen CS, Pain A, Christophides GK, et al.. A comprehensive survey of the Plasmodium life cycle by genomic, transcriptomic, and proteomic analyses. Science 2005; 307:82-6; PMID:15637271; http://dx.doi.org/ 10.1126/science.1103717 [DOI] [PubMed] [Google Scholar]
- 44.Lacsina JR, LaMonte G, Nicchitta CV, Chi JT. Polysome profiling of the malaria parasite Plasmodium falciparum. Mol Biochem Parasitol 2011; 179:42-6; PMID:21605599; http://dx.doi.org/ 10.1016/j.molbiopara.2011.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li J, Cox LS. Isolation and characterisation of a cAMP-dependent protein kinase catalytic subunit gene from Plasmodium falciparum. Mol Biochem Parasitol 2000; 109:157-63; PMID:10960174; http://dx.doi.org/ 10.1016/S0166-6851(00)00242-5 [DOI] [PubMed] [Google Scholar]
- 46.Trager W, Gill GS. Plasmodium falciparum gametocyte formation in vitro: its stimulation by phorbol diesters and by 8-bromo cyclic adenosine monophosphate. J Protozool 1989; 36:451-4; PMID:2681714; http://dx.doi.org/ 10.1111/j.1550-7408.1989.tb05822.x [DOI] [PubMed] [Google Scholar]
- 47.Boothroyd JC, Cross GA. Transcripts coding for variant surface glycoproteins of Trypanosoma brucei have a short, identical exon at their 5′ end. Gene 1982; 20:281-9; PMID:7166234; http://dx.doi.org/ 10.1016/0378-1119(82)90046-4 [DOI] [PubMed] [Google Scholar]
- 48.Sutton RE, Boothroyd JC. Evidence for trans splicing in trypanosomes. Cell 1986; 47:527-35; PMID:3022935; http://dx.doi.org/ 10.1016/0092-8674(86)90617-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van der Ploeg LH, Liu AY, Michels PA, De Lange T, Borst P, Majumder HK, Weber H, Veeneman GH, Van Boom J. RNA splicing is required to make the messenger RNA for a variant surface antigen in trypanosomes. Nucleic Acids Res 1982; 10:3591-604; PMID:6287414; http://dx.doi.org/ 10.1093/nar/10.12.3591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kolev NG, Franklin JB, Carmi S, Shi H, Michaeli S, Tschudi C. The transcriptome of the human pathogen Trypanosoma brucei at single-nucleotide resolution. PLoS Pathog 2010; 6:e1001090; PMID:20838601; http://dx.doi.org/ 10.1371/journal.ppat.1001090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nilsson D, Gunasekera K, Mani J, Osteras M, Farinelli L, Baerlocher L, Roditi I, Ochsenreiter T. Spliced leader trapping reveals widespread alternative splicing patterns in the highly dynamic transcriptome of Trypanosoma brucei. PLoS Pathog 2010; 6:e1001037; PMID:20700444; http://dx.doi.org/ 10.1371/journal.ppat.1001037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Siegel TN, Hekstra DR, Wang X, Dewell S, Cross GA. Genome-wide analysis of mRNA abundance in two life-cycle stages of Trypanosoma brucei and identification of splicing and polyadenylation sites. Nucleic Acids Res 2010; 38:4946-57; PMID:20385579; http://dx.doi.org/ 10.1093/nar/gkq237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blaxter M, Liu L. Nematode spliced leaders–ubiquity, evolution and utility. Int J Parasitol 1996; 26:1025-33; PMID:8982784; http://dx.doi.org/ 10.1016/S0020-7519(96)00060-4 [DOI] [PubMed] [Google Scholar]
- 54.Davis RE. Surprising diversity and distribution of spliced leader RNAs in flatworms. Mol Biochem Parasitol 1997; 87:29-48; PMID:9233671; http://dx.doi.org/ 10.1016/S0166-6851(97)00040-6 [DOI] [PubMed] [Google Scholar]
- 55.Regev-Rudzki N, Wilson DW, Carvalho TG, Sisquella X, Coleman BM, Rug M, Bursac D, Angrisano F, Gee M, Hill AF, et al.. Cell-cell communication between malaria-infected red blood cells via exosome-like vesicles. Cell 2013; 153:1120-33; PMID:23683579; http://dx.doi.org/ 10.1016/j.cell.2013.04.029 [DOI] [PubMed] [Google Scholar]
- 56.Mantel PY, Hjelmqvist D, Walch M, Kharoubi-Hess S, Nilsson S, Ravel D, Ribeiro M, Gruring C, Ma S, Padmanabhan P, et al.. Infected erythrocyte-derived extracellular vesicles alter vascular function via regulatory Ago2-miRNA complexes in malaria. Nat Commun 2016; 7:12727; PMID:27721445; http://dx.doi.org/ 10.1038/ncomms12727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nantakomol D, Dondorp AM, Krudsood S, Udomsangpetch R, Pattanapanyasat K, Combes V, Grau GE, White NJ, Viriyavejakul P, Day NP, et al.. Circulating red cell-derived microparticles in human malaria. J Infect Dis 2011; 203:700-6; PMID:21282195; http://dx.doi.org/ 10.1093/infdis/jiq104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Buck AH, Coakley G, Simbari F, McSorley HJ, Quintana JF, Le Bihan T, Kumar S, Abreu-Goodger C, Lear M, Harcus Y, et al.. Exosomes secreted by nematode parasites transfer small RNAs to mammalian cells and modulate innate immunity. Nat Commun 2014; 5:5488; PMID:25421927; http://dx.doi.org/ 10.1038/ncomms6488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garcia-Silva MR, das Neves RF, Cabrera-Cabrera F, Sanguinetti J, Medeiros LC, Robello C, Naya H, Fernandez-Calero T, Souto-Padron T, de Souza W, et al.. Extracellular vesicles shed by Trypanosoma cruzi are linked to small RNA pathways, life cycle regulation, and susceptibility to infection of mammalian cells. Parasitol Res 2014; 113:285-304; PMID:24241124; http://dx.doi.org/ 10.1007/s00436-013-3655-1 [DOI] [PubMed] [Google Scholar]
- 60.Cheng G, Luo R, Hu C, Cao J, Jin Y. Deep sequencing-based identification of pathogen-specific microRNAs in the plasma of rabbits infected with Schistosoma japonicum. Parasitology 2013; 140:1751-61; PMID:23942009; http://dx.doi.org/ 10.1017/S0031182013000917 [DOI] [PubMed] [Google Scholar]
- 61.Quintana JF, Makepeace BL, Babayan SA, Ivens A, Pfarr KM, Blaxter M, Debrah A, Wanji S, Ngangyung HF, Bah GS, et al.. Extracellular Onchocerca-derived small RNAs in host nodules and blood. Parasit Vectors 2015; 8:58; PMID:25623184; http://dx.doi.org/ 10.1186/s13071-015-0656-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mayoral JG, Hussain M, Joubert DA, Iturbe-Ormaetxe I, O'Neill SL, Asgari S. Wolbachia small noncoding RNAs and their role in cross-kingdom communications. Proc Natl Acad Sci U S A. 2014; 111:18721-6; PMID:25512495; http://dx.doi.org/ 10.1073/pnas.1420131112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hedrick PW. Population genetics of malaria resistance in humans. Heredity (Edinb) 2011; 107:283-304; PMID:21427751; http://dx.doi.org/ 10.1038/hdy.2011.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kwiatkowski DP. How Malaria Has Affected the Human Genome and What Human Genetics Can Teach Us about Malaria. Am J Hum Genet 77:171-92; PMID:16001361; http://dx.doi.org/ 10.1086/432519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saki N, Abroun S, Soleimani M, Kavianpour M, Shahjahani M, Mohammadi-Asl J, Hajizamani S. MicroRNA Expression in beta-Thalassemia and Sickle Cell Disease: A Role in The Induction of Fetal Hemoglobin. Cell J 2016; 17:583-92; PMID:26862517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Winter F, Edaye S, Huttenhofer A, Brunel C. Anopheles gambiae miRNAs as actors of defence reaction against Plasmodium invasion. Nucleic Acids Res 2007; 35:6953-62; PMID:17933784; http://dx.doi.org/ 10.1093/nar/gkm686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science 2005; 309:1577-81; PMID:16141076; http://dx.doi.org/ 10.1126/science.1113329 [DOI] [PubMed] [Google Scholar]


