An overview of progress in chemical genetics in plants, with a focus on the discoveries of small molecules in screens designed for the discovery of biological processes.
Abstract
The treatment of a biological system with small molecules to specifically perturb cellular functions is commonly referred to as chemical biology. Small molecules are used commercially as drugs, herbicides, and fungicides in different systems, but in recent years they are increasingly exploited as tools for basic research. For instance, chemical genetics involves the discovery of small-molecule effectors of various cellular functions through screens of compound libraries. Whereas the drug discovery field has largely been driven by target-based screening approaches followed by drug optimization, chemical genetics in plant systems tends to be fueled by more general phenotype-based screens, opening the possibility to identify a wide range of small molecules that are not necessarily directly linked to the process of interest. Here, we provide an overview of the current progress in chemical genetics in plants, with a focus on the discoveries regarding small molecules identified in screens designed with a basic biology perspective. We reflect on the possibilities that lie ahead and discuss some of the potential pitfalls that might be encountered upon adopting a given chemical genetics approach.
Living organisms, from the simplest and smallest to the largest and most complex ones on Earth, are an intricate balance of building blocks that act together to form a working system. Our ability to perturb living systems in a controlled manner has led to the definition of the central dogma in molecular biology, i.e. that DNA functions as the encoded information for protein structure and function through RNA intermediates. This central framework is used to increase our understanding of living systems and, along the way, to discover additional layers of complexity as part of the interactions at play in and around living organisms. One such layer is based on the interactions of small molecules with proteins and other biological macromolecules. Consisting of hormones, metabolites, and molecules found in the surrounding environment, these small molecules are an essential aspect of biological processes and their elaborate fine tuning.
Although the use of small molecules to alter biological systems has a rich history, for example through the medicinal use of plant compounds, its true potential has been recognized only recently. The discovery of penicillin in 1928 marked the onset of an era of increasing ability to utilize, design, and repurpose small molecules to alter biological processes. Well-known examples are medicinal drugs and various herbicides and fungicides, but in the last decade, small molecules have become valuable tools in basic plant research (Surpin and Raikhel, 2004; Hicks and Raikhel, 2009, 2010; Tresch, 2013; Dejonghe and Russinova, 2014; Serrano et al., 2015). Especially, the potential to overcome the obstacles of gene essentiality or high redundancy in gene families has made chemical genetics an attractive alternative to classical genetics approaches. An additional benefit is that the small molecules can be applied in a conditional, reversible, and dose-dependent fashion, thus allowing a temporary perturbation of a biological system. The publicly available small-molecule libraries, and the publication of primary and secondary screens of these libraries (Drakakaki et al., 2011), provide a wide variety of small molecules that plant researchers can choose to characterize in the context of the biological process of interest. Furthermore, for application in plants, small molecules can be adopted from other systems, provided that the target is well conserved.
A major challenge in chemical genetics approaches has been the identification of the target(s) of the small molecules of interest and, thus, elucidation of their mode of action (MoA). One commonly employed method to identify candidate targets is screening, in which a mutagenized plant population, for instance, is monitored for resistance against the selected small molecule. Over the years, other target identification approaches have been adopted, including affinity purification, but the plant field still lags behind the drug discovery field in expertise and applications (Ziegler et al., 2013; Dejonghe and Russinova, 2014). Here, our aim is to provide an overview of the recent progress in plant chemical genetics, including details regarding the tools and approaches used as well as the biological questions addressed and the insights gained. We discuss exciting opportunities and possible future directions and reflect on the challenges that are inherent to adopting a chemical genetics approach in plants.
SMALL-MOLECULE LIBRARIES
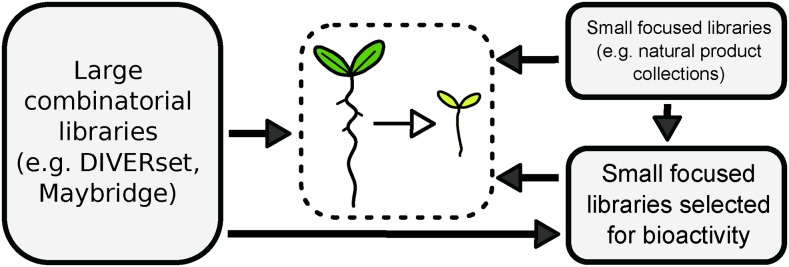
Chemical genetics approaches require carefully designed screening procedures and adequate screening libraries, in terms of compound origin and quantity, followed by a suitable target identification strategy. The different aspects and points to consider in designing a screen have been well reviewed elsewhere (Serrano et al., 2015). Accordingly, we will begin with an overview of the types of small-molecule libraries used over the past years in the plant field (Fig. 1; Box 1). In general, two types of libraries can be distinguished: large, often combinatorial libraries, such as the Chembridge DIVERSet library; and more focused collections, such as the LATCA (Hicks and Raikhel, 2012). By far, the most used library in plant chemical biology is the Chembridge DIVERSet (Armstrong et al., 2004; Zouhar et al., 2004; Surpin et al., 2005; DeBolt et al., 2007; Rojas-Pierce et al., 2007; Christian et al., 2008; Gendron et al., 2008; Savaldi-Goldstein et al., 2008; De Rybel et al., 2009, 2012; Lin et al., 2010; Tsuchiya et al., 2010; Kim et al., 2011; Kerchev et al., 2014; Hu et al., 2016; Van de Wouwer et al., 2016). Chembridge also provides focused or targeted small-molecule libraries (Poretska et al., 2016). Additional libraries include the Maybridge Hitfinder (Nishimura et al., 2012, 2014), the Life Chemicals, Inc., collection (Zhao, 2012; Ye et al., 2016), the Korean Chemical Bank (Kim et al., 2010), and the RIKEN Natural Products Depository (Noutoshi et al., 2012a; Ito et al., 2015). Several smaller libraries also can be combined, resulting in screens of sometimes more than 50,000 small molecules (Drakakaki et al., 2011; Okamoto et al., 2013; Knoth and Eulgem, 2014).
Figure 1.
Relationships between the different types of libraries used in plant chemical genetics. The Library of Active Compounds on Arabidopsis (LATCA) is an example of a focused library, in which small molecules from other types of libraries have been selected for bioactivity.
Most collections adhere to Lipinski’s rule of five (Lipinski et al., 2001), which defines the characteristics ensuring the bioavailability of the small molecules, such as molecular mass and number of H-donor sites. However, these conditions can differ depending on the system in which the small molecules are used (Serrano et al., 2015). In addition, the bioavailability of small molecules does not equate with bioactivity. Large collections, such as the DIVERSet library, offer bioavailable small molecules, but the entire collection is not necessarily bioactive in a given system, because the emphasis in library construction is on providing chemical diversity rather than guaranteeing bioactivity. By contrast, natural product libraries and libraries focused on a given phenotype or biological process of interest are smaller and often selected for bioactivity in a given system. For instance, the LATCA collection consists of 3,600 active small molecules able to inhibit hypocotyl growth in etiolated Arabidopsis (Arabidopsis thaliana) seedlings (Yoneda et al., 2007; Zhao et al., 2007; Schreiber et al., 2008; Park et al., 2009; Abdel-Hamid et al., 2011; Forde et al., 2013; Carland et al., 2016; Okubo-Kurihara et al., 2016; Sakai et al., 2017). For such focused collections, small molecules can originate from a number of different, larger libraries. In the case of the LATCA collection, Chembridge, LOPAC (Sigma-Aldrich), Spectrum (MicroSource), small molecules bioactive in yeast (Maybridge), known herbicides and hormones, and novel compounds (http://www.thecutlerlab.org/2008/05/latca_30.html) are combined. Another example of a focused library is the collection of Plasma Membrane Recycling Set A and Set B (PMRA/B), which contains small molecules with bioactivity directed toward endomembrane trafficking in Arabidopsis derived from the DIVERSet, Novacore (Chembridge), Tim Tec Myria (Sigma-Aldrich), LATCA, and CLICKables (http://www.thecutlerlab.org/2008/05/latca_30.html) libraries (Drakakaki et al., 2011; Rivera-Serrano et al., 2012; Worden et al., 2015).
Other small dedicated libraries include known drugs and natural products, such as the MicroSource Spectrum library (Yoneda et al., 2007; Robert et al., 2008; He et al., 2011; Yoshimoto et al., 2012; Noutoshi et al., 2012a), collections from the Chemical Diversity Research Institute in Moscow (Chuprov-Netochin et al., 2016), and the Analyticon Discovery set (Serrano et al., 2010; Meesters et al., 2014), or molecules that are bioactive in a different model organism, such as the Yeast Active library (Holbrook-Smith et al., 2016). Alternatively, small molecules can be derived from focused libraries based on a chemical scaffold (Khersonsky et al., 2003; Jeong et al., 2015), as part of an industrial collaboration (Serrano et al., 2007), or selected from bacterial cultures (Hayashi et al., 2001, 2003; Yamazoe et al., 2004; Xia et al., 2014). Chemical screens initially carried out in yeast also have been a source for bioactive compounds in plants, such as the screens that identified the inhibitor of the sirtuin family of NAD-dependent deacetylases in Saccharomyces cerevisiae, designated sirtinol (Grozinger et al., 2001; Zhao et al., 2003), and the vacuolar sorting inhibitors, called sortins (Zouhar et al., 2004).
Focused libraries of strong candidates for screening also can be generated by means of computational methods. Recently, Kohonen-based self-organizing maps have been utilized to extract information from experimental data sources and databases of agrochemicals to identify potential candidate molecules (Bushkov et al., 2016). Although experimental validation is essential to select hit molecules, such methods reduce significantly the required chemical space to be assayed physically.
PHENOTYPE-BASED SCREENS IN PLANT CHEMICAL GENETICS
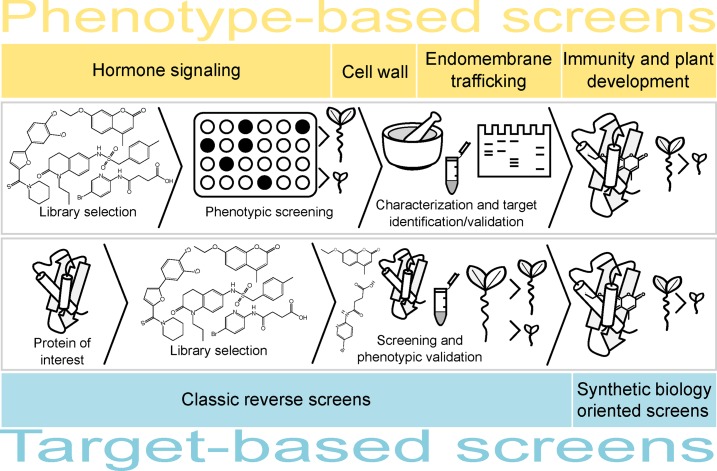
In the past decades, drug discovery and development have relied largely on target-based strategies, in which disease modeling and pathway analysis generate a list of candidate proteins, generally leading to high-throughput biochemical screening. By contrast, efforts to discover small molecules active in plants have been based mainly on empirical approaches, such as phenotype-based screens (Fig. 2; Table I), although in a few examples computational approaches have been explored as well (Schweitzer et al., 2002; Bushkov et al., 2016). Phenotype-based screens can be designed to be general and broadly specific, thus identifying compounds with different MoAs. For example, chemical screens for small molecules that inhibit or promote growth (of the hypocotyl or root) have revealed compounds affecting different hormonal pathways (Gendron et al., 2008; Savaldi-Goldstein et al., 2008). Similarly, a recent screen for compounds that alter leaf vein patterns in Arabidopsis produced chemical hits affecting hormone signaling, endomembrane trafficking, and MAPK function, among others (Carland et al., 2016). The more general phenotype-based screens have been especially valuable for the identification of small molecules acting in signaling cross talk (He et al., 2011; Kim et al., 2011; Ye et al., 2016). For instance, a chemical screen for compounds activating mitochondrial retrograde signaling uncovered an inhibitor of auxin responses, 2-furylacrylic acid (Armstrong et al., 2004; Sungur et al., 2007), exposing an unexpected link between mitochondrial function and auxin signaling (Kerchev et al., 2014).
Figure 2.
Work flow for phenotype- and target-based chemical screens. The size of the bars represents the number of reported small molecules relative to the total number of publications based on the indicated approach or topic.
Table I. Phenotype-based screens in plant chemical biology.
| Assay | Compound Name | MoA (Pathway/Direct Target) | Target Identification Strategy | References |
|---|---|---|---|---|
| Hormone signaling | ||||
| Inhibition of the PS-IAA4/5-GUS reporter in the presence of naphthaleneacetic acid | Yokonolide A, yokonolide B, terfestatin A, compounds A to C, 2-furylacrylic acid | Inhibitors of auxin signaling/not identified | – | Hayashi et al. (2001, 2003); Armstrong et al. (2004); Yamazoe et al. (2005); Sungur et al. (2007) |
| Activation of the pUGT74E2:LUC reporter | 2-Furylacrylic acid | Inhibitor of auxin signaling and mitochondrial function/not identified | – | Kerchev et al. (2014) |
| Inhibition of root elongation | WH1 to WH13 | Activators of auxin signaling/auxins | – | Christian et al. (2008) |
| Inhibition of growth | 2-[4-(Diethylamino)-2-hydroxybenzoyl]benzoic acid | Inhibitor of auxin signaling/ABCB19 | Candidate approach | Kim et al. (2010) |
| Aberrant root development | Rootin | Modifier of the PIN-mediated auxin distribution/not identified | – | Jeong et al. (2015) |
| Inhibition of the gravitropic curvature of maize coleoptiles | Yucasin | Inhibitor of IAA biosynthesis/YUC | Candidate approach | Nishimura et al. (2012, 2014) |
| Inhibition of gravitropism and localization of the GFP:δ-TIP | Gravacin | Auxin transport inhibitor/PGP19 | Compound-resistant screen | Surpin et al. (2005); Rojas-Pierce et al. (2007) |
| Promotion of hypocotyl elongation in det2-1 mutant/modifiers of acl5 mutant/rescue of the cat2 mutant | Proauxins | Activators of auxin signaling/proauxins | – | Savaldi-Goldstein et al. (2008); Yoshimoto et al. (2012); Kerchev et al. (2015) |
| Activation of the CYCB-GUS reporter in xylem pole pericycle cells | Naxillin | Promotion of IBA-to-IAA conversion/not identified | Compound- resistant screen | De Rybel et al. (2012) |
| Inhibition of the constitutive ethylene response phenotypes eto1-2 and ctr1-1 | l-Kynurenine | Inhibitor of the indole-3-pyruvic acid pathway of auxin biosynthesis/TAA1, TARs | Candidate approach | He et al. (2011) |
| Inhibition of the ethylene response in etiolated eto1-4 seedlings | Acsinones (9370, 9393, and 73033) | Inhibitors of ethylene biosynthesis/ACC | Candidate approach and compound- resistant screen | Lin et al. (2010); Chen et al. (2013) |
| Inhibition of hypocotyl elongation in dark | Brassinopride | Inhibitor of brassinosteroid biosynthesis/not identified | – | Gendron et al. (2008) |
| Promotion of hypocotyl elongation in light | Bikinin | Activating brassinosteroid signaling/BIN2 | Candidate approach | De Rybel et al. (2009) |
| Repressors of LOX2p < LUC expression | Jarin-1 | Inhibitor of jasmonate responses/JAR1 | Candidate approach | Meesters et al. (2014) |
| Inhibition of cotyledon expansion and greening after germination | Cotylimides | Inhibitors of strigolactone biosynthesis/not identified | – | Tsuchiya et al. (2010) |
| Suppression of hypocotyl inhibition by GR24 in light | Soporidine | Inhibition of strigolactone signaling/HTL, KAI2 | Candidate approach | Holbrook-Smith et al. (2016) |
| Inhibition of seed germination and hypocotyl growth in dark | Hypostatin/Glc-hypostatin | Inhibition of cell expansion/not identified | Compound-resistant screen | Zhao et al. (2007) |
| Inhibition of seed germination and hypocotyl growth in dark | Pyrabactin | Selective ABA agonist/PYR, PYLs | Compound-resistant screen | Zhao et al. (2007); Park et al. (2009) |
| Inhibition of seed germination | Germostatin | Activator of auxin signaling/not identified | Compound-resistant screen | Ye et al. (2016) |
| Cell wall homeostasis | ||||
| Organ swelling | Morlin | Altered movement of CESA/not identified | – | DeBolt et al. (2007) |
| Cell swelling in tobacco suspensions expressing GFP-α-tubulin | SS compounds, cobtorin, lasalocid sodium | Altered cell wall properties through affecting microtubule dynamic and enzymatic saccharification/not identified | Compound-resistant screen | Yoneda et al. (2007, 2010); Okubo-Kurihara et al. (2016) |
| Induction of polyploidy in H2B-YFP-expressing cells | C17 | Inhibition of cellulose biosynthesis/not identified | Compound-resistant screen | Hu et al. (2016) |
| Inhibition of pollen germination and growth | Cestrin | Altered movement of CESA/not identified | – | Drakakaki et al. (2011); Worden et al. (2015) |
| Inhibitors of lignin deposition | 39 compounds, p-iodobenzoic acid | Inhibitor of the phenylpropanoid pathway/Cinnamate 4-hydroxylase | Candidate approach | Van de Wouwer et al. (2016) |
| Endomembrane trafficking | ||||
| Inhibition of pollen germination and pollen tube growth/effectors of the circadian clock | ES1/(Prieurianin) | Actin cytoskeleton inhibitor/not identified | – | Robert et al. (2008); Tóth et al. (2012) |
| Inhibition of pollen germination and pollen tube growth | ES2 | Exocytosis inhibitor/EXO70 | Affinity pull down | Drakakaki et al. (2011); Zhang et al. (2016) |
| Inhibition of pollen germination and pollen tube growth | ES3, ES5 | Trafficking modifiers/not identified | – | Drakakaki et al. (2011) |
| Inhibition of pollen germination and pollen tube growth | ES7 | Late cytokinesis inhibitor/not identified | – | Drakakaki et al. (2011); Park et al. (2014) |
| Inhibition of pollen germination and pollen tube growth | ES8 | Basal polarity effector/not identified | – | Drakakaki et al. (2011); Doyle et al. (2015) |
| Inhibition of pollen germination and pollen tube growth | ES9 | Endocytosis inhibitor/protonophore | Candidate approach | Drakakaki et al. (2011); Dejonghe et al. (2016) |
| Inhibition of pollen germination and pollen tube growth | ES16 | Apical polarity effector/RabA | Candidate approach | Drakakaki et al. (2011); Li et al., (2017) |
| Inhibition of pollen germination and pollen tube growth | C834 | Vacuolar trafficking inhibitor/not identified | – | Drakakaki et al. (2011); Rivera-Serrano et al. (2012) |
| Inhibition of pollen germination and pollen tube growth | 22 compounds | Inhibitory or stimulatory effects in pollen tube and root growth assays/not identified | – | Chuprov-Netochin et al. (2016) |
| Affected gravitropism and localization of the GFP:δ-TIP | TE1 | Trafficking modifier/not identified | – | Surpin et al. (2005); Paudyal et al. (2014) |
| Affected gravitropism and localization of the GFP:δ-TIP | LDS-003655 | PTS1 and PTS2 peroxisome matrix import pathways/not identified/ | – | Surpin et al. (2005); Brown et al. (2011) |
| Immunity and cell death | ||||
| Modifiers of elicitor-responsive gene expression | Oxytriazine, fluazinam, cantharidin, enpiclonil | Pathogen-associated molecular pattern-triggered innate immune responses/not identified | – | Serrano et al. (2007) |
| Modifiers of elicitor-responsive gene expression | Triclosan | Pathogen-associated molecular pattern-triggered innate immune responses/MOD1 | Candidate approach | Serrano et al. (2007) |
| Suppressors of AvrRPM1-RPM1-dependent cell death | 4,15-Diacetoxyscirpenol, neosolaniol | Inhibition of AvrRpm1 synthesis/not identified | – | Serrano et al. (2010) |
| Enhanced Pst-avrRpm1-induced cell death | Imprimatin A and B | Salicylic acid metabolism/UGT74F1 and UGT76B1 | Candidate approach | Noutoshi et al. (2012b) |
| Activation of the pathogen-responsive reporter CaBP22p::GUS | 3,5-Dichloroanthranilic acid, 2-(5-bromo-2-hydroxy-phenyl)-thiazolidine-4-carboxylic acid | Activation of plant defense signaling/not identified | – | Knoth et al. (2009); Rodriguez-Salus et al. (2016) |
| Resistance to Pseudomonas syringae/enhanced Pst-avrRpm1-induced cell death | Sulfonamides | Not determined/not identified | – | Schreiber et al. (2008); Noutoshi et al. (2012a) |
| Suppression of the ABA-induced RAB18 reporter | [5-(3,4-Dichlorophenyl) furan-2-yl]-piperidine-1-ylmethanethione (DFPM) | Activation of plant immunity via Ca2+ signaling/not identified | – | Kim et al. (2011, 2012); Kunz et al. (2016) |
| Suppression of CYCLIC NUCLEOTIDE-GATED ION CHANNEL11 (AtCNGC11) and AtCNGC12 gain-of-function mutant cpr22-induced lethality | Diethylstilbestrol, erythrosin B, dibucaine | Ca2+ channel inhibition/not identified | – | Abdel-Hamid et al. (2011) |
| Plant development | ||||
| Vein pattern effectors | PATI-1 to PATI-4 | PATI-1 and PATI-2 inhibitors of PIN2 cycling/not identified | – | Carland et al. (2016) |
| Vein pattern effectors | HYVP-1 to HYVP-3 | Agonists of auxin transport/not identified | – | Carland et al. (2016) |
| Vein pattern effectors | OVP-1 and OVP-2 | OVP-1 targets BR signaling/not identified | – | Carland et al. (2016) |
| OVP-2 targets MAPKKK signaling/not identified | – | |||
| Rescue or mimic the amp1 phenotypes | Hyperphyllin | AMP1 regulatory pathway/not identified | – | Poretska et al.. (2016) |
| Stomatal patterning effectors | Bubblin | Inhibits stomatal asymmetric division/not identified | – | Sakai et al., (2017) |
In cases in which more specificity is desired in the screen, very often changes were monitored in either a particular reporter line or a specific mutant phenotype (Hayashi et al., 2001; De Rybel et al., 2012; Meesters et al., 2014). Examples of the latter are the discoveries of the compounds hyperphyllin and bubblin (Poretska et al., 2016; Sakai et al., 2017). Application of hyperphyllin mimicked the phenotype of the altered meristem program1 (amp1) mutant, whereas bubblin simulated stomatal clustering mutants. Although the targets of these compounds have not been fully characterized, hyperphyllin has an AMP1-related MoA, whereas bubblin affects the polar localization of BREAKING OF ASYMMETRY IN THE STOMATAL LINEAGE, making them useful tools for the study of the function of the M28 family of carboxypeptidases and cell polarity establishment during stomata formation in plants, respectively. Overall, screens for compounds that mirror a particular mutant have been beneficial in contributing small molecules with specific MoAs.
Hormone Signaling Pathways
A major area of interest in plant chemical genetics is the discovery of activators or inhibitors of different hormone signaling pathways (Fonseca et al., 2014; Rigal et al., 2014). Here, we review some important examples of phenotype-based screens that have led to the identification of small molecules affecting the signaling pathways for hormones, including auxin, abscisic acid (ABA), brassinosteroid (BR), ethylene, jasmonic acid (JA), and strigolactone (SL). One of the first phenotype-based screens relied on the ability of small molecules to suppress the Arabidopsis BA3 line harboring a GUS reporter controlled by the promoter of the indole-3-acetic acid (IAA)-inducible gene of pea (Pisum sativum), PS-IAA4/5. This line was used successfully to screen metabolites isolated from different Streptomyces species to uncover the specific auxin-signaling inhibitors yokonolide A, yokonolide B, and terfestatin A (Hayashi et al., 2001, 2003; Yamazoe et al., 2005). With the same strategy and reporter line, screening of a combinatorial library yielded structurally different auxin response inhibitors (Armstrong et al., 2004). Phenotypic screens based on the inhibition of growth or gravitropic responses in either Arabidopsis roots or maize (Zea mays) coleoptiles have been used to discover not only auxin-like molecules (Christian et al., 2008), but also inhibitors of auxin transport (gravacin and rootin; Rojas-Pierce et al., 2007; Jeong et al., 2015), signaling (2-[4-(diethylamino)-2-hydroxybenzoyl]benzoic acid; Kim et al., 2010), and biosynthesis (yucasin; Nishimura et al., 2014).
Screens for small molecules that inhibit seed germination or early seedling development have been instrumental in the discovery of chemical modifiers of ABA and SL signaling pathways, including the ABA agonist pyrabactin (Zhao et al., 2007; Park et al., 2009) and the SL biosynthesis inhibitors cotylimides (Tsuchiya et al., 2010). Seed germination inhibitory screens have led to the identification of hypostatins, cell expansion inhibitors with an unknown MoA (Zhao et al., 2007), and auxin response-promoting compounds, such as germostatin (Ye et al., 2016).
Other hormonal pathways have been the subjects of inhibitory screens. The small molecule brassinopride was found as an nontriazole BR biosynthesis inhibitor in a screen for small molecules that inhibit the hypocotyl elongation in dark-grown seedlings and activate the expression of the CONSTITUTIVE PHOTOMORPHOGENESIS AND DWARFISM-GUS reporter construct (Gendron et al., 2008). The predominant screening strategy for the ethylene pathway focuses on the suppression of the dark photomorphogenic phenotypes of mutants displaying constitutive ethylene responses, such as ethylene overproducer1-4 (eto1-4), eto1-2, and constitutive triple response1-1 (ctr1-1), which has enabled the detection of several inhibitors of ethylene biosynthesis and signaling, named acsinones (Lin et al., 2010), and the auxin biosynthesis inhibitor l-kynurenine (He et al., 2011). A screening strategy based on repression of the inducible expression of the JA marker gene LIPOXYGENASE2 (LOX2) identified the JA signaling inhibitor jarin-1 (Meesters et al., 2014).
Phenotypic screen designs focused on restoring or promoting a particular growth phenotype or transcriptional readout have contributed to the discovery of useful small-molecule modifiers of hormonal signaling pathways. For instance, the plant GSK3 kinase inhibitor bikinin was identified by screening for the promotion of constitutive BR responses in light (De Rybel et al., 2009). A very similar screen for small molecules inducing hypocotyl elongation in the BR-deficient mutant deetiolated2-1 (det2-1) in the dark revealed the growth-promoting compounds proauxins (Savaldi-Goldstein et al., 2008), which have been found in two unrelated chemical screens, one for modulators of xylem differentiation in the acaulis5 (acl5) mutant (Yoshimoto et al., 2012) and one for inhibitors of the photorespiratory phenotype of the catalase2 (cat2) mutant under photorespiration-promoting conditions (Kerchev et al., 2015). A screen for small molecules promoting lateral root development based on the ability to activate the B1-type cyclin (CYCB1)-GUS reporter specifically in xylem pole pericycle cells revealed the nonauxin small molecule naxillin. Naxillin enables the conversion of the auxin precursor indole-3-butyric acid (IBA) into IAA in the root cap and promotes lateral root formation (De Rybel et al., 2012). Screening for small molecules that restore the elongated hypocotyl phenotype in light caused by the ectopic expression of CONSTITUTIVE PHOTOMORPHOGENIC1 in the presence of SLs yielded a group of small molecules, designated as REDUCED GERMINATION (RG) compounds (Holbrook-Smith et al., 2016). The most potent of these RG compounds, soporidine, acted as an antagonist of SL signaling and SL-mediated Striga hermonthica germination. In summary, chemical genetics has contributed to the identification of numerous small molecules affecting most of the hormone signaling pathways in plants through phenotype-based screens designed to either suppress or promote selected phenotypes. In all cases, increasing the screening specificity through the use of specialized reporters has yielded compounds with preferential MoAs.
Endomembrane Trafficking
Because endomembrane trafficking plays a key role in plant growth, development, and adaptation to different stresses through regulation of hormone homeostasis and signaling, chemical genetics has been extensively used to identify small molecule modifiers of different trafficking routes (Hicks and Raikhel, 2010). In addition, the complex organization of the endomembrane system points to a need for chemical tools that can specifically and reversibly probe different vesicle transport pathways and overcome redundancy and lethality (Mishev et al., 2013). One successful screening strategy was based on the inhibition of tobacco (Nicotiana tabacum) pollen germination and pollen tube growth, both processes requiring active trafficking and allowing screening in a high-throughput fashion (Robert et al., 2008; Drakakaki et al., 2011; Chuprov-Netochin et al., 2016). These screens provided the community with 360 small molecules (PMRA/B sets) altering different aspects of endomembrane trafficking (Drakakaki et al., 2011) and other potentially plant growth-modulating compounds (Chuprov-Netochin et al., 2016). Many of these small molecules affecting endomembrane trafficking carry the Endosidin (ES) moniker (Robert et al., 2008). ES1, also named Prieurianin, was identified as an early endosomal compartment inhibitor (Robert et al., 2008) and later, also as stabilizer of the actin cytoskeleton (Tóth et al., 2012). The small molecules ES3, ES5, and ES7 affect cell polarity, vacuolar targeting and recycling, and callose deposition during cell plate maturation, respectively (Drakakaki et al., 2011; Park et al., 2014). Recently, ES2 has been found to inhibit exocytosis in plants and human cells and to target the EXO70 subunit of the exocyst complex (Zhang et al., 2016). ES8 affects secretory pathways, exclusively toward the basal plasma membrane of the cell, thereby affecting PIN-FORMED1 trafficking and auxin distribution (Doyle et al., 2015), whereas ES16 specifically perturbs apically localized proteins through regulation of the small GTPase RabA proteins (Li et al., 2017). ES9, which was identified as an inhibitor of endocytosis in different systems (Dejonghe et al., 2016), affected endomembrane dynamics, such as Golgi compartment movements, and depleted cellular ATP. These observations led to the classification of ES9 as a protonophoric small molecule that disrupts the proton balance throughout the cell. Secondary screens of the PMRA/B sets yielded the small molecules Cestrin (Worden et al., 2015) and C834 (Rivera-Serrano et al., 2012). Another screening strategy that revealed chemical effectors of the plant endomembrane trafficking is represented by a screen for small molecules affecting gravitropic responses of Arabidopsis seedlings, followed by selection of compounds that alter the localization of the GFP:δ-TIP marker (Surpin et al., 2005). This screen identified the endocytosis inhibitor TENin1 (Paudyal et al., 2014) and the peroxisomal protein import inhibitor LDS-003655 (Brown et al., 2011). By and large, the identification of chemical modifiers of plant endomembranes requires robust primary screens, most often designed to inhibit phenotypes dependent of intercellular trafficking (Surpin et al., 2005; Robert et al., 2008; Drakakaki et al., 2011). These screens are then followed by an image-based examination of different fluorescently labeled endomembrane markers to visualize the effect of the compound on various intracellular compartments. The meaningful use of such small molecules in research strongly requires knowledge of the MoAs.
Cell Wall Homeostasis
The use of small molecules to modify plant cell wall properties is an attractive strategy for increasing the plant biomass that relies on the accumulation of biopolymers, such as cellulose, hemicellulose, and lignin in the cell wall (Himmel et al., 2007). Additionally, cellulose biosynthesis inhibitors, found in diverse screens of combinatorial and natural chemical libraries of microbial agents, have been applied in agriculture as herbicides (Tateno et al., 2016). Some of these inhibitors either target cellulose biosynthesis directly or affect the cortical cytoskeleton by inhibiting microtubule or actin dynamics (Brabham and DeBolt, 2013). Recent screens for chemical cellulose synthesis inhibitors have been designed based on the observation that the depletion of cellulose microfibrils results in a decreased anisotropy of cell wall expansion and an inability to achieve a differentiated cell shape (DeBolt et al., 2007; Yoneda et al., 2007). Consequently, screens for compounds that caused the swelling of Arabidopsis seedling organs or altered the cell morphology of Bright Yellow-2 tobacco cells uncovered the cell wall modifier morlin, affecting microtubule dynamics (DeBolt et al., 2007), the spherical swelling (SS) compounds (Yoneda et al., 2007), and the carboxylic acid ionophore lasalocid sodium (Okubo-Kurihara et al., 2016). Whereas one of the SS compounds, designated cobtorin, perturbs the parallel relationship between cortical microtubules and nascent cellulose microfibrils (Yoneda et al., 2010), lasalocid sodium induces cell wall loosening by enzymatic saccharification enhancement (Okubo-Kurihara et al., 2016). The endomembrane inhibitor cestrin disrupts cellulose synthase complex trafficking and affects cellulose deposition (Worden et al., 2015). A phenotypic screen utilizing ploidy level measurements to find novel cell division-interfering compounds identified the compound C17, which increased polyploidy as a result of cytokinesis inhibition. Interestingly, C17 was further characterized as a cellulose synthase inhibitor that also interfered with mitochondrial retrograde signaling (Hu et al., 2016). Another chemical screen for inhibitors of the lignin biosynthetic pathway based on reduced lignin accumulation in the presence of the cellulose synthesis inhibitor isoxaben revealed 39 small molecules that cause major perturbations in the phenylpropanoid pathway. One compound was processed in plant cells to p-iodobenzoic acid, which was further characterized as a new inhibitor of cinnamate 4-hydroxylase, a key enzyme of the phenylpropanoid pathway for the synthesis of the lignin polymer building blocks (Van de Wouwer et al., 2016). Overall, chemical screens designed to identify cell wall modifiers have yielded useful molecules, although several interesting compounds have been discovered through unrelated phenotype-based screens, such as screening for trafficking or ploidy modifiers (Worden et al., 2015; Hu et al., 2016).
Immunity and Cell Death
Chemical phenotype-based screens have been designed to discover substances that interfere (via inhibition or induction) with defense reactions related to pattern-triggered immunity (Jones and Dangl, 2006). These screens have isolated many small molecules synthesized by Fusarium spp. and other fungal species, namely oxytriazine, triclosan, fluazinam, cantharidin, enpiclonil, two trichothecene-type mycotoxins, 4,15-diacetoxyscirpenol, and neosolaniol (Serrano et al., 2007, 2010). A high-throughput screen for compounds that facilitate pathogen-activated cell death in cultured suspension cells of Arabidopsis revealed the imprimatins that prime immune responses and enhance disease resistance against Pseudomonas syringae in Arabidopsis through the possible inhibition of salicylic acid glucosyltransferases (Noutoshi et al., 2012b).
Different classes of sulfonamides were discovered through two other screens for small molecules that either protect Arabidopsis from infection by P. syringae or enhance the avirulent P. syringae-induced cell death of Arabidopsis suspension cell cultures (Schreiber et al., 2008; Noutoshi et al., 2012a), with still unknown MoAs. The induction of the pathogen-responsive reporter CaBP222−333::GUS was used to identify the compounds 3,5-dicholoroanthranilic acid and 2-(5-bromo-2-hydroxy-phenyl)-thiazolidine-4-carboxylic acid, both triggering disease resistance against pathogens in Arabidopsis (Knoth et al., 2009; Rodriguez-Salus et al., 2016). The small molecule [5-(3,4-dichlorophenyl)furan-2-yl]-piperidine-1-ylmethanethione (DFPM) was detected via a chemical screen for the inhibition of ABA-dependent gene expression and ABA-induced stomatal closure. DFPM activates plant immunity to disrupt ABA signal transduction at the Ca2+ signaling level (Kim et al., 2011). Another screen for suppression of the lethality of constitutive expresser of PR genes22 (cpr22; a gain-of-function mutation of AtCNGC11 and AtCNGC12 of Arabidopsis) also uncovered Ca2+ channel blockers and compounds that probably indirectly affect the Ca2+ channel activity (Abdel-Hamid et al., 2011). Altogether, chemical genetics has been useful for the study of plant-pathogen interactions and for the plant immunity field. The identified compounds are attractive for basic research because they allow functional dissection of the plant immune system and for applied purposes because they can protect crop plants from diseases (Mott et al., 2014).
TARGET IDENTIFICATION
Although small molecules can be useful tools for dissecting a particular biochemical or signal transduction pathway without knowledge of the direct target, as demonstrated by the use of naxillin (De Rybel et al., 2012), the development of specific chemical probes with applications in basic research and commercial approaches requires a thorough understanding of the MoA and target protein(s). The recent example of the endocytosis inhibitor tyrphostin A23 (TyrA23) highlights the necessity for detailed characterization and target identification (Dejonghe et al., 2016). TyrA23, originally identified as a Tyr kinase inhibitor of the epidermal growth factor receptor, was later described as a specific inhibitor of the interaction between the receptor and the clathrin machinery (Yaish et al., 1988; Banbury et al., 2003). As such, TyrA23 has been used as a specific inhibitor of clathrin-mediated endocytosis in plant cells (Dejonghe et al., 2016). However, the endocytosis-inhibiting activity of TyrA23 has been found to be due to its protonophoric characteristics, causing cytosolic acidification and endocytic block (Dejonghe et al., 2016). Therefore, TyrA23 cannot be used as a specific inhibitor of clathrin-mediated endocytosis.
Even though phenotypic screenings present several advantages, such as compliance with Lipinski’s rule of 5 in the detected compounds, ensuring their bioavailability, and the potential to uncover small molecules with novel, previously unknown MoAs, this approach is more useful when combined with robust MoA analysis. MoA studies are a critical complement to phenotypic screening and can be developed at the molecular (such as target identification), biochemical, or cellular level (Swinney, 2013). A serious disadvantage in plant chemical genetics is the lack of implementation of robust MoA technologies, as in the drug discovery field (Wagner and Schreiber, 2016). Most target identification strategies in plant chemical genetics adopt a candidate approach or screening for compound-resistant mutants (Tresch, 2013; Dejonghe and Russinova, 2014). Although screening for compound-resistant mutants often is the preferred strategy, surprisingly, thus far, the most successful target identification has been obtained with the candidate approach. This approach relies on the careful characterization of phenotypes that will point to a testable number of candidates and on deep knowledge of either the signaling pathway or the process of interest. In this manner, the direct targets of small molecules such as yucasin, l-kynurenine, bikinin, acsinones, jarin-1, and soporidine have been identified (Table I). Very often, these targets are enzymes that can be produced easily in vitro, and their activity toward known substrates can be tested in the presence of the compound. Although proven to be successful, this strategy focuses on targets involved in or related to the phenotype of interest. A conceivable shortfall of this approach is the potential failure to uncover off-targets (Moffat et al., 2014), especially for small molecules originating from combinatorial libraries.
Compound-insensitive screens with ethyl methanesulfonate-mutagenized Arabidopsis populations have been informative in identifying the compound MoA at the biochemical pathway level, but they do not always reveal the direct target. For instance, the screen for germostatin-resistant mutants revealed a mutation in the GERMOSTATIN RESISTANCE1 (GRS1) gene. This GRS1 allele encodes a mutation in the PHD finger protein GSR1, which interacts physically with IAA17 and ADP-RIBOSYLATION FACTOR10 (ARF10)/ARF16 to form a corepressor complex and to enhance auxin signaling, but it is not a direct target of germostatin (Ye et al., 2016). A screen for naxillin-resistant mutants uncovered the NAXILLIN RESISTANT1 locus containing INDOLE-3-BUTYRIC ACID RESPONSE3, which codes for a protein implicated in the peroxisomal β-oxidation pathway in which IBA is converted into IAA17, confirming that a defect in this step is responsible for the naxillin resistance phenotype (De Rybel et al., 2012). In another screen, hypostatin resistance led to the identification of the glycoactivating enzymes but not to the direct target of the glycosylated hypostatin (Zhao et al., 2007). A screen for resistance to the ethylene biosynthesis inhibitor acsinone7303 detected 19 Arabidopsis mutants, designated revert to eto1, of which two carried mutations in the CELLULOSE SYNTHASE6 (CESA6) and DET2 genes, but none were direct targets (Chen et al., 2013). Similarly, in addition to mutations in CESA1 and CESA3, a forward genetic screen found two independent defective genes that encode pentatricopeptide repeat-like proteins and confer tolerance to C17 (Hu et al., 2016).
In the case of cobtorin, a full-length cDNA overexpression gene library from Arabidopsis was used to screen for the suppression of the cobtorin-induced phenotypes. Overexpression of two pectin modulation enzymes led to cobtorin resistance, but cobtorin did not bind any of them directly (Yoneda et al., 2010). Direct targets of only two compounds, gravicin and pyrabactin, have been identified successfully through compound-insensitive screens. In a screen for gravacin-resistant mutants, a member of the superfamily of ATP-binding cassette transporters, P-GLYCOPROTEIN19 (PGP19) was detected. Studies with PGP19-containing membranes indicated that gravacin binds PGP19 and inhibits auxin transport (Rojas-Pierce et al., 2007). Similarly, the pyrabactin targets PYRABACTIN RESISTANT1 (PYR1) and PYR1-LIKEs (PYLs) also were discovered in a pyrabactin insensitivity screen (Park et al., 2009).
Biochemical affinity purification is the most direct approach to find target and off-target proteins, given that the small-molecule hit can be modified without activity loss. This method often is used in drug discovery (Ziegler et al., 2013), but is less popular in plant chemical genetics (Dejonghe and Russinova, 2014). One of the few recent examples of affinity-based target identification in plant chemical biology was obtained from the ES2 MoA study, in which a biotin-tagged ES2 analog was used to identify EXO70 as an ES2 target (Zhang et al., 2016). Despite recent progress, direct target identification still remains the most challenging, and painstaking, step in chemical genetics biology projects. Although no single method is satisfactory, future success will require vigorous implementation of affinity-based and label-free methods in combination with genetics and exploit existing detailed knowledge of cellular pathways and processes.
TARGET-BASED SCREENS AND SYNTHETIC BIOLOGY
Although phenotype-based screens allow the identification of a wide range of small-molecule hits, often with an equally broad range of MoAs, they are less suitable for finding new analogs or hit molecules aimed at one specific target or target group. For these purposes, target-based screens or reverse-chemical genetics approaches are more appropriate, but only a few have been reported in plants; for instance, a screen for small-molecule binders of an Arabidopsis protein with unknown function was combined with in silico screening of the SPECS compound database and surface plasmon resonance for hit selection (Yoshitani et al., 2005). A notable advantage of initial in silico screening is the size of the small-molecule libraries that can be screened without the need for physical handling of the small molecules in a high-throughput fashion. Another example concerns the search for small-molecule inhibitors of Glc incorporation in cell wall polysaccharides (Zabotina et al., 2008). As most enzymes involved in polysaccharide synthesis are located in the Golgi apparatus, the screening procedure has been focused on its oligosaccharide content rather than on a particular enzyme or group of enzymes.
Several reverse screens have been based on the ABA receptor PYR1 and closely related PYLs. In a search for novel ABA agonists and antagonists (Ito et al., 2015), close to 25,000 small molecules from the RIKEN Natural Products Depository database were screened for interactions with recombinant Arabidopsis PYR1 fused to either glutathione S-transferase or His tags through a chemical array technique (MacBeath et al., 1999). A secondary screen for activation or inactivation of the clade III Ser/Thr-related protein kinase 2 confirmed hits from the primary screen as agonists or antagonists of ABA signaling.
In another screen, PYR1 and the dimeric ABA receptors PYL1, PYL2, and PYL3 were found to be targets of the ABA agonist quinabactin through a receptor activation-reporting yeast two-hybrid screen (Okamoto et al., 2013). Quinabactin application complemented the ABA-deficient phenotypes at the levels of seed germination, vegetative growth, and drought tolerance in various plant species, indicating that the activation of the dimeric receptors accounts for the majority of the ABA-mediated responses. The effects of quinabactin highlight the requirement for the dimeric receptors in ABA-mediated plant water use. Thus, activators of PYR1 and its close relatives could potentially be developed into agrochemicals to control the water use of crops.
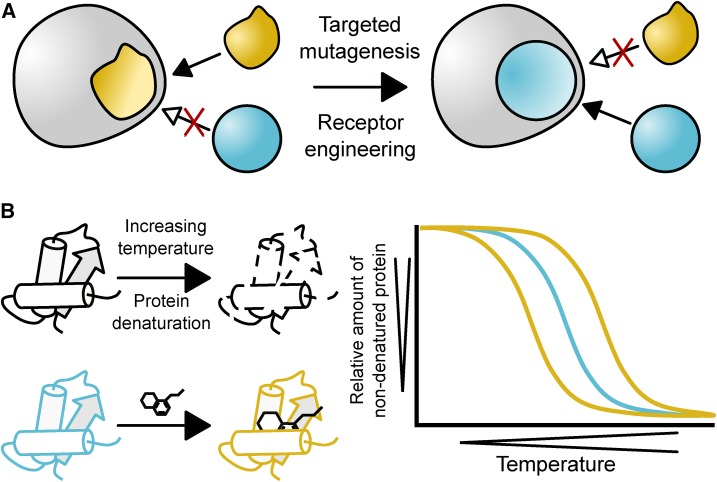
Importantly, instead of screening a large library of small molecules, a collection of receptor mutants could be screened for their activation by nonherbicidal agrochemicals. This idea was the impetus for a PYR1-focused screen involving a mutant collection covering all possible amino acid combinations for the residues lining the PYR1 ABA-binding pocket (Park et al., 2015). The fungicide mandipropamid bound with low affinity to a particular mutant that was further improved by targeted mutagenesis, resulting in a nanomolar affinity of mandipropamid in the engineered PYR1 receptor. Crucially, the conserved and essential Lys residue needed for ABA responsiveness was substituted by an Arg, rendering the engineered PYR1 receptor responsive to mandipropamid, but not to ABA, and creating an orthogonal ligand-receptor system (Park et al., 2015). In addition, because overexpression of wild-type ABA receptors results in decreased yields (Kim et al., 2014), substitution of the essential Lys residue ensured that the overexpressed engineered PYR1 failed to have negative consequences for plant growth (Park et al., 2015). Orthogonal receptor-ligand pairs are designed to become unresponsive to endogenous signaling cues, such as ABA in the case of the engineered PYR1 receptor (Fig. 3A). Yet, the engineered receptor remains capable of activating its endogenous downstream targets upon binding its orthogonal ligand, which, in turn, is inactive in all other pathways within the biological system of interest (Voß et al., 2015).
Figure 3.
Principle of orthogonal receptor-ligand pairs and the cellular thermal shift assay (CETSA). A, Orthogonal receptor-ligand pairs can be designed from an endogenous receptor, which is capable of binding the endogenous ligand (yellow) but not the ligand of interest (blue), which lacks activity in the biological system of interest. Receptor engineering results in the inability of the endogenous ligand to activate the engineered receptor, which is now activated by the ligand of interest. B, The CETSA principle is based on the small molecule-mediated stabilization or destabilization of the protein target (yellow cartoon and curves) relative to the unbound protein target (blue cartoon and curve), as measured by thermal denaturation of the protein of interest (black cartoons, curves).
Such small molecule-based orthogonal systems represent a well-known and successful approach in mammalian systems and offer a plethora of new possibilities in engineering and controlling biological systems (Erhart et al., 2013; Voß et al., 2015; Kim et al., 2016). Recently, the small-molecule control of therapeutic T cell functions has been studied (Wu et al., 2015). As T cells can recognize and kill cancer cells, they become valuable options as agents in cancer therapy, but the administration of T cells directed toward cancer cells poses the risk of excessive activity and off-target effects with potentially lethal outcomes. Engineered T cells with chimeric antigen receptors controlled by a small molecule allow the chemical regulation of T cell activities in a spatiotemporal manner, thereby importantly reducing the risks of excessive and off-target activity (Wu et al., 2015). Such applications of orthogonal ligand-receptor pairs highlight the potential of synthetic chemical biology approaches, for which the engineered PYR1 receptor is currently the lone example in plant chemical biology.
Although such methods are emerging in plant systems, including fluorescently tagged hormones and metabolites to visualize signaling events (Nemhauser and Torii, 2016), very few small molecule-based orthogonal systems have been described. However, GA and ABA have been used as small molecules in mammalian-based orthogonal chemically induced dimerization (CID) systems (Liang et al., 2011; Miyamoto et al., 2012). Taken together, target-based screens offer the possibility to identify a small molecule ligand for a specific protein of interest and to further improve on small molecule binding. This, in turn, can fuel the development of molecular tools, such as orthogonal receptor-ligand pairs.
FUTURE PERSPECTIVES
Well-characterized small molecules can be valuable tools to temporarily disturb biological systems, to act as important survival-promoting agents, or to enhance overall performance. Although plant chemical genetics has experienced important advances in recent years, certain aspects could benefit from studying chemical biology approaches used in other systems.
One part of the pipeline to advance by application of the current trends in drug discovery is the available chemical space for screening. Theoretically, the chemical space is vast and probably will never be fully exploited. The actual chemical space is variable, but is restricted to the size of libraries that can be handled conveniently in a high-throughput manner, whereas the data sets often consist of a particular kind of bioavailable small molecules, such as diversity-oriented combinatorial libraries (Bajorath, 2016). Yet, in these types of small-molecule libraries, the number of hit molecules that result from screening efforts is disappointingly low. As a consequence, interest in combinatorial libraries is waning and focus is shifting toward fragment-based and natural product libraries (Harvey et al., 2015; Bajorath, 2016). Attempts to purify new natural product-based hit molecules, including small molecules derived from marine sources (Gerwick and Moore, 2012), have gained a renewed attention after a period of relative neglect, thanks to some success stories (Gerwick and Moore, 2012; Harvey et al., 2015). Natural product libraries are particularly interesting in the search for small-molecule inhibitors of protein-protein interactions (Harvey et al., 2015); such libraries could potentially be valuable resources for small-molecule probes of endomembrane trafficking in plants.
Although several libraries provide natural products for chemical screens in plants, most small molecules originate from the DIVERSet library. Despite valuable hits can still be delivered by such libraries, the often planar overall structure of the chemicals due to enrichment in aromatic groups has been recognized as a limiting factor in hit discovery. Current efforts are focused on the identification or generation of small molecules with an increased degree of stereochemistry and overall three-dimensional structure instead of combinatorial collections (Lovering et al., 2009; Harvey et al., 2015; Bajorath, 2016), because such natural product collections have the added advantage of being enriched in bioavailable compounds. An increase in the diversity of various types of libraries, including natural products, would be beneficial for plant chemical biology in the future.
Target identification in plant chemical genetics is another area that would profit from the adoption of different approaches when compared with the standard strategies currently used. Notably, biochemical approaches, such as affinity purification, or label-free approaches, such as the cellular thermal shift assay (CETSA; Martinez Molina et al., 2013), are well suited to identify direct targets, especially at a proteome-wide level. Numerous examples of affinity purifications exist in mammalian systems (Ziegler et al., 2013). The CETSA approach, which is based on the principle that small molecule-protein interactions either stabilize or destabilize the protein, is emerging as a valuable label-free approach capable of identifying off-targets (Savitski et al., 2014; Becher et al., 2016). Stabilization or destabilization can be assessed through the thermal profile of the protein of interest, which reflects the thermal stability and indicates how much protein is not yet denatured at certain temperatures (Fig. 3B). Thus, CETSA provides a label-free and accessible means to assess small-molecule binding to a protein of interest. However, each positive interaction according to the CETSA approach should be carefully confirmed with complementary approaches.
Chemical genetics projects often tend to be long and expensive endeavors, especially when including target identification, validation, and optimization. However, thorough understanding of the MoA and knowledge of the target proteins allow the anticipation of undesirable phenotypes and, eventually, improvement of the small molecule of interest, so that a specific probe can be developed through structure-activity relationship analysis. Current estimates in drug discovery are that small combinatorial compounds have six target proteins on average (Arooj et al., 2015). Thus, small molecules from, for example, the DIVERSet library will most probably have more than one target, potentially complicating the interpretation of observed phenotypes upon small-molecule application. Moreover, the recent example of TyrA23 (Dejonghe et al., 2016) highlights the need for an in-depth analysis of the MoA, which is not necessarily linked to protein targets. Similarly, examples from the mammalian drug discovery field, in which small-molecule hits have been found to additionally affect mitochondrial function (Moffat et al., 2014; Wallace, 2015), emphasize the need for a thorough analysis of off-target effects. The study of multitarget small molecules, or polypharmacology, can highlight possible off-target and toxic effects as part of the MoA and redirect small molecules as well. Currently, in silico attempts show great potential in the identification of multitarget small molecules (Lavecchia and Cerchia, 2016) and can be complemented by biochemical methods, such as CETSA and affinity purification. Taken together, full characterization and target identification should be considered indispensable for small molecules to become valuable and to be widely used research tools in the future.
Finally, thorough understanding of the MoA and identification of target proteins, together with crystallographic data on the target proteins, can promote the development of orthogonal ligand-receptor pairs, such as the engineered PYR1-mandipropamid pair (Park et al., 2015). Several small molecules have been used in CID to initiate the dimerization of two protein domains in mammalian systems (DeRose et al., 2013; Voß et al., 2015; Kim et al., 2016), providing blueprints for similar setups in plant systems. One of the best-known examples is the rapamycin-based CID, in which rapamycin acts as the mediator to link the FK506-binding protein (FKBP) and the FKBP rapamycin-binding domain of mTOR (FRB) together. When fused to different proteins of interest, the rapamycin-induced dimerization of FKBP and FRB can be used to relocate proteins or reconstitute signaling cascades in a spatiotemporally controlled manner. As rapamycin application is difficult to reverse and affects endogenous targets, other systems have been developed (DeRose et al., 2013; Voß et al., 2015; Kim et al., 2016), including light-controlled GA3 (Schelkle et al., 2015) and photocaged ABA (Wright et al., 2015). Such orthogonal systems, including light-controlled small molecules, represent a new frontier in chemical biology. They could become invaluable tools in agriculture, as hinted by the engineered PYR1-mandipropamid system (Park et al., 2015), or could act as a specific switch for signaling pathways and transcriptional responses. Taken together, small-molecule application opens up a plethora of possibilities to control biological processes, and in coming years, the use of well-characterized and defined chemical tools will become indispensable in plant research and beyond.
Acknowledgments
We thank Martine De Cock for help in preparing the manuscript.
Glossary
- MoA
mode of action
- LATCA
Library of Active Compounds on Arabidopsis
- ABA
abscisic acid
- BR
brassinosteroid
- JA
jasmonic acid
- SL
strigolactone
- IBA
indole-3-butyric acid
- SS
spherical swelling
- TyrA23
tyrphostin A23
- CID
chemically induced dimerization
Footnotes
Articles can be viewed without a subscription.
References
- Abdel-Hamid H, Chin K, Moeder W, Yoshioka K (2011) High throughput chemical screening supports the involvement of Ca2+ in cyclic nucleotide-gated ion channel-mediated programmed cell death in Arabidopsis. Plant Signal Behav 6: 1817–1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong JI, Yuan S, Dale JM, Tanner VN, Theologis A (2004) Identification of inhibitors of auxin transcriptional activation by means of chemical genetics in Arabidopsis. Proc Natl Acad Sci USA 101: 14978–14983; erratum Armstrong JI, Yuan S, Dale JM, Tanner VN, Theologis A (2004) Proc Natl Acad Sci USA 101: 17565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arooj M, Sakkiah S, Cao GP, Kim S, Arulalapperumal V, Lee KW (2015) Finding off-targets, biological pathways, and target diseases for chymase inhibitors via structure-based systems biology approach. Proteins 83: 1209–1224 [DOI] [PubMed] [Google Scholar]
- Bajorath J. (2016) Extending accessible chemical space for the identification of novel leads. Expert Opin Drug Discov 11: 825–829 [DOI] [PubMed] [Google Scholar]
- Banbury DN, Oakley JD, Sessions RB, Banting G (2003) Tyrphostin A23 inhibits internalization of the transferrin receptor by perturbing the interaction between tyrosine motifs and the medium chain subunit of the AP-2 adaptor complex. J Biol Chem 278: 12022–12028 [DOI] [PubMed] [Google Scholar]
- Becher I, Werner T, Doce C, Zaal EA, Tögel I, Khan CA, Rueger A, Muelbaier M, Salzer E, Berkers CR, et al. (2016) Thermal profiling reveals phenylalanine hydroxylase as an off-target of panobinostat. Nat Chem Biol 12: 908–910 [DOI] [PubMed] [Google Scholar]
- Brabham C, DeBolt S (2013) Chemical genetics to examine cellulose biosynthesis. Front Plant Sci 3: 309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LA, O’Leary-Steele C, Brookes P, Armitage L, Kepinski S, Warriner SL, Baker A (2011) A small molecule with differential effects on the PTS1 and PTS2 peroxisome matrix import pathways. Plant J 65: 980–990 [DOI] [PubMed] [Google Scholar]
- Bushkov NA, Veselov MS, Chuprov-Netochin RN, Marusich EI, Majouga AG, Volynchuk PB, Shumilina DV, Leonov SV, Ivanenkov YA (2016) Computational insight into the chemical space of plant growth regulators. Phytochemistry 122: 254–264 [DOI] [PubMed] [Google Scholar]
- Carland F, Defries A, Cutler S, Nelson T (2016) Novel vein patterns in Arabidopsis induced by small molecules. Plant Physiol 170: 338–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I-J, Lo W-S, Chuang J-Y, Cheuh C-M, Fan Y-S, Lin L-C, Wu S-J, Wang L-C (2013) A chemical genetics approach reveals a role of brassinolide and cellulose synthase in hypocotyl elongation of etiolated Arabidopsis seedlings. Plant Sci 209: 46–57 [DOI] [PubMed] [Google Scholar]
- Christian M, Hannah WB, Lüthen H, Jones AM (2008) Identification of auxins by a chemical genomics approach. J Exp Bot 59: 2757–2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuprov-Netochin R, Neskorodov Y, Marusich E, Mishutkina Y, Volynchuk P, Leonov S, Skryabin K, Ivashenko A, Palme K, Touraev A (2016) Novel small molecule modulators of plant growth and development identified by high-content screening with plant pollen. BMC Plant Biol 16: 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBolt S, Gutierrez R, Ehrhardt DW, Melo CV, Ross L, Cutler SR, Somerville C, Bonetta D (2007) Morlin, an inhibitor of cortical microtubule dynamics and cellulose synthase movement. Proc Natl Acad Sci USA 104: 5854–5859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejonghe W, Kuenen S, Mylle E, Vasileva M, Keech O, Viotti C, Swerts J, Fendrych M, Ortiz-Morea FA, Mishev K, et al. (2016) Mitochondrial uncouplers inhibit clathrin-mediated endocytosis largely through cytoplasmic acidification. Nat Commun 7: 11710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejonghe W, Russinova E (2014) Target identification strategies in plant chemical biology. Front Plant Sci 5: 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRose R, Miyamoto T, Inoue T (2013) Manipulating signaling at will: chemically-inducible dimerization (CID) techniques resolve problems in cell biology. Pflugers Arch 465: 409–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rybel B, Audenaert D, Vert G, Rozhon W, Mayerhofer J, Peelman F, Coutuer S, Denayer T, Jansen L, Nguyen L, et al. (2009) Chemical inhibition of a subset of Arabidopsis thaliana GSK3-like kinases activates brassinosteroid signaling. Chem Biol 16: 594–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rybel B, Audenaert D, Xuan W, Overvoorde P, Strader LC, Kepinski S, Hoye R, Brisbois R, Parizot B, Vanneste S, et al. (2012) A role for the root cap in root branching revealed by the non-auxin probe naxillin. Nat Chem Biol 8: 798–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle SM, Haeger A, Vain T, Rigal A, Viotti C, Łangowska M, Ma Q, Friml J, Raikhel NV, Hicks GR, et al. (2015) An early secretory pathway mediated by GNOM-LIKE 1 and GNOM is essential for basal polarity establishment in Arabidopsis thaliana. Proc Natl Acad Sci USA 112: E806–E815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drakakaki G, Robert S, Szatmari AM, Brown MQ, Nagawa S, Van Damme D, Leonard M, Yang Z, Girke T, Schmid SL, et al. (2011) Clusters of bioactive compounds target dynamic endomembrane networks in vivo. Proc Natl Acad Sci USA 108: 17850–17855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhart D, Zimmermann M, Jacques O, Wittwer MB, Ernst B, Constable E, Zvelebil M, Beaufils F, Wymann MP (2013) Chemical development of intracellular protein heterodimerizers. Chem Biol 20: 549–557 [DOI] [PubMed] [Google Scholar]
- Fonseca S, Rosado A, Vaughan-Hirsch J, Bishopp A, Chini A (2014) Molecular locks and keys: the role of small molecules in phytohormone research. Front Plant Sci 5: 709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde BG, Cutler SR, Zaman N, Krysan PJ (2013) Glutamate signalling via a MEKK1 kinase-dependent pathway induces changes in Arabidopsis root architecture. Plant J 75: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron JM, Haque A, Gendron N, Chang T, Asami T, Wang ZY (2008) Chemical genetic dissection of brassinosteroid-ethylene interaction. Mol Plant 1: 368–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerwick WH, Moore BS (2012) Lessons from the past and charting the future of marine natural products drug discovery and chemical biology. Chem Biol 19: 85–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grozinger CM, Chao ED, Blackwell HE, Moazed D, Schreiber SL (2001) Identification of a class of small molecule inhibitors of the sirtuin family of NAD-dependent deacetylases by phenotypic screening. J Biol Chem 276: 38837–38843 [DOI] [PubMed] [Google Scholar]
- Harvey AL, Edrada-Ebel R, Quinn RJ (2015) The re-emergence of natural products for drug discovery in the genomics era. Nat Rev Drug Discov 14: 111–129 [DOI] [PubMed] [Google Scholar]
- Hayashi K, Jones AM, Ogino K, Yamazoe A, Oono Y, Inoguchi M, Kondo H, Nozaki H (2003) Yokonolide B, a novel inhibitor of auxin action, blocks degradation of AUX/IAA factors. J Biol Chem 278: 23797–23806 [DOI] [PubMed] [Google Scholar]
- Hayashi K, Ogino K, Oono Y, Uchimiya H, Nozaki H (2001) Yokonolide A, a new inhibitor of auxin signal transduction, from Streptomyces diastatochromogenes B59. J Antibiot (Tokyo) 54: 573–581 [DOI] [PubMed] [Google Scholar]
- He W, Brumos J, Li H, Ji Y, Ke M, Gong X, Zeng Q, Li W, Zhang X, An F, et al. (2011) A small-molecule screen identifies l-kynurenine as a competitive inhibitor of TAA1/TAR activity in ethylene-directed auxin biosynthesis and root growth in Arabidopsis. Plant Cell 23: 3944–3960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks GR, Raikhel NV (2009) Opportunities and challenges in plant chemical biology. Nat Chem Biol 5: 268–272 [DOI] [PubMed] [Google Scholar]
- Hicks GR, Raikhel NV (2010) Advances in dissecting endomembrane trafficking with small molecules. Curr Opin Plant Biol 13: 706–713 [DOI] [PubMed] [Google Scholar]
- Hicks GR, Raikhel NV (2012) Small molecules present large opportunities in plant biology. Annu Rev Plant Biol 63: 261–282 [DOI] [PubMed] [Google Scholar]
- Himmel ME, Ding S-Y, Johnson DK, Adney WS, Nimlos MR, Brady JW, Foust TD (2007) Biomass recalcitrance: engineering plants and enzymes for biofuels production. Science 315: 804–807 [DOI] [PubMed] [Google Scholar]
- Holbrook-Smith D, Toh S, Tsuchiya Y, McCourt P (2016) Small-molecule antagonists of germination of the parasitic plant Striga hermonthica. Nat Chem Biol 12: 724–729 [DOI] [PubMed] [Google Scholar]
- Hu Z, Vanderhaeghen R, Cools T, Wang Y, De Clercq I, Leroux O, Nguyen L, Belt K, Millar AH, Audenaert D, et al. (2016) Mitochondrial defects confer tolerance against cellulose deficiency. Plant Cell 28: 2276–2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Kondoh Y, Yoshida K, Umezawa T, Shimizu T, Shinozaki K, Osada H (2015) Novel abscisic acid antagonists identified with chemical array screening. ChemBioChem 16: 2471–2478 [DOI] [PubMed] [Google Scholar]
- Jeong S, Kim JY, Choi H, Kim H, Lee I, Soh MS, Nam HG, Chang YT, Lim PO, Woo HR (2015) Rootin, a compound that inhibits root development through modulating PIN-mediated auxin distribution. Plant Sci 233: 116–126 [DOI] [PubMed] [Google Scholar]
- Jones JD, Dangl JL (2006) The plant immune system. Nature 444: 323–329 [DOI] [PubMed] [Google Scholar]
- Kerchev P, Mühlenbock P, Denecker J, Morreel K, Hoeberichts FA, Van Der Kelen K, Vandorpe M, Nguyen L, Audenaert D, Van Breusegem F (2015) Activation of auxin signalling counteracts photorespiratory H2O2-dependent cell death. Plant Cell Environ 38: 253–265 [DOI] [PubMed] [Google Scholar]
- Kerchev PI, De Clercq I, Denecker J, Mühlenbock P, Kumpf R, Nguyen L, Audenaert D, Dejonghe W, Van Breusegem F (2014) Mitochondrial perturbation negatively affects auxin signaling. Mol Plant 7: 1138–1150 [DOI] [PubMed] [Google Scholar]
- Khersonsky SM, Jung DW, Kang TW, Walsh DP, Moon HS, Jo H, Jacobson EM, Shetty V, Neubert TA, Chang YT (2003) Facilitated forward chemical genetics using a tagged triazine library and zebrafish embryo screening. J Am Chem Soc 125: 11804–11805 [DOI] [PubMed] [Google Scholar]
- Kim AK, DeRose R, Ueno T, Lin B, Komatsu T, Nakamura H, Inoue T (2016) Toward total synthesis of cell function: reconstituting cell dynamics with synthetic biology. Sci Signal 9: re1. [DOI] [PubMed] [Google Scholar]
- Kim H, Lee K, Hwang H, Bhatnagar N, Kim DY, Yoon IS, Byun MO, Kim ST, Jung KH, Kim BG (2014) Overexpression of PYL5 in rice enhances drought tolerance, inhibits growth, and modulates gene expression. J Exp Bot 65: 453–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Henrichs S, Bailly A, Vincenzetti V, Sovero V, Mancuso S, Pollmann S, Kim D, Geisler M, Nam HG (2010) Identification of an ABCB/P-glycoprotein-specific inhibitor of auxin transport by chemical genomics. J Biol Chem 285: 23309–23317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, Hauser F, Ha T, Xue S, Böhmer M, Nishimura N, Munemasa S, Hubbard K, Peine N, Lee BH, et al. (2011) Chemical genetics reveals negative regulation of abscisic acid signaling by a plant immune response pathway. Curr Biol 21: 990–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, Kunz HH, Bhattacharjee S, Hauser F, Park J, Engineer C, Liu A, Ha T, Parker JE, Gassmann W, et al. (2012) Natural variation in small molecule-induced TIR-NB-LRR signaling induces root growth arrest via EDS1- and PAD4-complexed R protein VICTR in Arabidopsis. Plant Cell 24: 5177–5192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoth C, Eulgem T (2014) High-throughput screening of small-molecule libraries for inducers of plant defense responses. Methods Mol Biol 1056: 45–49 [DOI] [PubMed] [Google Scholar]
- Knoth C, Salus MS, Girke T, Eulgem T (2009) The synthetic elicitor 3,5-dichloroanthranilic acid induces NPR1-dependent and NPR1-independent mechanisms of disease resistance in Arabidopsis. Plant Physiol 150: 333–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz HH, Park J, Mevers E, García AV, Highhouse S, Gerwick WH, Parker JE, Schroeder JI (2016) Small molecule DFPM derivative-activated plant resistance protein signaling in roots is unaffected by EDS1 subcellular targeting signal and chemical genetic isolation of victr R-protein mutants. PLoS ONE 11: e0155937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavecchia A, Cerchia C (2016) In silico methods to address polypharmacology: current status, applications and future perspectives. Drug Discov Today 21: 288–298 [DOI] [PubMed] [Google Scholar]
- Li R, Rodriguez-Furlan C, Wang J, van de Ven W, Gao T, Raikhel NV, Hicks GR (2017) Different endomembrane trafficking pathways establish apical and basal polarities. Plant Cell 29: 90–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang F-S, Ho WQ, Crabtree GR (2011) Engineering the ABA plant stress pathway for regulation of induced proximity. Sci Signal 4: rs2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L-C, Hsu J-H, Wang L-C (2010) Identification of novel inhibitors of 1-aminocyclopropane-1-carboxylic acid synthase by chemical screening in Arabidopsis thaliana. J Biol Chem 285: 33445–33456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ (2001) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 46: 3–26 [DOI] [PubMed] [Google Scholar]
- Lovering F, Bikker J, Humblet C (2009) Escape from flatland: increasing saturation as an approach to improving clinical success. J Med Chem 52: 6752–6756 [DOI] [PubMed] [Google Scholar]
- MacBeath G, Koehler AN, Schreiber SL (1999) Printing small molecules as microarrays and detecting protein—ligand interactions en masse. J Am Chem Soc 121: 7967–7968 [Google Scholar]
- Martinez Molina D, Jafari R, Ignatushchenko M, Seki T, Larsson EA, Dan C, Sreekumar L, Cao Y, Nordlund P (2013) Monitoring drug target engagement in cells and tissues using the cellular thermal shift assay. Science 341: 84–87 [DOI] [PubMed] [Google Scholar]
- Meesters C, Mönig T, Oeljeklaus J, Krahn D, Westfall CS, Hause B, Jez JM, Kaiser M, Kombrink E (2014) A chemical inhibitor of jasmonate signaling targets JAR1 in Arabidopsis thaliana. Nat Chem Biol 10: 830–836 [DOI] [PubMed] [Google Scholar]
- Mishev K, Dejonghe W, Russinova E (2013) Small molecules for dissecting endomembrane trafficking: a cross-systems view. Chem Biol 20: 475–486 [DOI] [PubMed] [Google Scholar]
- Miyamoto T, DeRose R, Suarez A, Ueno T, Chen M, Sun T, Wolfgang MJ, Mukherjee C, Meyers DJ, Inoue T (2012) Rapid and orthogonal logic gating with a gibberellin-induced dimerization system. Nat Chem Biol 8: 465–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat JG, Rudolph J, Bailey D (2014) Phenotypic screening in cancer drug discovery: past, present and future. Nat Rev Drug Discov 13: 588–602 [DOI] [PubMed] [Google Scholar]
- Mott GA, Middleton MA, Desveaux D, Guttman DS (2014) Peptides and small molecules of the plant-pathogen apoplastic arena. Front Plant Sci 5: 677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemhauser JL, Torii KU (2016) Plant synthetic biology for molecular engineering of signalling and development. Nat Plants 2: 16010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T, Hayashi K, Suzuki H, Gyohda A, Takaoka C, Sakaguchi Y, Matsumoto S, Kasahara H, Sakai T, Kato J, et al. (2014) Yucasin is a potent inhibitor of YUCCA, a key enzyme in auxin biosynthesis. Plant J 77: 352–366 [DOI] [PubMed] [Google Scholar]
- Nishimura T, Matano N, Morishima T, Kakinuma C, Hayashi K, Komano T, Kubo M, Hasebe M, Kasahara H, Kamiya Y, et al. (2012) Identification of IAA transport inhibitors including compounds affecting cellular PIN trafficking by two chemical screening approaches using maize coleoptile systems. Plant Cell Physiol 53: 1671–1682 [DOI] [PubMed] [Google Scholar]
- Noutoshi Y, Ikeda M, Saito T, Osada H, Shirasu K (2012a) Sulfonamides identified as plant immune-priming compounds in high-throughput chemical screening increase disease resistance in Arabidopsis thaliana. Front Plant Sci 3: 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noutoshi Y, Okazaki M, Kida T, Nishina Y, Morishita Y, Ogawa T, Suzuki H, Shibata D, Jikumaru Y, Hanada A, et al. (2012b) Novel plant immune-priming compounds identified via high-throughput chemical screening target salicylic acid glucosyltransferases in Arabidopsis. Plant Cell 24: 3795–3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M, Peterson FC, Defries A, Park SY, Endo A, Nambara E, Volkman BF, Cutler SR (2013) Activation of dimeric ABA receptors elicits guard cell closure, ABA-regulated gene expression, and drought tolerance. Proc Natl Acad Sci USA 110: 12132–12137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo-Kurihara E, Ohtani M, Kurihara Y, Kakegawa K, Kobayashi M, Nagata N, Komatsu T, Kikuchi J, Cutler S, Demura T, et al. (2016) Modification of plant cell wall structure accompanied by enhancement of saccharification efficiency using a chemical, lasalocid sodium. Sci Rep 6: 34602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E, Díaz-Moreno SM, Davis DJ, Wilkop TE, Bulone V, Drakakaki G (2014) Endosidin 7 specifically arrests late cytokinesis and inhibits callose biosynthesis, revealing distinct trafficking events during cell plate maturation. Plant Physiol 165: 1019–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S-Y, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, Lumba S, Santiago J, Rodrigues A, Chow TF, et al. (2009) Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 324: 1068–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S-Y, Peterson FC, Mosquna A, Yao J, Volkman BF, Cutler SR (2015) Agrochemical control of plant water use using engineered abscisic acid receptors. Nature 520: 545–548 [DOI] [PubMed] [Google Scholar]
- Paudyal R, Jamaluddin A, Warren JP, Doyle SM, Robert S, Warriner SL, Baker A (2014) Trafficking modulator TENin1 inhibits endocytosis, causes endomembrane protein accumulation at the pre-vacuolar compartment and impairs gravitropic response in Arabidopsis thaliana. Biochem J 460: 177–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poretska O, Yang S, Pitorre D, Rozhon W, Zwerger K, Uribe MC, May S, McCourt P, Poppenberger B, Sieberer T (2016) The small molecule hyperphyllin enhances leaf formation rate and mimics shoot meristem integrity defects associated with AMP1 deficiency. Plant Physiol 171: 1277–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigal A, Ma Q, Robert S (2014) Unraveling plant hormone signaling through the use of small molecules. Front Plant Sci 5: 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Serrano EE, Rodriguez-Welsh MF, Hicks GR, Rojas-Pierce M (2012) A small molecule inhibitor partitions two distinct pathways for trafficking of tonoplast intrinsic proteins in Arabidopsis. PLoS ONE 7: e44735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert S, Chary SN, Drakakaki G, Li S, Yang Z, Raikhel NV, Hicks GR (2008) Endosidin1 defines a compartment involved in endocytosis of the brassinosteroid receptor BRI1 and the auxin transporters PIN2 and AUX1. Proc Natl Acad Sci USA 105: 8464–8469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Salus M, Bektas Y, Schroeder M, Knoth C, Vu T, Roberts P, Kaloshian I, Eulgem T (2016) The synthetic elicitor 2-(5-bromo-2-hydroxy-phenyl)-thiazolidine-4-carboxylic acid links plant immunity to hormesis. Plant Physiol 170: 444–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas-Pierce M, Titapiwatanakun B, Sohn EJ, Fang F, Larive CK, Blakeslee J, Cheng Y, Cutler SR, Peer WA, Murphy AS, et al. (2007) Arabidopsis P-glycoprotein19 participates in the inhibition of gravitropism by gravacin. Chem Biol 14: 1366–1376; erratum Rojas-Pierce M, Titapiwatanakun B, Sohn EJ, Fang F, Larive CK, Blakeslee J, Cheng Y, Cutler SR, Peer WA, Murphy AS, et al (2007) Chem Biol 15: 87 [DOI] [PubMed] [Google Scholar]
- Sakai Y, Sugano SS, Kawase T, Shirakawa M, Imai Y, Kawamoto Y, Sugiyama H, Nakagawa T, Hara-Nishimura I, Shimada T (2017) The chemical compound bubblin induces stomatal mispatterning in Arabidopsis by disrupting the intrinsic polarity of stomatal lineage cells. Development 144: 499–506 [DOI] [PubMed] [Google Scholar]
- Savaldi-Goldstein S, Baiga TJ, Pojer F, Dabi T, Butterfield C, Parry G, Santner A, Dharmasiri N, Tao Y, Estelle M, et al. (2008) New auxin analogs with growth-promoting effects in intact plants reveal a chemical strategy to improve hormone delivery. Proc Natl Acad Sci USA 105: 15190–15195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitski MM, Reinhard FBM, Franken H, Werner T, Savitski MF, Eberhard D, Martinez Molina D, Jafari R, Dovega RB, Klaeger S, et al. (2014) Tracking cancer drugs in living cells by thermal profiling of the proteome. Science 346: 1255784. [DOI] [PubMed] [Google Scholar]
- Schelkle KM, Griesbaum T, Ollech D, Becht S, Buckup T, Hamburger M, Wombacher R (2015) Light-induced protein dimerization by one- and two-photon activation of gibberellic acid derivatives in living cells. Angew Chem Int Ed Engl 54: 2825–2829 [DOI] [PubMed] [Google Scholar]
- Schreiber K, Ckurshumova W, Peek J, Desveaux D (2008) A high-throughput chemical screen for resistance to Pseudomonas syringae in Arabidopsis. Plant J 54: 522–531 [DOI] [PubMed] [Google Scholar]
- Schweitzer BA, Loida PJ, CaJacob CA, Chott RC, Collantes EM, Hegde SG, Mosier PD, Profeta S (2002) Discovery of imidazole glycerol phosphate dehydratase inhibitors through 3-D database searching. Bioorg Med Chem Lett 12: 1743–1746 [DOI] [PubMed] [Google Scholar]
- Serrano M, Hubert DA, Dangl JL, Schulze-Lefert P, Kombrink E (2010) A chemical screen for suppressors of the avrRpm1-RPM1-dependent hypersensitive cell death response in Arabidopsis thaliana. Planta 231: 1013–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M, Kombrink E, Meesters C (2015) Considerations for designing chemical screening strategies in plant biology. Front Plant Sci 6: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M, Robatzek S, Torres M, Kombrink E, Somssich IE, Robinson M, Schulze-Lefert P (2007) Chemical interference of pathogen-associated molecular pattern-triggered immune responses in Arabidopsis reveals a potential role for fatty-acid synthase type II complex-derived lipid signals. J Biol Chem 282: 6803–6811 [DOI] [PubMed] [Google Scholar]
- Sungur C, Miller S, Bergholz J, Hoye RC, Brisbois RG, Overvoorde P (2007) The small molecule 2-furylacrylic acid inhibits auxin-mediated responses in Arabidopsis thaliana. Plant Cell Physiol 48: 1693–1701 [DOI] [PubMed] [Google Scholar]
- Surpin M, Raikhel N (2004) Traffic jams affect plant development and signal transduction. Nat Rev Mol Cell Biol 5: 100–109 [DOI] [PubMed] [Google Scholar]
- Surpin M, Rojas-Pierce M, Carter C, Hicks GR, Vasquez J, Raikhel NV (2005) The power of chemical genomics to study the link between endomembrane system components and the gravitropic response. Proc Natl Acad Sci USA 102: 4902–4907; erratum Surpin M, Rojas-Pierce M, Carter C, Hicks GR, Vasquez J, Raikhel NV (2005) Proc Natl Acad Sci USA 102: 10752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinney DC. (2013) Phenotypic vs. target-based drug discovery for first-in-class medicines. Clin Pharmacol Ther 93: 299–301 [DOI] [PubMed] [Google Scholar]
- Tateno M, Brabham C, DeBolt S (2016) Cellulose biosynthesis inhibitors: a multifunctional toolbox. J Exp Bot 67: 533–542 [DOI] [PubMed] [Google Scholar]
- Tóth R, Gerding-Reimers C, Deeks MJ, Menninger S, Gallegos RM, Tonaco IAN, Hübel K, Hussey PJ, Waldmann H, Coupland G (2012) Prieurianin/endosidin 1 is an actin-stabilizing small molecule identified from a chemical genetic screen for circadian clock effectors in Arabidopsis thaliana. Plant J 71: 338–352 [DOI] [PubMed] [Google Scholar]
- Tresch S. (2013) Strategies and future trends to identify the mode of action of phytotoxic compounds. Plant Sci 212: 60–71 [DOI] [PubMed] [Google Scholar]
- Tsuchiya Y, Vidaurre D, Toh S, Hanada A, Nambara E, Kamiya Y, Yamaguchi S, McCourt P (2010) A small-molecule screen identifies new functions for the plant hormone strigolactone. Nat Chem Biol 6: 741–749 [DOI] [PubMed] [Google Scholar]
- Van de Wouwer D, Vanholme R, Decou R, Goeminne G, Audenaert D, Nguyen L, Höfer R, Pesquet E, Vanholme B, Boerjan W (2016) Chemical genetics uncovers novel inhibitors of lignification, including p-iodobenzoic acid targeting CINNAMATE-4-HYDROXYLASE. Plant Physiol 172: 198–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voß S, Klewer L, Wu Y-W (2015) Chemically induced dimerization: reversible and spatiotemporal control of protein function in cells. Curr Opin Chem Biol 28: 194–201 [DOI] [PubMed] [Google Scholar]
- Wagner BK, Schreiber SL (2016) The power of sophisticated phenotypic screening and modern mechanism-of-action methods. Cell Chem Biol 23: 3–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace KB. (2015) Multiple targets for drug-induced mitochondrial toxicity. Curr Med Chem 22: 2488–2492 [DOI] [PubMed] [Google Scholar]
- Worden N, Wilkop TE, Esteve VE, Jeannotte R, Lathe R, Vernhettes S, Weimer B, Hicks G, Alonso J, Labavitch J, et al. (2015) CESA TRAFFICKING INHIBITOR inhibits cellulose deposition and interferes with the trafficking of cellulose synthase complexes and their associated proteins KORRIGAN1 and POM2/CELLULOSE SYNTHASE INTERACTIVE PROTEIN1. Plant Physiol 167: 381–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CW, Guo Z-F, Liang F-S (2015) Light control of cellular processes by using photocaged abscisic acid. ChemBioChem 16: 254–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C-Y, Roybal KT, Puchner EM, Onuffer J, Lim WA (2015) Remote control of therapeutic T cells through a small molecule-gated chimeric receptor. Science 350: aab4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Lei L, Brabham C, Stork J, Strickland J, Ladak A, Gu Y, Wallace I, DeBolt S (2014) Acetobixan, an inhibitor of cellulose synthesis identified by microbial bioprospecting. PLoS ONE 9: e95245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaish P, Gazit A, Gilon C, Levitzki A (1988) Blocking of EGF-dependent cell proliferation by EGF receptor kinase inhibitors. Science 242: 933–935 [DOI] [PubMed] [Google Scholar]
- Yamazoe A, Hayashi K, Kepinski S, Leyser O, Nozaki H (2005) Characterization of terfestatin A, a new specific inhibitor for auxin signaling. Plant Physiol 139: 779–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazoe A, Hayashi K, Kuboki A, Ohira S, Nozaki H (2004) The isolation, structural determination, total synthesis of terfestatin A, a novel auxin signaling inhibitor from Streptomyces sp. Tetrahedron Lett 45: 8359–8362 [Google Scholar]
- Ye Y, Gong Z, Lu X, Miao D, Shi J, Lu J, Zhao Y (2016) Germostatin resistance locus 1 encodes a PHD finger protein involved in auxin-mediated seed dormancy and germination. Plant J 85: 3–15 [DOI] [PubMed] [Google Scholar]
- Yoneda A, Higaki T, Kutsuna N, Kondo Y, Osada H, Hasezawa S, Matsui M (2007) Chemical genetic screening identifies a novel inhibitor of parallel alignment of cortical microtubules and cellulose microfibrils. Plant Cell Physiol 48: 1393–1403 [DOI] [PubMed] [Google Scholar]
- Yoneda A, Ito T, Higaki T, Kutsuna N, Saito T, Ishimizu T, Osada H, Hasezawa S, Matsui M, Demura T (2010) Cobtorin target analysis reveals that pectin functions in the deposition of cellulose microfibrils in parallel with cortical microtubules. Plant J 64: 657–667 [DOI] [PubMed] [Google Scholar]
- Yoshimoto K, Noutoshi Y, Hayashi K, Shirasu K, Takahashi T, Motose H (2012) A chemical biology approach reveals an opposite action between thermospermine and auxin in xylem development in Arabidopsis thaliana. Plant Cell Physiol 53: 635–645 [DOI] [PubMed] [Google Scholar]
- Yoshitani N, Satou K, Saito K, Suzuki S, Hatanaka H, Seki M, Shinozaki K, Hirota H, Yokoyama S (2005) A structure-based strategy for discovery of small ligands binding to functionally unknown proteins: combination of in silico screening and surface plasmon resonance measurements. Proteomics 5: 1472–1480 [DOI] [PubMed] [Google Scholar]
- Zabotina O, Malm E, Drakakaki G, Bulone V, Raikhel N (2008) Identification and preliminary characterization of a new chemical affecting glucosyltransferase activities involved in plant cell wall biosynthesis. Mol Plant 1: 977–989 [DOI] [PubMed] [Google Scholar]
- Zhang C, Brown MQ, van de Ven W, Zhang Z-M, Wu B, Young MC, Synek L, Borchardt D, Harrison R, Pan S, et al. (2016) Endosidin2 targets conserved exocyst complex subunit EXO70 to inhibit exocytosis. Proc Natl Acad Sci USA 113: E41–E50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y. (2012) A chemical genetics method to uncover small molecules for dissecting the mechanism of ABA responses in Arabidopsis seed germination. Methods Mol Biol 876: 107–116 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Chow TF, Puckrin RS, Alfred SE, Korir AK, Larive CK, Cutler SR (2007) Chemical genetic interrogation of natural variation uncovers a molecule that is glycoactivated. Nat Chem Biol 3: 716–721 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Dai X, Blackwell HE, Schreiber SL, Chory J (2003) SIR1, an upstream component in auxin signaling identified by chemical genetics. Science 301: 1107–1110 [DOI] [PubMed] [Google Scholar]
- Ziegler S, Pries V, Hedberg C, Waldmann H (2013) Target identification for small bioactive molecules: finding the needle in the haystack. Angew Chem Int Ed Engl 52: 2744–2792 [DOI] [PubMed] [Google Scholar]
- Zouhar J, Hicks GR, Raikhel NV (2004) Sorting inhibitors (sortins): chemical compounds to study vacuolar sorting in Arabidopsis. Proc Natl Acad Sci USA 101: 9497–9501 [DOI] [PMC free article] [PubMed] [Google Scholar]