Genetic variance for mesophyll conductance among cultivars in both chambers and the field indicate photosynthesis, but not water use efficiency, would be improved by enhancing mesophyll conductance.
Abstract
Photosynthetic efficiency is a critical determinant of crop yield potential, although it remains below the theoretical optimum in modern crop varieties. Enhancing mesophyll conductance (i.e. the rate of carbon dioxide diffusion from substomatal cavities to the sites of carboxylation) may increase photosynthetic and water use efficiencies. To improve water use efficiency, mesophyll conductance should be increased without concomitantly increasing stomatal conductance. Here, we partition the variance in mesophyll conductance to within- and among-cultivar components across soybean (Glycine max) grown under both controlled and field conditions and examine the covariation of mesophyll conductance with photosynthetic rate, stomatal conductance, water use efficiency, and leaf mass per area. We demonstrate that mesophyll conductance varies more than 2-fold and that 38% of this variation is due to cultivar identity. As expected, mesophyll conductance is positively correlated with photosynthetic rates. However, a strong positive correlation between mesophyll and stomatal conductance among cultivars apparently impedes positive scaling between mesophyll conductance and water use efficiency in soybean. Contrary to expectations, photosynthetic rates and mesophyll conductance both increased with increasing leaf mass per area. The presence of genetic variation for mesophyll conductance suggests that there is potential to increase photosynthesis and mesophyll conductance by selecting for greater leaf mass per area. Increasing water use efficiency, though, is unlikely unless there is simultaneous stabilizing selection on stomatal conductance.
Historical increases in crop productivity are attributable primarily to the optimization of two out of the four parameters contributing to yield potential. Yield potentials are a function of incoming solar radiation, the interception efficiency of that radiation by the canopy, the efficiency of converting intercepted radiation into biomass, and the proportion of biomass partitioned to harvestable product (i.e. harvest index; Monteith, 1977). Beginning with the green revolution, major advances in crop yield potential have been realized through maximizing canopy radiation interception efficiency (Evans, 1993) and harvest indices (Hay, 1995). In soybean (Glycine max), interception efficiency has increased through a combination of later maturation leading to longer growing seasons and a decreased susceptibility to lodging (Koester et al., 2014). Likewise, the harvest index is optimized in many modern crops, including soybean, and regularly accounts for 50% or more of aboveground biomass (Hay, 1995). The optimization of harvest indices and interception efficiencies in many of the most widely cultivated crops is nearing its upper limit (Zhu et al., 2010). However, we can improve upon the remaining determinant of yield potential: the efficiency of converting absorbed light to biomass (Beadle and Long, 1985; Slattery et al., 2013; Koester et al., 2014, 2016; Slattery and Ort, 2015).
Because photosynthesis is the primary determinant of conversion efficiency, several routes to improving crop photosynthetic rates have been identified (for review, see Long et al., 2006; von Caemmerer and Evans, 2010; Zhu et al., 2010; Evans, 2013; Ort et al., 2015). Some strategies rely on biological engineering of photosynthetic enzymes: for example, altering Rubisco to reduce photorespiration or to increase carboxylation (Whitney et al., 2011; Betti et al., 2016; Prins et al., 2016) or up-regulating other rate-limiting Calvin cycle enzymes (Lefebvre et al., 2005; Zhu et al., 2007). Another even more ambitious approach aims to reengineer the entire photosynthetic pathway by introducing C4-type carbon-concentrating mechanisms into C3 crops (Mitchell and Sheehy, 2006; Sage and Sage, 2009; Sage and Zhu, 2011). An alternative, and potentially more readily achievable, strategy that could improve photosynthetic rates, and possibly water use efficiency (WUE), is to enhance the mesophyll conductance to CO2 (gm; Flexas et al., 2013a). Several analyses show that gm can limit photosynthetic rates at magnitudes similar to stomatal conductance (gs; Grassi and Magnani, 2005; Galmés et al., 2013; Tomás et al., 2013), and simulations indicate that a doubling of gm in C3 crops would result in a nearly 20% boost to photosynthetic rates (Zhu et al., 2010). gm alters the CO2 concentration gradient from the substomatal cavity (Ci) to the chloroplast stroma (Cc) and is presumed to be independent of water loss from the leaf. Therefore, an additional advantage of improving photosynthesis through enhancing gm is the potential for concurrent improvement of WUE.
The expectation that increasing gm will improve intrinsic WUE (steady-state assimilation [AN]/gs) must be tempered by the possibility of a correlation between gm and gs (Flexas et al., 2016). A positive relationship between gm and gs has been reported in several studies (Barbour et al., 2010; Gu et al., 2012; Flexas et al., 2013a, 2013b), although two studies in wheat (Triticum aestivum) detected no relationship between gm and gs (Jahan et al., 2014; Barbour et al., 2016). It is not yet clear if the coordination between gm and gs is associated with the independent scaling of both diffusional conductances with the overall physiological activity of leaves or if there is an underlying mechanistic relationship we have yet to appreciate (e.g. reliance on the same aquaporins in both the diffusion path of CO2 and the outside-xylem portion of hydraulic flow; Flexas et al., 2013b). Given the potential for coordination between gm and gs, the absolute value of gm may be less of a determinant to improving WUE than is the relative value of gm to gs (i.e. the ratio gm/gs). However, few studies have investigated intraspecific variation in gm, and fewer still have resolved how gm and gs covary.
While theory demonstrates that improving gm will result in greater photosynthetic rates, the available empirical data on gm to this point has focused primarily on cross-species comparisons. Surprisingly, less attention has been paid to intraspecific comparisons, where genetic variation in gm, should it exist, would provide both the genetic material and a guide to select for a reduced diffusional limitation. Across species, gm varies at least 24-fold in seed plants (Tomás et al., 2013), with the lowest values observed in evergreen trees and shrubs and upper values seen in grasses and herbaceous dicots (Flexas et al., 2008). From the reports currently available, it appears that gm varies within genera and species, although most attention has focused on grasses. In studies of barley (Hordeum vulgare) and rice (Oryza sativa), where only four cultivars of each were compared, gm varied among them by ∼30% (Barbour et al., 2010; Adachi et al., 2013). Another study in rice found nearly 60% variation among 11 inbred lines (Gu et al., 2012). In wheat, 2-fold variation was found among 10 genotypes (Jahan et al., 2014) and 3-fold variation among 150 mapping population lines (Barbour et al., 2016). Clearly, genetic variation for gm exists in the monocots, although in 2014, half of the world’s 10 most planted (as total area) crops were eudicots (FAOSTAT 2016; http://faostat3.fao.org/download/Q/QC/E). Tomato (Solanum lycopersicum) and grape (Vitis vinifera) are the only eudicot food crops for which gm has been studied at the intrageneric (Muir et al., 2014, 2017) or intraspecific (Galmés et al., 2011; Tomás et al., 2014) level, and estimates of genetic variation for gm in these and other eudicot crops are lacking, revealing a substantial knowledge gap.
gm is an emergent trait influenced by many independent leaf properties, with contributions from both structural and biochemical traits (Flexas et al., 2012). Anatomically, higher gm is associated with thinner cell walls and greater mesophyll cell surface area exposed to intercellular air spaces per unit of leaf area (SMES; Evans et al., 2009; Terashima et al., 2011). Cell wall thickness does influence the resistance of CO2 diffusion into cells, but the small variance in cell wall thickness expected among genotypes of short-lived crop leaves developing under uniform conditions should exert little influence on the variance of gm (Giuliani et al., 2013). However, SMES partially determines the number of parallel diffusion paths for CO2 into mesophyll cells and differs among species within genera (Giuliani et al., 2013) and genotypes within species (Galmés et al., 2013), providing a key trait underlying variation in gm. Leaf density (LD) and leaf thickness (LT) combine to determine leaf mass per area (LMA). Across species, the relationship between gm and LMA is negative (Hassiotou et al., 2009; Niinemets et al., 2009), because species with higher LMA have thicker cell walls and greater cell densities leading to reduced SMES. Within species, particularly those with relatively thin leaves such as soybean, only the upper limit of gm seems controlled by LMA (Flexas et al., 2008), which may reflect an altered LMA-SMES relationship at lower values of LMA (Milla-Moreno et al., 2016). For species with low LMA leaves, at the intraspecific level, the components of LMA (i.e. LD and LT) or LMA itself may provide meaningful predictors of gm as proxies for SMES.
We used soybean as a model eudicot crop to assess how gm varied across cultivars and covaried with leaf physiological and structural traits associated with photosynthesis (Table I). We addressed several questions. (1) Does AN scale with gm as strongly across cultivars of soybean, a thin-leafed eudicot, as reported for other crops and cross-species comparisons? (2) If gm does scale with AN, then does genetic variation exist for this trait? (3) Is WUE greater in cultivars with greater gm, or (4) is scaling between gm and WUE precluded by coordination between gm and gs? And (5) is LMA, or are its components, predictive of gm? We hypothesized that (1) the role of gm on carbon supply would lead to coordination with AN, (2) any correlation between gm and gs would result in no detectable relationship between WUE and gm, and (3) leaves with greater LMA, thickness, and density would exhibit lower gm. These hypotheses were tested on 12 cultivars of edamame soybean grown under controlled conditions and then examined further in eight of those cultivars at three growth stages under field conditions. Analyses in two environments and across three growth stages gave us a robust design with which to assess the variation and covariation of gm among cultivars, providing confidence in results that were consistent across these groups.
Table I. List of all traits estimated in this study, the symbols used throughout the text, and their units.
| Category and Trait | Symbol | Units |
|---|---|---|
| Leaf function | ||
| Apparent photorespiratory CO2 compensation point | Ci* | Pa |
| Mitochondrial respiration in the light | Rd | µmol CO2 m−2 s−1 |
| Absorptance at 470 and 665 nm | α | unitless |
| Chlorophyll content | SPAD | unitless |
| Net assimilation rate | AN | µmol CO2 m−2 s−1 |
| Maximum light- and CO2-saturated assimilation rate | Amax | µmol CO2 m−2 s−1 |
| Stomatal conductance to water vapor | gs | mol water m−2 s−1 |
| Stomatal conductance to CO2 | gs-co2 | mol CO2 m−2 s−1 |
| Mesophyll conductance | gm | µmol CO2 m−2 s−1 Pa−1 |
| Ratio of mesophyll to stomatal conductance | gm/gs-co2 | mol CO2 mol−1 CO2 |
| Intrinsic WUE | AN/gs | µmol CO2 mol−1 water |
| Integrated WUE, the ratio of 13C to 12C of leaf tissue | δ13C | ‰ |
| Calibrated linear electron transport rate | Jcal | µmol electrons m−2 s−1 |
| CO2 concentration gradient between intercellular air spaces and chloroplast stroma | Ci-Cc | µmol CO2 mol−1 air |
| Nitrogen concentration | Nmass | % (w/w) |
| Nitrogen concentration per area | Narea | mg N m−2 leaf |
| Leaf structure | ||
| Leaf thickness | LT | mm |
| Leaf density | LD | g cm−3 |
| Leaf mass per area | LMA | g m−2 |
| Leaf dry matter content | LDMC | g dry weight g−1 fresh weight |
RESULTS
Parameters Used to Estimate gm
To improve the accuracy of our variable-J estimates of gm, we determined the apparent photorespiratory CO2 compensation point (Ci*) and day respiration rate (Rd) for all soybean cultivars. Ci* and Rd were estimated from Laisk curves on chamber-grown plants (n = 5–8 and n = 72). Mean Ci* was 4.08 ± 0.058 (se) Pa CO2, and mean Rd was 0.937 ± 0.036 µmol CO2 m−2 s−1. Both Ci* and Rd differed among cultivars (P < 0.05; Table II; Supplemental Fig. S1), although only cultivar-specific Rd vales were used, under the presumption that the true photorespiratory compensation point (Γ*) should not vary across such closely related plants. A sensitivity analysis examining how variable Γ* alters estimates of genetic variance for gm among cultivars revealed that gm does depend on Γ*, but the effects of Γ* on genetic variance in gm were minimal (Supplemental Fig. S2). The mean Ci* here falls between the value of Γ* from tobacco (Nicotiana tabacum; Bernacchi et al., 2002) commonly used for soybean (Bernacchi et al., 2005; Rosenthal et al., 2014; Köhler et al., 2017) and a value calculated from soybean Rubisco kinetic properties (Gallé et al., 2013), and it further matches exactly the Ci* estimated for soybean with similar methodology (Walker and Ort, 2015). Thus, the mean Ci* value was used as a proxy for Γ* in all calculations. Cultivar-specific Rd values were used here and ranged from 0.63 to 1.32 µmol CO2 m−2 s−1 (Supplemental Fig. S1). Since these Rd values were estimated from plants grown in a controlled environment only, we performed a sensitivity analysis to assess how variation in Rd would alter estimates of genetic variance for gm from field-grown plants (Supplemental Fig. S3) by recalculating gm for all field measurements using an unvarying Rd for all cultivars ranging from 0.5 to 1.5 µmol m−2 s−1. Estimates of genetic variance in gm were nearly unresponsive to this magnitude variation in Rd (Supplemental Fig. S3).
Table II. Variance partitioning within and between cultivars for traits measured as part of the controlled environment experiment and those used for both experiments (i.e. Ci* and Rd).
Variance attributed to cultivar provides an upper limit estimate of the contribution of genetics to observed trait variability. Trait abbreviations are as in Table II. Min and Max are the minimum and maximum trait values. The variance components were estimated by partitioning variance within and between cultivars with restricted maximum likelihood (REML).
| Trait | Range |
Variance Components |
Variance Attributed to Cultivar | ||
|---|---|---|---|---|---|
| Min | Max | Cultivar | Residual | ||
| % | |||||
| Ci* | 3.07 | 5.60 | 4.172 × 10−2 | 0.20434 | 17.00 |
| Rd | 0.327 | 1.57 | 1.904 × 10−2 | 7.773 × 10−2 | 19.68 |
| α | 0.843 | 0.917 | 8.876 × 10−5 | 1.965 × 10−4 | 31.12 |
| SPAD | 28.0 | 41.5 | 1.909 | 5.341 | 26.33 |
| AN | 15.9 | 27.8 | 2.905 | 4.772 | 37.84 |
| Amax | 19.5 | 38.9 | 6.113 | 20.415 | 23.04 |
| gs | 0.155 | 0.421 | 6.174 × 10−4 | 3.1206 × 10−3 | 16.52 |
| gs-co2 | 0.0966 | 0.263 | |||
| gm | 1.22 | 2.71 | 4.224 × 10−2 | 6.66 × 10−2 | 38.81 |
| gm/gs-co2 | 0.410 | 1.21 | 0 | 0.5102 | 0 |
| Ci-Cc | 90.2 | 144 | 18.33 | 97.64 | 15.81 |
| AN/gs | 55.1 | 109 | 0 | 170.4 | 0 |
| Jcal | 135 | 219 | 127.2 | 283.4 | 31.00 |
| LT | 0.163 | 0.285 | 1.025 × 10−4 | 6.704 × 10−4 | 13.26 |
| LD | 0.0771 | 0.181 | 1.035 × 10−4 | 5.356 × 10−4 | 16.19 |
| LMA | 20.1 | 39.1 | 1.113 × 10−16 | 27.14 | 0 |
| LDMC | 0.163 | 0.241 | 4.402 × 10−5 | 2.963 × 10−4 | 12.93 |
Leaf absorptance at 470 and 665 nm (α; Table I) was measured on all plants to constrain the calibration of electron transport rates used in the estimation of gm. Absorptance averaged 0.89 (range, 0.82–0.96; Tables II and III; Supplemental Fig. S4). Under both controlled and field conditions, cultivar identity was a significant source of variation in absorptance (P < 0.01) primarily attributable to one cultivar that also had consistently greater relative chlorophyll content, as assessed with a clamp-on chlorophyll meter. Absorptance increased with plant growth stage in the field (P < 0.001) and overall was greater for field-grown plants than for those grown in chambers. Relative chlorophyll content differed among cultivars under both field and controlled environment conditions (P < 0.01). Nitrogen concentrations were determined for the field-grown plants; these did not differ among cultivars on a leaf-area or leaf-mass (w/w) basis. Nitrogen content per leaf area increased with growth stage in the field (Table III; P < 0.001), while nitrogen per leaf mass peaked at the early reproductive growth stage (P < 0.001).
Table III. ANOVA statistics from linear mixed-effects models for traits measured in the field experiment.
For each trait, the growth stage of measurement was treated as a fixed effect, and cultivar identity was treated as a random effect to partition phenotypic variance to within and between cultivars. Trait abbreviations are as in Table II. Min and Max are the minimum and maximum trait values. Model degrees of freedom are type III Satterthwaite approximates. Variance components were estimated by partitioning variance within and between cultivars with REML. Significance levels are as follows: n.s., P > 0.05, and ***, P < 0.001 after adjusting for multiple comparisons. Effects were not estimated for gs-co2, since it is a direct transformation of gs.
| Trait | Range |
Growth Stage, Fixed Effect |
Variance Component |
Variance Attributed to Cultivar | |||
|---|---|---|---|---|---|---|---|
| Min | Max | F2,78-86 | P | Cultivar | Residual | ||
| % | |||||||
| α | 0.815 | 0.955 | 59.5 | *** | 8.007 × 10−5 | 3.445 × 10−4 | 18.86 |
| SPAD | 25 | 54.8 | 129.1 | *** | 4.291 | 11.848 | 26.59 |
| AN | 7.8 | 33.4 | 3.0 | n.s. | 4.84 | 18.08 | 21.12 |
| gs | 0.0988 | 0.585 | 35.2 | *** | 8.88 × 10−4 | 7.29 × 10−3 | 10.86 |
| gs-co2 | 0.0618 | 0.365 | |||||
| gm | 0.39 | 2.42 | 8.7 | *** | 0.0178 | 0.1357 | 11.60 |
| gm/gs-co2 | 0.445 | 1.389 | 33.4 | *** | 0.00 | 2.186 × 10−2 | 0.00 |
| Ci-Cc | 62.0 | 229.8 | 14.0 | *** | 76.91 | 553.91 | 12.19 |
| AN/gs | 41.5 | 114.0 | 67.1 | *** | 0.00 | 116 | 0.00 |
| δ13C | −31.31 | −26.2 | 107.8 | *** | 0.1842 | 0.3746 | 32.96 |
| Jcal | 95.0 | 300.4 | 1.1 | n.s. | 346.7 | 2164.3 | 13.81 |
| Narea | 0.380 | 3.163 | 55.5 | *** | 9.855 × 10−3 | 0.1702 | 5.47 |
| Nmass | 1.937 | 5.891 | 19.9 | *** | 0.00 | 0.4463 | 0.00 |
| LT | 0.145 | 0.3025 | 39.8 | *** | 1.602 × 10−5 | 6.741 × 10−4 | 2.32 |
| LD | 0.105 | 0.281 | 184.2 | *** | 1.355 × 10−4 | 5.387 × 10−4 | 20.10 |
| LMA | 19.6 | 80.8 | 147.4 | *** | 5.001 | 67.21 | 6.93 |
| LDMC | 0.172 | 0.374 | 244.6 | *** | 1.308 × 10−4 | 5.5574 × 10−4 | 19.01 |
Physiological and Structural Trait Variations among Cultivars
When grown in controlled environment chambers, phenotypic variance in leaf structure and physiology varied to similar extents across the 12 cultivars studied. Among cultivars, variance was greater for physiological than for structural traits (Table II). Calibrated electron transport rate (Jcal) ranged 1.6-fold with cultivar, explaining 31% of the variance. Cultivar identity contributed little to the 2.7-fold variance in gs, while gm ranged 2.2-fold, with 38.8% of total phenotypic variance in gm found among cultivars. Differences in gm led to variation in the CO2 concentration gradient between Ci and Cc (Ci-Cc), where 15.8% of the variance was explained by cultivar. A 1.7-fold range in AN was observed, with 37.8% of the variance among cultivars. Little of the phenotypic variance in structural traits was explained by cultivar (Table II).
Eight of the 12 cultivars also were grown in the field and measured at three growth stages that corresponded to late vegetative (V4 and V5), early reproductive (R2–R4), and late reproductive (R6) periods. Cultivar identity and growth stage both affected phenotypic trait variation in the field (Table III). Cultivar tended to have a greater effect on physiological traits, while growth stage tended to have stronger effects on structural traits (Table III). For instance, among cultivars, variance was 13.8% for Jcal and 21.1% for AN; neither trait differed by growth stage (Table III; Supplemental Fig. S5, A and D). gs was highest during vegetative growth in July at 425 ± 18 mmol m−2 s−1 (mean ± se), then declined through reproductive growth to a low of 236 ± 18.3 mmol m−2 s−1 during the late reproductive stage in September (Supplemental Fig. S5B), seemingly tracking precipitation (Supplemental Fig. S6). gm (Supplemental Fig. S5C) and Ci-Cc (data not shown) behaved similarly; both gm and Ci-Cc were indistinguishable between vegetative and early reproductive growth, then gm declined at the late reproductive stage, resulting in an increase of Ci-Cc (Table III). Phenotypic variance attributed to cultivar for gs, gm, and Ci-Cc, was lower in the field than in the controlled environment (compare Tables II and III). Intrinsic (AN/gs) and integrated (δ13C) WUE tracked gs throughout the season (i.e. AN/gs increased steadily while δ13C became less negative from late vegetative to late reproductive growth). None of the variance in AN/gs was contributed by cultivar identity, while one-third of the variance in δ13C was among cultivars (Table III).
Leaf structural traits differed among cultivars and more strongly between growth stages. Although the youngest fully expanded top-canopy leaves were always sampled, the leaves sampled at the late reproductive stage were clearly older and more robust than those sampled earlier in the season. LT differed negligibly among cultivars, but it did increase at the late vegetative growth stage (Table III). Among cultivars, variance explained some variation in LD and leaf dry matter content (LDMC), although again, the largest differences were observed at the late reproductive growth stage (R6). Environmental variance and development dominated variation in LMA, which increased slightly between vegetative and early reproductive growth and 1.8-fold in the late reproductive stage, with no detectable among-cultivar contribution (Table III).
Trait Correlations with gm
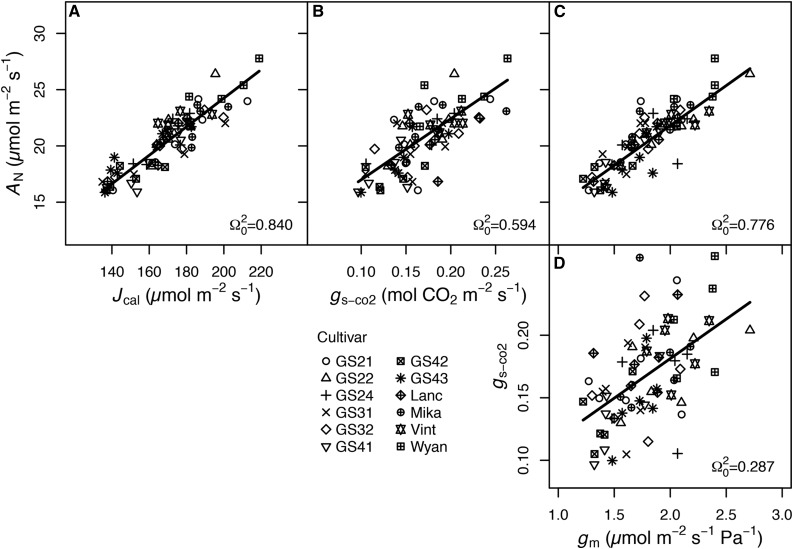
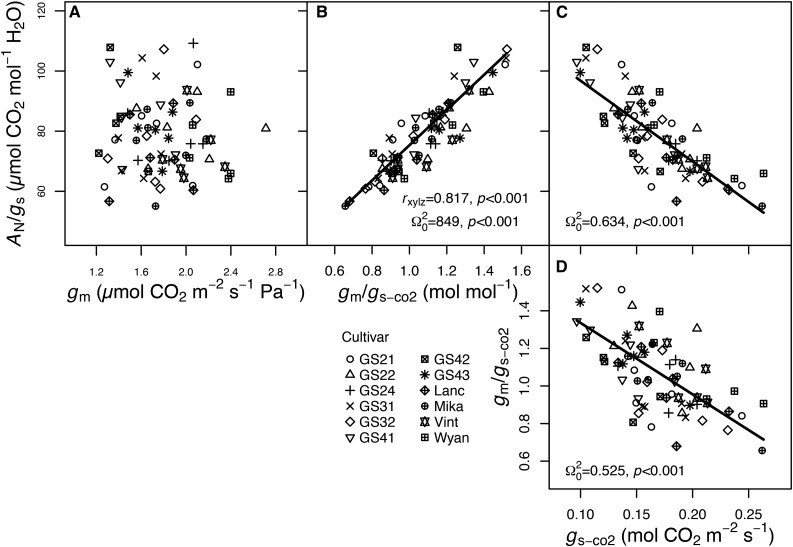
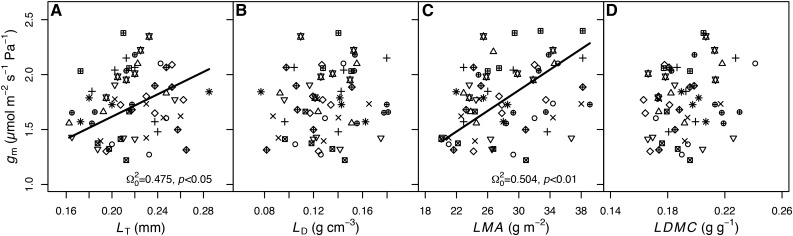
In the controlled environment, most physiological traits were correlated with one another, and cultivar identity often explained modest amounts of variance in these relationships (Table IV). Steady-state photosynthetic rate was closely coupled with Jcal (coefficient of determination [Ω20] = 0.84, P < 0.001), gs-co2 (Ω20 = 0.594, P < 0.001), and gm (Ω20 = 0.776, P < 0.001; Fig. 1, A–C), with 14.5%, 11.3%, and 10.4%, respectively, of the variance in these relationships found among cultivars. The two diffusional conductances to CO2, gm and gs-co2, were correlated (Ω20 = 0.287, P < 0.001; Fig. 1D), and cultivar identity was responsible for 23.3% of the variation in this relationship. No relationship between intrinsic WUE (AN/gs) and gm was detected (P > 0.1; Fig. 2A). We separated the effects of gm on AN/gs without the confounding correlation of gm with gs-co2 by analyzing the relationship between the ratio of gm/gs-co2 and AN/gs. There was a strong positive relationship between gm/gs-co2 and AN/gs (Ω20 = 0.849, P < 0.001; Fig. 2B), with 19.7% of the variance among cultivars. The correlation between gm/gs-co2 and AN/gs may be spurious, because gs is in the denominator of both terms (i.e. both AN/gs and gm/gs-co2 declined with increasing gs or gs-co2; Fig. 2, C and D). However, when controlling for gs, the partial correlation of AN/gs and gm/gs-co2 was still significant (rxy|z = 0.817, P < 0.001). gm was positively correlated with LMA (Ω20 = 0.504, P < 0.01; Fig. 3C) and not correlated with LDMC. Of the components of LMA, LT, and LD, only LT was significantly associated with gm (Ω20 = 0.475, P < 0.05; Fig. 3A).
Table IV. Estimates of cultivar effect on bivariate relationships for the controlled environment and field experiments.
Variance components were estimated with REML. Significance of the cultivar effect was determined with likelihood ratio tests, and P values were adjusted for multiple testing. Significance levels are as follows: n.s., P > 0.05; *, P < 0.05; and **, P < 0.01. Abbreviations are as in Table II.
| Experiment | Trait 1 | Trait 2 | Variance Explained by Cultivar | P |
|---|---|---|---|---|
| % | ||||
| Controlled environment | AN | gs | 11.3 | n.s. |
| AN | gm | 10.4 | n.s. | |
| AN | Jcal | 14.5 | * | |
| gm | gsco2 | 23.3 | * | |
| gm | Jcal | 24.5 | ** | |
| AN/gs | gm | 0 | n.s. | |
| AN/gs | Ci-Cc | 0 | n.s. | |
| AN | LMA | 33.6 | ** | |
| gm | LT | 41.1 | ** | |
| gm | LD | 32.8 | ** | |
| gm | LMA | 36.1 | ** | |
| gm | LDMC | 33.4 | ** | |
| Field | AN | gs | 13.6 | n.s. |
| AN | gm | 1.97 | n.s. | |
| AN | Jcal | 0 | n.s. | |
| gm | gsco2 | 0 | n.s. | |
| gm | Jcal | 0 | n.s. | |
| AN/gs | gm | 1.11 | n.s. | |
| δ13C | gm | 27.3 | ** | |
| δ13C | Ci/Ca | 41.3 | ** | |
| AN/gs | Ci-Cc | 0 | n.s. | |
| AN | LMA | 11.2 | n.s. | |
| gm | LT | 9.20 | n.s. | |
| gm | LD | 6.33 | n.s. | |
| gm | LMA | 6.91 | n.s. | |
| gm | LDMC | 11.6 | n.s. |
Figure 1.
Relationships between light-saturated AN and total Jcal (A), gs-co2 (B), and gm (C) and between gs-co2 and gm (D) for the 12 soybean cultivars grown in the controlled environment experiment. Symbols for the different cultivars are indicated (n = 5–6 replicates). Regression lines and Ω20 are from linear mixed-effects models (in all cases, P < 0.01).
Figure 2.
Relationships between AN/gs and gm (A), gm/gs-co2 (B), and gs-co2 (C) and between gm/gs-co2 and gs-co2 (D) for the 12 soybean cultivars in the controlled environment experiment. Different symbols represent the different cultivars as indicated (n = 5–6 replicates). Regression lines, Ω20, and P values are from linear mixed-effects models. In B, the partial correlation (rxy|z) of AN/gs with gm/gs-co2 after accounting for gs also is presented.
Figure 3.
Relationships between gm and leaf structural traits, LMA (A), LT (B), LD (C), and LDMC (D), for chamber-grown soybean from the controlled environment experiment. Different symbols indicate different cultivars as indicated in Figures 1 and 2 (n = 5–6 replicates). Regression lines, Ω20, and P values are from linear mixed-effects models with cultivar as a random effect.
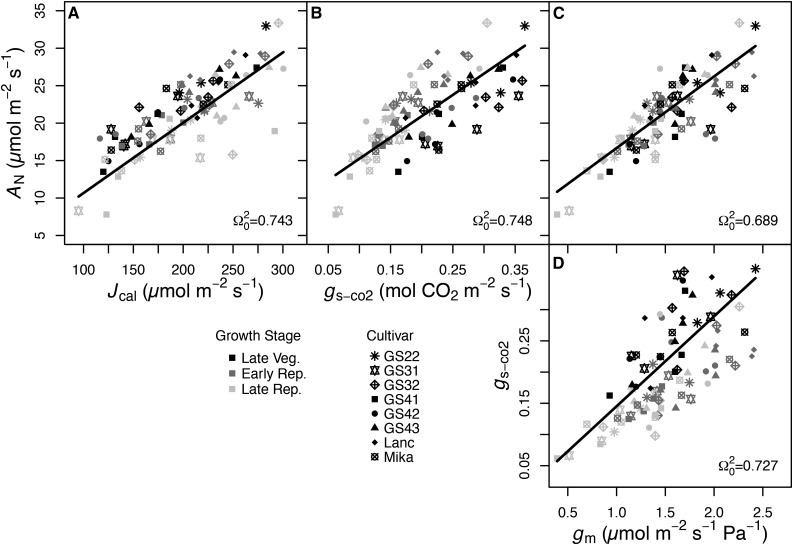
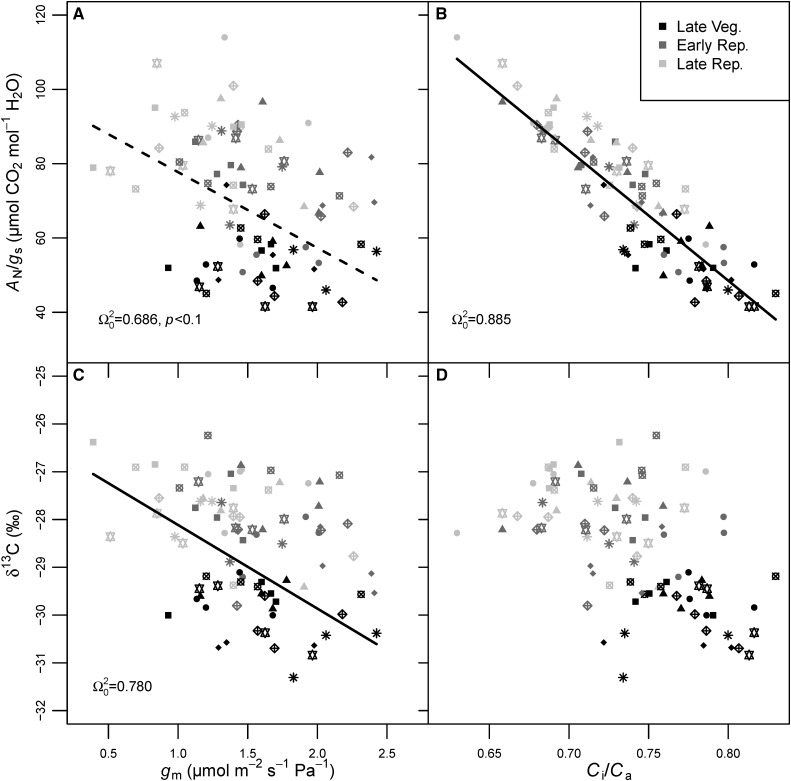
In the field, correlations among physiological traits were generally strong and often modified across growth stages. To examine the effects of among-cultivar trait variation independent of growth stage, linear mixed-effects models were fit treating both the predictor trait and growth stage as fixed effects and cultivar identity as a random effect. Steady-state assimilation was coordinated with CO2 supply as gs-co2 (Ω20 = 0.748, P < 0.001), gm (Ω20 = 0.689, P < 0.001), or total CO2 conductance (Ω20 = 0.795; data not shown), and with reductant supply as Jcal (Ω20 = 0.743, P < 0.001; Fig. 4, A–C). Bivariate relationships of gs-co2 and Jcal with AN differed among growth stages (P < 0.001 for each), and the relationship between AN and gm was not modified by growth stage. gm and gs-co2 were correlated (Ω20 = 0.611, P < 0.001) and differed by growth stage (P < 0.001; Fig. 4D). Intrinsic WUE was not significantly related to gm, but δ13C was negatively correlated with gm (Ω20 = 0.780, P < 0.05; Fig. 5, A and C), and 27% of the variance in this relationship was among cultivars (Table IV). The ratio of intercellular to ambient [CO2] (Ci/Ca) was negatively correlated with AN/gs (P < 0.001), but not δ13C (Fig. 5, B and C), although, in both cases, growth stage modified the relationships (P < 0.001). A strong positive relationship existed between AN/gs and gm/gs-co2 (Ω20 = 0.762, P < 0.001; Supplemental Fig. S7A). As in the controlled environment, this is due partly to spurious correlation with gs in both denominators (Supplemental Fig. S7, B and C). After controlling for spurious correlation with a partial correlation analysis, the effect size was reduced, but the positive AN/gs-gm/gs-co2 relationship did remain significant (rxy|z = 0.605, P < 0.001).
Figure 4.
Relationships between steady-state AN and total Jcal (A), AN and gs-co2 (B), AN and gm (C), and gs-co2 and gm (D). Data are from the eight soybean cultivars in the field experiment and measured at the late vegetative (V5 and V6; black symbols), early reproductive (R2–R4; dark gray symbols), and late reproductive (R6; light gray symbols) growth stages. Different symbols represent different cultivars as indicated, with n = 4 replicates for each cultivar-growth stage combination except GS22, where n = 3. Ω20 and regression lines are from linear mixed-effects models with the x variable and growth stage as predictors and cultivar treated as a random effect (P < 0.001 for all).
Figure 5.
Relationships between AN/gs and gm (A), AN/gs and Ci/Ca (B), δ13C and gm (C), and δ13C and Ci/Ca (D) from the field experiment. Different symbols represent different cultivars (see Fig. 4 legend) with n = 4 replicates, and shading represents late vegetative (V4 and V5; black symbols), early reproductive (R2–R4; dark gray symbols), and late reproductive (R6; light gray symbols) growth stages. The dashed line is marginally significant (P < 0.1), while the solid lines are significant (P < 0.05), according to linear mixed-effects models with growth stage and the variable on the x axis as fixed effect predictors and cultivar identity as a random effect. In all cases, growth stage was a significant predictor (P < 0.001). Cultivar identity significantly improved model fit in C and D (P < 0.001).
Covariation between structural and physiological traits was generally low, resulting from the substantially higher LMA and LD at the late reproductive stage. Within the late vegetative and early reproductive growth stages, positive relationships were observed between gm and LMA or LD (Supplemental Fig. S8), leading to marginally significant (P < 0.1) relationships for gm versus LMA and gm versus LD across all stages after correcting for multiple comparisons. gm was not associated with LT or LDMC (Supplemental Fig. S8). Development had a strong effect on all these relationships: that is, without accounting for growth stage, the correlation of gm with these structural traits becomes negative (positive above) because of a significant decrease in gm and increase in LMA, LT, and LD observed at the late reproductive (R6) stage. As expected, across all three growth stages, LT, LD, and LMA were each positively correlated with AN (P < 0.01; data not shown).
Trait Correlations Were Consistent between Experiments
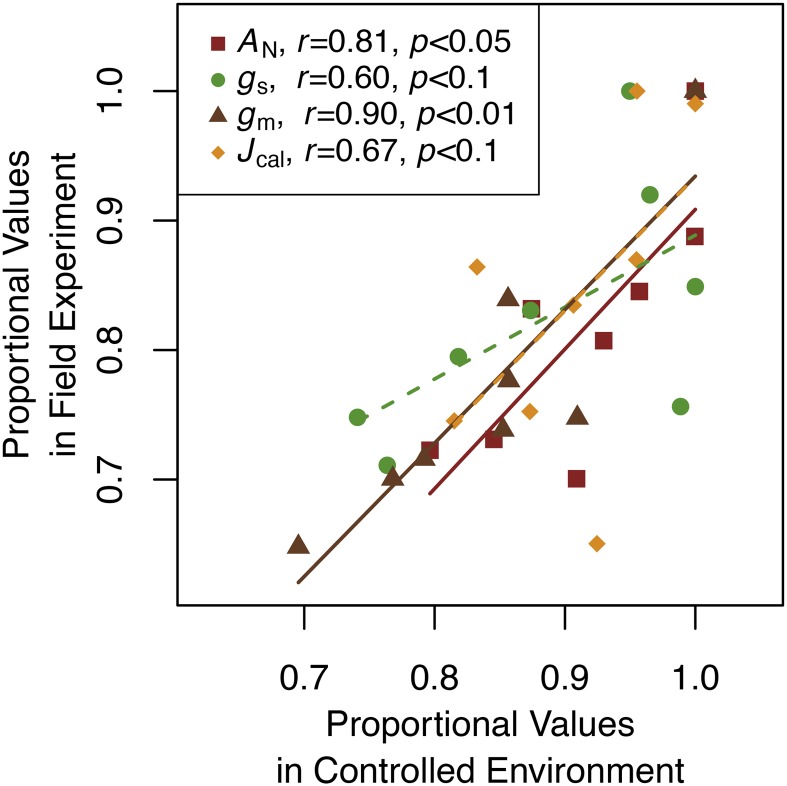
To test the consistency of key trait rankings between the controlled environment and field experiments, we compared standardized (see “Materials and Methods”) cultivar-mean AN, Jcal, gm, and gs from the late vegetative growth stage from the field with those measured in the controlled environment (all chamber plants were measured at the late vegetative stage). Using Spearman’s rank correlations, AN (r = 0.81) and gm (r = 0.9) were consistent between experiments (after adjusting for multiple comparisons; P < 0.05 for both; Fig. 6). Electron transport (r = 0.67) and gs (r = 0.6) were somewhat consistent between chambers and field environments (P < 0.1; Fig. 6). Regression slopes also were tested for consistency across both environments and growth stages using standardized major axis regression (Supplemental Table S1). Of the 42 possible comparisons, slopes differed in just nine cases, all of which were comparisons of slopes from chamber-grown plants with field-grown plants (Supplemental Table S1), indicating that trait correlations were similar between environments and were especially stable among growth stages in the field.
Figure 6.
Consistency among estimates of AN, gs, gm, and Jcal for late vegetative (V2–V4) growth stage soybean plants grown in a controlled environment or in the field. Symbols are cultivar-mean values standardized as a proportion of maximum cultivar-mean trait values. Correlations and P values are Spearman-rank correlations adjusted for multiple comparisons. Solid lines indicate significant relationships (P < 0.05), and the dashed line is marginally significant (P < 0.1).
Trait Coordination
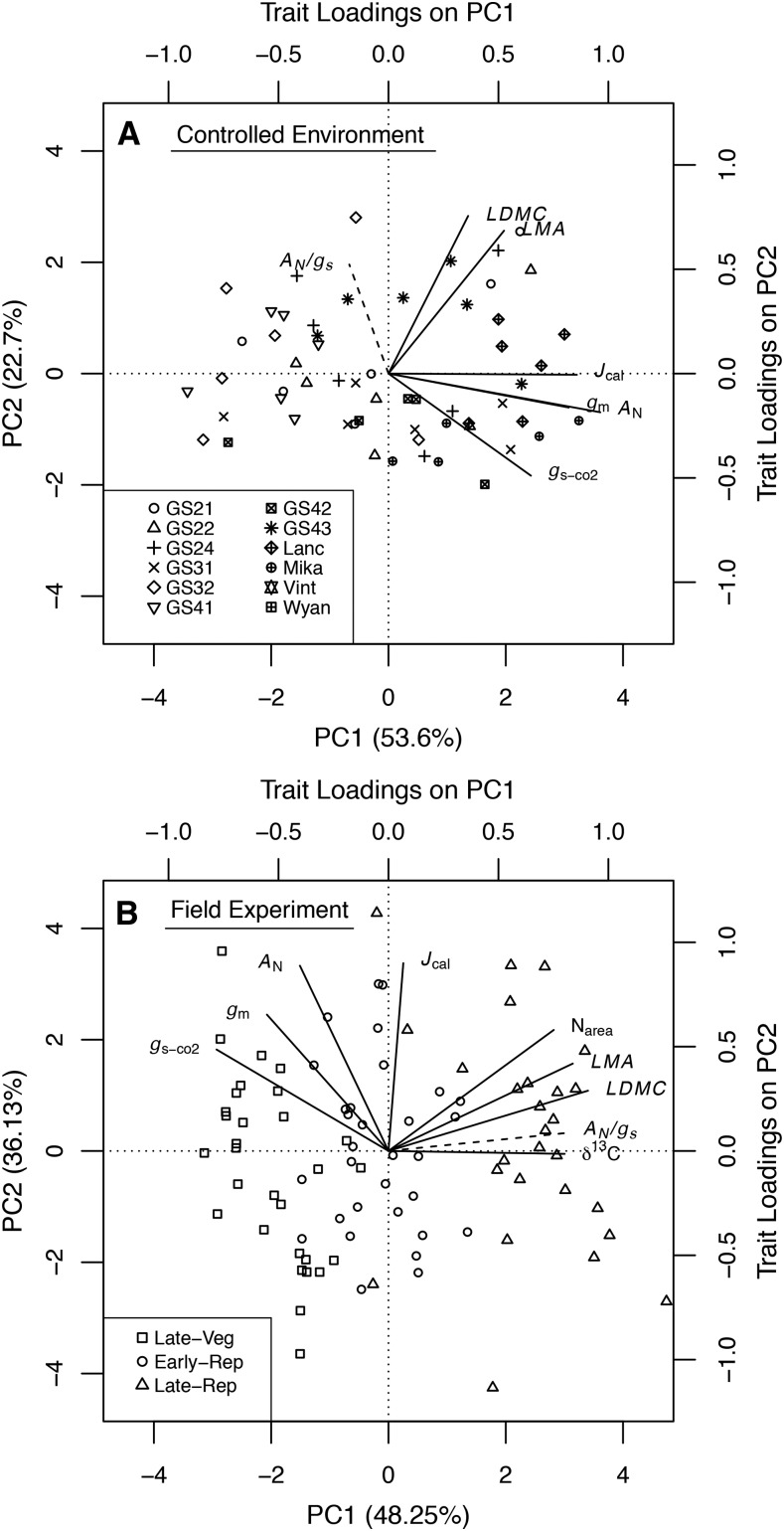
To better understand multivariate trait coordination, we performed principal component analyses with all major traits for both controlled environment and field experiments. Of particular interest were the relationships of intrinsic (AN/gs) and integrated (δ13C) WUE with gs-co2, gm, and AN as well as the relationships between physiological and structural traits. For the controlled environment experiment, the first two principal components cumulatively explained 76.3% of the variance (53.6% and 22.7%, respectively). Physiological traits loaded most heavily with the first principal component (PC1) and structural traits with the second principal component (PC2; Fig. 7A). All traits loaded positively with PC1, indicating coordination between greater photosynthetic activity and more structurally robust leaves. On PC2, the structural traits loaded positively while AN, gm, and especially gs-co2 had slightly negative loadings, suggesting a tradeoff in the positive scaling of structure and physiology. Because AN/gs is a direct combination of other traits, it was not used in fitting the principal component analysis but was mapped after fitting (see “Materials and Methods”). When mapped, AN/gs plotted nearly orthogonal to the gs-co2 loading in the trait space of the first two principal components (Fig. 7A), indicating a CO2 supply-water loss tradeoff and that, under chamber conditions, intrinsic WUE was primarily influenced by gs.
Figure 7.
Principal component analyses for the main traits investigated here from the controlled environment experiment on 12 cultivars of soybean (A) and the field experiment on eight of the same cultivars (B). AN, gs-co2, gm, Jcal, LMA, and LDMC were used in the fitting in A and B. Additionally, in B, Narea and δ13C were used in the fitting. AN/gs (dashed lines) was mapped in the trait space in both A and B. In A, different symbols indicate different cultivars, while in B, they indicate different soybean growth stages.
In the field experiment, the first two principal components accounted for 84.4% of the variation (48.3% and 36.1%, respectively). A tradeoff between CO2 supply (gs-co2 and gm) and leaf structural robustness and water loss was present on PC1, where structural traits (LMA, LDMC, and nitrogen content per leaf area [Narea]) and δ13C loaded positively while AN, gm, and especially gs-co2 loaded negatively (Fig. 7B). Leaves with greater LMA and higher LDMC had lower gs-co2 and, in turn, greater δ13C. There was positive coordination among all traits except δ13C along PC2. Intrinsic (AN/gs) WUE mapped to nearly the same location as δ13C, and both were inversely located relative to AN, gm, and especially gs-co2 on PC1. Individual observations within each growth stage were scattered widely across PC2. A clear pattern was apparent along PC1, with late vegetative observations clustering on the negative end (i.e. with greater physiological trait values), early reproductive observations clustering around zero, and late reproductive observations clustering on the positive end with greater leaf structural robustness.
Important similarities in trait coordination and tradeoffs emerged under both controlled and field environments. Physiological traits associated with carbon uptake (i.e. AN and Jcal) loaded heavily on one principal component and structural traits or WUE traits with the other principal component. In both cases, gs and gm had the same directionality of loadings on the first two principal components, but gs-co2 loaded more strongly along the second principal component, indicating some variance in gs-co2 independent of gm (Fig. 7). For both experiments, AN and gm were more closely associated with one another than either was with gs-co2, and mapping of AN/gs was to a greater extent in opposition to gs-co2 than in accordance with AN. Thus, in both chamber- and field-grown plants, there was some coordination and some tradeoff between leaf structure (as LMA or LDMC) and the physiological traits AN and gm.
DISCUSSION
Enhancing gm can improve carbon assimilation and may improve intrinsic WUE (Flexas et al., 2013a, 2016) and yield potential in C3 crops (Slattery et al., 2013). Consistent with some recent studies (Barbour et al., 2010, 2016; Galmés et al., 2011; Gu et al., 2012; Giuliani et al., 2013; Jahan et al., 2014), we found strong support for the relationship between AN and gm. gm is reported to respond to environmental stimuli and stress at time scales ranging from seconds to seasons (Bernacchi et al., 2002; Grassi and Magnani, 2005; Galmés et al., 2007; von Caemmerer and Evans, 2015; Sorrentino et al., 2016). Here, the relationship between AN and gm among 12 soybean cultivars spanning five maturity groups is consistent between growth chamber and field experiments and across developmental stages (Figs. 1 and 4). The consistent relative rankings of cultivars for gm and AN indicates that there is genetic differentiation for gm in soybean. The degree to which gm is genetically determined remains to be fully quantified (Barbour et al., 2016), but our results indicate that there is potential for selection on gm to improve carbon assimilation.
Our second hypothesis was partially supported. We found no significant relationship between AN/gs and gm (Figs. 2A and 5A); this is consistent with results in rice (Giuliani et al., 2013) and wheat (Jahan et al., 2014), while a negative correlation was found in well-watered and water-stressed tomato (see Supplemental Table S1 in Galmés et al., 2011). The disparity between the prevailing assumption that enhancing gm will improve AN/gs (Flexas et al., 2013a, 2016) and data inconsistent with this idea stems from the correlation between gm and gs reported here (Figs. 1 and 4) and elsewhere (Barbour et al., 2010; Galmés et al., 2011; Gu et al., 2012; Giuliani et al., 2013). The similarity of responses of gm and gs to water stress, salt stress, light, and CO2 led to the hypothesis that gm and gs are inextricably coregulated (Flexas et al., 2008; Vrábl et al., 2009; Sorrentino et al., 2016). Yet, gm and gs can vary independently. Tazoe et al. (2011) elegantly demonstrated that gm was unchanged while gs differed in wild-type compared with ost1 Arabidopsis (Arabidopsis thaliana) mutants with stomata unresponsive to abscisic acid (ABA) or drought (Mustilli et al., 2002). Likewise, Vrábl et al. (2009) concluded that the link between gm and gs in wheat is flexible, since gs but not gm declined after ABA treatment, although others have observed that gs and gm decline in unison following ABA treatment (Sorrentino et al., 2016). gs also is highly sensitive to the leaf-to-air vapor pressure deficit (VPD), and Warren (2008) reported that gs could be altered by varying VPD without affecting gm. Outside of two studies in wheat (Jahan et al., 2014; Barbour et al., 2016), either no relationship, or a negative relationship, has been found between gm and AN/gs. The extent of coordination between gm and gs seems to vary within and among species and even between the cultivars measured here (Supplemental Fig. S5, B and C). And given the potential for gs to respond to environmental stimuli independent of gm, we speculate that inconsistencies in the AN/gs versus gm relationship among studies may arise simply from variation in measurement conditions or may indicate that these relationships are species or genotype specific (Perez-Martin et al., 2009). Finally, the tendency for a negative AN/gs versus gm relationship also was apparent among aquaporin transformants expressing altered gm (Flexas et al., 2016), raising the possibility that variation in aquaporin expression (Perez-Martin et al., 2014) might explain genotypic, environmental, or developmental differences in gm reported here and in the literature.
Do dynamic tradeoffs between gm and AN/gs affect WUE throughout the growing season? Seasonally integrated WUE can be estimated from leaf carbon isotope ratios (δ13C; Farquhar et al., 1989). When stomata close, AN/gs increases and substomatal [CO2] (Ci) decreases relative to atmospheric [CO2] (Ca). Consistently lowered Ci leads to lower discrimination (Δ) against 13C by Rubisco. Therefore, Δ is proportional to the Ci/Ca ratio, and δ13C in leaf dry matter is interpreted as resulting from the mean leaf lifetime Ci/Ca ratio, which can be related to AN/gs. Larger values of δ13C can be interpreted as higher integrated WUE as long as the leaf-to-air VPD does not vary among samples (Farquhar et al., 1989). From our gas exchange, we see that gm and gs-co2 were correlated (Fig. 4); thus, we observed both greater Ci and Cc with greater gm, allowing Rubisco to discriminate against 13CO2 to a greater extent, yielding more negative δ13C values. Since the δ13C of leaves from the field-grown plants was inversely associated with gm (Fig. 5C), we conclude that cultivars with higher gm had lower integrated WUE. The only other study measuring δ13C and gm in an intraspecific crop comparison (Giuliani et al., 2013) found a nonsignificant, but also negative, relationship between the traits. We recognize that gm itself alters carbon isotope fractionation, since it partly determines Cc. No direct relationship was seen between Ci/Ca and δ13C, indicating that some variation in δ13C was unrelated to WUE and likely was a result of variation in gm (Seibt et al., 2008).
Can we simultaneously increase assimilation and water efficiency in soybean? Optimizing both WUE and AN will require selection for a greater gm-to-gs-co2 ratio and not gm in isolation (Flexas et al., 2013a). We did find a consistent positive relationship between AN/gs and gm/gs-co2 and, in the field, a positive relationship between δ13C and gm/gs-co2. Similar positive intraspecific scaling of AN/gs and gm/gs has been found in tomato (Galmés et al., 2011), rice (Giuliani et al., 2013), and grape (Tomás et al., 2014). Together, these results indicate that WUE could be improved by increasing gm, but only at a common gs. After controlling for gs in the denominator of both AN/gs and gm/gs-co2 using partial correlations, the strength of these relationships was greatly reduced but still significant (Fig. 2; Supplemental Fig. S7). Corroborating evidence from the principal component analyses indicates that the variation in AN/gs was driven more by variation in gs than in AN. While the AN/gs-gm/gs correlation is clearly significant with meaningful effects in other studies (Flexas et al., 2013a), the spurious nature of the relationship in our data set indicates that care should be taken when interpreting this relationship in future studies. Ultimately, our data support a framework of enhancing AN through selection for increased cultivar-mean gm but not simultaneous improvements in AN/gs.
Despite the increased environmental heterogeneity, the reduction in the number of cultivars, and the inclusion of multiple developmental stages, the results from the field experiment were consistent with the chamber study. With the sole exception of gm/gs-co2, trait values had a smaller minimum and a greater maximum in the field relative to controlled chambers. The increase in phenotypic variance in the field systematically reduced among-cultivar variance for physiological traits (compare Tables II and III), which is consistent with theory and previous reports (Conner et al., 2003). If trait variance is partitioned for the controlled environment experiment including only the cultivars grown in the field, the among-cultivar variance for gm drops from 38.8% to 25.7% and that for AN drops from 37.8% to 25.6%, values closer to those from the field experiment (11.6% and 21.1%, respectively). Despite low power for detection, cultivar-mean AN and gm rankings were consistent between controlled environment and field growth conditions (Fig. 6). Bivariate trait relationships also were quite consistent across experiments. All significant relationships observed in the controlled environment, with the exception of the gm-LT, also were found in the field. Further evidence of bivariate consistency is provided by the abundance of overlap in the slopes of bivariate trait relationships between environments and across growth stages (Supplemental Table S1). These consistencies highlight the role of genetic control over these traits.
Traits potentially contributing to the variation of gm are generally time consuming and complex to quantify (e.g. anatomical leaf traits). This recognition provides incentive to find strong correlates of gm that are more tractable and that can be used as proxies for gm itself. Because gm is an emergent trait, we quantified LMA, LT, LD, and LDMC to test if these traits would provide predictive power to detect variance in gm across cultivars. These traits varied little across cultivars grown in chambers, with only LT and LMA significantly related to gm, or AN for that matter, and the variance in gm explained by LMA was modest (Ω20 = 0.5). This result held in measurements of field-grown plants, although with a much-reduced share of the variance in gm explained (Supplemental Fig. S8). To our knowledge, the gm-LMA relationship across crop genotypes has only been reported in two other studies, and both report a negative correlation among genotypes (Galmés et al., 2011; Gu et al., 2012). If the effect of growth stage had not been accounted for in our analysis of the field data set, the gm-LMA relationship also would be interpreted as negative, while the true relationship among cultivars in our study was always positive.
When is the gm-LMA relationship positive? Light gradients may explain the positive relationship between these two traits in forest canopies, where, for example, LMA in beech (Fagus sylvatica) trees scaled positively with both gm and photosynthetic capacity (Montpied et al., 2009). A positive association of gm with LMA also was observed in populations of Populus balsamifera trees (Soolanayakanahally et al., 2009). Milla-Moreno et al. (2016) extended this work on P. balsamifera, revealing a positive relationship between LMA and the thickness of the palisade mesophyll layer. The surface area of mesophyll cells exposed to intercellular air space, known to scale with gm, increased with palisade thickness, providing a mechanistic link between gm and LMA (Milla-Moreno et al., 2016). Whether the positive scaling in soybean presented here can be explained by a similar mechanism is an open question worth exploring. The reported negative correlation of LMA and gm reported by Galmés et al. (2011) was likely due to differences between watering treatments, where stressed plants had higher LMA and lower gm, rather than to inherent differences among genotypes. The contrast between our positive gm-LMA relationship and that found by Gu et al. (2012) is harder to explain, since they saw a negative relationship between LMA and gm in rice regardless of plant water status. A recent study in tomato and tomato wild relatives, bridging the gap between these intraspecific studies and broad taxonomic surveys where the gm-LMA relationship is consistently negative, demonstrates that, at finer taxonomic scales, this relationship is more labile, resulting from low coordination between LMA and leaf physiological activity (Muir et al., 2017). In any event, if the observed scaling of gm with LMA holds, it suggests an appealing trait for preliminary selection of candidate soybean genotypes with fast photosynthetic physiologies.
CONCLUSION
To artificially select and improve agronomically relevant traits, genotypic variation must exist for those traits and that variation must be heritable (Falconer and Mackay, 1996). In this study, we show that there is genetic variation for gm and that variation is highly coordinated with leaf photosynthetic physiology and, to a lesser extent, with coarse leaf structure across soybean cultivars. Genetic variation for gm (Jahan et al., 2014) and the recent detection of the only known quantitative trait locus associated with gm hints at the genetic basis for gm in wheat (Barbour et al., 2016). Whether that quantitative trait locus can be identified in other taxa remains to be seen. Indeed, the genetic basis, magnitude, and nature of genetic variation in gm must continue to be evaluated in wheat and other taxa, as well as in variable environments, to fully grasp its potential to improve crop productivity. However, in soybean, unlike in wheat (Barbour et al., 2016), the coupling of gm and gs may interfere with the potential to improve WUE through selection on gm.
MATERIALS AND METHODS
Controlled Environment Experiment
Twelve cultivars of soybean (Glycine max) with varying maturity group status were obtained from a number of sources (Supplemental Table S2) and grown under controlled conditions in growth chambers. Seeds were sown directly in 3.75-L pots containing Pro-Mix HP medium (Premier Tech Horticulture). Plants were maintained at 25°C/21°C in a 16/8-h light/dark cycle with an irradiance of approximately 550 µmol m−2 s−1 at the top of the canopy. All plants were well watered and fertilized weekly with a solution of Plantex (Plant Products). Pots were rotated within chambers every 4th day and between chambers weekly. Gas exchange was measured before flowering on the middle leaflet of the fourth or fifth trifoliate, whichever was the youngest and fully expanded. The relative chlorophyll content of leaves was estimated with a SPAD chlorophyll meter (SPAD 502 Plus; Spectrum Technologies) prior to measurement, and an effort was made to only select leaves within a range of 30 to 40 relative chlorophyll units. Three leaves were below 30, and one was above 40. Chamber-grown plants were used for AN-Ci curves to estimate gm and for Laisk curves to estimate biochemical properties.
Field Experiment
Two plants of each cultivar from the controlled environment experiment were transferred to a glasshouse and grown for seed collection. Plants were well watered, fertilized weekly, and allowed to grow until they naturally senesced. Adequate seed was collected from eight cultivars to allow planting in the field the following summer (Table I). The field plot consisted of 13 10-m-long rows. Cultivars were planted within rows in 1-m-long groupings with 30 cm between groups and eight groups per row. The eight cultivars of edamame soybean were confined to four rows, interspersed among the remaining nine rows that were planted with soybean from other experiments. Seeds were sown to a depth of 3 cm and separated by 7.5 cm on June 4, 2015. The plot is located in a river flood plain and is underlain by Haymond silt loam soil of alluvial origin (Hay1AO; U.S. Department of Agriculture Natural Resources Conservation Service). Total precipitation throughout the growing season (June through September) was 432 mm, the mean daily temperature was 21.7°C, and absolute minimum/maximum temperatures were 7°C/33°C (Supplemental Fig. S6). Precipitation immediately preceding and during seed sowing was low (Supplemental Fig. S6). To improve germination and seedling establishment, we irrigated the field three times during the week after planting. The field was rainfed thereafter. Gas exchange was measured on plants at three times throughout the season (see next paragraph). Precipitation was high preceding the first gas-exchange campaign and relatively lower preceding the second and third campaigns (Supplemental Figs. S6).
Leaves were collected for gas-exchange measurements in the laboratory at three developmental stages: late vegetative (plants at V4 and V5), early reproductive (R2–R4), and late reproductive (R6). As is common with soybean (Bernacchi et al., 2005; Ainsworth et al., 2007), the youngest completely expanded trifoliates were excised predawn in the field and stored in the dark with petioles in water until they could be recut under water in the laboratory. Leaves were removed from the dark 25 min before measurement and allowed to acclimate under a light-emitting diode panel at approximately 1,650 µmol m−2 s−1. AN-Ci curves were measured on all leaves.
Gas-Exchange Measurements
Determination of Rd and Ci*
So-called Laisk curves were measured on plants from growth chambers to calculate the apparent Ci* and Rd of each cultivar (Laisk, 1977; Brooks and Farquhar, 1985). Leaves were acclimated at 25°C, a CO2 concentration of 400 µmol mol−1, a photosynthetic photon flux density (PPFD) of 1,200 µmol m−2 s−1, and a VPD of less than 1.5 with an open gas-exchange system (LI-6400; LiCor) and a 2- × 3-cm broadleaf chamber (LI-6400-02B). Subambient CO2-response curves (CO2 set points of 90, 75, 55, and 42 µmol mol−1) were measured at three subsaturating PPFDs (235, 175, and 125 µmol m−2 s−1). Leaves were reacclimated at ambient conditions between curves. To control for the large differential in CO2 concentrations between the ambient air and the leaf chamber and the low fluxes at subambient [CO2], the entire chamber head was loosely sealed in a plastic bag immediately before and during the response curves, allowing the exhaust air from the leaf chamber to surround the outside of the chamber. The first CO2 set point was not used in the analyses, since it took nearly that length of time for the air in the bag to turn over completely at the low flow rate (250 µmol s−1) used for Laisk curves. Laisk curves were measured on five to eight replicates of each cultivar.
Ci* and Rd were calculated by the modified slope-intercept method of Walker and Ort (2015). For each cultivar, a linear regression was performed on the aggregated CO2-response curves for a given PPFD. The slopes and intercepts from the three regressions were extracted. A second linear regression was performed with the slopes from the first regressions as the x values and intercepts as the y values. Ci* was taken as the absolute value of the slope from the second regression, while Rd was taken as the y intercept. We found no differences between Ci* and Rd calculated by the slope-intercept method of Walker and Ort (2015) and the traditional common-slope method (paired Student’s t tests, P > 0.1 for both) or the sd values of these estimates. The procedure followed here for measuring Laisk curves, though, did follow several of the recommended procedures of Walker and Ort (2015). Measuring only four subambient CO2 partial pressures ensured that curves at each irradiance were completed rapidly and minimized the deactivation of Rubisco. Maximum CO2 partial pressures used in the fittings also were consistently below 10 Pa. Note that Walker and Ort (2015) further recommend the use of at least four irradiances, and additional recommendations have since been proposed (Hanson et al., 2016).
AN-Ci Curves
Combined CO2-response and fluorescence curves were measured on five to six replicates of each cultivar for the controlled environment experiment and on one plant from each row-by-cultivar combination from the field experiment, providing four replicates of each cultivar per growth stage (with the exception of one cultivar that had only three replicates per stage due to poor germination). Leaves were clamped into the 6400-40 fluorescence head cuvette and allowed to acclimate to ambient conditions for at least 30 min. Ambient conditions for AN-Ci curves were equivalent to those for Laisk curves except that a flow rate of 300 µmol s−1 was used, and for leaves from the field the PPFD was set to 1,800 µmol m−2 s−1. Once steady-state conditions were reached, a point was logged and the [CO2] was iteratively changed in the sequence 400, 325, 250, 175, 100, 50, 400, 400, 500, 650, 950, 1,250, 1,600, and 2,000 µmol mol−1. At each CO2 set point, gas-exchange parameters and steady state fluorescence (Fs) were logged, and a multiphase flash chlorophyll fluorescence routine was executed following the recommended procedures of Loriaux et al. (2013) to determine the maximum fluorescence (Fm′). Following this response curve, the leaf was allowed to reacclimate to ambient conditions until net assimilation and gs returned to steady-state conditions. The air stream supplied to the leaf was then switched to a humidified tank of N2 with 1% oxygen, and a second CO2-response curve was executed with only subambient CO2 concentrations (400, 325, 250, 175, 100, and 50 µmol mol−1). Again, Fs and Fm′ were estimated with the multiphase flash routine at each set point.
Diffusional leaks during CO2-response curves resulting from the large differentials in [CO2] between the inside of the leaf chamber and the ambient air can cause substantial error in leaf flux rates (Flexas et al., 2007; Rodeghiero et al., 2007). We estimated diffusional leaks by measuring identical curves on heat-inactivated leaves (n = 12) according to Flexas et al. (2007). The apparent photosynthetic rate of heat-inactivated leaves was subtracted from the photosynthetic rate of experimental leaves, followed by correction of Ci following the equations used by the LI-6400 (Flexas et al., 2007, 2012).
Variable-J gm Calculation
Several methods exist to estimate gm, and their relative strengths and weaknesses are reviewed elsewhere (Pons et al., 2009; Gu and Sun, 2014). Here, we employed methods and protocols to minimize errors that can arise using the variable-J method (Pons et al., 2009). gm was estimated with the variable-J equation of Harley et al. (1992):
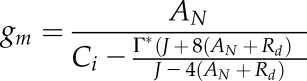 |
(1) |
where AN and Ci were the leak-corrected gas-exchange values from the LI-6400, Rd values were cultivar-specific values taken from Laisk curves, and Γ* was approximated by the mean of all Ci* values also obtained from Laisk curves (see above or below). The electron transport rate (J) was estimated as follows. First the quantum yield of PSII (ΦPSII) and the quantum yield of CO2 fixation (ΦCO2) were quantified as:
| (2) |
| (3) |
where α was the leaf absorptance measured at 470 ± 5 and 665 ± 5 nm (the peak wavelengths emitted by the 6400-40 light source) immediately following gas exchange using a spectroradiometer and leaf-clamp integrating sphere (Jaz Spectroclip; Ocean Optics). Absorptance at the two wavelengths was weighted to account for the gas-exchange measurement light being 10% blue and 90% red. The average of three measures of absorptance made across the leaf blade, avoiding major veins, was used in the calculations. Using the CO2-response curve measured at 1% oxygen, a linear regression of ΦPSII on ΦCO2 was performed, and the regression coefficients were then used to calibrate the ΦPSII values at 21% oxygen (Valentini et al., 1995; Long and Bernacchi, 2003) as:
| (4) |
| (5) |
A Jcal was then calculated as:
| (6) |
and used in the variable-J equation for estimating gm. CO2-response curves were measured at 1% oxygen, and the above electron transport rate corrections were performed, for all plants except the field sampling at the early reproductive stage. In order to facilitate more rapid sampling at this stage, thus standardizing maturity as much as was possible, the 1% oxygen curves on these plants were omitted. The mean slope and intercept values from Equation 4 resulting from measurements at the late vegetative stage were used in this instance to correct the electron transport rate following Equations 5 and 6, with the addition of PPFD being corrected with measured α values.
Values of gm reported throughout this article are those calculated from measurements where the ambient [CO2] (Ca) in the reference air stream was 325 µmol mol−1. Estimating gm with a Ca of 250, 325, and 400 µmol mol−1 resulted in an inverse relationship between gm and Ca. The correlation of gm calculated at each of these three Ca values was high (r > 0.9, P < 0.001), and the mean of the three estimates was not significantly different from the value calculated at Ca = 325 µmol mol−1. These values were ultimately used, as we assume a larger drop in [CO2] across the boundary layer of leaves outside the well-mixed gas-exchange chamber; therefore, the gm estimates at Cs = 325 µmol mol−1 are likely more representative of the average values under growth conditions.
The CO2 concentration in the chloroplast (Cc) was calculated with the estimated gm values according to Fick’s first law:
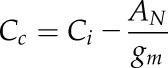 |
(7) |
and used to calculate the Ci-Cc and the ratios Ci/Ca and Cc/Ci.
Leaf Morphology
After gas-exchange and absorptance measurements, seven 1-cm-diameter punches were taken from the leaf lamina avoiding primary veins. Lamina thickness was measured on four punches with digital calipers and averaged to determine LT. The punches were then weighed for fresh mass, dried for more than 72 h at 65°C, and weighed again for dry mass. LMA was calculated from dry mass and the cumulative area of the punches. Dividing LMA by LT yielded LD. Then, LDMC was calculated as the ratio of dry mass to fresh mass. After massing, the dry leaf tissue from plants in the field experiment was ground to a fine powder and analyzed for carbon, nitrogen, and 13C content (δ13C) at the University of Illinois.
Statistical Analysis
All analyses and visualizations were performed in R version 3.3.0 (R Core Team, 2015). To estimate the influence of genetics on cultivar trait differentiation, variation among cultivars for traits of interest from the controlled environment experiment was assessed by partitioning total phenotypic variance within and among cultivars with REML. For field-grown plants, all cultivars were replicated in four blocks within a larger soybean field. Cultivar and block were treated as random effects with growth stage as a fixed effect using the lmer function of the lme4 package (Bates et al., 2015). Then, the genetic component of phenotypic variance was partitioned using REML. Bivariate trait relationships were analyzed with linear mixed-effects models where one of the traits was treated as a fixed effect predictor and block and cultivar were treated as random effects, also with lmer. For mixed-effects models, the significance of fixed effects was determined with Student’s t tests using Satterthwaite-approximated degrees of freedom. The significance of random effects was determined with likelihood ratio tests comparing models with and without the random effect. Effect sizes for bivariate relationships are reported as Ω20 values, a mixed-model analog to r2 calculated as 1 minus the variance in residuals divided by the variance in the fitted response variable (Xu, 2003). The significance level of all tests was adjusted to reflect multiple testing on nonindependent observation by controlling for the false discovery rate and adjusting P values accordingly (Benjamini and Hochberg, 1995).
To test for univariate cultivar trait consistencies across experiments, we compared the values of key traits (AN, gs, gm, and Jcal) as measured in the controlled environment (measured during vegetative growth) with the late vegetative stage from the field experiment for all cultivars measured in both experiments. We divided cultivar-mean trait values by the maximum cultivar-mean value in each experiment to produce standardized proportional rankings. Then, Spearman’s rank correlations were performed on the standardized traits between experiments using a one-tailed test to determine if the relative rankings of cultivars were consistent in chamber- and field-grown plants. To examine if bivariate relationships were consistent across experiments, we compared the slopes of the primary relationships from the controlled environment experiment and all three growth stages from the field experiment with standardized major-axis regression using the smatr package (Warton et al., 2012). Slopes were considered different at α = 0.05 after correcting for multiple comparisons.
Multivariate trait coordination was assessed with principal component analysis. Of all the traits measured in the controlled environment experiment, we included AN, gs-co2, gm, Jcal, LMA, and LDMC as primary variables in fitting the analysis. Since the calculation of AN/gs is a linear combination of other variables, it was included as a supplementary variable that was mapped in the principal component trait space but not used in the fitting. For the field experiment, we included AN, gs-co2, gm, Jcal, Narea, δ13C, LMA, and LDMC. In addition to again mapping AN/gs as a supplementary continuous variable, growth stage was included as a supplementary factor variable to investigate any seasonal shifts in the positioning of observations. Principal component analyses were fit with the FactoMineR package (Lê et al., 2008).
All data for reproducing the figures and analyses in this article are available from the Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.2gd3b).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Mean ± se cultivar Rd and Ci* as determined from Laisk curves on five to eight chamber-grown plants.
Supplemental Figure S2. The reliance of gm estimates on the value of Γ*.
Supplemental Figure S3. Comparisons of gm calculated using cultivar-specific and a range of unvarying Rd estimates for the late vegetative, early reproductive, and late reproductive growth stages of field-grown soybean cultivars indicated along the x axis.
Supplemental Figure S4. Mean ± se α at 470 and 665 nm for each cultivar from the controlled environment experiment and the field experiment.
Supplemental Figure S5. Mean AN, gs, gm, and Jcal for cultivars grown in the field.
Supplemental Figure S6. Daily precipitation, 15-d cumulative precipitation, and mean temperature across the growing season from a meteorological tower located ∼200 m from the field experiment.
Supplemental Figure S7. Relationships between AN/gs to gm/gs-co2, AN/gs to gs-co2, and gm/gs-co2 to gs-co2.
Supplemental Figure S8. Relationships between gm and LMA, LT, LD, and LDMC from the field experiment.
Supplemental Table S1. Comparisons of standardized major axis regression slopes from primary bivariate relationships presented for all possible comparisons of the three growth stages.
Supplemental Table S2. Cultivars used in this study along with their maturity group status and the source of seeds.
Supplementary Material
Acknowledgments
We thank Ryan Dorkoski, Harold Blazier, and Arthur Trese for their logistical support maintaining plants as well as Christopher D. Muir and two anonymous reviewers for thoughtful comments and critiques that substantially improved the article.
Glossary
- WUE
water use efficiency
- LMA
leaf mass per area
- LDMC
leaf dry matter content
- PC1
first principal component
- PC2
second principal component
- ABA
abscisic acid
- VPD
vapor pressure deficit
- PPFD
photosynthetic photon flux density
- REML
restricted maximum likelihood
Footnotes
This work was supported by Ohio University through a research grant and fellowship to N.J.T.
Articles can be viewed without a subscription.
References
- Adachi S, Nakae T, Uchida M, Soda K, Takai T, Oi T, Yamamoto T, Ookawa T, Miyake H, Yano M, et al. (2013) The mesophyll anatomy enhancing CO2 diffusion is a key trait for improving rice photosynthesis. J Exp Bot 64: 1061–1072 [DOI] [PubMed] [Google Scholar]
- Ainsworth EA, Rogers A, Leakey ADB, Heady LE, Gibon Y, Stitt M, Schurr U (2007) Does elevated atmospheric [CO2] alter diurnal C uptake and the balance of C and N metabolites in growing and fully expanded soybean leaves? J Exp Bot 58: 579–591 [DOI] [PubMed] [Google Scholar]
- Barbour MM, Bachmann S, Bansal U, Bariana H, Sharp P (2016) Genetic control of mesophyll conductance in common wheat. New Phytol 209: 461–465 [DOI] [PubMed] [Google Scholar]
- Barbour MM, Warren CR, Farquhar GD, Forrester G, Brown H (2010) Variability in mesophyll conductance between barley genotypes, and effects on transpiration efficiency and carbon isotope discrimination. Plant Cell Environ 33: 1176–1185 [DOI] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67: 1–48 [Google Scholar]
- Beadle CL, Long SP (1985) Photosynthesis: is it limiting to biomass production? Biomass 8: 119–168 [Google Scholar]
- Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc 57: 289–300 [Google Scholar]
- Bernacchi CJ, Morgan PB, Ort DR, Long SP (2005) The growth of soybean under free air [CO2] enrichment (FACE) stimulates photosynthesis while decreasing in vivo Rubisco capacity. Planta 220: 434–446 [DOI] [PubMed] [Google Scholar]
- Bernacchi CJ, Portis AR, Nakano H, von Caemmerer S, Long SP (2002) Temperature response of mesophyll conductance: implications for the determination of Rubisco enzyme kinetics and for limitations to photosynthesis in vivo. Plant Physiol 130: 1992–1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betti M, Bauwe H, Busch FA, Fernie AR, Keech O, Levey M, Ort DR, Parry MAJ, Sage R, Timm S, et al. (2016) Manipulating photorespiration to increase plant productivity: recent advances and perspectives for crop improvement. J Exp Bot 67: 2977–2988 [DOI] [PubMed] [Google Scholar]
- Brooks A, Farquhar GD (1985) Effect of temperature on the CO2/O2 specificity of ribulose-1,5-bisphosphate carboxylase/oxygenase and the rate of respiration in the light: estimates from gas-exchange measurements on spinach. Planta 165: 397–406 [DOI] [PubMed] [Google Scholar]
- Conner JK, Franks R, Stewart C (2003) Expression of additive genetic variances and covariances for wild radish floral traits: comparison between field and greenhouse environments. Evolution 57: 487–495 [DOI] [PubMed] [Google Scholar]
- Evans JR. (2013) Improving photosynthesis. Plant Physiol 162: 1780–1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JR, Kaldenhoff R, Genty B, Terashima I (2009) Resistances along the CO2 diffusion pathway inside leaves. J Exp Bot 60: 2235–2248 [DOI] [PubMed] [Google Scholar]
- Evans LT. (1993) Crop Evolution, Adaptation and Yield. Cambridge University Press, Cambridge, UK [Google Scholar]
- Falconer DS, Mackay TFC (1996) Introduction to Quantitative Genetics, Ed 4 Pearson; Longman Group Ltd., Essex, England [Google Scholar]
- Farquhar GD, Ehleringer JR, Hubick KT (1989) Carbon isotope descrimination and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 40: 503–537 [Google Scholar]
- Flexas J, Barbour MM, Brendel O, Cabrera HM, Carriquí M, Díaz-Espejo A, Douthe C, Dreyer E, Ferrio JP, Gago J, et al. (2012) Mesophyll diffusion conductance to CO2: an unappreciated central player in photosynthesis. Plant Sci 193-194: 70–84 [DOI] [PubMed] [Google Scholar]
- Flexas J, Díaz-Espejo A, Berry JA, Cifre J, Galmés J, Kaldenhoff R, Medrano H, Ribas-Carbó M (2007) Analysis of leakage in IRGA’s leaf chambers of open gas exchange systems: quantification and its effects in photosynthesis parameterization. J Exp Bot 58: 1533–1543 [DOI] [PubMed] [Google Scholar]
- Flexas J, Díaz-Espejo A, Conesa MA, Coopman RE, Douthe C, Gago J, Gallé A, Galmés J, Medrano H, Ribas-Carbo M, et al. (2016) Mesophyll conductance to CO2 and Rubisco as targets for improving intrinsic water use efficiency in C3 plants. Plant Cell Environ 39: 965–982 [DOI] [PubMed] [Google Scholar]
- Flexas J, Niinemets U, Gallé A, Barbour MM, Centritto M, Diaz-Espejo A, Douthe C, Galmés J, Ribas-Carbo M, Rodriguez PL, et al. (2013a) Diffusional conductances to CO2 as a target for increasing photosynthesis and photosynthetic water-use efficiency. Photosynth Res 117: 45–59 [DOI] [PubMed] [Google Scholar]
- Flexas J, Ribas-Carbó M, Diaz-Espejo A, Galmés J, Medrano H (2008) Mesophyll conductance to CO2: current knowledge and future prospects. Plant Cell Environ 31: 602–621 [DOI] [PubMed] [Google Scholar]
- Flexas J, Scoffoni C, Gago J, Sack L (2013b) Leaf mesophyll conductance and leaf hydraulic conductance: an introduction to their measurement and coordination. J Exp Bot 64: 3965–3981 [DOI] [PubMed] [Google Scholar]
- Food and Agriculture Organization of the United Nations (2016) FAOSTAT Database. Rome, Italy: FAO. http://faostat3.fao.org/download/Q/QC/E
- Gallé A, Lautner S, Flexas J, Ribas-Carbo M, Hanson D, Roesgen J, Fromm J (2013) Photosynthetic responses of soybean (Glycine max L.) to heat-induced electrical signalling are predominantly governed by modifications of mesophyll conductance for CO2. Plant Cell Environ 36: 542–552 [DOI] [PubMed] [Google Scholar]
- Galmés J, Conesa MÀ, Ochogavía JM, Perdomo JA, Francis DM, Ribas-Carbó M, Savé R, Flexas J, Medrano H, Cifre J (2011a) Physiological and morphological adaptations in relation to water use efficiency in Mediterranean accessions of Solanum lycopersicum. Plant Cell Environ 34: 245–260 [DOI] [PubMed] [Google Scholar]
- Galmés J, Medrano H, Flexas J (2007) Photosynthetic limitations in response to water stress and recovery in Mediterranean plants with different growth forms. New Phytol 175: 81–93 [DOI] [PubMed] [Google Scholar]
- Galmés J, Ochogavía JM, Gago J, Roldán EJ, Cifre J, Conesa MÀ (2013) Leaf responses to drought stress in Mediterranean accessions of Solanum lycopersicum: anatomical adaptations in relation to gas exchange parameters. Plant Cell Environ 36: 920–935 [DOI] [PubMed] [Google Scholar]
- Giuliani R, Koteyeva N, Voznesenskaya E, Evans MA, Cousins AB, Edwards GE (2013) Coordination of leaf photosynthesis, transpiration, and structural traits in rice and wild relatives (genus Oryza). Plant Physiol 162: 1632–1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi G, Magnani F (2005) Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant Cell Environ 28: 834–849 [Google Scholar]
- Gu J, Yin X, Stomph TJ, Wang H, Struik PC (2012) Physiological basis of genetic variation in leaf photosynthesis among rice (Oryza sativa L.) introgression lines under drought and well-watered conditions. J Exp Bot 63: 5137–5153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu L, Sun Y (2014) Artefactual responses of mesophyll conductance to CO2 and irradiance estimated with the variable J and online isotope discrimination methods. Plant Cell Environ 37: 1231–1249 [DOI] [PubMed] [Google Scholar]
- Hanson DT, Stutz SS, Boyer JS (2016) Why small fluxes matter: the case and approaches for improving measurements of photosynthesis and (photo)respiration. J Exp Bot 67: 3027–3039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley PC, Loreto F, Di Marco G, Sharkey TD (1992) Theoretical considerations when estimating the mesophyll conductance to CO2 flux by analysis of the response of photosynthesis to CO2. Plant Physiol 98: 1429–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassiotou F, Ludwig M, Renton M, Veneklaas EJ, Evans JR (2009) Influence of leaf dry mass per area, CO2, and irradiance on mesophyll conductance in sclerophylls. J Exp Bot 60: 2303–2314 [DOI] [PubMed] [Google Scholar]
- Hay RKM. (1995) Harvest index: a review of its use in plant breeding and crop physiology. Ann Appl Biol 126: 197–216 [Google Scholar]
- Jahan E, Amthor JS, Farquhar GD, Trethowan R, Barbour MM (2014) Variation in mesophyll conductance among Australian wheat genotypes. Funct Plant Biol 41: 568–580 [DOI] [PubMed] [Google Scholar]
- Koester RP, Nohl BM, Diers BW, Ainsworth EA (2016) Has photosynthetic capacity increased with 80 years of soybean breeding? An examination of historical soybean cultivars. Plant Cell Environ 39: 1058–1067 [DOI] [PubMed] [Google Scholar]
- Koester RP, Skoneczka JA, Cary TR, Diers BW, Ainsworth EA (2014) Historical gains in soybean (Glycine max Merr.) seed yield are driven by linear increases in light interception, energy conversion, and partitioning efficiencies. J Exp Bot 65: 3311–3321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler IH, Ruiz-Vera UM, VanLoocke A, Thomey ML, Clemente T, Long SP, Ort DR, Bernacchi CJ (2017) Expression of cyanobacterial FBP/SBPase in soybean prevents yield depression under future climate conditions. J Exp Bot 68: 715–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laisk AK. (1977) Kinetics of Photosynthesis and Photorespiration in C3 Plants. Nauk, Moscow: (in Russian) [Google Scholar]
- Lê S, Josse J, Husson F (2008) FactoMineR: an R package for multivariate analysis. J Stat Softw 25: 1–18 [Google Scholar]
- Lefebvre S, Lawson T, Zakhleniuk OV, Lloyd JC, Raines CA, Fryer M (2005) Increased sedoheptulose-1,7-bisphosphatase activity in transgenic tobacco plants stimulates photosynthesis and growth from an early stage in development. Plant Physiol 138: 451–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SP, Bernacchi CJ (2003) Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. J Exp Bot 54: 2393–2401 [DOI] [PubMed] [Google Scholar]
- Long SP, Zhu XG, Naidu SL, Ort DR (2006) Can improvement in photosynthesis increase crop yields? Plant Cell Environ 29: 315–330 [DOI] [PubMed] [Google Scholar]
- Loriaux SD, Avenson TJ, Welles JM, McDermitt DK, Eckles RD, Riensche B, Genty B (2013) Closing in on maximum yield of chlorophyll fluorescence using a single multiphase flash of sub-saturating intensity. Plant Cell Environ 36: 1755–1770 [DOI] [PubMed] [Google Scholar]
- Milla-Moreno EA, McKown AD, Guy RD, Soolanayakanahally RY (2016) Leaf mass area predicts palisade structural properties linked to mesophyll conductance in balsam poplar. Botany 94: 225–239 [Google Scholar]
- Mitchell PL, Sheehy JE (2006) Supercharging rice photosynthesis to increase yield. New Phytol 171: 688–693 [DOI] [PubMed] [Google Scholar]
- Monteith JL. (1977) Climate and the efficiency of crop production in Britain. Philos Trans R Soc Lond B Biol Sci 281: 277–294 [Google Scholar]
- Montpied P, Granier A, Dreyer E (2009) Seasonal time-course of gradients of photosynthetic capacity and mesophyll conductance to CO2 across a beech (Fagus sylvatica L.) canopy. J Exp Bot 60: 2407–2418 [DOI] [PubMed] [Google Scholar]
- Muir CD, Conesa MÀ, Roldán EJ, Molins A, Galmés J (2017) Weak coordination between leaf structure and function among closely related tomato species. New Phytol 213: 1642–1653 [DOI] [PubMed] [Google Scholar]
- Muir CD, Hangarter RP, Moyle LC, Davis PA (2014) Morphological and anatomical determinants of mesophyll conductance in wild relatives of tomato (Solanum sect. Lycopersicon, sect. Lycopersicoides; Solanaceae). Plant Cell Environ 37: 1415–1426 [DOI] [PubMed] [Google Scholar]
- Mustilli AC, Merlot S, Vavasseur A, Fenzi F, Giraudat J (2002) Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell 14: 3089–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinemets U, Díaz-Espejo A, Flexas J, Galmés J, Warren CR (2009) Role of mesophyll diffusion conductance in constraining potential photosynthetic productivity in the field. J Exp Bot 60: 2249–2270 [DOI] [PubMed] [Google Scholar]
- Ort DR, Merchant SS, Alric J, Barkan A, Blankenship RE, Bock R, Croce R, Hanson MR, Hibberd JM, Long SP, et al. (2015) Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proc Natl Acad Sci USA 112: 8529–8536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Martin A, Flexas J, Ribas-Carbó M, Bota J, Tomás M, Infante JM, Diaz-Espejo A (2009) Interactive effects of soil water deficit and air vapour pressure deficit on mesophyll conductance to CO2 in Vitis vinifera and Olea europaea. J Exp Bot 60: 2391–2405 [DOI] [PubMed] [Google Scholar]
- Perez-Martin A, Michelazzo C, Torres-Ruiz JM, Flexas J, Fernández JE, Sebastiani L, Diaz-Espejo A (2014) Regulation of photosynthesis and stomatal and mesophyll conductance under water stress and recovery in olive trees: correlation with gene expression of carbonic anhydrase and aquaporins. J Exp Bot 65: 3143–3156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons TL, Flexas J, von Caemmerer S, Evans JR, Genty B, Ribas-Carbo M, Brugnoli E (2009) Estimating mesophyll conductance to CO2: methodology, potential errors, and recommendations. J Exp Bot 60: 2217–2234 [DOI] [PubMed] [Google Scholar]
- Prins A, Orr DJ, Andralojc PJ, Reynolds MP, Carmo-Silva E, Parry MAJ (2016) Rubisco catalytic properties of wild and domesticated relatives provide scope for improving wheat photosynthesis. J Exp Bot 67: 1827–1838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2015) R: A Language and Environment for Statistical Computing, Vienna, Austria. http://www.R-project.org/
- Rodeghiero M, Niinemets U, Cescatti A (2007) Major diffusional leaks of clamp-on leaf cuvettes still unaccounted: How erroneous are the estimates of Faquhar et al. model parameters? Plant Cell Environ 30: 1006–1022 [DOI] [PubMed] [Google Scholar]
- Rosenthal DM, Ruiz-Vera UM, Siebers MH, Gray SB, Bernacchi CJ, Ort DR (2014) Biochemical acclimation, stomatal limitation and precipitation patterns underlie decreases in photosynthetic stimulation of soybean (Glycine max) at elevated [CO2] and temperatures under fully open air field conditions. Plant Sci 226: 136–146 [DOI] [PubMed] [Google Scholar]
- Sage RF, Zhu XG (2011) Exploiting the engine of C4 photosynthesis. J Exp Bot 62: 2989–3000 [DOI] [PubMed] [Google Scholar]
- Sage TL, Sage RF (2009) The functional anatomy of rice leaves: implications for refixation of photorespiratory CO2 and efforts to engineer C4 photosynthesis into rice. Plant Cell Physiol 50: 756–772 [DOI] [PubMed] [Google Scholar]
- Seibt U, Rajabi A, Griffiths H, Berry JA (2008) Carbon isotopes and water use efficiency: sense and sensitivity. Oecologia 155: 441–454 [DOI] [PubMed] [Google Scholar]
- Slattery RA, Ainsworth EA, Ort DR (2013) A meta-analysis of responses of canopy photosynthetic conversion efficiency to environmental factors reveals major causes of yield gap. J Exp Bot 64: 3723–3733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery RA, Ort DR (2015) Photosynthetic energy conversion efficiency: setting a baseline for gauging future improvements in important food and biofuel crops. Plant Physiol 168: 383–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soolanayakanahally RY, Guy RD, Silim SN, Drewes EC, Schroeder WR (2009) Enhanced assimilation rate and water use efficiency with latitude through increased photosynthetic capacity and internal conductance in balsam poplar (Populus balsamifera L.). Plant Cell Environ 32: 1821–1832 [DOI] [PubMed] [Google Scholar]
- Sorrentino G, Haworth M, Wahbi S, Mahmood T, Zuomin S, Centritto M (2016) Abscisic acid induces rapid reductions in mesophyll conductance to carbon dioxide. PLoS ONE 11: e0148554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazoe Y, von Caemmerer S, Estavillo GM, Evans JR (2011) Using tunable diode laser spectroscopy to measure carbon isotope discrimination and mesophyll conductance to CO2 diffusion dynamically at different CO2 concentrations. Plant Cell Environ 34: 580–591 [DOI] [PubMed] [Google Scholar]
- Terashima I, Hanba YT, Tholen D, Niinemets Ü (2011) Leaf functional anatomy in relation to photosynthesis. Plant Physiol 155: 108–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomás M, Flexas J, Copolovici L, Galmés J, Hallik L, Medrano H, Ribas-Carbó M, Tosens T, Vislap V, Niinemets Ü (2013) Importance of leaf anatomy in determining mesophyll diffusion conductance to CO2 across species: quantitative limitations and scaling up by models. J Exp Bot 64: 2269–2281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomás M, Medrano H, Brugnoli E, Escalona JM, Martorell S, Pou A, Ribas-Carbó M, Flexas J (2014) Variability of mesophyll conductance in grapevine cultivars under water stress conditions in relation to leaf anatomy and water use efficiency. Aust J Grape Wine Res 20: 272–280 [Google Scholar]
- Valentini R, Epron D, Angelis PDE, Matteucci G, Dreyer E (1995) In situ estimation of net CO2 assimilation, photosynthetic electron flow and photorespiration in Turkey oak (Q. cerris L.) leaves: diurnal cycles under different levels of water supply. Plant Cell Environ 18: 631–640 [Google Scholar]
- von Caemmerer S, Evans JR (2010) Enhancing C3 photosynthesis. Plant Physiol 154: 589–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Caemmerer S, Evans JR (2015) Temperature responses of mesophyll conductance differ greatly between species. Plant Cell Environ 38: 629–637 [DOI] [PubMed] [Google Scholar]
- Vrábl D, Vasková M, Hronková M, Flexas J, Šantruček J (2009) Mesophyll conductance to CO2 transport estimated by two independent methods: effect of variable CO2 concentration and abscisic acid. J Exp Bot 60: 2315–2323 [DOI] [PubMed] [Google Scholar]
- Walker BJ, Ort DR (2015) Improved method for measuring the apparent CO2 photocompensation point resolves the impact of multiple internal conductances to CO2 to net gas exchange. Plant Cell Environ 38: 2462–2474 [DOI] [PubMed] [Google Scholar]
- Warren CR. (2008) Soil water deficits decrease the internal conductance to CO2 transfer but atmospheric water deficits do not. J Exp Bot 59: 327–334 [DOI] [PubMed] [Google Scholar]
- Warton DI, Duursma RA, Falster DS, Taskinen S (2012) SMATR 3: an R package for estimation and inference about allometric lines. Methods Ecol Evol 3: 257–259 [Google Scholar]
- Whitney SM, Houtz RL, Alonso H (2011) Advancing our understanding and capacity to engineer nature’s CO2-sequestering enzyme, Rubisco. Plant Physiol 155: 27–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R. (2003) Measuring explained variation in linear mixed effects models. Stat Med 22: 3527–3541 [DOI] [PubMed] [Google Scholar]
- Zhu XG, de Sturler E, Long SP (2007) Optimizing the distribution of resources between enzymes of carbon metabolism can dramatically increase photosynthetic rate: a numerical simulation using an evolutionary algorithm. Plant Physiol 145: 513–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XG, Long SP, Ort DR (2010) Improving photosynthetic efficiency for greater yield. Annu Rev Plant Biol 61: 235–261 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.