pTAC10, a key component of the plastid-encoded RNA polymerase complex, interacts with other components through its C-terminal region downstream of the S1 RNA-binding domain.
Abstract
Regulation of photosynthetic gene expression by plastid-encoded RNA polymerase (PEP) is essential for chloroplast development. The activity of PEP largely relies on at least 12 PEP-associated proteins (PAPs) encoded in the nuclear genome of plant cells. A recent model proposed that these PAPs regulate the establishment of the PEP complex through broad PAP-PEP or PAP-PAP interactions. In this study, we identified the Arabidopsis (Arabidopsis thaliana) seedling-lethal mutant ptac10-1, which has defects in chloroplast development, and found that the mutant phenotype is caused by the suppression of PLASTID S1 RNA-BINDING DOMAIN PROTEIN (pTAC10/PAP3). Analysis of the heterozygous mutant and pTAC10-overexpressing transgenic plants indicated that the expression level of pTAC10 is tightly linked to chloroplast development. Characterization of the interaction of pTAC10 with PAPs revealed that pTAC10 interacts with other PAPs, such as FSD2, FSD3, TrxZ, pTAC7, and pTAC14, but it does not interact with PEP core enzymes, such as rpoA and rpoB. Analysis of pTAC10 interactions using truncated pTAC10 proteins showed that the pTAC10 carboxyl-terminal region downstream of the S1 domain is involved in the pTAC10-PAP interaction. Furthermore, overexpression of truncated pTAC10s lacking the C-terminal regions downstream of the S1 domain could not rescue the ptac10-1 mutant phenotype and induced an abnormal whitening phenotype in Columbia-0 plants. Our observations suggested that these pTAC10-PAP interactions are essential for the formation of the PEP complex and chloroplast development.
Plant growth and development rely largely on the development of chloroplasts, which are responsible for photosynthesis and the biosynthesis of amino acids, lipids, and phytohormones (Peltier et al., 2006; Biswal et al., 2013; Buchanan et al., 2015). Plastids were symbiotically acquired by heterotrophic unicellular eukaryotes, and a massive transfer of plastid genes to the host nucleus during evolution transformed plastids into semiautonomous organelles (Sagan, 1967; Martin et al., 2002; Pfalz and Pfannschmidt, 2013). The plastid genome of land plants contains genes related to photosynthesis and gene expression. The expression of these plastid genes involves two distinct types of RNA polymerase, nucleus-encoded RNA polymerase and plastid-encoded RNA polymerase (PEP; Sugiura, 1992; Hajdukiewicz et al., 1997; Yagi and Shiina, 2014; Yu et al., 2014).
PEP is the major RNA polymerase responsible for the formation of fully active chloroplasts in response to environmental signals (Link, 2003; Pfannschmidt and Liere, 2005). The core subunits of multimeric PEP are encoded by rpoA, rpoB, rpoC1, and rpoC2, and PEP is essential for chloroplast development, as it promotes the expression of photosynthetic genes. For example, mutant plants in which the expression of these rpo genes is disrupted have defects in photosynthetic gene expression and chloroplast development (Allison et al., 1996; De Santis-MacIossek et al., 1999; Krause et al., 2000).
PEP exists as a complex involving interaction with at least 12 PEP-associated proteins (PAPs). Steiner et al. (2011) identified 10 PAPs, and recently, pTAC7 and MurE-like were categorized as PAPs based on the phenotypes of their mutant plants (Steiner et al., 2011; Pfalz and Pfannschmidt, 2013). All PAPs also have been identified in the proteomes of the chloroplast nucleoid or TRANSCRIPTIONALLY ACTIVE CHROMOSOME (TAC; Pfalz et al., 2006; Majeran et al., 2012; Melonek et al., 2016). Genetic approaches have demonstrated the essential role of PAPs in the regulation of PEP activity. Mutant plants that lack the expression of PAPs exhibit the suppression of photosynthetic gene expression, resulting in defects in chloroplast development and leaf greening (Pfalz et al., 2006; Garcia et al., 2008; Myouga et al., 2008; Arsova et al., 2010; Chen et al., 2010; Schröter et al., 2010; Gao et al., 2011; Steiner et al., 2011; Yagi et al., 2012; Huang et al., 2013; Pfalz and Pfannschmidt, 2013; Williams-Carrier et al., 2014). PAPs are categorized into four groups based on their function/predicted function (Kindgren and Strand, 2015). Group 1 PAPs, including pTAC2/PAP2, pTAC3/PAP1, pTAC7/PAP12, pTAC10/PAP3, pTAC12/PAP5, and pTAC14/PAP7, are involved in DNA and RNA metabolism. Group 2 PAPs, including pTAC7/PAP12, FLN1/PAP6, FLN2, and TrxZ/PAP10, are involved in the fine-tuning of chloroplast gene transcription. Group 3 PAPs, including FSD2/PAP9 and FSD3/PAP4, are involved in the protection of the PEP complex against reactive oxygen species. The functions of group 4 PAPs, including pTAC6/PAP8 and MurE-like/PAP11, remain unknown.
Each PAP interacts extensively with other PAPs or PEP core proteins. For instance, the group 1 PAPs pTAC3 and pTAC14 interact with the PEP α-core subunit (Yagi et al., 2012) and pTAC12 of group 1 (Gao et al., 2011), respectively. FLN1, belonging to group 2, interacts with TrxZ and FLN2 of the same group (Arsova et al., 2010; Huang et al., 2013), and FSD3 of group 3 interacts with FSD2 of the same group (Myouga et al., 2008). pTAC7 binds to pTAC10, pTAC12, pTAC14, and FLN1 belonging to both groups 1 and 2 (Yu et al., 2013). Establishment of the PEP complex through these PAP-PAP or PAP-PEP interactions may be a key developmental bottleneck governing the activity of the PEP complex and chloroplast development (Pfalz and Pfannschmidt, 2013).
The pTAC10 protein, which contains an S1 RNA-binding domain, is an essential PAP responsible for the regulation of PEP activity and chloroplast development (Jeon et al., 2012; Williams-Carrier et al., 2014). A recent study by Pfalz et al. (2015) suggested that pTAC10, as a key subunit of the PEP complex, is involved in the assembly of the complete PEP complex in maize (Zea mays; Pfalz et al., 2015). However, the interaction of pTAC10 with other PAPs or PEP core enzymes is largely unknown. In this study, we identified ptac10-1, a mutant with defects in chloroplast development. Phenotypic analysis of the homozygous and heterozygous mutants, as well as pTAC10-overexpressing transgenic plants, indicated that pTAC10 is a key regulator for chloroplast development. Characterization of the interaction of pTAC10 with other subunits of the PEP complex revealed that pTAC10 interacts with at least five PAPs through its C-terminal regions downstream of the S1 RNA-binding domain. Thus, this study finds an important role for pTAC10 in chloroplast development, likely through effects on the PEP complex.
RESULTS
Characterization of Seedling-Lethal Mutants with Defects in Chloroplast Development
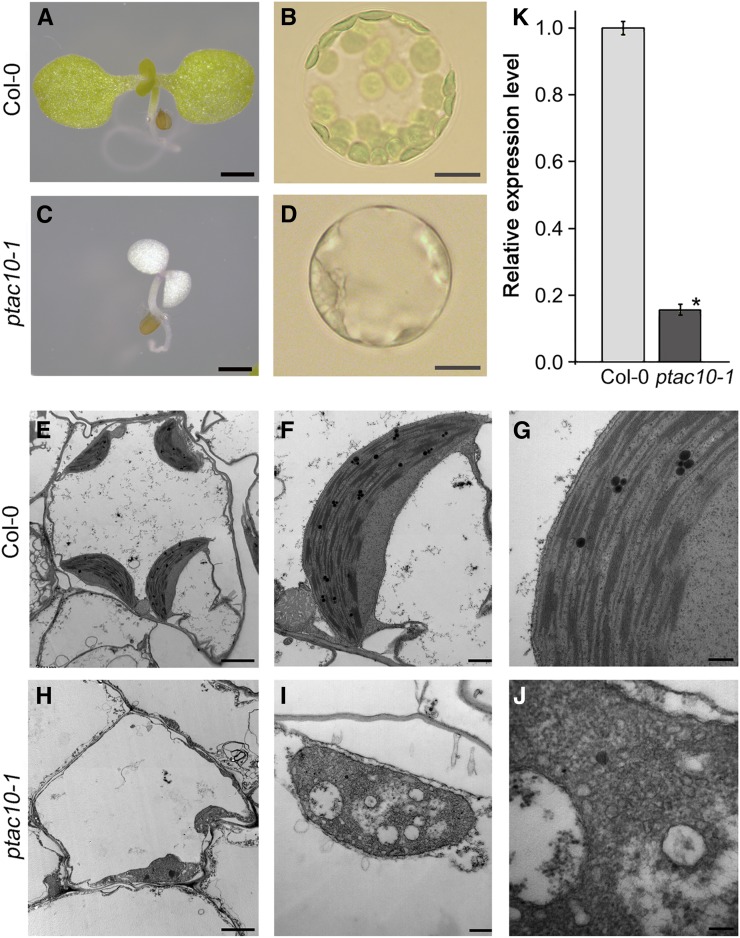
We isolated a T-DNA insertion mutant with defects in leaf greening (Fig. 1, A–D). The mutant plant showed a whitening phenotype by 3 d after germination, and the growth of this mutant was arrested at around 7 d (Supplemental Fig. S1, A–C). Morphological analysis of the protoplasts showed that this mutant plant has plastids but no chloroplasts, indicating that chloroplast development was severely compromised in the mutants. Transmission electron microscopy supported this. Unlike the Col-0 chloroplasts with well-organized thylakoid membranes, the plastids of this mutant plant did not exhibit proper stacking of thylakoid membranes (Fig. 1, E–J; Supplemental Fig. S2). These results suggested that these mutants may contain a mutation in one of the genes involved in chloroplast development.
Figure 1.
Characterization of the ptac10-1 mutants. A to D, Images of 7-d-old Columbia-0 (Col-0) and ptac10-1 mutant seedlings (left) and the protoplasts isolated from these plants (right). Bars = 1 mm in the seedling images and 5 μm in the protoplast images. E to J, Chloroplast ultrastructure of 7-d-old Col-0 (E–G) and ptac10-1 mutants (H–J). Transmission electron microscopy images showed that the ptac10-1 mutants are defective in chloroplast development. Bars = 2 μm in the left images, 0.5 μm in the middle images, and 200 nm in the right images. K, Analysis of the expression levels of pTAC10 in the ptac10-1 mutant and Col-0 by quantitative reverse transcription (qRT)-PCR. Error bars indicate sd. The asterisk indicates a statistically significant difference between the corresponding samples and their control (P < 0.01, Student’s t test). All experiments were repeated at least three times with similar results.
To identify the mutated gene responsible for this phenotype, we performed flanking sequence tag analysis and identified the candidate gene, pTAC10. We then performed genotyping and genetic analysis on the mutants for further verification (Supplemental Fig. S3, A and B). The pale-white mutant plants were homozygous for the T-DNA insertion. The seeds harvested from the selfed heterozygous mutants developed normal green and white seedlings at an approximately 3:1 ratio (green:white seedlings = 185:56). The genotypes of randomly selected green seedlings segregated at an approximately 1:2 ratio (homozygous wild-type seedlings:heterozygous mutant seedlings = 5:11), suggesting that the mutant phenotype is linked to the genotype (Supplemental Fig. S3C). We analyzed the expression level of pTAC10 in the homozygous mutants, which was around 5-fold lower than that in the Col-0 plants (Fig. 1K).
In the homozygous mutant, the expression of PEP-dependent genes, such as photosynthetic genes, was strongly suppressed, but the expression of nucleus-encoded RNA polymerase-dependent genes, such as rpoA, rpoB, rpoC1, and rpoC2, increased (Supplemental Fig. S3D; Supplemental Table S1), supporting the hypothesis that this mutant phenotype is caused by the suppressed expression of pTAC10, which encodes a subunit of the PEP complex. To examine this, we conducted a complementation experiment. To this end, we generated pTAC10 overexpression lines and found that overexpression of pTAC10 in the 35s::pTAC10 transgenic lines rescued the pale-white phenotype of the ptac10-1 homozygous mutant (Supplemental Fig. S3E). Collectively, these results indicated that the mutant we isolated is a ptac10 mutant (we named this allele ptac10-1), and pTAC10, as a key component of the PEP complex, plays a pivotal role in chloroplast development and leaf greening.
pTAC10 Expression Affects Chloroplast Development and Leaf Greening
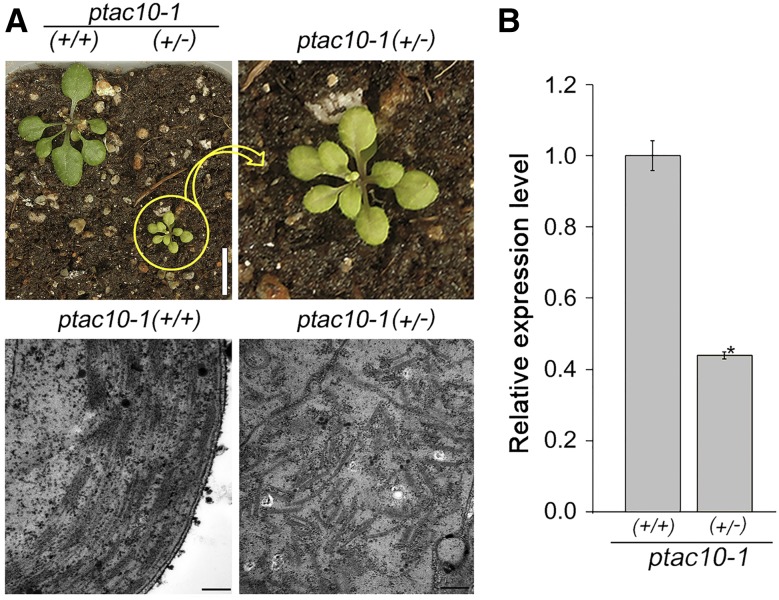
The essential function of pTAC10 in chloroplast development also was shown by the phenotype of the ptac10-1 heterozygous mutants. Heterozygous ptac10-1 (+/−) mutants displayed a pale-green phenotype compared with the wild-type plants (+/+; Fig. 2A). Unlike the homozygous mutants, which completely lacked stacked thylakoid membranes, the heterozygous mutants showed some stacks of thylakoid membranes. However, the organization of the thylakoid membranes was poor compared with the wild-type plants. In the heterozygous mutants, the expression level of pTAC10 was around half that of the wild-type plants (Fig. 2B; Supplemental Fig. S4). Together, these results indicated that the expression of pTAC10 is essential for chloroplast development and leaf greening.
Figure 2.
Chloroplast structure in ptac10-1 heterozygous mutants. A, Phenotypes of 3-week-old ptac10-1 heterozygous mutants (+/−) and wild-type plants (+/+; top) and the chloroplast ultrastructure in these plants (bottom). Bars = 1 cm in the top images and 200 nm in the bottom images. B, Analysis of the expression level of pTAC10 in the ptac10-1 heterozygous mutants (+/−) and wild-type plants (+/+) by qRT-PCR. Error bars indicate sd. The asterisk indicates a statistically significant difference between the corresponding sample and its control (P < 0.01, Student’s t test). All experiments were repeated at least three times with similar results.
Light-Dependent pTAC10 Expression Increases during Leaf Development
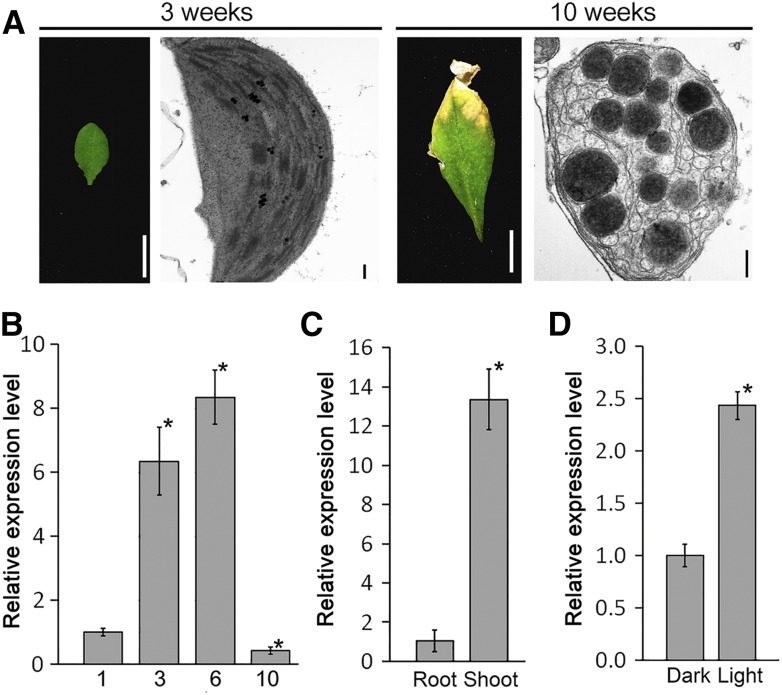
To understand how the expression of pTAC10 is regulated during leaf development, we analyzed the expression of pTAC10 in various leaf developmental stages in the Col-0 background (Fig. 3, A and B). The expression level of pTAC10 in the cotyledons of 1-week-old Col-0 seedlings was approximately 6-fold lower than that in rosettes of 3-week-old Col-0 plants. The expression level was highest in the leaves of 6-week-old plants but was lowest in 10-week-old leaves with senescent chloroplasts (Fig. 3B). The expression of pTAC10 was much higher in leaves than in roots (Fig. 3C), suggesting that pTAC10 expression occurs mainly in leaves and that its expression is correlated with chloroplast development and leaf greening.
Figure 3.
Regulation of pTAC10 expression with leaf development and in response to light. A and B, Leaf and chloroplast morphology (A) and the expression level of pTAC10 (B) at the indicated stages. Cotyledons of 1-week-old Col-0 seedlings and seventh or eighth rosettes of 3-, 6-, and 10-week-old Col-0 plants were analyzed. Bars = 0.5 cm in leaf images and 200 nm in chloroplast images. C, Tissue-specific expression of pTAC10 in 1-week-old Col-0 by qRT-PCR. D, Light-dependent expression of pTAC10 in 1-week-old Col-0 seedlings grown in continuous dark or light conditions. Error bars indicate sd. Asterisks indicate statistically significant differences between the corresponding samples and their controls (P < 0.01, Student’s t test). The expression analyses were repeated twice with similar results.
To further understand the regulation of pTAC10 expression in response to light, we analyzed changes in pTAC10 expression in light and dark conditions. The expression level of pTAC10 in plants grown in light conditions was approximately 2.5-fold higher than that in plants grown in dark conditions (Fig. 3D). These results suggested that pTAC10 expression is involved in chloroplast development.
Overexpression of pTAC10 Promotes Chloroplast Development and Affects Plant Development
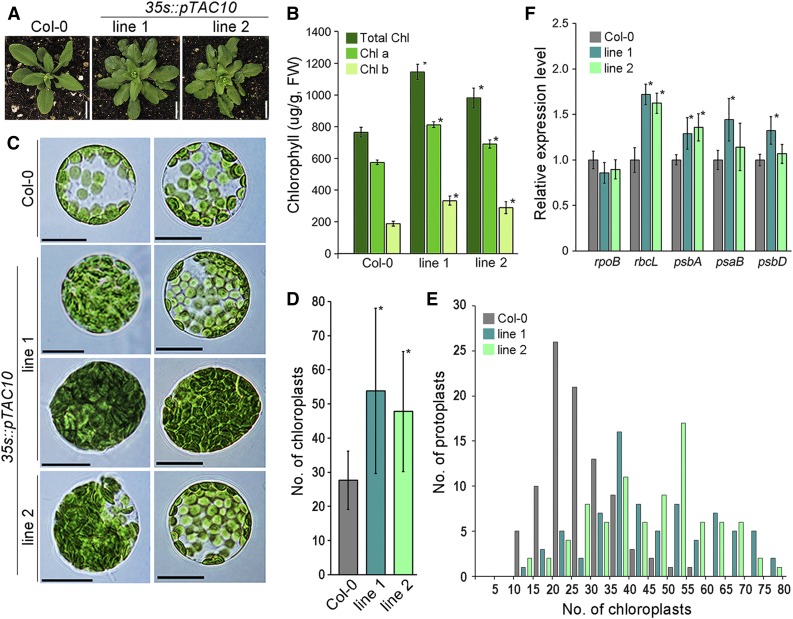
To understand the function of pTAC10 in chloroplast development and leaf greening, we analyzed the effect of the overexpression of pTAC10 on chloroplast development in the Col-0 background. Overexpression of pTAC10 caused leaves to have darker green color than in Col-0 control plants grown under the same conditions (Fig. 4A). Indeed, quantification of the chlorophyll contents showed a 30% to 40% increase in chlorophyll contents in the 35s::pTAC10 transgenic plants compared with the Col-0 control plants (Fig. 4B). The higher chlorophyll contents in the transgenic plants also were observed in young seedlings: 35s::pTAC10 transgenic plants grown in soil for 2 weeks showed a 17% to 22% increase in chlorophyll contents compared with Col-0 grown under the same conditions (Supplemental Fig. S5). When etiolated seedlings of 35s::pTAC10 and Col-0 were exposed to light and chlorophyll contents in these seedlings were analyzed in time-course manner, we found that pTAC10-overexpressing seedlings started to show higher chlorophyll contents than Col-0 by 12 h. These findings suggested that leaf greening is faster in pTAC10-overexpressing transgenic plants than in wild-type plants (Supplemental Fig. S6).
Figure 4.
pTAC10 overexpression promotes chloroplast development. The effect of pTAC10 overexpression on chloroplast development was analyzed. A, Phenotypes of pTAC10-overexpressing transgenic plants (35s::pTAC10) grown in soil for 5 weeks. Bars = 1 cm. B, Chlorophyll contents in 35s::pTAC10 transgenic plants and Col-0 plants grown in soil for 6 weeks. FW, Fresh weight. C, Morphology of the protoplasts isolated from 6-week-old Col-0, 35s::pTAC10 line 1, and 35s::pTAC10 line 2. Bars = 10 μm. D and E, Measurement of the number of chloroplasts in Col-0, 35s::pTAC10 line 1, and 35s::pTAC10 line 2. Values are means of chloroplast numbers in D and number of protoplasts in E (n > 80). F, Analysis of chloroplast gene expression level in 35s::pTAC10 and Col-0 plants grown in soil for 6 weeks. Error bars indicate sd. Asterisks indicate statistically significant differences between the corresponding samples and their controls (P < 0.01, Student’s t test). Analyses of morphology, chlorophyll content, and gene expression were repeated at least twice with similar results.
To investigate chloroplast development in these plants, we isolated protoplasts from the transgenic plants and Col-0 control plants and compared their morphology (Fig. 4, C–E). The 35s::pTAC10 transgenic plants had more chloroplasts than the Col-0 plants. The protoplasts isolated from 5-week-old Col-0 plants contained around 28 chloroplasts, whereas the protoplasts from 35s::pTAC10 contained around 50 chloroplasts (n > 80). When the number of chloroplasts was analyzed through frequency distribution, 92% of protoplasts contained fewer than 40 chloroplasts and only 8% of protoplasts contained more than 40 chloroplasts in Col-0 plants. However, in 35s::pTAC10 transgenic plants, approximately 60% of protoplasts contained more than 40 chloroplasts, indicating that the number of chloroplasts increased in the pTAC10-overexpressing plants. Furthermore, some protoplasts in the 35s::pTAC10 transgenic plants were fully filled with chloroplasts, which was not observed in the Col-0 control plants. However, there was no apparent difference in chloroplast ultrastructure between the 35s::pTAC10 transgenic plants and the Col-0 plants (Supplemental Fig. S7).
The expression of PEP-dependent genes, such as rbcL and psbA, increased significantly in the transgenic plants (Fig. 4F). To further explore this, we analyzed the expression of rbcL in the transgenic plants with the various expression levels of the pTAC10 transgene (Supplemental Fig. S8). In all independent lines of the pTAC10-overexpressing transgenic plants we tested, the expression level of rbcL was higher than that of wild-type plants, and with the increase of the pTAC10 expression level, the rbcL expression level tended to be up-regulated.
To test whether these effects are specific to pTAC10, we next examined transgenic plants overexpressing another PAP gene, FSD3. Unlike pTAC10-overexpressing transgenic plants, the phenotype and chlorophyll contents of the FSD3-overexpressing transgenic plants were almost identical to those of wild-type plants. Moreover, the increase in rbcL expression was not observed in the FSD3-overexpressing transgenic plants with various expression levels of the FSD3 transgene. These observations suggested that pTAC10 is a limiting factor for the regulation of PEP activity.
The promotion of chloroplast development in pTAC10-overexpressing plants seemed to affect plant biomass and productivity (Table I). The transgenic plants showed enhanced biomass and productivity: total dry weight and seed weight increased by 10% to 25% in the transgenic lines compared with the Col-0 plants grown under the same conditions. However, there was little or no difference in apical growth between the Col-0 plants and the 35s::pTAC10 transgenic plants.
Table I. Biomass and productivity of 35s::pTAC10 transgenic plants.
The biomass and productivity of 35s::pTAC10 transgenic plants were analyzed by measuring height, dry weight, and total seed weight. For this analysis, Col-0 and 35s::pTAC10 transgenic plants were grown in the same growth conditions for 4 months (n > 10). Lines 1, 2, and 3 indicate independent lines of 35s::pTAC10 transgenic plants. The experiment was repeated twice with similar results.
| Parameter | Plant | Average | sd | △% | P |
|---|---|---|---|---|---|
| Height (cm) | |||||
| Wild type | Col-0 | 29.482 | 2.449 | – | – |
| 35s::pTAC10 | Line 1 | 29.800 | 2.757 | 1.079 | 0.778 |
| Line 2 | 28.400 | 1.927 | −3.669 | 0.264 | |
| Line 3 | 29.773 | 2.661 | 0.987 | 0.792 | |
| Dry weight (g) | |||||
| Wild type | Col-0 | 1.653 | 0.230 | – | – |
| 35s::pTAC10 | Line 1 | 2.090 | 0.339 | 26.437 | 0.002 |
| Line 2 | 1.960 | 0.230 | 18.561 | 0.005 | |
| Line 3 | 1.897 | 0.229 | 14.745 | 0.021 | |
| Total seed weight (g) | |||||
| Wild type | Col-0 | 0.080 | 0.013 | – | – |
| 35s::pTAC10 | Line 1 | 0.097 | 0.015 | 21.178 | 0.010 |
| Line 2 | 0.093 | 0.013 | 16.421 | 0.027 | |
| Line 3 | 0.089 | 0.009 | 11.438 | 0.073 |
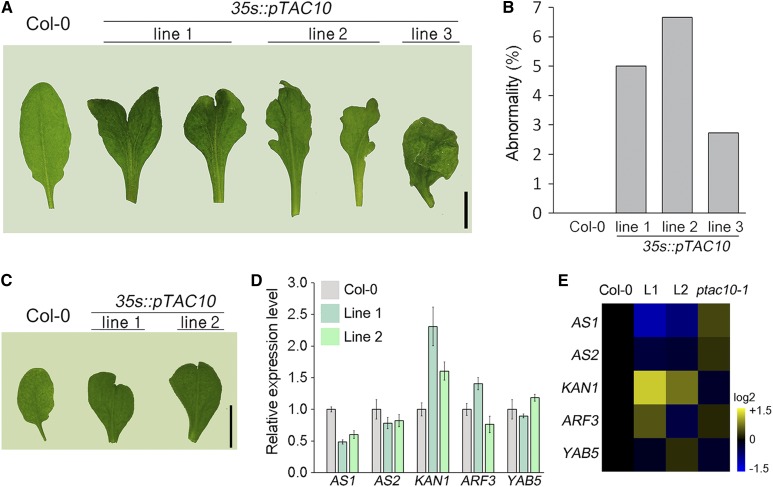
The 35s::pTAC10 transgenic plants formed abnormal leaves (Fig. 5, A and B). A snake tongue-like or severely wrinkled shape was detected in around 2% to 7% of leaves of the pTAC10-overexpressing transgenic plants, while no leaf abnormalities were observed in the Col-0 control plants grown under the same conditions. Chloroplast development is involved in leaf morphology in Arabidopsis (Arabidopsis thaliana; Moschopoulos et al., 2012; Mateo-Bonmatí et al., 2015). Therefore, these results suggested that the regulation of chloroplast development by the overexpression of pTAC10 might affect leaf morphology. To understand possible mechanisms underlying this, expression levels of the genes that encode key regulators of leaf morphology, such as AS1, AS2, KAN1, ARF3, and YAB5 (Machida et al., 2015), were analyzed in the leaves with abnormal morphology (Fig. 5, C–E). We found that the expression of AS1 and AS2 was down-regulated but the expression of KAN1 was up-regulated in the abnormal leaves collected from two independent lines of pTAC10-overexpressing plants. The expression of these genes was differentially regulated in ptac10-1 mutants: AS1 and AS2 expression tended to be up-regulated but KAN1 expression tended to be down-regulated in ptac10-1 mutants. These findings suggested that the regulation of AS and KAN1 expression is possibly involved in the formation of abnormal leaf morphology in pTAC10-overexpressing transgenic plants.
Figure 5.
Leaves with abnormal morphology in 35s::pTAC10 plants. A and B, Images of abnormal leaves collected from 8-week-old 35s::pTAC10 transgenic plants (A) and their ratios (B). The total numbers of leaves observed were 120 (line 1, five plants), 105 (line 2, five plants), and 110 (line 3, five plants). C, Images of abnormal leaves collected from 4-week-old 35s::pTAC10 transgenic plants. D, Expression levels of the genes related to leaf morphology in these leaves. Error bars indicate sd. The experiment was repeated twice with similar results. E, Heat map showing the expression pattern of the indicated genes in the pTAC10-overexpressing and ptac10-1 mutant plants. Expression levels of these genes in ptac10-1 mutants were collected from the RNA sequencing results. L1 and L2 indicate the abnormal leaves collected from 35s::pTAC10 line 1 and 35s::pTAC10 line 2 plants. Scale bar = 1 cm in A and C.
Interaction of pTAC10 with PAPs
Mutant plants in which the expression of PAPs is knocked out display whitening of the leaves and a strong reduction in PEP activity (Pfalz et al., 2006; Garcia et al., 2008; Myouga et al., 2008; Arsova et al., 2010; Chen et al., 2010; Schröter et al., 2010; Gao et al., 2011; Steiner et al., 2011; Yagi et al., 2012; Williams-Carrier et al., 2014). This indicates that the regulation of PEP activity and chloroplast development relies largely on the expression of PAPs. However, the expression of PAPs tended to be up-regulated in the ptac10-1 mutants (Fig. 6A; Supplemental Table S2). In contrast, the expression of PAPs was down-regulated in the pTAC10-overexpressing transgenic plants (Supplemental Fig. S9). These results indicated that the phenotypes of the ptac10-1 mutants and the 35s::pTAC10 transgenic plants were not caused by the transcriptional regulation of PAP expression.
Figure 6.
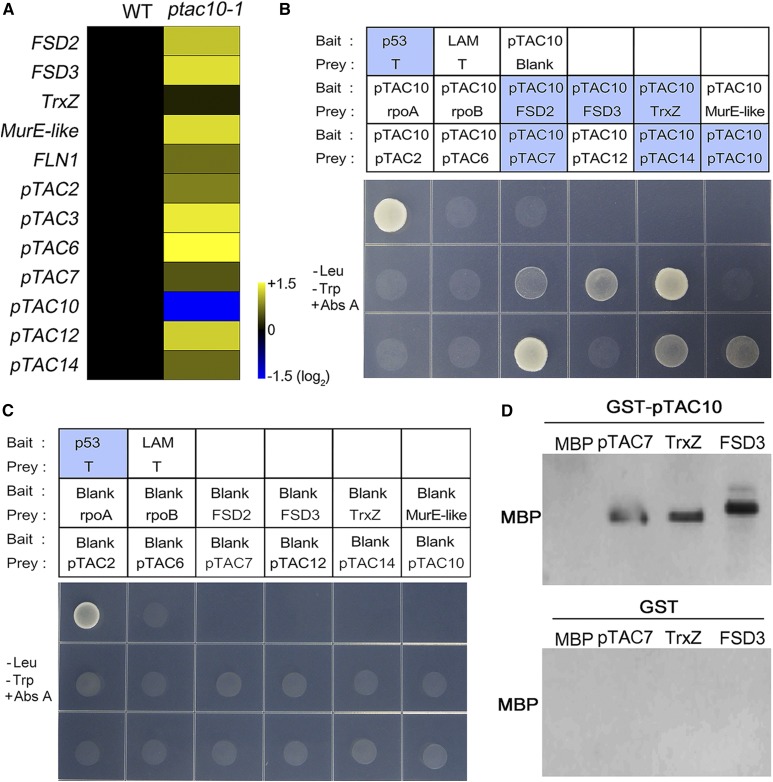
pTAC10 interacts with PAPs. The expression patterns of PAPs in the ptac10-1 mutants and the interactions of pTAC10 with PAPs were analyzed. A, Heat map showing the expression pattern of 12 PAPs in the ptac10-1 mutants and wild-type Col-0 plants (WT). Expression patterns of these genes were collected from RNA sequencing results using 1-week-old Col-0 and ptac10-1 mutant plants. B, Yeast two-hybrid assay showing that pTAC10 interacts with subunits of the PEP complex. The yeast line cotransformed with the p53 bait and T prey plasmids was used for a positive control, and the yeast line transformed with the LAM bait and T prey plasmids was used for a negative control. The yeast lines transformed with blank prey plasmids together with pTAC10 bait plasmids were used for the test of self-activation. –Leu –Trp +Abs A indicates Abs A-containing double dropout (DDO) medium (–Leu –Trp) for the test of pTAC10 interaction. Blue boxes indicate the yeast lines that survived in the Abs A-containing medium. C, Blank bait plasmid analysis to verify the interaction of pTAC10-PAPs in the yeast two-hybrid assay. D, GST pull-down assay showing that pTAC10 interacts with the PAPs. MBP and GST proteins were used as negative controls for the pTAC10-PAP interaction. Interaction analyses were repeated twice with similar results.
Establishment of the PEP complex is essential for PEP activity, and a recent study by Pfalz et al. (2015) suggested that pTAC10 is involved in the formation of the PEP complex. Although a previous study showed that pTAC10 interacts with pTAC7 (Yu et al., 2013), the interaction of pTAC10 with other PAPs or PEP core subunits has largely remained unknown. To investigate the interaction of pTAC10 with other subunits of the PEP complex, we isolated 12 genes encoding PEP complex subunits and analyzed the interactions of the encoded proteins with pTAC10 through yeast two-hybrid assays. The yeast (Saccharomyces cerevisiae Y2H GOLD) cotransformed with the pTAC10 bait plasmid and the prey plasmids encoding other PAPs, such as FSD2, FSD3, TrxZ, pTAC7, pTAC10, or the pTAC14 prey plasmid, survived in Leu-, Trp-, adenosine-, and His-deficient Quadruple Dropout medium (QDO), while the yeast lines cotransformed with the pTAC10 bait plasmid and prey plasmids encoding rpoA, rpoB, pTAC2, pTAC6, pTAC12, or the MurE-like prey plasmids did not survive these growth conditions (Supplemental Fig. S10). This result was further verified using the aureobasidin A (Abs A) system in which only the yeast lines with bait-prey protein interactions survive. Consistent with the QDO results, the yeast lines cotransformed with the pTAC10 bait plasmid and prey plasmids encoding FSD2, FSD3, TrxZ, pTAC7, pTAC10, or the pTAC14 prey plasmid survived in the Abs A condition. These results indicated that pTAC10 binds to pTAC7, pTAC10, and pTAC14 belonging to group 1, TrxZ belonging to group 2, and FSD2 and FSD3 belonging to group 3, suggesting that pTAC10 interacts with the PAPs belonging to groups 1, 2, and 3 but not to a single group (Fig. 6, B and C). The GST pull-down assay partially supported this: the GST-fused pTAC10 protein extracted from Escherichia coli interacted with MBP-fused pTAC7, TrxZ, and FSD3 but not with MBP (Fig. 6D). These results suggested that pTAC10 exhibits a broad range of interactions with other PAPs.
Recent work suggested that PAPs belonging to group 1 might be involved in the prokaryotic linkage of the transcription and translation system (Kindgren and Strand, 2015). To test the possible involvement of pTAC10 in this system, we cloned several genes encoding components of the plastid translation machinery, such as 30S ribosomal protein S3, 50S ribosomal protein L12-1, 50S ribosomal protein L29, and plastid elongation factor Tu, in Arabidopsis (Ristic et al., 2004; Fleischmann et al., 2011; Pfalz and Pfannschmidt, 2013) and analyzed the interaction between pTAC10 and these translation components through the yeast two-hybrid assay (Supplemental Fig. S11). The yeast lines transformed with the pTAC10 bait plasmid and the prey plasmids did not survive in the medium including Abs A. This suggested that pTAC10 proteins do not interact with these four components of the translational machinery.
The pTAC10 N-Terminal Regions, Including the S1 Domain, Are Not Involved in the Interactions of pTAC10 with PAPs
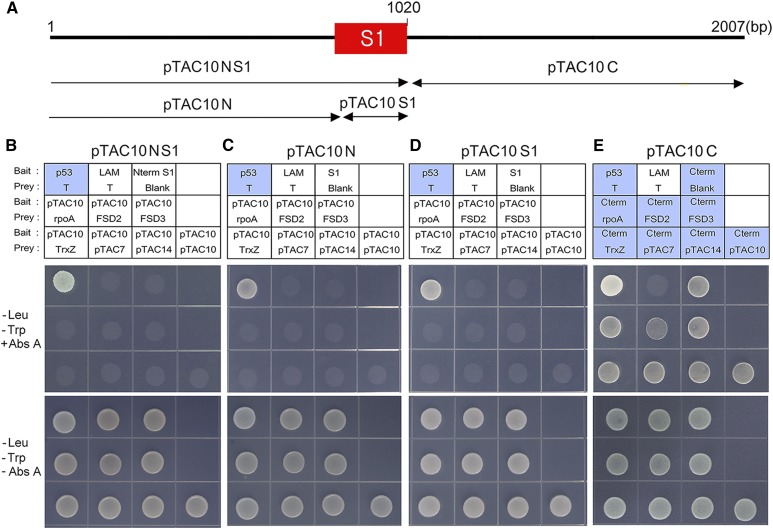
pTAC10 contains an S1 RNA-binding domain in the middle of the protein (Fig. 7A). A recent study using tobacco (Nicotiana tabacum) pTAC10 suggested that the S1 domain with RNA-binding activity plays an essential role in the regulation of photosynthetic gene expression (Jeon et al., 2012). This suggested that the S1 domain might be involved in the interaction of pTAC10 with other PAPs. To test this hypothesis, we generated truncated pTAC10 bait plasmids with or without the S1 domain and performed the yeast two-hybrid assay (Fig. 7, B–E). The truncated pTAC10NS1 protein carrying the pTAC10 N-terminal regions and S1 domain did not interact with other PAPs (Fig. 7B). None of the yeast lines cotransformed with this truncated pTAC10 bait and other PAP prey plasmids survived in Leu- or Trp-deficient medium including Abs A. To further investigate this, we performed yeast two-hybrid assays using two different pTAC10 bait plasmids, one carrying the N-terminal regions without the S1 domain (pTAC10N) and the other carrying the S1 domain alone (pTAC10S1; Fig. 7, C and D). Consistent with the result of the pTAC10NS1 protein, none of the yeast lines carrying these bait and target prey plasmids survived in the Abs A conditions, suggesting that the N-terminal regions, including the S1 domain, are not involved in the interaction of pTAC10 with other PAPs and that the C-terminal regions downstream of the S1 domain might mediate the interactions between pTAC10 and PAPs. To test this hypothesis, we cloned the truncated pTAC10 protein carrying the pTAC10 C-terminal regions downstream of the S1 domain (pTAC10C) and performed the yeast two-hybrid assay (Fig. 7E). Although the yeast lines cotransformed with this bait plasmid and target prey plasmids survived in the Abs A conditions, the yeast line transformed with the blank prey plasmid also survived in this condition, indicating that removal of the N-terminal region induces self-activation.
Figure 7.
The pTAC10 N-terminal regions including the S1 domain are not involved in the pTAC10-PAP interaction. A, Schematic of truncated pTAC10 protein structures. B to D, Yeast two-hybrid assays using the pTAC10NS1 (B), pTAC10N (C), and pTAC10S1 (D) plasmids. None of the yeast lines transformed with pTAC10NS1, pTAC10N, or pTAC10S1 bait plasmid together with the indicated prey plasmid survived in the Abs A-containing DDO medium. E, Yeast two-hybrid assay using the pTAC10C bait plasmid. Regardless of prey, all yeast lines carrying this bait plasmid survived in the Abs A-containing DDO medium. The rpoA prey plasmid was used as a negative control for pTAC10 interaction. DDO medium without Abs A was used for the validation of yeast transformation and equal dropping. Blue boxes indicate the yeast lines that survived in the Abs A-containing medium. All experiments were repeated at least twice with similar results.
pTAC10 Interacts with PAP Components through Its C-Terminal Regions
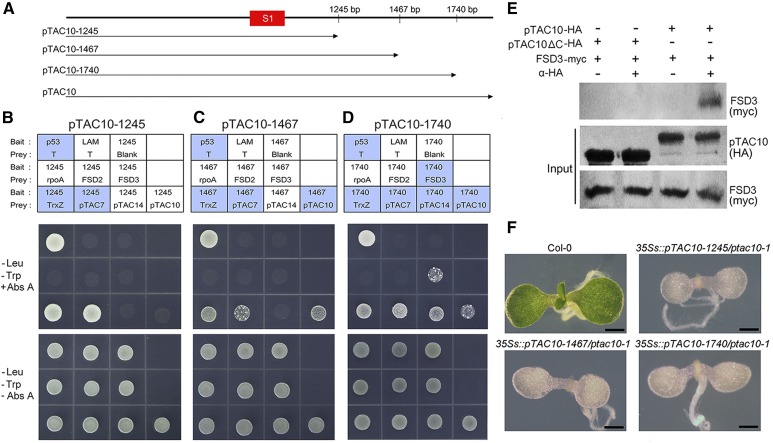
To further investigate the role of the pTAC10 C-terminal regions in the interaction of pTAC10 with PAPs, we cloned a series of truncated pTAC10 bait plasmids that contained the N-terminal region, the S1 domain, and additional parts of the C-terminal regions (Fig. 8A). Yeast two-hybrid assays using these pTAC10 bait plasmids did not self-activate and showed interactions with target prey proteins. When the pTAC10-1245 bait plasmid was used, the yeast lines carrying the TrxZ or pTAC7 prey plasmid survived in the Abs A conditions, suggesting that the pTAC10 C-terminal region containing amino acids 341 to 415 is involved in the pTAC10 interaction with TrxZ and pTAC7 (Fig. 8B). As the length of the C-terminal regions increased, additional interactions were observed. pTAC10-1467 showed an interaction with full-length pTAC10 as well as with TrxZ and pTAC7 (Fig. 8C). In the case of pTAC10-1740, an additional interaction was observed with pTAC14 and FSD3 (Fig. 8D). These results indicated that the pTAC10 C-terminal region containing amino acids 341 to 415 can bind to pTAC7 and TrxZ, the region containing amino acids 416 to 489 can bind to pTAC10, the region containing amino acids 490 to 580 can bind to pTAC14 and FSD3, and the region containing amino acids 581 to 668 can bind to FSD2. Collectively, these results suggested that the pTAC10 C-terminal region downstream of the S1 domain is responsible for the interactions of pTAC10 with PAPs. Coimmunoprecipitation analysis of the pTAC10-FSD3 interaction partially supported this. Full-length pTAC10 interacted with FSD3, while the truncated pTAC10 without its C-terminal region did not interact with FSD3 (Fig. 8E).
Figure 8.
The pTAC10 C-terminal region downstream of the S1 domain mediates the pTAC10-PAP interactions. A, Schematic of the truncated pTAC10 protein structure. B and D, Yeast two-hybrid assay results using pTAC10-1245 (B), pTAC10-1467 (C), and pTAC10-1740 (D) as bait plasmids. Blue boxes indicate the yeast lines that survived in the Abs A-containing medium. E, Coimmunoprecipitation analysis of the pTAC10 and FSD3 interaction. pTAC10-HA and pTAC10ΔC-HA indicate intact pTAC10 and pTAC10 lacking their C-terminal regions downstream of the S1 region fused with the HA epitope, respectively. FSD3-myc indicates FSD3 proteins fused with the myc epitope. F, Morphology of the ptac10-1 mutant transformed with the 35s::pTAC10-1245, 35s::pTAC10-1467, or 35s::pTAC10-1740 plasmid. Introduction of the 35s::pTAC10-1245, 35s::pTAC10-1467, or 35s::pTAC10-1740 plasmid did not rescue the ptac10-1 mutant phenotype, leading to seedling lethality. Interaction analyses were repeated twice with similar results. Bars = 1 mm.
To understand whether these pTAC10-PAP interactions are essential for chloroplast development, we introduced the 35s::pTAC10-1245, 35s::pTAC10-1467, and 35s::pTAC10-1740 plasmids into the ptac10-1 mutants to determine if they could rescue the ptac10-1 mutant phenotype (Fig. 8F). Expression of these truncated pTAC10 genes could not rescue the ptac10-1 mutant phenotype. This suggested that the complete C-terminal region of pTAC10 is essential for its interaction with PAPs and its functions in chloroplast development.
If the C-terminal region of pTAC10 mediates the pTAC10-PAP interaction, we expected that overexpression of truncated pTAC10 might affect PEP complex formation and chloroplast development by disrupting the interaction of endogenous pTAC10 with other PAPs. To address this, we checked the phenotypes of 35s::pTAC10/Col-0, 35s::pTAC10-1245/Col-0, 35s::pTAC10-1467/Col-0, and 35s::pTAC10-1740/Col-0 plants and found that overexpression of pTAC10-1467 or pTAC10-1740 in the Col-0 background induced an abnormal whitening phenotype (Fig. 9, A–J). The abnormal whitening phenotype was observed in three independent lines out of 12 lines of 35s::pTAC10-1467/Col-0 (25%) and four independent lines out of 13 lines of 35s::pTAC10-1740/Col-0 (31%), whereas 35s::pTAC10/Col-0 (eight lines) and 35s::pTAC10-1245/Col-0 (15 lines) did not display the abnormal phenotype. The abnormal whitening phenotype suddenly emerged in rosettes, petioles, cauline leaves, and stems at various developmental stages. The morphology of plastids differed between the green and white parts of the leaves with the whitening phenotype (Fig. 9, K–O; Supplemental Fig. S12). The plastids in the green part contained well-organized thylakoid membranes similar to the chloroplasts of wild-type plants, but the plastids in the whitening tissues did not show the proper stacking of thylakoid membranes. Analysis of the expression levels of the truncated pTAC10 transgene and the endogenous pTAC10 gene indicated that their expression levels were similar between the transgenic plants with and without the abnormal whitening phenotype (Supplemental Fig. S13, A and B). Furthermore, the expression level of endogenous pTAC10 in the green part also was similar or slightly higher compared with that in the whitening part of the leaves with the abnormal whitening phenotype (Supplemental Fig. S13, C and D). These observations suggested that the whitening phenotype might be caused by the disruption of endogenous pTAC10 function rather than by pTAC10 gene silencing. Collectively, these observations suggested that the pTAC10-PAP interaction through its C-terminal regions is crucial for PEP complex formation and that the assembly of the PEP complex is involved in plastid signaling.
Figure 9.
Abnormal whitening phenotypes in pTAC10-1467- or pTAC10-1740-overexpressing plants. A to C, Four-week-old Col-0 (A), 35s::pTAC10-1467/Col-0 (B), and 35s::pTAC10-1740/Col-0 (C) with the abnormal whitening phenotype. D, Seven-week-old Col-0 (left), 35s::pTAC10-1467/Col-0 (middle), and 35s::pTAC10-1740/Col-0 (right) with the abnormal whitening phenotype. 1467 and 1740 indicate 35s::pTAC10-1467/Col-0 and 35s::pTAC10-1740/Col-0, respectively. E to J, High-resolution images of Col-0 (E and H), 35s::pTAC10-1467/Col-0 (F and I), and 35s::pTAC10-1740/Col-0 (G and J). K, Image of a 35s::pTAC10-1467/Col-0 leaf with the abnormal whitening phenotype. L and M, Ultrastructure of the plastid in the green part of K and its high-resolution image. N and O, Ultrastructure of the plastid in the whitening part of K and its high-resolution image. Transmission electron microscopy analysis was repeated twice with similar results. Bars = 1 cm (A–D) and 200 nm (L–O).
DISCUSSION
PAPs play an essential role in the regulation of the activity of the PEP complex. A recent model proposed that the structural establishment of the PEP complex is a key developmental bottleneck governing the activity of the PEP complex and chloroplast development (Pfalz and Pfannschmidt, 2013). This model is supported by many studies showing PAP-PAP and PAP-PEP interactions (Arsova et al., 2010; Gao et al., 2011; Yagi et al., 2012; Huang et al., 2013; Yu et al., 2013). Although previous studies had suggested the potential importance of pTAC10 in the formation of the PEP complex, the interaction of pTAC10 with other subunits of the PEP complex has remained largely unknown (Yu et al., 2013; Pfalz et al., 2015). In this study, we found that pTAC10 proteins, which are required for chloroplast development, show a broad range of interactions with other PAPs but do not bind to core subunits of the PEP complex such as rpoA and rpoB. Among the 10 PAPs isolated in this study, pTAC10 interacted with five of them, pTAC7, pTAC14, FSD2, FSD3, and TrxZ. Based on the recent categorization of the PAPs (Kindgren and Strand, 2015), pTAC10 showed interactions with the PAPs belonging to three different groups: pTAC7 and pTAC14 of group 1, TrxZ of group 2, and FSD2 and FSD3 of group 3. Together with the results described by Pfalz et al. (2015) showing that pTAC10 is involved in the formation of the fully assembled PEP complex in maize, this result supports the idea that pTAC10 plays a pivotal role in the proper assembly of the PEP complex.
pTAC10 contains an S1 RNA-binding domain in the middle region of its protein structure. The S1 domain, conserved in a large number of RNA-associated proteins, was first identified in ribosomal protein S1 in E. coli (Escherichia coli; Subramanian, 1983; Bycroft et al., 1997). Previous biochemical and structural studies in bacteria showed that the S1 domain is responsible for the interaction with RNA (Boni et al., 1991). The direct interaction between the S1 domain and RNA also was shown in the plastid transcription system: the S1 domain of tobacco pTAC10 binds to RNA in a sequence-nonspecific manner (Jeon et al., 2012). This study also revealed that the S1 domain is crucial for pTAC10 function in PEP-dependent photosynthetic gene expression. However, it is likely that the S1 domain does not participate in the pTAC10 interaction with other PAPs, as we showed that the S1 domain of pTAC10 did not mediate its interaction with 11 subunits of the PEP complex cloned in this study. Truncated pTAC10 proteins carrying only the S1 domain or the N-terminal region with the S1 domain did not bind to the PAPs. Instead, the C-terminal region downstream of the S1 domain is responsible for the interactions. Unlike the truncated pTAC10 proteins lacking the C-terminal regions downstream of the S1 domain, the series of truncated pTAC10 proteins carrying the N-terminal region, the S1 domain, and additional C-terminal regions interacted with the PAPs. Together with the essential role of the S1 domain in interactions with RNAs, these results suggest that pTAC10 plays a key role in chloroplast development by mediating interactions with RNA and other PAPs through its S1 domain and C-terminal regions downstream of the S1 domain.
Since PEP complex formation, which is required for PEP activity, is mediated through PAP-PEP and PAP-PAP interactions, the pTAC10-PAP interaction through its C-terminal region might be essential for the establishment of the PEP complex and chloroplast development. The importance of the pTAC10-PAP interactions was partially supported by the ptac10-1 mutant complementation test using the truncated pTAC10 proteins. Expression of the truncated pTAC10 cDNAs, such as pTAC10-1245, pTAC10-1467, and pTAC10-1740, did not rescue the ptac10-1 mutant phenotype, whereas the full-length pTAC10 did rescue the phenotype. We also found that overexpression of truncated pTAC10s, such as pTAC10-1467 and pTAC10-1740, induced an abnormal whitening phenotype in the Col-0 background. The morphology of the plastids in the whitening tissue was very different from that of the chloroplasts, and proper stacking of thylakoid membranes was not observed in the plastids of the whitening tissues. Transcript levels of endogenous pTAC10 were similar between the transgenic plants with and without the whitening phenotype or between the whitening and the green parts of the leaves with the whitening phenotype. These observations suggest that the whitening phenotype might be caused by the potential for the truncated pTAC10 proteins to compete with endogenous pTAC10 proteins for the interaction with other PAPs, leading to disruption of the proper assembly of the PEP complex. Together with the result that overexpression of full-length pTAC10 increases the number of chloroplasts, these results suggest that the composition of the PEP complex might affect plastid signaling.
In this study, we showed that overexpression of full-length pTAC10 promotes the expression of PEP-dependent photosynthetic genes and chlorophyll contents. Unlike pTAC10, overexpression of another PAP, FSD3, did not affect the expression of photosynthetic genes and chlorophyll contents. These findings suggest that pTAC10, with its broad range of interactions with other PAPs, is a limiting factor for the formation of the PEP complex. Despite the essential role of the PEP complex in chloroplast development, it remains largely unknown when the active PEP complex assembles during plant development. In this study, we showed that pTAC10 expression increases gradually along with leaf development and that pTAC10 overexpression activates the expression of photosynthetic genes and chlorophyll contents even in young plants. These results suggest that the assembly of active PEP complex might be promoted along with leaf development and that pTAC10 plays an essential role in the assembly of the PEP complex.
Finally, we showed here that overexpression of full-length pTAC10 cDNA also enhanced plant growth and productivity. These observations suggest that plant growth and productivity can be improved by the regulation of chloroplast development. Further molecular and genetic approaches will advance our knowledge of the functions of pTAC10 in PEP complex formation and chloroplast development and inform strategies for creating transgenic crops with high yields.
MATERIALS AND METHODS
Plant Materials, Growth Conditions, and Treatments
Arabidopsis (Arabidopsis thaliana) ecotype Col-0 was used as a control in this study. Seeds were surface sterilized and plated on one-half-strength Murashige and Skoog solid medium. After 2 d of vernalization at 4°C in darkness, plants were grown in a growth chamber with a light regime of 16/8 h (light/dark) at 22°C. These seedlings were transferred into soil for tests on mature plants.
Construction of the 35s::pTAC10 and 35s::FSD3 Plasmids
The Gateway system (Invitrogen) was used for construction of the 35s::pTAC10 and 35s::FSD3 plasmids. Full-length pTAC10 and FSD3 cDNA fragments were amplified by reverse transcription-PCR using total RNA extracted from 6-week-old Arabidopsis leaves, respectively. These cDNAs were inserted into the pDONR221 vector (Invitrogen) by the BP reaction. Then, these pENTRY clones were recombined into the modified pMDC plant binary vector carrying the 35s promoter by the LR reaction (for primer information, see Supplemental Table S3). For the construction of 35s::pTAC10-1245, 35s::pTAC10-1467, and 35s::pTAC10-1740, each truncated pTAC10 cDNA was amplified by PCR using full-length pTAC10 cDNA as a template. These cDNAs were introduced into the pMDC vector through the BP and LR reactions.
Ultramicrosectioning and Transmission Electron Microscopy
For the visualization of chloroplast ultrastructure, ultramicrosectioning was performed as described previously by Motohashi et al. (2001) with slight modifications. Leaves collected from 1-, 3-, 6- and 10-week-old plants were fixed for 24 h at room temperature using fixing solution 1 (0.86 m Na-P [pH 7.2], 1% paraformaldehyde [w/w], and 1% glutaraldehyde [w/w]). These samples were washed three times using washing solution (0.137 m Na-P [pH 7.2]) and then fixed again for 1 h at room temperature using fixing solution 2 (0.86 m Na-P [pH 7.2] and 2% osmium tetroxide [w/w]). After washing three times, dehydration was performed with a series of acetone gradients (25%, 50%, 75%, and 100% in distilled, deionized water [v/v]) for 30 min each and then incubated in absolute acetone overnight. The dehydrated samples were sequentially incubated in a series of Spurr resin gradients (Sigma; 25%, 50%, 75%, and 100% in acetone [v/v]) for 1 h each and absolute Spurr overnight. For solidification, these samples were placed in a mold at 65°C for 2 d. Sections (70 nm) were taken with an ultramicrotome (EM UC7; Leica). Images were captured with a transmission electron microscope (JEM1010; JEOL).
Yeast Two-Hybrid Assay
The yeast two-hybrid assay was performed using the Matchmaker Gold Yeast Two-Hybrid System (Clontech). For construction of the yeast two-hybrid assay, pGBKT7 carrying a Trp biosynthetic TRP1 and pGADT7 carrying a Leu biosynthetic LEU2 were used for the bait and prey plasmids, respectively. The PAP cDNAs were amplified by reverse transcription-PCR from total RNA extracted from 6-week-old Arabidopsis leaves, and rpoA and rpoB were amplified from chloroplast genomic DNA isolated from the chloroplasts of 6-week-old Arabidopsis leaves. pGBKT7 was used for the construction of the recombinant bait plasmid, and pGADT7 was used for the recombinant prey plasmid. These cDNAs were inserted into the SmaI site of pGBKT7 and pGADT7 using the gene assembly system (Gibson assembly; New England Biolabs) in frame with its GAL4 DNA-binding domain and activation domain. The pTAC10 bait and PAP prey plasmids were cotransformed into Y2H Gold yeast (Saccharomyces cerevisiae) strain with the Abs A antibiotic resistance gene to test protein-protein interactions. Yeast carrying both the bait and prey plasmids were selected in minimal yeast growth medium without Trp and Leu (DDO). To screen for the interaction between the indicated proteins, the selected yeast (OD = 0.05) were inoculated on the DDO medium including 250 ng mL−1 Abs A. After a 3- to 4-d incubation in dark conditions at 30°C, the growth of yeast was captured using a digital camera (Coolpix p300; Nikon). Sequences of the primers used for the bait and prey plasmids are given in Supplemental Table S3.
In Vitro Pull-Down Assay
pTAC10 cDNA was inserted into pGEX-DC for GST fusion, and rpoA, FSD2, FSD3, and TrxZ cDNA was inserted into pMAL-DC for MBP fusion (Zhang et al., 2005). All constructs were introduced into the Escherichia coli BL21 (DE3) pLysS strain, and recombinant protein expression was induced by 0.5 mm isopropyl-β-d-thiogalactoside at 18°C overnight. For in vitro pull-down assay, GST and GST-pTAC10 fusion proteins were extracted using homogenization buffer (25 mm Tris-HCl [pH 7.5], 0.5% Triton X-100, 150 mm NaCl, and 2 mm EDTA) and incubated for 4 h at 4°C with glutathione-Sepharose 4B beads (GE Healthcare) with rotation. After washing three times with the homogenization buffer without Triton X-100, MBP-fused prey proteins were incubated together with the beads at 4°C overnight. After washing three times, proteins were eluted by boiling with 2× sample buffer. Eluted proteins were loaded on 10% SDS-polyacrylamide gels and then transferred to a polyvinylidene fluoride membrane. Immunoblotting was performed with MBP polyclonal antibodies (Santa Cruz) and anti-rabbit HRP-linked secondary antibody (Thermo Fisher). Western-blot signals were detected with Amersham ECL prime (GE Healthcare).
Coimmunoprecipitation Assay
For coimmunoprecipitation analysis, 35s::pTAC10-HA, 35s::pTAC10ΔC-HA, and 35s::FSD3-myc plasmids were generated. pTAC10 and pTAC10ΔC cDNAs were introduced into the pE2C plasmid (Addgene) for pTAC10-HA and pTAC10ΔC-HA. FSD3 was introduced into the pE3C plasmid for FSD3-myc. These entry clones were inserted into the pMDC plasmid by the LR reaction. Intact or truncated pTAC10 proteins fused with a C-terminal HA tag (pTAC10-HA and pTAC10-ΔC-HA) and FSD3 fused with a C-terminal myc epitope tag (FSD3-myc) were coproduced in leaves of Nicotiana benthamiana by agroinfiltration using Agrobacterium tumefaciens strain GV3101 (Llave et al., 2000). Total proteins were extracted with immunoprecipitation buffer (50 mm Tris-HCl [pH 7.5], 150 mm NaCl, 10 mm MgCl2, 0.1% Nonidet P-40 [v/v], 1 mm phenylmethylsulfonyl fluoride, 10% glycerol [v/v], 1 mm DTT, and protease inhibitor cocktail). After centrifugation at 15,000g for 15 min, the supernatant was incubated with HA polyclonal antibodies (Thermo Fisher) at 4°C for 2 h. Immune complexes were then pulled down by protein A agarose at 4°C overnight and washed four times with immunoprecipitation buffer without Nonidet P-40. Immunoblot analysis was performed with myc monoclonal antibodies (Thermo Fisher) and anti-mouse HRP-linked secondary antibodies (Thermo Fisher).
qRT-PCR Analysis
qRT-PCR analyses were carried out using total RNA extracted from Col-0, ptac10-1, and pTAC10-overexpressing plants. Extraction of total RNA was performed using the RNeasy plant mini-prep kit (Qiagen) according to the manufacturer’s instructions. For synthesis of the first-strand cDNA, 20 µL of reverse transcription reaction was performed using 2 µg of total RNA and SuperScript III reverse transcriptase (Invitrogen). For quantitative PCR, a master mix was prepared using the LightCycler 480 SYBR GREEN I Master Mix (Roche). PCR and fluorescence detection were performed using a Light Cycler NANO Real-Time PCR machine (Roche). PCR conditions were programmed according to the manufacturer’s instructions (initial denaturation at 95°C for 5 min followed by 45 cycles of denaturation at 95°C for 10 s, annealing at 58°C for 10 s, and extension at 72°C for 10 s). Expression levels were analyzed using three technical replicates. AtACT2 (At3g18780) was used as an internal control. Primer information is given in Supplemental Table S3.
Measurement of Chlorophyll Content
The chlorophyll contents in 6-week-old Col-0 and 35s::pTAC10 transgenic plants were measured as described previously by Sumanta et al. (2014). Fresh leaves (0.75 g) were homogenized with a plant tissue homogenizer with 15 mL of 95% (v/v) ethanol in distilled, deionized water. The homogenized samples were centrifuged at 12,000g for 15 min at 4°C. The supernatants were diluted 10-fold using 95% ethanol. Contents of chlorophyll were measured with a UV/visible spectrophotometer (OPTIZEN POP; Mecasys). An SPDA-502 chlorophyll meter (Konica Minolta) was used for direct measurements.
RNA Sequencing Analysis
Col-0 plants and ptac10-1 mutants were grown in one-half-strength Murashige and Skoog solid medium for 7 d. Total RNA was extracted from aerial parts of these plants using the RNeasy plant mini-prep kit (Qiagen). One microgram of total RNA was used to generate cDNA libraries with the TruSeq RNA library kit. This step consisted of mRNA purification, fragmentation, random hexamer-primed reverse transcription, and paired-end sequencing. The libraries were qualified using an Agilent Technologies 2100 Bioanalyzer. Enrichment of the transcript sequencing data was calculated as fragments per kilobase of exon model per million mapped fragments of each transcript in each sample by Cufflinks software. The transcripts with zero fragments per kilobase of exon model per million mapped fragment values were removed from each data set. The multiexperiment viewer MeV, part of the TM4 microarray software suite, was used for the generation of heat maps (Saeed et al., 2006). The accession number of these data sets is GSE90159 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE90159).
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: pTAC10 (At3G48500), pTAC2 (At1G74850), pTAC6 (At1G21600), pTAC7 (At5G24314), pTAC12 (At2G34640), pTAC14 (At4G20130), FSD2 (At5G51100), FSD3 (At5G23310), TrxZ (At3G06730), MurE-like (At1G63680), rpoA (AtCG00740), rpoB (AtCG00190), rbcL (AtCG00490), psbA (AtCG00020), psaB (AtCG00340), and psbD (AtCG00270).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Growth of ptac10-1 mutants.
Supplemental Figure S2. Plastid ultrastructure of ptac10-1 mutants.
Supplemental Figure S3. Genotyping of the ptac10-1 mutants.
Supplemental Figure S4. Expression level of pTAC10 in heterozygous ptac10-1 mutants.
Supplemental Figure S5. Chlorophyll contents in the 35s::pTAC10 plants.
Supplemental Figure S6. Leaf greening in the etiolated seedlings.
Supplemental Figure S7. Chloroplast ultrastructure of the 35s::pTAC10 plants.
Supplemental Figure S8. Expression levels of rbcL in pTAC10- or FSD3-overexpressing plants.
Supplemental Figure S9. PAP expression in the 35s::pTAC10 plant.
Supplemental Figure S10. Interaction of pTAC10 with the PEP complex subunits.
Supplemental Figure S11. Interaction of pTAC10 with components of the plastid translation machinery.
Supplemental Figure S12. Plastid ultrastructure in the whitening leaves of 35s::pTAC10-1467 plants.
Supplemental Figure S13. Analysis of expression levels of the truncated pTAC10 and endogenous pTAC10.
Supplemental Table S1. Expression pattern of chloroplast genes in the ptac10-1 mutant.
Supplemental Table S2. Expression pattern of PAPs in the ptac10-1 mutant.
Supplemental Table S3. Primers used in this study.
Supplementary Material
Acknowledgments
We thank Nam-Hai Chua for donating pGEX-DC and pMAL-DC plasmid for GST and MBP fusions.
Glossary
- Col-0
Columbia-0
- Abs A
aureobasidin A
- qRT
quantitative reverse transcription
- DDO
double dropout
Footnotes
This work was supported by the National Research Foundation of Korea funded by the Korean Government (grant no. NRF-2016R1D1A1B03931167), the Cooperative Research Program for Agriculture Science and Technology Development (grant no. PJ01121501 to Y.D.C.), the Rural Development Administration, Republic of Korea, through the National Center for GM Crops, and the Brain Korea 21 Plus project of the Korean Government (graduate research assistantship to T.Y.U.).
References
- Allison LA, Simon LD, Maliga P (1996) Deletion of rpoB reveals a second distinct transcription system in plastids of higher plants. EMBO J 15: 2802–2809 [PMC free article] [PubMed] [Google Scholar]
- Arsova B, Hoja U, Wimmelbacher M, Greiner E, Ustün S, Melzer M, Petersen K, Lein W, Börnke F (2010) Plastidial thioredoxin z interacts with two fructokinase-like proteins in a thiol-dependent manner: evidence for an essential role in chloroplast development in Arabidopsis and Nicotiana benthamiana. Plant Cell 22: 1498–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Krupinska K, Biswal UC (2013) Plastid Development in Leaves during Growth and Senescence. Springer, Dordrecht, The Netherlands [Google Scholar]
- Boni IV, Isaeva DM, Musychenko ML, Tzareva NV (1991) Ribosome-messenger recognition: mRNA target sites for ribosomal protein S1. Nucleic Acids Res 19: 155–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan BB, Gruissem W, Jones RL (2015) Biochemistry and Molecular Biology of Plants. John Wiley & Sons, Hoboken, New Jersey [Google Scholar]
- Bycroft M, Hubbard TJ, Proctor M, Freund SM, Murzin AG (1997) The solution structure of the S1 RNA binding domain: a member of an ancient nucleic acid-binding fold. Cell 88: 235–242 [DOI] [PubMed] [Google Scholar]
- Chen M, Galvão RM, Li M, Burger B, Bugea J, Bolado J, Chory J (2010) Arabidopsis HEMERA/pTAC12 initiates photomorphogenesis by phytochromes. Cell 141: 1230–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santis-MacIossek G, Kofer W, Bock A, Schoch S, Maier RM, Wanner G, Rüdiger W, Koop HU, Herrmann RG (1999) Targeted disruption of the plastid RNA polymerase genes rpoA, B and C1: molecular biology, biochemistry and ultrastructure. Plant J 18: 477–489 [DOI] [PubMed] [Google Scholar]
- Fleischmann TT, Scharff LB, Alkatib S, Hasdorf S, Schöttler MA, Bock R (2011) Nonessential plastid-encoded ribosomal proteins in tobacco: a developmental role for plastid translation and implications for reductive genome evolution. Plant Cell 23: 3137–3155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZP, Yu QB, Zhao TT, Ma Q, Chen GX, Yang ZN (2011) A functional component of the transcriptionally active chromosome complex, Arabidopsis pTAC14, interacts with pTAC12/HEMERA and regulates plastid gene expression. Plant Physiol 157: 1733–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M, Myouga F, Takechi K, Sato H, Nabeshima K, Nagata N, Takio S, Shinozaki K, Takano H (2008) An Arabidopsis homolog of the bacterial peptidoglycan synthesis enzyme MurE has an essential role in chloroplast development. Plant J 53: 924–934 [DOI] [PubMed] [Google Scholar]
- Hajdukiewicz PT, Allison LA, Maliga P (1997) The two RNA polymerases encoded by the nuclear and the plastid compartments transcribe distinct groups of genes in tobacco plastids. EMBO J 16: 4041–4048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Yu QB, Lv RH, Yin QQ, Chen GY, Xu L, Yang ZN (2013) The reduced plastid-encoded polymerase-dependent plastid gene expression leads to the delayed greening of the Arabidopsis fln2 mutant. PLoS ONE 8: e73092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon Y, Jung HJ, Kang H, Park YI, Lee SH, Pai HS (2012) S1 domain-containing STF modulates plastid transcription and chloroplast biogenesis in Nicotiana benthamiana. New Phytol 193: 349–363 [DOI] [PubMed] [Google Scholar]
- Kindgren P, Strand Å (2015) Chloroplast transcription, untangling the Gordian knot. New Phytol 206: 889–891 [DOI] [PubMed] [Google Scholar]
- Krause K, Maier RM, Kofer W, Krupinska K, Herrmann RG (2000) Disruption of plastid-encoded RNA polymerase genes in tobacco: expression of only a distinct set of genes is not based on selective transcription of the plastid chromosome. Mol Gen Genet 263: 1022–1030 [DOI] [PubMed] [Google Scholar]
- Link G. (2003) Redox regulation of chloroplast transcription. Antioxid Redox Signal 5: 79–87 [DOI] [PubMed] [Google Scholar]
- Llave C, Kasschau KD, Carrington JC (2000) Virus-encoded suppressor of posttranscriptional gene silencing targets a maintenance step in the silencing pathway. Proc Natl Acad Sci USA 97: 13401–13406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida C, Nakagawa A, Kojima S, Takahashi H, Machida Y (2015) The complex of ASYMMETRIC LEAVES (AS) proteins plays a central role in antagonistic interactions of genes for leaf polarity specification in Arabidopsis. Wiley Interdiscip Rev Dev Biol 4: 655–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeran W, Friso G, Asakura Y, Qu X, Huang M, Ponnala L, Watkins KP, Barkan A, van Wijk KJ (2012) Nucleoid-enriched proteomes in developing plastids and chloroplasts from maize leaves: a new conceptual framework for nucleoid functions. Plant Physiol 158: 156–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W, Rujan T, Richly E, Hansen A, Cornelsen S, Lins T, Leister D, Stoebe B, Hasegawa M, Penny D (2002) Evolutionary analysis of Arabidopsis, cyanobacterial, and chloroplast genomes reveals plastid phylogeny and thousands of cyanobacterial genes in the nucleus. Proc Natl Acad Sci USA 99: 12246–12251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo-Bonmatí E, Casanova-Sáez R, Quesada V, Hricová A, Candela H, Micol JL (2015) Plastid control of abaxial-adaxial patterning. Sci Rep 5: 15975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melonek J, Oetke S, Krupinska K (2016) Multifunctionality of plastid nucleoids as revealed by proteome analyses. Biochim Biophys Acta 1864: 1016–1038 [DOI] [PubMed] [Google Scholar]
- Moschopoulos A, Derbyshire P, Byrne ME (2012) The Arabidopsis organelle-localized glycyl-tRNA synthetase encoded by EMBRYO DEFECTIVE DEVELOPMENT1 is required for organ patterning. J Exp Bot 63: 5233–5243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motohashi R, Nagata N, Ito T, Takahashi S, Hobo T, Yoshida S, Shinozaki K (2001) An essential role of a TatC homologue of a ΔpH-dependent protein transporter in thylakoid membrane formation during chloroplast development in Arabidopsis thaliana. Proc Natl Acad Sci USA 98: 10499–10504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myouga F, Hosoda C, Umezawa T, Iizumi H, Kuromori T, Motohashi R, Shono Y, Nagata N, Ikeuchi M, Shinozaki K (2008) A heterocomplex of iron superoxide dismutases defends chloroplast nucleoids against oxidative stress and is essential for chloroplast development in Arabidopsis. Plant Cell 20: 3148–3162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier JB, Cai Y, Sun Q, Zabrouskov V, Giacomelli L, Rudella A, Ytterberg AJ, Rutschow H, van Wijk KJ (2006) The oligomeric stromal proteome of Arabidopsis thaliana chloroplasts. Mol Cell Proteomics 5: 114–133 [DOI] [PubMed] [Google Scholar]
- Pfalz J, Holtzegel U, Barkan A, Weisheit W, Mittag M, Pfannschmidt T (2015) ZmpTAC12 binds single-stranded nucleic acids and is essential for accumulation of the plastid-encoded polymerase complex in maize. New Phytol 206: 1024–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfalz J, Liere K, Kandlbinder A, Dietz KJ, Oelmüller R (2006) pTAC2, -6, and -12 are components of the transcriptionally active plastid chromosome that are required for plastid gene expression. Plant Cell 18: 176–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfalz J, Pfannschmidt T (2013) Essential nucleoid proteins in early chloroplast development. Trends Plant Sci 18: 186–194 [DOI] [PubMed] [Google Scholar]
- Pfannschmidt T, Liere K (2005) Redox regulation and modification of proteins controlling chloroplast gene expression. Antioxid Redox Signaling 7: 607–618 [DOI] [PubMed] [Google Scholar]
- Ristic Z, Wilson K, Nelsen C, Momcilovic I, Kobayashi S, Meeley R, Muszynski M, Habben J (2004) A maize mutant with decreased capacity to accumulate chloroplast protein synthesis elongation factor (EF-Tu) displays reduced tolerance to heat stress. Plant Sci 167: 1367–1374 [Google Scholar]
- Saeed AI, Bhagabati NK, Braisted JC, Liang W, Sharov V, Howe EA, Li J, Thiagarajan M, White JA, Quackenbush J (2006) TM4 microarray software suite. Methods Enzymol 411: 134–193 [DOI] [PubMed] [Google Scholar]
- Sagan L. (1967) On the origin of mitosing cells. J Theor Biol 14: 255–274 [DOI] [PubMed] [Google Scholar]
- Schröter Y, Steiner S, Matthäi K, Pfannschmidt T (2010) Analysis of oligomeric protein complexes in the chloroplast sub-proteome of nucleic acid-binding proteins from mustard reveals potential redox regulators of plastid gene expression. Proteomics 10: 2191–2204 [DOI] [PubMed] [Google Scholar]
- Steiner S, Schröter Y, Pfalz J, Pfannschmidt T (2011) Identification of essential subunits in the plastid-encoded RNA polymerase complex reveals building blocks for proper plastid development. Plant Physiol 157: 1043–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian AR. (1983) Structure and functions of ribosomal protein S1. Prog Nucleic Acid Res Mol Biol 28: 101–142 [DOI] [PubMed] [Google Scholar]
- Sugiura M. (1992) The chloroplast genome. In Schilperoort RA, Dure L, eds, 10 Years Plant Molecular Biology. Springer, Dordrecht, The Netherlands, pp 149–168 [Google Scholar]
- Sumanta N, Haque CI, Nishika J, Suprakash R (2014) Spectrophotometric analysis of chlorophylls and carotenoids from commonly grown fern species by using various extracting solvents. Research Journal of Chemical Sciences 4: 63–69 [Google Scholar]
- Williams-Carrier R, Zoschke R, Belcher S, Pfalz J, Barkan A (2014) A major role for the plastid-encoded RNA polymerase complex in the expression of plastid transfer RNAs. Plant Physiol 164: 239–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi Y, Ishizaki Y, Nakahira Y, Tozawa Y, Shiina T (2012) Eukaryotic-type plastid nucleoid protein pTAC3 is essential for transcription by the bacterial-type plastid RNA polymerase. Proc Natl Acad Sci USA 109: 7541–7546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi Y, Shiina T (2014) Recent advances in the study of chloroplast gene expression and its evolution. Front Plant Sci 5: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu QB, Huang C, Yang ZN (2014) Nuclear-encoded factors associated with the chloroplast transcription machinery of higher plants. Front Plant Sci 5: 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu QB, Lu Y, Ma Q, Zhao TT, Huang C, Zhao HF, Zhang XL, Lv RH, Yang ZN (2013) TAC7, an essential component of the plastid transcriptionally active chromosome complex, interacts with FLN1, TAC10, TAC12 and TAC14 to regulate chloroplast gene expression in Arabidopsis thaliana. Physiol Plant 148: 408–421 [DOI] [PubMed] [Google Scholar]
- Zhang X, Garreton V, Chua NH (2005) The AIP2 E3 ligase acts as a novel negative regulator of ABA signaling by promoting ABI3 degradation. Genes Dev 19: 1532–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.