Abstract
Background
Recent studies have suggested a potential increased risk of acute kidney injury (AKI) among proton-pump inhibitor (PPI) users. However, the present results are conflicting. Thus, we performed a meta-analysis to investigate the association between PPI therapy and the risk of AKI.
Methods
EMBASE, PubMed, Web of Science, and Cochrane Library databases (up to September 23, 2016) were systematically searched for any studies assessing the relationship between PPI use and risk of AKI. Studies that reported relevant risk ratios (RRs), odds ratios, or hazard ratios were included. We calculated the pooled RRs with 95% confidence intervals (CI) using a random-effects model of the meta-analysis. Subgroup analysis was conducted to explore the source of heterogeneity.
Results
Seven observational studies (five cohort studies and two case–control studies) were identified and included, and a total of 513,696 cases of PPI use among 2,404,236 participants were included in the meta-analysis. The pooled adjusted RR of AKI in patients with PPIs use was 1.61 (95% CI: 1.16–2.22; I2=98.1%). Furthermore, higher risks of AKI were found in the subgroups of cohort studies, participant’s average age <60 years, participants with and without baseline PPI excluded, sample size <300,000, and number of adjustments ≥11. Subgroup analyses revealed that participants with or without baseline PPI excluded might be a source of heterogeneity.
Conclusion
PPI use could be a risk factor for AKI and should be administered carefully. Nevertheless, some confounding factors might impact the outcomes. More well-designed prospective studies are needed to clarify the association.
Keywords: proton-pump inhibitor, acute kidney injury, risk, meta-analysis
Introduction
Since the introduction to the market in 1987, proton-pump inhibitor (PPI) utilization has increased rapidly. Now, PPIs are among the most widely used medication, in both prescription and over-the-counter (OTC) sales. A common mechanism of all agents in PPI class is the blocking of the H+/K+ ATPase (adenosine triphosphatase) to reduce acid production by the parietal cell.1 They are used dominantly to protect the gastrointestinal tract from acid-related disorders and the effects of glucocorticoid or non-steroidal anti-inflammatory drugs.2 In clinical settings, PPIs are perceived to be of a favorable safety profile.3,4
However, some severe adverse effects of PPIs have been reported in recent years,5,6 of which acute kidney injury (AKI) growingly aroused the vigilance of clinicians. Many case reports suggested PPI as a possible cause of kidney disorders since 1992.7–10 Several case–control and cohort studies explored the association between exposure to PPIs and AKI, but the outcomes remained inconsistent.11–16 Five studies demonstrated that PPIs use was significantly associated with increased risk of AKI,12–14,16 whereas no obvious relevance was found in other studies.11,15
Hence, we conducted this comprehensive meta-analysis to determine the association between PPI use and risk of AKI. This study might help clarify the controversial issues and provide clinical guidance.
Methods
Literature search strategy
We systematically searched EMBASE, PubMed, Web of Science, and Cochrane Library databases from inception to September 23, 2016, using the following terms: “proton pump inhibitor,” “proton pumps,” “PPI,” “anti-ulcer agent,” “antacid,” “esomeprazole,” “omeprazole,” “ilaprazole,” “dexlansoprazole,” “rabeprazole,” “lansoprazole,” “pantoprazole,” “acute kidney injury,” “acute renal injury,” “AKI,” “acute renal failure,” “acute renal dysfunction” (search strategies are available in detail in the Supplementary materials section). No language restriction was enhanced. Furthermore, we searched the reference lists of all included articles for additional eligible studies. The full text of a record was reviewed carefully if there was any doubt to the eligibility of it. Two of the authors (Yang and George) independently screened titles and abstracts, analyzed full-text articles, and ascertained the final eligible records. Divergences were resolved by discussion, or consulting a third author.
Inclusion and exclusion criteria
Eligible studies met the following criteria: 1) the study design was a case–control, cohort, or clinical trial study; 2) the exposure of interest was PPI use; 3) the outcome measured included AKI; and 4) odds ratio (OR) or hazard ratio (HR) or risk ratio (RR), and the corresponding 95% confidence interval (CI) were reported or could be calculated. Reviews, letters, case reports, abstracts, animal studies, and editorial materials were excluded.
Data extraction
We extracted ORs, RRs, or HRs, and each with a 95% CI from the included studies. Study characteristics were extracted by two authors (Yang and George) separately as follows: first author’s last name, publication year, country origin, study design, PPI use groups versus control groups size, mean age, proportion of men, control group restriction, length of follow-up, and definition of AKI.
Quality assessment
We evaluated the quality of studies using Newcastle–Ottawa Quality Assessment Scale (NOS) of observational studies.17 On this scale, points were given to a study based on three categories: participant’s selection (4 points), groups’ comparability (2 points), and ascertainment of exposure (3 points) for case–control study or ascertainment of outcome (3 points) for cohort study (the Supplementary materials section for details). Overall, study quality was graded as good (score, 7–9), fair (score, 4–6), or poor (score, 0–3). Two authors performed the quality assessment independently, and disagreements were resolved by discussion.
Statistical analyses
All meta-analyses were performed by STATA (version 10.0; Stata Corporation, College Station, TX, USA). Adjusted OR or RR was used to measure the association between the PPI use and the AKI risk across included studies. Considering the low incidence of AKI in PPI users, we assumed that ORs were similar to RRs.18 Therefore, the adjusted HRs and their corresponding 95% CIs were the effective values for all studies. Heterogeneity of HRs among studies was assessed using the chi-squared based on Q-statistic test (P<0.1) and quantified by I2 statistic. I2 values were considered to represent insignificant (0%–25%), low (26%–50%), moderate (51%–75%), and high (>75%) heterogeneity.19 Because of our high heterogeneity, the random-effects model was used to calculate the weighted mean, variance of the summary effect, associated 95% CI, and P-value. We conducted sensitivity analyses to assess the influence of a study on the pooled effect estimate by recalculating the pooled RR with the removal of one study in each turn. Subgroup analyses and univariable random-effects meta-regression were further conducted to explore the potential source of heterogeneity. Reporting bias was evaluated visually by a funnel plot, and Begg and Mazumdar’s rank correlation test and Egger’s regression test were performed to assess the asymmetry of the funnel plot (P<0.1).20 Except for Q-statistic test, Egger’s test, and Begg–Mazumdar’s test, P<0.05 in two-tailed test was considered to be statistically significant.
Results
Literature search, study characteristics, and quality
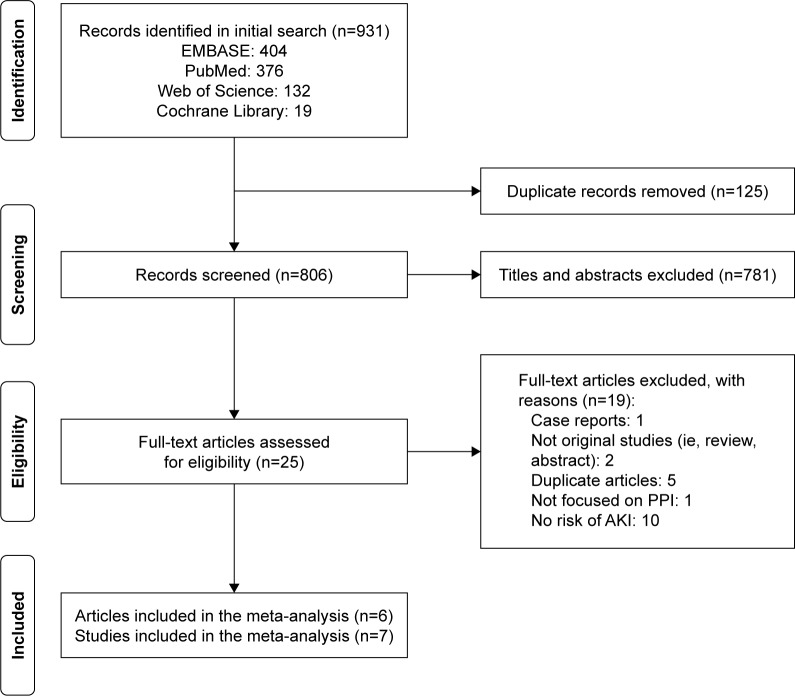
We retrieved 931 potentially relevant records through database searches, and 26 records were remained for full-text review. Eventually, six eligible articles were identified,11–16 of which one article included two independent cohort studies.14 Therefore, seven studies were included for our analysis of the association between the PPI use and the AKI risk. Figure 1 shows the study identification process.
Figure 1.
Flow chart of selection of studies.
Abbreviations: AKI, acute kidney injury; PPI, proton-pump inhibitor.
Table 1 shows the characteristics of the seven included studies. They were published from 2012 to 2016. Five articles were from the United States,12,14–16 one from Canada,13 and one from the United Kingdom.11
Table 1.
Characteristics of included studies
| Study | Leonard et al11 | Klepser et al12 | Antoniou et al13 | Lazarus et al14 (ARIC) | Lazarus et al14 (GHS) | Lee et al15 | Xie et al16 |
| Year | 2012 | 2013 | 2015 | 2016 | 2016 | 2016 | 2016 |
| Location | Britain | United States | Canada | United States | United States | United States | United States |
| Study design | Retrospective case–control | Retrospective case–control | Prospective cohort | Prospective cohort | Prospective cohort | Prospective cohort | Retrospective cohort |
| PPI/control (n) | 27,982/1,323,850 | 195/607 | 290,592/290,592 | 322/10,160 | 16,900/231,851 | 3,725/10,528 | 173,321/20,270 |
| Mean age (years) | 63.5 | 44.6 | 74.3 | 62.6 | 49.6 | 63.4 | 56.7 |
| Men (%) | 50.4 | 42.6 | 43.3 | 43.9 | 43.5 | 57.3 | 93.1 |
| Baseline PPI exclude | No | No | Yes | No | No | No | Yes |
| Controls restriction | No use of NSAIDS | Matched control | Matched control | Matched control | Matched control | No use of H2 blockers | Use of H2 blockers |
| Length of follow-up | NA | NA | 120 days | Median: 13.9 years | Median: 6.2 years | 7 days | 5 years |
| Definition of AKI | OXMIS | ICD-9 | ICD-10 | ICD-9-CM or ICD-10-CM | ICD-9-CM or National Death Index | KDIGO | Scr >0.3 mg/dL or 50% increase within 30 days |
| Number of adjustments | 16 | 10 | 5 | 15 | 14 | 7 | 14 |
| S, C, E/Oa | 3, 2, 3 | 3, 1, 3 | 4, 1, 2 | 4, 2, 3 | 4, 2, 3 | 4, 1, 1 | 4, 2, 3 |
Note:
Quality Assessment Newcastle–Ottawa Scale.
Abbreviations: AKI, acute kidney injury; ARIC, atherosclerosis risk in communities; C, comparability; E, exposure; GHS, Geisinger Health System; ICD-9/10-CM, International Classification of Diseases, Ninth/Tenth Revision, Clinical Modification; KDIGO, Kidney Disease: Improving Global Outcomes; NA, not applicable; NSAID, nonsteroidal anti-inflammatory drug; O, outcome; OXMIS, Oxford Medical Information System; PPI, proton-pump inhibitor; S, selection; Scr, serum creatinine.
Six included studies were graded as good quality, whereas one study15 was graded as fair quality according to NOS as shown in Table 2.
Table 2.
Subgroup meta-analysis
| Subgroup | No of studies | RR (95% CI) | I2 (%) | P-valuea | P-valueb |
|---|---|---|---|---|---|
| Study design | 0.596 | ||||
| Case–control | 2 | 1.41 (0.68–2.91) | 76.3 | 0.040 | |
| Cohort | 5 | 1.69 (1.18–2.42) | 98.1 | <0.001 | |
| Sample size | 0.967 | ||||
| <300,000 | 5 | 1.59 (1.09–2.33) | 97.2 | <0.001 | |
| ≥300,000 | 2 | 1.63 (0.69–3.83) | 99.4 | <0.001 | |
| Participant’s average age (years) | 0.623 | ||||
| <60 | 3 | 1.77 (1.14–2.75) | 96.7 | <0.001 | |
| ≥60 | 4 | 1.50 (0.92–2.47) | 98.5 | <0.001 | |
| Participants with baseline PPI excluded | 0.021 | ||||
| Yes | 2 | 2.32 (1.98–2.71) | 83.3 | 0.014 | |
| No | 5 | 1.21 (1.03–1.42) | 81.5 | <0.001 | |
| Number of adjustments | 0.730 | ||||
| <11 | 3 | 1.76 (0.84–3.70) | 98.6 | <0.001 | |
| ≥11 | 4 | 1.53 (1.02–2.29) | 98.3 | <0.001 | |
Notes:
P-value for heterogeneity among studies assessed with Cochran’s Q-test.
P-value for interaction evaluated by meta-regression models.
Abbreviations: CI, confidence interval; PPI, proton-pump inhibitor; RR, risk ratio.
Association between PPIs use and AKI risk
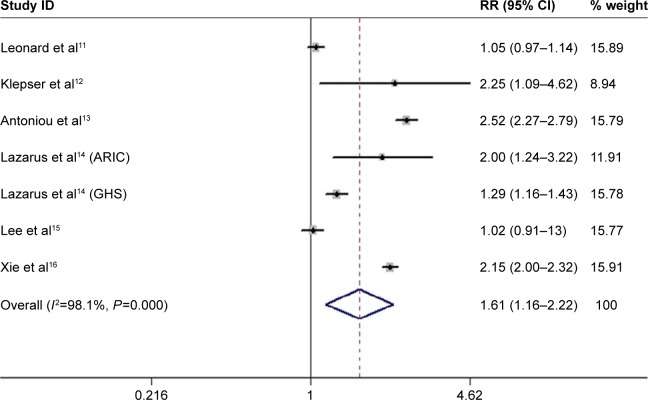
The analysis pooled data from seven studies with weights from 8.94% to 15.91%. Overall, PPIs use was associated with an increased risk of AKI. And, the adjusted pooled RR was 1.61 (95% CI: 1.16–2.22) using random-effects models with high heterogeneity (I2=98.1%) (Figure 2).
Figure 2.
Association between proton-pump inhibitors use and risk of acute kidney injury.
Note: Weights are from random-effects analysis.
Abbreviations: ARIC, atherosclerosis risk in communities; CI, confidence interval; GHS, Geisinger Health System; RR, risk ratio.
Subgroup and meta-regression analysis
We further conducted subgroup meta-analysis, and Table 2 shows the possible confounding factors and outcomes. In the subgroups of cohort studies, participants with and without baseline PPI excluded, participant’s average age <60 years, sample size <300,000, and number of adjustments ≥11, PPIs use was found to increase the risk of AKI. However, in the subgroups of case–control studies, sample size ≥300,000, participant’s average age ≥60 years, and number of adjustments <11, no significant association was observed.
Of note, the revealed positive association was more pronounced among studies with baseline PPI excluded compared with those without baseline PPI excluded. In these two subgroups, the association between PPIs use and increased risk of AKI significantly differed (P=0.021 for the interaction).
Sensitivity analysis
Removal of any single study did not change the overall RRs significantly. The pooled RRs varied from 1.47 (95% CI: 1.06–2.04) to 1.75 (95% CI: 1.23–2.49; Table S1 and Figure S1).
Publication bias
No evidence of bias publication was identified by the Begg–Mazumdar’s rank correlation test and Egger linear regression test (P=0.764 and 0.966, respectively).
Discussion
This is the first comprehensive meta-analysis of seven studies to assess the effect of PPIs on the risk of AKI in 2,404,236 participants. We demonstrated an association between PPIs use and an overall 61% higher adjusted risk of AKI. The association remains significant across sensitivity analyses. Subgroup analyses showed a more pronounced risk of AKI in participants with baseline PPI excluded. However, in the studies with larger sample size (≥300,000), older participants (≥60 years old), fewer numbers of adjustments (<11), and case–control studies, no significant effect was observed.
Considerable heterogeneity across studies was present in our study. Subgroup and meta-regression analyses showed participants with and without baseline PPI excluded might be a source of heterogeneity.
The mechanisms of the associations between PPIs use and AKI were not clear yet. The proposed mechanism could be through interstitial nephritis. Most AKI events were identified specifically in the form of acute interstitial nephritis (AIN), which was suggested to have an association with PPIs exposure by multiple studies.7,21–24 PPI-induced AIN might be a cell-mediated idiosyncratic immune response,25 a class effect as all PPIs could cause AIN. Kidney biopsy demonstrated a diffused interstitial cellular infiltrate of eosinophils and lymphocytes with and without tubulitis, whereas the glomeruli and vasculature were normal.24,26
It could be hypothesized that an individual’s prior exposure to PPIs could desensitize the kidney to developing PPI-induced AKI. Our subgroup analysis revealed that positive association was significantly more pronounced among studies with baseline PPI excluded compared with those without exclusion of the baseline PPI. Therefore, for those patients to whom PPI has never before been administered, careful assessment should be made by clinicians to determine the necessity of those drugs.
Our analysis revealed that younger patients might be more likely to develop AKI due to PPIs use than elderly patients. In older participants (≥60 years old), PPIs use had no significant association with AKI, but a significant association was found in <60-year-old participants. However, the difference was not maintained by meta-regression analysis. Our outcome might be explained by the fact that PPI-induced AKI was thought to be an immune hypersensitivity reaction, which was known to be more common in the young. A large case-series study reported that PPI-induced AKI was more common in elderly patients,27 but this case series had limitations of no control group and small sample size (n=15). Besides, three11,14,15 of four11,13–15 studies that included participants ≥60-year-old were not limited to new PPIs users, and these elderly patients could have had a history of exposure to PPIs, which might have hidden the risk of AKI. Meanwhile, Arora et al found that younger participants were more likely to develop chronic kidney disease (CKD) due to PPIs use, which was comparatively consistent with our outcome.28
An individual’s existing diseases and exposure to other drugs might impact the AKI risk assessment due to PPIs. Our subgroup analysis found no positive association between PPIs use and AKI in the studies with fewer adjustments, but for studies with more adjustments, the association turned significant. For example, Lee et al analyzed data with different models, which differed in number of confounders. When only demographics or/and cardiovascular comorbidities were considered, the association between PPIs use and increased risk was significant, but was not as significant when possible clinical indications for PPIs use, severity of illness, or the use of outpatient medication use were included.15
It was reported that AKI was associated with the development of CKD and progression to end-stage renal diseases (ESRDs).29–31 The association between PPIs use and CKD was shown by several studies,14,16,28 and the association was more pronounced in patients using higher doses of PPI.14 Lazarus et al14 and Xie et al16 conducted three cohort studies and found 1.22- to 1.50-fold increased risk of CKD for PPI users versus non-PPI users, and a 1.28- to 1.39-fold for PPI users versus H2 receptor antagonist users.14,16 Meanwhile, twice-daily PPI dosing was associated with a 15% higher risk than once-daily dosing.14 A case–control study had the similar risk of CKD (OR =1.10; 95% CI: 1.05–1.16) among patients taking PPIs versus those not on PPIs.28
In addition, an association between PPIs use and ESRD was also revealed by a cohort study (HR =1.96; 95% CI: 1.21–3.18)14 and a case–control study (adjusted OR =1.88; 95% CI: 1.71–2.06).32
PPI-related CKD might be explained by the following reasons: First, interstitial fibrosis might develop rapidly once the acute inflammatory process in AIN patients begins.25,26 About 30%–70% biopsy-proven AIN patients would not recover fully and had severe sequelae, including chronic interstitial nephritis, CKD, ESRD, and dialysis, especially for those without timely diagnosis or treatment.24,27 Second, Xie et al16 conducted a 5-year cohort in which it was observed that even though adjusting for AKI, PPIs use was significantly associated with renal insufficiency. This suggested the existence of unrecognized AKI or chronic latent renal injury. Third, PPIs use was reported to be associated with hypomagnesemia, which can cause endothelial cell dysfunction, oxidative stress, and inflammation, and is related to renal interstitial tubular injury and causes decline of renal function.33–36
Our study provides important implications for public health. Millions of individuals take PPIs each year, more than half of which may not be medically indicated.37–39 PPIs are generally perceived as benign and well tolerated. Our analysis revealed that PPIs use might be linked to untoward effects on the kidneys. More consideration should be taken for patients with the following characteristics: first, young patients (<60 years old); second, patients who have never had exposure to PPIs; third, those on high PPIs dosage; and finally, patients with existing kidney diseases26,40 or taking other nephrotoxic drugs. A study showed that PPIs use might increase the risk of AKI (adjusted HR =2.15; 95% CI: 2.00–2.32), CKD, and ESRD compared with H2 blockers,16 which suggested that H2 blockers could be a better choice in some cases. But, this result needs more relevant studies for support.
Although our meta-analysis included studies with larger sample size and higher quality, there were still several limitations. First, the heterogeneity was high in both total population and subgroup. Second, studies included were observational and could not provide evidence of causality. Case–control and cohort studies had the opposite outcomes. Third, there were no exact and uniform restrictions of PPIs use indications, dosage, and duration in included studies, which might have impacted the risk of AKI. Finally, restriction on control groups and considered confounders taken into account had many differences that affected the outcomes. In light of these limitations, further well-designed prospective and interventional studies were required.
Our study revealed that PPIs use might be related to the increased risk of AKI, and the risk was more pronounced in young patients and those never exposed to PPIs. Although our findings will not prevent clinicians from prescribing PPIs to patients with definite evidences, our study emphasized the importance of curtailing the indiscriminating use of PPIs and the need to exercise more caution when prescribing these drugs. Health care providers should closely monitor patients taking PPIs by urinalysis and renal function tests to recognize any renal injury in time.
Supplementary materials
Search strategy to identify studies evaluating proton-pump inhibitors use and risk of acute kidney injury.
On PubMed
Search ((((((acute kidney injury) OR acute renal injury) OR AKI) OR acute renal failure) OR acute renal dysfunction)) AND ((((((((((((proton pump inhibitor) OR proton pumps) OR PPI) OR anti-ulcer agent) OR antacid) OR esomeprazole) OR omeprazole) OR ilaprazole) OR dexlansoprazole) OR rabeprazole) OR lansoprazole) OR pantoprazole).
On Cochrane
#1 proton pump inhibitor or proton pumps or PPI or anti-ulcer agent or antacid (word variations have been searched)
#2 esomeprazole or omeprazole or dexlansoprazole or ilaprazole or rabeprazole (word variations have been searched)
#3 lansoprazole or pantoprazole (word variations have been searched)
#4 acute kidney injury or acute renal injury or AKI or acute renal failure or acute renal dysfunction (word variations have been searched)
#5 #1 or #2 or #3
#6 #4 and #5
Choose #6
Choose trials
On Web of Science™
#1 proton pump inhibitor
#2 proton pumps
#3 PPI
#4 anti-ulcer agent
#5 antacid
#6 esomeprazole
#7 omeprazole
#8 ilaprazole
#9 dexlansoprazole
#10 rabeprazole
#11 lansoprazole
#12 pantoprazole
#13 acute kidney injury
#14 acute renal injury
#15 AKI
#16 acute renal failure
#17 acute renal dysfunction
#18 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12
#19 #13 OR #14 OR #15 OR #16 OR #17
#20 #18 AND #19
Choose #20
On EMBASE
proton pump inhibitor
proton pumps
PPI
anti-ulcer agent
antacid
esomeprazole
omeprazole
ilaprazole
dexlansoprazole
rabeprazole
lansoprazole
pantoprazole
acute kidney injury
acute renal injury
AKI
acute renal failure
acute renal dysfunction
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12
13 or 14 or 15 or 16 or 17
18 and 19
Choose 20
Supplemental item
Standard for evaluation of NOS.
For case–control studies
Selection included: 1) the case definition is adequate, 2) the cases are consecutive or obviously representative of cases, 3) controls are from community, but not hospital or without description, and 4) controls do have history of AKI.
Comparability included: 1) study controls for age and sex and 2) study controls for ≥11 additional risk factors.
Exposure included: 1) ascertainment of exposure is with secure record, 2) ascertainment for cases and controls is by same method, and 3) cases and controls groups have same non-response rate.
For cohort study
Selection included: 1) exposed cohort truly or somewhat representative, 2) non-exposed cohort drawn from the same community as the exposed cohort, 3) ascertainment of exposure, and 4) outcome of interest not present at start.
Comparability included: 1) study controls for age and sex and 2) study controls for ≥11 additional risk factors.
Outcome included: 1) assessment of outcome (independent blind assessment or record linkage), 2) follow-up ≥5 years, and 3) complete accounting for cohorts or subjects lost to follow-up unlikely to introduce bias.
Sensitivity analysis.
Abbreviations: ARIC, atherosclerosis risk in communities; GHS, Geisinger Health System.
Table S1.
Sensitivity analysis
| Study omitted | RR (95% CI) | I2 (%) | P-valuea |
|---|---|---|---|
| Leonard et al11 | 1.74 (1.24–2.44) | 97.6 | <0.001 |
| Klepser et al12 | 1.55 (1.11–2.18) | 98.4 | <0.001 |
| Antoniou et al13 | 1.47 (1.06–2.04) | 97.7 | <0.001 |
| Lazarus et al14 (ARIC) | 1.56 (1.10–2.21) | 98.4 | <0.001 |
| Lazarus et al14 (GHS) | 1.68 (1.14–2.47) | 98.4 | <0.001 |
| Lee et al15 | 1.75 (1.23–2.49) | 98.1 | <0.001 |
| Xie et al16 | 1.52 (1.07–2.15) | 97.6 | <0.001 |
Note:
P-value for heterogeneity among studies assessed with Cochrane’s Q-test.
Abbreviations: ARIC, atherosclerosis risk in communities; CI, confidence interval; GHS, Geisinger Health System; RR, risk ratio.
Acknowledgments
This work was supported by the National Nature Science Foundation of China (NSFC) (Nos 81470948, 81670633, and 81270770 for GX; Nos 81200531 and 81570667 for SWG) and the National Key Technology R&D Program (Grants 2013BAI09B06) for GX.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Robinson M, Horn J. Clinical pharmacology of proton pump inhibitors: what the practising physician needs to know. Drugs. 2003;63(24):2739–2754. doi: 10.2165/00003495-200363240-00004. [DOI] [PubMed] [Google Scholar]
- 2.Grace Newsome E, American College of Rheumatology Guidelines for the management of rheumatoid arthritis: 2002 update. J Am Acad Nurse Pract. 2002;14(10):432–437. doi: 10.1111/j.1745-7599.2002.tb00072.x. [DOI] [PubMed] [Google Scholar]
- 3.Bateman DN, Colin-Jones D, Hartz S, et al. Mortality study of 18,000 patients treated with omeprazole. Gut. 2003;52(7):942–946. doi: 10.1136/gut.52.7.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boparai V, Rajagopalan J, Triadafilopoulos G. Guide to the use of proton pump inhibitors in adult patients. Drugs. 2008;68(7):925–947. doi: 10.2165/00003495-200868070-00004. [DOI] [PubMed] [Google Scholar]
- 5.Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296(24):2947–2953. doi: 10.1001/jama.296.24.2947. [DOI] [PubMed] [Google Scholar]
- 6.Johnstone J, Nerenberg K, Loeb M. Meta-analysis: proton pump inhibitor use and the risk of community-acquired pneumonia. Aliment Pharmacol Ther. 2010;31(11):1165–1177. doi: 10.1111/j.1365-2036.2010.04284.x. [DOI] [PubMed] [Google Scholar]
- 7.Ruffenach SJ, Siskind MS, Lien YH. Acute interstitial nephritis due to omeprazole. Am J Med. 1992;93(4):472–473. doi: 10.1016/0002-9343(92)90181-a. [DOI] [PubMed] [Google Scholar]
- 8.Christensen PB, Albertsen KE, Jensen P. Renal failure after omeprazole. Lancet. 1993;341(8836):55. doi: 10.1016/0140-6736(93)92531-w. [DOI] [PubMed] [Google Scholar]
- 9.O’Donnell D. Acute renal failure due to omeprazole. Med J Aust. 1996;165(4):234–235. doi: 10.5694/j.1326-5377.1996.tb124940.x. [DOI] [PubMed] [Google Scholar]
- 10.Vera Rivera M, Pou Potau M, Botey Puig A. Omeprazole and interstitial nephritis: a reversible cause of acute renal failure. Med Clin (Barc) 2002;118(1):39. doi: 10.1016/s0025-7753(02)72274-4. [DOI] [PubMed] [Google Scholar]
- 11.Leonard CE, Freeman CP, Newcomb CW, et al. Proton pump inhibitors and traditional nonsteroidal anti-inflammatory drugs and the risk of acute interstitial nephritis and acute kidney injury. Pharmacoepidemiol Drug Saf. 2012;21(11):1155–1172. doi: 10.1002/pds.3329. [DOI] [PubMed] [Google Scholar]
- 12.Klepser DG, Collier DS, Cochran GL. Proton pump inhibitors and acute kidney injury: a nested case-control study. BMC Nephrology. 2013;14:150. doi: 10.1186/1471-2369-14-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antoniou T, Hollands S, Gomes T, et al. Proton pump inhibitors and the risk of acute kidney injury in older patients: a population-based cohort study. CMAJ Open. 2015;3(2):E166–E171. doi: 10.9778/cmajo.20140074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med. 2016;176(2):238–246. doi: 10.1001/jamainternmed.2015.7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee J, Mark RG, Celi LA, Danziger J. Proton pump inhibitors are not associated with acute kidney injury in critical illness. J Clin Pharmacol. 2016;56(12):1500–1506. doi: 10.1002/jcph.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie Y, Bowe B, Li T, Xian H, Balasubramanian S, Al-Aly Z. Proton pump inhibitors and risk of incident CKD and progression to ESRD. J Am Soc Nephrol. 2016;27(10):3153–3163. doi: 10.1681/ASN.2015121377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walls GA, Shee B, O’Connell D, Peterson J, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [Accessed 15 March, 2017]. Available from http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 18.Davies HT, Crombie IK, Tavakoli M. When can odds ratios mislead? BMJ. 1998;316(7136):989–991. doi: 10.1136/bmj.316.7136.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sierra F, Suarez M, Rey M, Vela MF. Systematic review: proton pump inhibitor-associated acute interstitial nephritis. Aliment Pharmacol Ther. 2007;26(4):545–553. doi: 10.1111/j.1365-2036.2007.03407.x. [DOI] [PubMed] [Google Scholar]
- 22.Harmark L, van der Wiel HE, de Groot MC, van Grootheest AC. Proton pump inhibitor-induced acute interstitial nephritis. Br J Clin Pharmacol. 2007;64(6):819–823. doi: 10.1111/j.1365-2125.2007.02927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geevasinga N, Coleman PL, Roger SD. Rabeprazole-induced acute interstitial nephritis. Nephrology (Carlton) 2005;10(1):7–9. doi: 10.1111/j.1440-1797.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 24.Geevasinga N, Coleman PL, Webster AC, Roger SD. Proton pump inhibitors and acute interstitial nephritis. Clin Gastroenterol Hepatol. 2006;4(5):597–604. doi: 10.1016/j.cgh.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Praga M, Gonzalez E. Acute interstitial nephritis. Kidney Intern. 2010;77(11):956–961. doi: 10.1038/ki.2010.89. [DOI] [PubMed] [Google Scholar]
- 26.Perazella MA, Brewster UC. Proton pump inhibitors and the kidney: critical review. Clin Nephrol. 2007;68(2):65–72. doi: 10.5414/cnp68065. [DOI] [PubMed] [Google Scholar]
- 27.Simpson IJ, Marshall MR, Pilmore H, et al. Proton pump inhibitors and acute interstitial nephritis: report and analysis of 15 cases. Nephrology. 2006;11(5):381–385. doi: 10.1111/j.1440-1797.2006.00651.x. [DOI] [PubMed] [Google Scholar]
- 28.Arora P, Gupta A, Golzy M, et al. Proton pump inhibitors are associated with increased risk of development of chronic kidney disease. BMC Nephrol. 2016;17(1):112. doi: 10.1186/s12882-016-0325-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Intern. 2012;81(5):442–448. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okusa MD, Chertow GM, Portilla D; Acute Kidney Injury Advisory Group of the American Society of N. The nexus of acute kidney injury, chronic kidney disease, and World Kidney Day 2009. Clin J Am Soc Nephrol. 2009;4(3):520–522. doi: 10.2215/CJN.06711208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishani A, Xue JL, Himmelfarb J, et al. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009;20(1):223–228. doi: 10.1681/ASN.2007080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peng YC, Lin CL, Yeh HZ, Chang CS, Wu YL, Kao CH. Association between the use of proton pump inhibitors and the risk of ESRD in renal diseases: a population-based, case-control study. Medicine (Baltimore) 2016;95(15):e3363. doi: 10.1097/MD.0000000000003363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mazur A, Maier JA, Rock E, Gueux E, Nowacki W, Rayssiguier Y. Magnesium and the inflammatory response: potential physiopathological implications. Arch Biochem Biophys. 2007;458(1):48–56. doi: 10.1016/j.abb.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 34.Wolf FI, Trapani V, Simonacci M, Ferre S, Maier JA. Magnesium deficiency and endothelial dysfunction: is oxidative stress involved? Magnes Res. 2008;21(1):58–64. [PubMed] [Google Scholar]
- 35.Matsuzaki H, Ohdachi J, Fuchigami M, et al. Changes in N-acetyl-beta-D-glucosaminidase activity in the urine and urinary albumin excretion in magnesium deficient rats. Biosci Biotechnol Biochem. 2002;66(1):192–194. doi: 10.1271/bbb.66.192. [DOI] [PubMed] [Google Scholar]
- 36.Kieboom BC, Kiefte-de Jong JC, Eijgelsheim M, et al. Proton pump inhibitors and hypomagnesemia in the general population: a population-based cohort study. Am J Kidney Dis. 2015;66(5):775–782. doi: 10.1053/j.ajkd.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 37.Forgacs I, Loganayagam A. Overprescribing proton pump inhibitors. BMJ. 2008;336(7634):2–3. doi: 10.1136/bmj.39406.449456.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choudhry MN, Soran H, Ziglam HM. Overuse and inappropriate prescribing of proton pump inhibitors in patients with Clostridium difficile-associated disease. QJM. 2008;101(6):445–448. doi: 10.1093/qjmed/hcn035. [DOI] [PubMed] [Google Scholar]
- 39.Strid H, Simren M, Bjornsson ES. Overuse of acid suppressant drugs in patients with chronic renal failure. Nephrol Dial Transplant. 2003;18(3):570–575. doi: 10.1093/ndt/18.3.570. [DOI] [PubMed] [Google Scholar]
- 40.Bell JS, Blacker N, Leblanc VT, et al. Prescribing for older people with chronic renal impairment. Aust Fam Physician. 2013;42(1–2):24–28. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sensitivity analysis.
Abbreviations: ARIC, atherosclerosis risk in communities; GHS, Geisinger Health System.
Table S1.
Sensitivity analysis
| Study omitted | RR (95% CI) | I2 (%) | P-valuea |
|---|---|---|---|
| Leonard et al11 | 1.74 (1.24–2.44) | 97.6 | <0.001 |
| Klepser et al12 | 1.55 (1.11–2.18) | 98.4 | <0.001 |
| Antoniou et al13 | 1.47 (1.06–2.04) | 97.7 | <0.001 |
| Lazarus et al14 (ARIC) | 1.56 (1.10–2.21) | 98.4 | <0.001 |
| Lazarus et al14 (GHS) | 1.68 (1.14–2.47) | 98.4 | <0.001 |
| Lee et al15 | 1.75 (1.23–2.49) | 98.1 | <0.001 |
| Xie et al16 | 1.52 (1.07–2.15) | 97.6 | <0.001 |
Note:
P-value for heterogeneity among studies assessed with Cochrane’s Q-test.
Abbreviations: ARIC, atherosclerosis risk in communities; CI, confidence interval; GHS, Geisinger Health System; RR, risk ratio.