Abstract
Objectives
DWP05195 is a transient receptor potential vanilloid 1 (TRPV1) antagonist developed for managing pain. The purpose of this study was to evaluate the pharmacodynamics pharmacokinetics, safety, and tolerability of DWP05195 in healthy subjects. This was a first-in-human randomized, double-blinded, placebo-controlled, dose escalation study.
Subjects and methods
DWP05195 or placebo was administered as a single dose of 10–600 mg in the single-dose study and as 100–400 mg once daily for 8 days in the multiple-dose studies. Each study group consisted of 10 subjects (study drug-to-placebo ratio was 8:2). For pharmacodynamics assessment, the heat pain threshold (HPtr), heat pain tolerance (HPtol), perfusion intensity, and flare area ratio of cutaneous blood flow were measured. Safety and tolerability were evaluated throughout the study.
Results
The maximum plasma concentrations and area under the plasma concentration–time curve from zero to the last measurable time dose-dependently increased. HPtr and HPtol tended to increase more after DWP05195 administration than after placebo administration. HPtr and HPtol tended to dose-dependently increase after administration of DWP05195. Cutaneous blood flow was reduced as the dose of DWP05195 increased during the multiple-dose study. DWP05195 was well tolerated up to 600 and 400 mg single- and multiple-dose administrations, respectively.
Conclusion
The pharmacological activity of DWP05195, measured using HPtr and HPtol, increased as expected in a dose-dependent manner owing to increased systemic exposure, indicating that DWP05195 can be used as a TRPV1 antagonist for pain management.
Keywords: DWP05195, TRPV1 antagonist, pain tolerance, pain threshold, capsaicin
Introduction
Pain is a common reason for visiting a physician.1 Nonsteroidal anti-inflammatory drugs (NSAIDs) and opioids are the currently available medications for the management of pain.2,3 However, NSAIDs are not efficacious in treating neuropathic pain.4 Furthermore, an effective dosage of opioids cannot be used to manage neuropathic pain in clinical settings. This is because opioids cause detrimental physiological problems, such as physical dependence, addiction, and tolerance, which decrease patients’ quality of life.5,6 The abovementioned limitations of NSAIDs and opioids are related to their pharmacological actions within the human body.7 Management of neuropathic pain is therefore an unmet medical need. Thus, it is necessary to develop an analgesic with a novel mechanism of action that can serve as a more effective alternative for the treatment of neuropathic pain.8,9
Transient receptor potential vanilloid 1 (TRPV1) has been reported to have a role in the development of neuropathic pain in several animal studies.10–12 TRPV1 is expressed in C fibers and A-delta fibers, which are responsible for the transmission of pain signals.13,14 The expression of TRPV1 has been reported to increase after nerve injury.15 Sensory neurons from mice lacking TRPV1 showed selective deficiency in their responses to noxious stimuli.2
DWP05195 is a TRPV1 antagonist that is under development by Daewoong Pharmaceutical Co. Ltd., Seoul, South Korea. DWP05195 has been reported to competitively inhibit transduction of the pain signal evoked by typical TRPV1 agonists such as capsaicin, endovanilloid, anandamide, and N-arachidonoyl dopamine. DWP05195 also generates analgesic effects in animal models with nerve injury and in animal models of diabetic neuropathy. Based on these findings, DWP05195 might be beneficial for pain management.
This study aimed to evaluate the pharmacodynamics (PD), pharmacokinetics (PKs), safety, and tolerability of DWP05195 after single and multiple oral administrations in healthy subjects.
Subjects and methods
Study subjects
The eligible subjects were Korean healthy male volunteers aged between 20 and 45 years whose body mass index was in the range of 19.0–27.0 kg/m2. Subjects were screened before enrollment to determine health based on previous medical history, physical examinations, 12-lead electrocardiograms (ECGs), vital signs, and laboratory tests. All subjects provided written informed consent before entering the study. Subjects were excluded if there was a history or evidence of any of the following: significant disease of the respiratory, cardiovascular, renal, gastrointestinal, hepatic, endocrine, hematologic, neurologic, or psychiatric systems; alcoholism or drug abuse; the use of any prescription drug; the use of any over-the-counter medication or herbal medication; and participation in any other clinical trial within 12 weeks before the scheduled administration of study drug. Subjects who had the following vital signs were also excluded: low blood pressure (systolic blood pressure ≤85 mmHg) and high blood pressure (systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg).
This study was performed in accordance with the principles of the Declaration of Helsinki and Korean Good Clinical Practice. The study protocol was reviewed and approved by the Ministry of Food and Drug Safety (Republic of Korea) and the Institutional Review Board of Seoul National University Hospital. The ClinicalTrials.gov registration numbers are NCT00969787 and NCT01094834 for the single- and multiple-dose studies, respectively.
Study design
The trial was conducted as a double-blinded, randomized, placebo-controlled, dose escalation study in Seoul National University Hospital. The trial consisted of two parts: a single ascending dose study and a multiple ascending dose study. A total of 121 subjects (single-dose study: 80 subjects and multiple-dose study: 40 subjects) were involved in the trial. In the single-dose study, subjects were assigned to one of eight groups receiving 10, 20, 50, 100, 150, 250, 400, or 600 mg DWP05195. In the multiple-dose study, subjects were assigned to one of four groups receiving 100, 200, 300, or 400 mg DWP05195, and the study drug was administered once daily for 8 days. Each treatment group consisted of 10 subjects (study drug-to-placebo ratio was 8:2). All subjects were randomized to treatment, and a randomization code was generated by an independent external provider.
Experimental pain (PD) assessments
Heat pain threshold (HPtr) and heat pain tolerance (HPtol) were measured using a Thermal NeuroSensory Analyzer (TSA; Medoc Ltd., Udim, Israel) to evaluate DWP05195 PDs.16 HPtr was defined as the temperature at which the subjects first felt a painful stimulus. HPtol was defined as the temperature at which the subjects first felt maximum tolerable pain. The temperature of the TSA tip, which was attached to the measurement site of the lower arm, increased with constant velocity until 50°C. The HPtr and HPtol were measured by the subject pushing a button on a response unit when the temperature of the TSA tip caused the first sensation of heat pain for HPtr and maximum tolerable pain for HPtol. The border of the measurement site was marked with a circle having a radius of 2.4 cm on the volar aspect of the lower arm. HPtr and HPtol measurements were repeated three times, and average values were used in the analyses.
HPtr and HPtol were measured in all groups, including the placebo group. HPtr and HPtol were measured on the normal skin before (0 h) and 3 and 8 h after DWP05195 administration in the single-dose study. In contrast, HPtr and HPtol were measured at 0 and 3 h on days 1 and 8, respectively, after administration of the study drug in the multiple-dose study. On day 8, HPtr and HPtol were additionally measured on capsaicin-sensitized skin after measurements were taken on the normal skin before the first dose and 3 h after the eighth dose (skin sensitization test). To apply capsaicin cream, an engraved sticker with a 3 cm diameter circular hole was attached on the measurement site before application of capsaicin cream, and then, ~1 mL of capsaicin cream (0.025% capsaicin) was applied to the measurement site. The cream was left on the skin under occlusion for 30 min and then gently wiped off.
The cutoff temperature for either test was 50°C to prevent skin injury. In addition, PD values that were obtained at temperatures >50°C before administering the drug were excluded from the analyses.17,18 Perfusion intensity (PI) and flare area ratio (FAR) were measured as the PD parameters of peripheral perfusion by using a PeriScan PIM 3 System (Perimed, Stockholm, Sweden). PD parameters were measured on non-sensitized skin (HPtrnon and HPtolnon) and on capsaicin-sensitized skin (HPtrcap and HPtolcap) in the multiple-dose study. To estimate interindividual variability more accurately, the actual values of HPtr and HPtol were individually corrected using baseline PD values of HPtr and HPtol, respectively, per the following equations: corrected HPtr = HPtr after study drug administration − HPtr before study drug administration and corrected HPtol = HPtol after study drug administration − HPtol before study drug administration where each value is the average of three measurements.
PK assessments
In the single-dose study, venous blood samples for the measurement of plasma DWP05195 concentrations were collected before administration and 0.33, 0.67, 1, 1.5, 2, 3, 4, 6, 10, 16, 24, 36, 48, and 72 h after administration. Urine samples were collected just before study drug administration and 0–6, 6–12, 12–24, 24–36, 36–48, and 48–72 h after study drug administration.
In the multiple-dose study, venous blood samples were collected at the following time points to evaluate the PK of DWP05195: before administration and 0.33, 0.67, 1, 1.5, 2, 3, 4, 6, 10, 16, and 24 h after administration on day 1. On days 3, 4, 5, 6, and 7, blood samples were taken before drug administration. On day 8, blood samples were taken before drug administration and at 0.33, 0.67, 1, 1.5, 2, 3, 4, 6, 10, 16, 24 36, 48, 72, and 96 h after administration. Urine samples were collected just before administering the study drug and at 0–6, 6–12, and 12–24 h on days 1 and 8 after administration of the drug. Blood samples were collected into heparinized tubes and centrifuged immediately to obtain plasma, which was stored at −70°C until analysis.
The plasma and urine concentrations of DWP05195 were analyzed using high-performance liquid chromatography (Shimadzu System, Shimadzu, Japan) and mass spectrometry (API 4000 mass spectrometer; SCIEX, Framingham, MA, USA). A mixture of methanol and water (70:30) was used as the mobile phase, and the detection wavelength was set at 340 nm. The concentration of DWP05195 was calculated using the ratio of the peak area of DWP05195 to the peak area of the internal standard. The lower limit of quantitation of DWP05195 was 5 μg/L. The assay method was validated over ranges of 5–1,000 and 5–5,000 μg/L for DWP05195 in plasma and urine, respectively. In the single-dose study, the precision of the assay method (using 2, 20, 600, and 1,000 μg/L quality control samples) was <10% and the accuracy was 93.1%–104.9%. In the multiple-dose study, the precision of the assay method (using 5, 50, 1,500, and 5,000 μg/L quality control samples) was <5.3% and the accuracy was 94.4%–109.6%.
A non-compartmental method was used to analyze the individual PK parameters using Phoenix® software (ver 6.4; Pharsight Corp., Mountain View, CA, USA). PK analyses were performed using the actual time of blood sample collection. The maximum plasma concentration (Cmax) of DWP05195 and the time at which Cmax was observed (Tmax) were directly obtained from the plasma concentration–time profiles. The accumulation ratio of multiple dosing regimen was defined as the ratio of the area under the plasma concentration–time curve (AUC) within a dosing interval in a steady state (AUCτ,ss) to AUC0–24h (the AUC on day 1). Renal clearance and the amount of unchanged drug excreted in urine were evaluated from the urine samples.
Safety and tolerability assessments
Safety and tolerability assessments were conducted in all subjects who received one or more doses of the study drug. Safety and tolerability were evaluated based on data from physical examination, including vital signs such as systolic and diastolic blood pressures, pulse rate and temperature, ECG, and laboratory test results (hematology, clinical chemistry, thyroid function test, coagulation, and urinalysis) obtained throughout the study. Adverse events (AEs) were recorded from voluntary reports made by the subjects or by asking the subjects general health-related questions.
Statistical analysis
SAS® software (ver 9.3; SAS Institute Inc., Cary, NC, USA) was used for the statistical analyses, and the level of significance was set at 0.05. To evaluate changes in PD parameters between the DWP05195-treated and placebo groups, analysis of covariance was performed using a mixed model with a covariate of the baseline values of those parameters. Because the HPtr or HPtol of some individuals was >50°C, the ratios between the numbers of the measurements >50°C to all measurements were additionally compared among the various treatment groups using logistic regression to complement the limitation of left-censored data. The relationship between each PD parameter and AUC0–24h (after single-dose administrations) or AUCτ,ss (after multiple-dose administrations) was analyzed using linear regression.
PK parameters and demographic characteristics were summarized using descriptive statistics. Dose proportionality was tested using Cmax, AUC0–24h, Cmax,ss, and AUCτ,ss with linear regression by using log-transformed values based on the power model.19 Fisher’s exact test was used to compare the continuous variables of the safety test among the treatment groups.
Results
Study subjects
Of the 121 subjects recruited for this study, 120 completed it. In the multiple-dose study, one subject who had received 200 mg DWP05195 withdrew his informed consent on day 5 and dropped out; however, he was replaced with a new subject. The data for one subject in the 100 mg DWP05195 multiple-dose group who did not have a peak drug concentration after the last dosing on day 8 were excluded from the PK and PD assessments.
The average age, height, and weight of subjects who completed the study were 27.1±5.1 years, 173.3±5.4 cm, and 68.8±7.2 kg, respectively, in the DWP05195 treatment groups (n=96) and 27.4±5.0 years, 173.1±5.6 cm, and 68.0±6.3 kg, respectively, in the placebo group (n=24). There were no significant differences in these characteristics between the placebo and DWP05195-treated groups in either the single- or multiple-dose studies.
Experimental pain (PD) assessment results
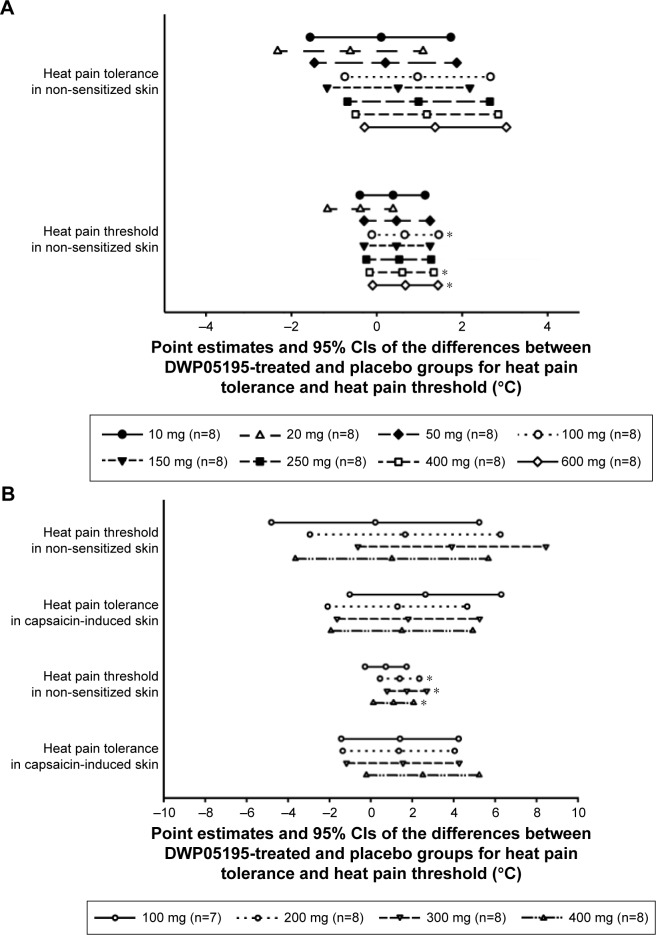
HPtr and HPtol maximally increased after 3 h, but tended to decrease 8 h after a single-dose administration of DWP05195 >150 mg (Figure S1). Based on these results, blood sampling times for the PD analyses were set at 0 h on day 1 and at 1 and 3 h on day 8 in the multiple-dose study. Changes in HPtr and HPtol from the baseline values were larger in the DWP05195 treatment groups at a dose of 50 mg (1.1°C–3.3°C and 0.9°C–1.5°C, respectively) than those in the placebo group (0.6°C and −0.1°C, respectively; Table 1 and Figure S2).
Table 1.
Summary of experimental pain (pharmacodynamic) assessment results after single- and multiple-dose administrations of DWP05195 to healthy male subjects
| Dose group | ΔHPtrnorm,3h | ΔHPtrcap,3h | ΔHPtolnorm,3h | ΔHPtolcap,3h |
|---|---|---|---|---|
| Single dose | ||||
| Placebo (n=16) | 0.6±2.0 | – | −0.1±0.9 | – |
| 10 mg (n=8) | 0.4±2.9 | – | 0.8±1.3 | – |
| 20 mg (n=8) | −1.2±3.7 | – | −0.5±1.4 | – |
| 50 mg (n=8) | 1.1±1.7 | – | 0.9±0.8 | – |
| 100 mg (n=8) | 2.1±1.8 | – | 1.3±0.9 | – |
| 150 mg (n=8) | 1.0±1.8 | – | 0.9±1.1 | – |
| 250 mg (n=8) | 2.5±1.9 | – | 1.4±1.2 | – |
| 400 mg (n=8) | 2.0±2.9 | – | 1.2±1.0 | – |
| 600 mg (n=8) | 3.3±3.3 | – | 1.5±1.4 | – |
| Multiple dose | ||||
| Placebo (n=8) | 1.9±4.4 | 1.0±1.0 | 1.4±1.5 | 2.3±1.8 |
| 100 mg (n=8) | 0.8±3.5 | 2.9±2.8 | 0.9±0.9 | 2.8±2.5 |
| 200 mg (n=8) | 2.9±2.1 | 5.1±2.2 | 2.6±1.2 | 3.5±1.9 |
| 300 mg (n=8) | 6.0±3.0 | 2.6±2.8 | 3.9±2.6 | 3.5±2.4 |
| 400 mg (n=8) | 2.2±1.5 | 2.3±3.5 | 1.4±1.5 | 4.4±2.9 |
Abbreviations: HPtrnorm, heat pain threshold in normalized skin; HPtrcap, heat pain threshold in capsaicin-sensitized skin; HPtolnorm, heat pain tolerance in normalized skin; HPtolcap, heat pain tolerance in capsaicin-sensitized skin.
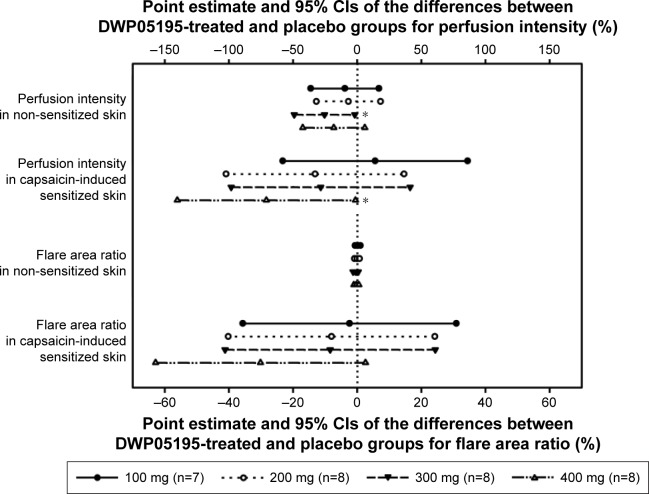
Similarly, after multiple administrations of DWP05195, the HPtr and HPtol of non-sensitized (HPtrnorm and HPtolnorm) and capsaicin-sensitized (HPtrcap and HPtolcap) skin increased in all treatment groups within 3 h of administering the study drug. HPtr and HPtol values, adjusted to their respective baseline values, tended to be higher in all the DWP05195-treated groups (doses over 200 mg) than those in the placebo group (Table 1 and Figure S3). HPtr or HPtol was sometimes measured at 50°C in some subjects. The number of HPtr and HPtol values reaching 50°C on normal (non-sensitized) skin increased significantly as the dose of the study drug increased (P<0.05, logistic regression). The PIs and FARs obtained after multiple administrations of DWP05195 (100 mg) were lower than those obtained after placebo administration. However, only differences in PIs of normal skin between the 300 mg DWP05195 dose and placebo groups were statistically significant.
PK–PD relationship of DWP05195
In the DWP05195-treated groups, HPtr and HPtol tended to increase with dose increments in both the single- and multiple-dose studies (Figure 1). In contrast, PI significantly decreased as the DWP0595 dose increased (Figure 2).
Figure 1.
Point estimates and 95% confidence intervals (CIs) of the differences between DWP05195-treated and placebo groups for heat pain threshold and heat pain tolerance after (A) a single-dose administration and (B) multiple-dose administrations of DWP05195 in healthy male subjects.
Note: *Indicates significant difference (P<0.05).
Figure 2.
Point estimates and 95% confidence intervals (CIs) of differences between DWP05195-treated and placebo groups for perfusion intensity and flare area ratio after multiple-dose administrations of DWP05195 in healthy male subjects.
Note: *Indicates significant difference (P<0.05).
HPtr and HPtol significantly increased (P<0.05) from their respective baseline values as DWP05195 exposure (AUC0–24h) increased after a single-dose administration (Figure S4). Correlations among the HPtr or HPtol of both non-sensitized and capsaicin-sensitized skins to DWP05195 and AUCτ,ss values trended in a positive direction; however, the values were not significant. A positive correlation between the AUCτ,ss of DWP05195 and HPtr or HPtol in both non-sensitized and capsaicin-sensitized skins was obtained; however, it was not statistically significant.
PK of DWP05195
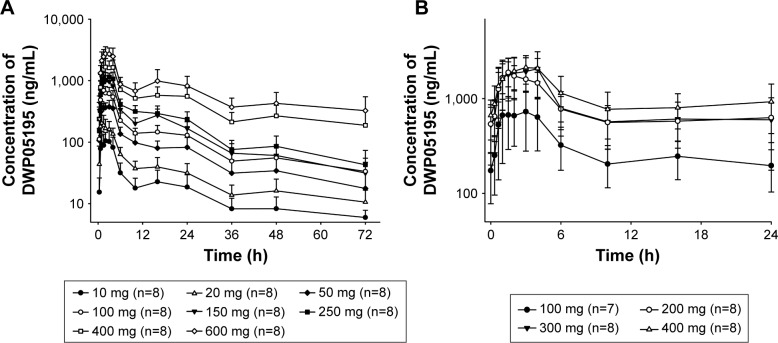
DWP05195 was rapidly absorbed after both single- and multiple- dose administrations, reaching Cmax in ~1.5–3.0 h post-dose administration (Figure 3 and Table 2). The plasma concentrations of DWP05159 were eliminated with a mean terminal elimination half-life of 35.3–49.1 h after the double peaks within 24 h. In both the single- and multiple-dose studies, Cmax and AUClast increased as the dose of DWP05195 was increased. In the single-dose study, Cmax and AUClast were not proportional to the administered dose, as the 95% confidence intervals (CIs) of the slope gradients did not include 1.0. However, the AUClast was nearly dose proportional as the 95% CIs ranged from 0.81 to 0.95. In the multiple-dose study, both Cmax,ss and AUCτ,ss values confirmed dose proportionality based on the finding that the 95% CIs of the slopes of the log-transformed Cmax,ss and AUCτ,ss included 1.0 (Cmax,ss: 0.55–1.26 and AUCτ,ss: 0.54–1.25) in the power model. The mean accumulation ratio was 1.16–1.57 after multiple doses of DWP05195 (100–400 mg) were administered, and no trend was observed as the dose was increased. The fraction of unchanged drug excreted in urine was <0.1% in both the single- and multiple-dose studies.
Figure 3.
Mean plasma concentration–time profiles of DWP05195 (A) from 0 to 72 h after a single oral dose administration and (B) at steady state after multiple oral doses from 168 to 192 h.
Note: The error bars represent standard deviations.
Table 2.
Summary of PK parameters of DWP05195 after single- and multiple-dose administrations of DWP05195 to healthy male subjects
| Dose group | PK parameters
|
|||||
|---|---|---|---|---|---|---|
| Doses | Tmax (h) | Cmax (μg/L) | AUClast (μg·h/L) | AUCinf (μg·h/L) | t1/2 (h) | |
| Single dose | 10 mg (n=8) | 1.5 (1.0–3.0) | 142.1±42.4 | 1,172.8±547.3 | 1,427.7±715.4 | 31.1±13.5 |
| 20 mg (n=8) | 1.0 (0.7–3.0) | 245.8±94.4 | 2,253.3±745.8 | 2,968.0±1,072.4 | 47.8±22.6 | |
| 50 mg (n=8) | 2.3 (0.7–4.0) | 449.0±63.8 | 5,023.9±1,786.6 | 5,961.2±2,725.7 | 31.5±12.2 | |
| 100 mg (n=8) | 1.5 (1.0–4.0) | 743.9±201.5 | 8,227.6±2,150.6 | 10,740.9±4,300.6 | 42.2±19.6 | |
| 150 mg (n=8) | 2.0 (1.0–4.0) | 1,202.4±211.6 | 11,733.8±2,354.1 | 13,203.1±2,945.4 | 31.7±6.4 | |
| 250 mg (n=8) | 2.3 (1.0–4.0) | 1,329.0±177.0 | 14,459.0±2,837.3 | 17,093.5±4,144.9 | 31.3±22.1 | |
| 400 mg (n=8) | 2.0 (1.1–4.0) | 2,106.7±340.8 | 30,291.0±10,520.5 | 43,867.4±22,980.2 | 45.3±7.7 | |
| 600 mg (n=8) | 3.0 (2.0–4.0) | 3,012.4±823.0 | 47,522.3±17,555.2 | 83,942.0±50,324.3 | 62.8±30.1 | |
| Doses | Tmax,ss (h) | Cmax,ss (μg/L) | AUCτ,ss (μg⋅h/L) | Rac | t1/2,ss (h) | |
| Steady state | 100 mg (n=7) | 3.0 (1.0–4.0) | 939.1±423.9 | 8,327.2±2,679.6 | 1.3±0.3 | 36.7±16.5 |
| 200 mg (n=8) | 1.5 (1.0–4.1) | 1,975.1±602.4 | 20,040.6±5,871.6 | 1.5±0.4 | 49.1±35.8 | |
| 300 mg (n=8) | 3.5 (0.7–4.0) | 2,311.7±856.9 | 20,941.1±10,412.2 | 1.2±0.4 | 37.6±44.9 | |
| 400 mg (n=8) | 2.5 (1.0–6.0) | 2,348.2±615.0 | 24,081.6±9,393.3 | 1.4±0.3 | 36.4±13.3 | |
Note: All values presented as mean ± SD, except for Tmax for which the median (range) is presented.
Abbreviations: PK, pharmacokinetic; Tmax, time to reach peak plasma drug concentrations; Cmax, maximum plasma drug concentration; AUClast, area under the plasma concentration–time curve from 0 to 72 h; AUCinf, area under the plasma concentration–time curve from time 0 to infinity; t1/2, elimination half-life; Tmax,ss, time to reach peak plasma drug concentrations at steady state; Cmax,ss, maximum plasma drug concentration at steady state; AUCτ,ss, area under the plasma concentration–time curve from 0 to 24 h at steady state; Rac, accumulation ratio of steady state to first dose at regular administration for AUCτ,ss/AUC0–24h; AUC0–24h, area under the plasma concentration–time curve on day 1; SD, standard deviation; t1/2,ss, elimination half-life at steady state.
Safety and tolerability of DWP05195
In the single-dose study, 31 out of 80 subjects experienced 51 drug-related AEs (ie, adverse drug reactions [ADRs]) throughout the study. No serious events were reported during the study. Increases in body temperatures (to 38°C and >38.0°C–38.3°C) were reported in two and three subjects in the 400 and 600 mg dose groups, respectively. The body temperature tended to increase with the DWP05195 dose as shown in Figure S5. In the multiple-dose study, 36 out of 41 subjects experienced 305 ADRs throughout the study (Table S1). Hyperthermia (a temperature >38°C) was reported once (38.3°C) in the 400 mg dose group.
In both single- and multiple-dose studies, the percentage of ADR cases significantly increased as the dose of DWP05195 increased (P<0.05). All reported AEs were mild to moderate in severity and resolved without medication or therapy. There were no abnormal results from the laboratory tests, physical examinations, or ECGs.
Discussion
This is the first study to evaluate the PD, PK, safety, and tolerability of different doses of DWP05195 in human subjects. Increases in HPtr and HPtol were higher in the DWP05195-treated groups than in the placebo group. PI and FAR decreased as the dose of DWP05195 increased. Increasing trends in HPtr and HPtol after multiple doses of DWP05195 are administered have also been observed in previous preclinical studies. However, HPtr and HPtol also increased from their respective baseline values after placebo administration. The increase is anticipated to have been caused by diurnal variations in pain perception and thermal sensitivity.20–22 Increased HPtr and HPtol have been consistently reported in previous studies with other TRPV1 antagonists such as SB-705498 (GlaxoSmithKline, London, UK) and AZD-1386 (AstraZeneca, London, UK).23
In the present study, the application of capsaicin proved to be methodologically beneficial for reducing the incidence of censored data by decreasing the overall HPtol and HPtr.24–26 To prevent skin injury while measuring HPtr and HPtol, the cutoff temperature was set at 50°C and measurements were suspended when the 50°C limit was reached. The measured HPtr and HPtol values reached the 50°C limit as the dose of DWP05195 increased; however, this occurred more often in the normal skin than in the capsaicin-sensitized skin. This indicates that a more accurate evaluation of HPtr and HPtol was achieved because capsaicin was applied to the skin.26
TRPV1 is involved in thermoregulation. This may be the reason for the large number of thermoregulation-related AEs observed in the present study, such as “feeling cold”, “feeling hot”, and peripheral coldness. Changes in thermoregulatory function were also observed in some previous preclinical studies on TRPV1.27,28 The TRPV1 channel is present in neurons in the preopticohypothalamic area, which is involved in thermoregulatory pathways mediated by medial preoptic nucleus cells or gamma-aminobutyric acid (GABA)ergic myeloperoxidase cells.29,30
In the present study, an increasing trend in body temperature after administration of DWP05195 was observed, and five cases of hyperthermia (maximum 38.3°C) were reported. This finding has been reported in previous studies with other TRPV1 antagonists. A previously reported clinical study with AMG-517 (Amgen, Thousand Oaks, CA, USA) resulted in plasma concentration-dependent hyperthermia; the maximal body temperature was recorded as 39.9°C.31 A Phase 1 study of AZD-1386 (doses ranging from 3 to 190 mg and 20 to 150 mg in a single-dose and multiple-dose study, respectively) reported an induction of hyperthermia in healthy subjects. When compared to previous studies, the hyperthermia cases in this study were mild and less frequently observed. However, careful investigation of hyperthermia is required for further evaluation of DWP05195.
Since this is a first-in-human study, the administered doses of DWP05195 were decided based on no observed adverse effect levels (NOAELs) derived from a 4-week preclinical trial conducted in Sprague Dawley rats and beagle dogs (75 and 50 mg/kg, respectively). The NOAELs were then converted to human equivalent doses in line with the guidelines specified by the US Food and Drug Administration32,33 and adjusted using safety factors. Consequently, a 72 mg dose was set as the maximum recommended starting dose (MRSD) to be administered to healthy adult male subjects weighing 60 kg. In addition, in a preclinical efficacy trial conducted in a rat model of diabetic neuropathy, a minimal effective dose (MED) of 1 mg/kg was obtained for DWP05195, which converts to 9.6 mg for a healthy adult male subject weighing 60 kg. Considering the abovementioned MRSD and MED values derived from nonclinical studies, the starting dose of DWP05195 was set at 10 mg. In addition, dose increments were planned from 10 to 20, 50, 100, 150, 250, 400, and 600 mg (unpublished internal data).
Conclusion
Subjects who were administered DWP05195 showed relatively higher HPtr and HPtol as well as lower PI and FAR than did those who were administered the placebo. This indicates that DWP05195 is a TRPV1 antagonist. Given the near dose-proportional systemic exposure observed in the single- and multiple-dose studies, systemic exposure is expected to increase as the dose of DWP05195 is increased. DWP05195 was well tolerated at 10–600 mg after a single oral dose and at 100–400 mg/day for 8 days after multiple oral doses were administered to healthy volunteers.
Supplementary materials
Baseline-adjusted (A) heat pain threshold and (B) heat pain tolerance among dose groups after a single oral dose of DWP05195 was administered to healthy male subjects.
Note: The error bars represent the standard deviation at each sampling time.
Experimental pain assessment results of the single-dose study.
Notes: HPtr and HPtol in normalized skin 3 h after a single-dose administration are shown. The error bars denote standard deviations.
Abbreviations: HPtr, heat pain threshold; HPtol, heat pain tolerance.
Experimental pain assessment results from the multiple-dose studies.
Notes: (A) HPtr in normalized skin and in capsaicin-sensitized skin 3 h after drug administration is shown. (B) HPtol in normalized skin and in capsaicin-sensitized skin 3 h after administration of multiple doses is shown. The error bars denote the standard deviations.
Abbreviations: HPtr, heat pain threshold; HPtol, heat pain tolerance.
Correlation between baseline-adjusted changes in (A) HPtr and (B) HPtol 3 h after a single dose of DWP05195 or placebo was administered to healthy male subjects.
Abbreviations: HPtr, heat pain threshold; HPtol, heat pain tolerance; AUC0–24h, area under the plasma concentration–time curve on day 1.
Body temperature–time profiles after multiple administrations of study drug.
Notes: (A) Placebo, (B) DWP05195 100 mg, (C) DWP05195 200 mg, (D) DWP05195 300 mg, and (E) DWP05195 400 mg. The error bars represent the standard deviation at each sampling time.
Table S1.
Summary of AEs
| Dose group | Drug-related AEs | Common drug-related AEs* |
|---|---|---|
| Single-dose study | ||
| DWP05195 | ||
| 10 mg (n=8) | 1 (1) | Feeling hot (12.5%) |
| 20 mg (n=8) | 0 (0) | |
| 50 mg (n=8) | 2 (2) | Feeling cold (25.0%) |
| 100 mg (n=8) | 0 (0) | |
| 150 mg (n=8) | 2 (2) | Feeling cold (12.5%) |
| 250 mg (n=8) | 11 (7) | Feeling cold (50.0%), feeling hot (37.5%) |
| 400 mg (n=8) | 13 (7) | Feeling cold (62.5%), paresthesia (37.5%), pyrexia (25.0%), headache (25.0%), feeling hot (12.5%) |
| 600 mg (n=8) | 17 (8) | Feeling cold (100.0%), pyrexia (37.5%), headache (25.0%), feeling hot (12.5%), paresthesia (12.5%) |
| Placebo (n=16) | 4 (5) | Headache (12.5%), feeling hot (6.25%) |
| Multiple-dose study | ||
| DWP05195 | ||
| 100 mg (n=8) | 8 (8) | Feeling cold (37.5%), feeling hot (25.0%) |
| 200 mg (n=9) | 71 (9) | Feeling cold (100.0%), feeling hot (55.6%), headache (22.2%) |
| 300 mg (n=8) | 83 (8) | Feeling cold (100.0%), headache (37.5%), hyperhidrosis (25.0%), feeling hot (25.0%) |
| 400 mg (n=8) | 115 (8) | Feeling cold (100.0%), feeling hot (50.0%) |
| Hyperhidrosis (50.0%), headache (25.0%) | ||
| Placebo (n=8) | 16 (5) | Peripheral coldness (25.0%), headache (25.0%) |
Notes: All values are presented as the number of AEs (number of subjects with AEs). The AE was countered as drug related when its causality assessment was certain, probable, or possible.
Common AEs (>5% incidence for single-dose study and >20% incidence for multiple-dose study) were presented with incidence, (number of subjects with AEs/number of subjects) ×100, in each dosage group.
Abbreviation: AEs, adverse events.
Acknowledgments
This study was sponsored by Daewoong Pharmaceutical Co., Ltd. (Seoul, South Korea). An abstract of this paper was presented at the ASCPT 2016 Annual Meeting as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in Clinical Pharmacology and Therapeutics: http://onlinelibrary.wiley.com/doi/10.1002/cpt.310/full. The actual paper, however, has never been published.
Footnotes
Disclosure
Hee Sun Kim and Ji Duck Kim are employees of Daewoong Pharmaceutical Co., Ltd. (Seoul, Republic of Korea). The other authors report no conflicts of interest in this work.
References
- 1.Mäntyselkä P, Kumpusalo E, Ahonen R, et al. Pain as a reason to visit the doctor: a study in Finnish primary health care. Pain. 2001;89(2–3):175–180. doi: 10.1016/s0304-3959(00)00361-4. [DOI] [PubMed] [Google Scholar]
- 2.Gøtzsche PC. NSAIDs. BMJ Clin Evid. 2010;2010:1108. [Google Scholar]
- 3.Potter MB. NSAIDs alone or with opioids as therapy for cancer pain. Am Fam Physician. 2005;72(3):436–437. [PubMed] [Google Scholar]
- 4.Moore RA, Chi CC, Wiffen PJ, Derry S, Rice AS. Oral nonsteroidal anti-inflammatory drugs for neuropathic pain. Cochrane Database Syst Rev. 2015;10:CD010902. doi: 10.1002/14651858.CD010902.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cepeda MS, Farrar JT, Baumgarten M, Boston R, Carr DB, Strom BL. Side effects of opioids during short-term administration: effect of age, gender, and race. Clin Pharmacol Ther. 2003;74(2):102–112. doi: 10.1016/S0009-9236(03)00152-8. [DOI] [PubMed] [Google Scholar]
- 6.Palos GR. Opioids and cancer survivors: issues in side-effect management. Oncol Nurs Forum. 2008;35(suppl):13–19. doi: 10.1188/08.ONF.S1.13-19. [DOI] [PubMed] [Google Scholar]
- 7.Berde C, Nurko S. Opioid side effects – mechanism-based therapy. N Engl J Med. 2008;358:2400–2402. doi: 10.1056/NEJMe0801783. [DOI] [PubMed] [Google Scholar]
- 8.Kamei T, Miyauchi M, Oyamada Y, Shimizu I. Novel therapeutic approach to neuropathic pain: “Hot” and “Cool” TRP-channel family. Nihon Yakurigaku Zasshi. 2012;140(5):196–200. doi: 10.1254/fpj.140.196. [DOI] [PubMed] [Google Scholar]
- 9.Rashid MH, Inoue M, Kondo S, Kawashima T, Bakoshi S, Ueda H. Novel expression of vanilloid receptor 1 on capsaicin-insensitive fibers accounts for the analgesic effect of capsaicin cream in neuropathic pain. J Pharmacol Exp Ther. 2003;304(3):940–948. doi: 10.1124/jpet.102.046250. [DOI] [PubMed] [Google Scholar]
- 10.Caterina MJ, Leffler A, Malmberg AB, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288(5464):306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 11.Staaf S, Oerther S, Lucas G, Mattsson JP, Ernfors P. Differential regulation of TRP channels in a rat model of neuropathic pain. Pain. 2009;144(1–2):187–199. doi: 10.1016/j.pain.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 12.Dyachenko IA, Andreev YA, Logashina YA, Murashev AN, Grishin EV. Biological activity of a polypeptide modulator of TRPV1 receptor. Dokl Biol Sci. 2015;465(1):279–281. doi: 10.1134/S0012496615060034. [DOI] [PubMed] [Google Scholar]
- 13.Appendino G, De Petrocellis L, Trevisani M, et al. Development of the first ultra-potent “capsaicinoid” agonist at transient receptor potential vanilloid type 1 (TRPV1) channels and its therapeutic potential. J Pharmacol Exp Ther. 2005;312(2):561–570. doi: 10.1124/jpet.104.074864. [DOI] [PubMed] [Google Scholar]
- 14.Cortright DN, Crandall M, Sanchez JF, Zou T, Krause JE, White G. The tissue distribution and functional characterization of human VR1. Biochem Biophys Res Commun. 2001;281(5):1183–1189. doi: 10.1006/bbrc.2001.4482. [DOI] [PubMed] [Google Scholar]
- 15.Hudson LJ, Bevan S, Wotherspoon G, Gentry C, Fox A, Winter J. VR1 protein expression increases in undamaged DRG neurons after partial nerve injury. Eur J Neurosci. 2001;13(11):2105–2114. doi: 10.1046/j.0953-816x.2001.01591.x. [DOI] [PubMed] [Google Scholar]
- 16.Shukla G, Bhatia M, Behari M. Quantitative thermal sensory testing – value of testing for both cold and warm sensation detection in evaluation of small fiber neuropathy. Clin Neurol Neurosurg. 2005;107(6):486–490. doi: 10.1016/j.clineuro.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 17.Ravn P, Frederiksen R, Skovsen AP, Christrup LL, Werner MU. Prediction of pain sensitivity in healthy volunteers. J Pain Res. 2012;5:313–326. doi: 10.2147/JPR.S33925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tjølsen A, Rosland JH, Berge OG, Hole K. The increasing-temperature hot-plate test: an improved test of nociception in mice and rats. J Pharmacol Methods. 1991;25(3):241–250. doi: 10.1016/0160-5402(91)90014-v. [DOI] [PubMed] [Google Scholar]
- 19.Smith BP, Vandenhende FR, DeSante KA, et al. Confidence interval criteria for assessment of dose proportionality. Pharm Res. 2000;17(10):1278–1283. doi: 10.1023/a:1026451721686. [DOI] [PubMed] [Google Scholar]
- 20.Koch HJ, Raschka C, Fischer-Barnicol D, Lanquillon S, Ibach B. Diurnal variation of pain perception and heart rate in the human tourniquet pain model in healthy volunteers. Psychiatr Prax. 2004;31(suppl 1):S155–S157. doi: 10.1055/s-2004-828471. [DOI] [PubMed] [Google Scholar]
- 21.Krøigård T, Sothynathan I, Sindrup SH. Intraindividual variability and long-term changes of thermal quantitative sensory testing. J Clin Neurophysiol. 2015;32(4):352–356. doi: 10.1097/WNP.0000000000000193. [DOI] [PubMed] [Google Scholar]
- 22.Strian F, Lautenbacher S, Galfe G, Hölzl R. Diurnal variations in pain perception and thermal sensitivity. Pain. 1989;36(1):125–131. doi: 10.1016/0304-3959(89)90120-6. [DOI] [PubMed] [Google Scholar]
- 23.Gomtsyan A, Brederson JD. Clinical and preclinical experience with TRPV1 antagonists as potential analgesic agents. In: Szallasi A, editor. TRP Channels as Therapeutic Targets: From Basic Science to Clinical Use. New York: Academic Press; 2015. pp. 129–139. [Google Scholar]
- 24.Cavallone LF, Frey K, Montana MC, et al. Reproducibility of the heat/capsaicin skin sensitization model in healthy volunteers. J Pain Res. 2013;6:771–784. doi: 10.2147/JPR.S53437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dirks J, Petersen KL, Dahl JB. The heat/capsaicin sensitization model: a methodologic study. J Pain. 2003;4(3):122–128. doi: 10.1054/jpai.2003.10. [DOI] [PubMed] [Google Scholar]
- 26.Flühr K, Neddermeyer TJ, Lötsch J. Capsaicin or menthol sensitization induces quantitative but no qualitative changes to thermal and mechanical pain thresholds. Clin J Pain. 2009;25(2):128–131. doi: 10.1097/AJP.0b013e3181817aa2. [DOI] [PubMed] [Google Scholar]
- 27.Garami A, Pakai E, Oliveira DL, et al. Thermoregulatory phenotype of the Trpv1 knockout mouse: thermoeffector dysbalance with hyper-kinesis. J Neurosci. 2011;31(5):1721–1733. doi: 10.1523/JNEUROSCI.4671-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gavva NR, Bannon AW, Hovland DN, Jr, et al. Repeated administration of vanilloid receptor TRPV1 antagonists attenuates hyperthermia elicited by TRPV1 blockade. J Pharmacol Exp Ther. 2007;323(1):128–137. doi: 10.1124/jpet.107.125674. [DOI] [PubMed] [Google Scholar]
- 29.Morrison SF, Nakamura K. Central neural pathways for thermoregulation. Front Biosci (Landmark Ed) 2011;16:74–104. doi: 10.2741/3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romanovsky AA, Almeida MC, Garami A, et al. The transient receptor potential vanilloid-1 channel in thermoregulation: a thermosensor it is not. Pharmacol Rev. 2009;61(3):228–261. doi: 10.1124/pr.109.001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chizh BA, O’Donnell MB, Napolitano A, et al. The effects of the TRPV1 antagonist SB-705498 on TRPV1 receptor-mediated activity and inflammatory hyperalgesia in humans. Pain. 2007;132(1–2):132–141. doi: 10.1016/j.pain.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Imam MT, Venkateshan SP, Tandon M, Saha N, Pillai KK. Comparative evaluation of US Food and Drug Administration and pharmacologically guided approaches to determine the maximum recommended starting dose for first-in-human clinical trials in adult healthy men. J Clin Pharmacol. 2011;51(12):1655–1664. doi: 10.1177/0091270010387429. [DOI] [PubMed] [Google Scholar]
- 33.Chan G, Gray P, Glue P. An evaluation of the FDA draft guidance for estimating the maximum recommended starting dose (MRSD) for first-in-human (FIH) studies. Clin Pharmacol Ther. 2004;75(2):8. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Baseline-adjusted (A) heat pain threshold and (B) heat pain tolerance among dose groups after a single oral dose of DWP05195 was administered to healthy male subjects.
Note: The error bars represent the standard deviation at each sampling time.
Experimental pain assessment results of the single-dose study.
Notes: HPtr and HPtol in normalized skin 3 h after a single-dose administration are shown. The error bars denote standard deviations.
Abbreviations: HPtr, heat pain threshold; HPtol, heat pain tolerance.
Experimental pain assessment results from the multiple-dose studies.
Notes: (A) HPtr in normalized skin and in capsaicin-sensitized skin 3 h after drug administration is shown. (B) HPtol in normalized skin and in capsaicin-sensitized skin 3 h after administration of multiple doses is shown. The error bars denote the standard deviations.
Abbreviations: HPtr, heat pain threshold; HPtol, heat pain tolerance.
Correlation between baseline-adjusted changes in (A) HPtr and (B) HPtol 3 h after a single dose of DWP05195 or placebo was administered to healthy male subjects.
Abbreviations: HPtr, heat pain threshold; HPtol, heat pain tolerance; AUC0–24h, area under the plasma concentration–time curve on day 1.
Body temperature–time profiles after multiple administrations of study drug.
Notes: (A) Placebo, (B) DWP05195 100 mg, (C) DWP05195 200 mg, (D) DWP05195 300 mg, and (E) DWP05195 400 mg. The error bars represent the standard deviation at each sampling time.
Table S1.
Summary of AEs
| Dose group | Drug-related AEs | Common drug-related AEs* |
|---|---|---|
| Single-dose study | ||
| DWP05195 | ||
| 10 mg (n=8) | 1 (1) | Feeling hot (12.5%) |
| 20 mg (n=8) | 0 (0) | |
| 50 mg (n=8) | 2 (2) | Feeling cold (25.0%) |
| 100 mg (n=8) | 0 (0) | |
| 150 mg (n=8) | 2 (2) | Feeling cold (12.5%) |
| 250 mg (n=8) | 11 (7) | Feeling cold (50.0%), feeling hot (37.5%) |
| 400 mg (n=8) | 13 (7) | Feeling cold (62.5%), paresthesia (37.5%), pyrexia (25.0%), headache (25.0%), feeling hot (12.5%) |
| 600 mg (n=8) | 17 (8) | Feeling cold (100.0%), pyrexia (37.5%), headache (25.0%), feeling hot (12.5%), paresthesia (12.5%) |
| Placebo (n=16) | 4 (5) | Headache (12.5%), feeling hot (6.25%) |
| Multiple-dose study | ||
| DWP05195 | ||
| 100 mg (n=8) | 8 (8) | Feeling cold (37.5%), feeling hot (25.0%) |
| 200 mg (n=9) | 71 (9) | Feeling cold (100.0%), feeling hot (55.6%), headache (22.2%) |
| 300 mg (n=8) | 83 (8) | Feeling cold (100.0%), headache (37.5%), hyperhidrosis (25.0%), feeling hot (25.0%) |
| 400 mg (n=8) | 115 (8) | Feeling cold (100.0%), feeling hot (50.0%) |
| Hyperhidrosis (50.0%), headache (25.0%) | ||
| Placebo (n=8) | 16 (5) | Peripheral coldness (25.0%), headache (25.0%) |
Notes: All values are presented as the number of AEs (number of subjects with AEs). The AE was countered as drug related when its causality assessment was certain, probable, or possible.
Common AEs (>5% incidence for single-dose study and >20% incidence for multiple-dose study) were presented with incidence, (number of subjects with AEs/number of subjects) ×100, in each dosage group.
Abbreviation: AEs, adverse events.