Abstract
Urinary tract infections are one of the most common infectious diseases diagnosed in the community and in the hospital setting. Their treatment is complicated by drug-resistant pathogens and the colonization by microbes of indwelling urinary catheters. This study assessed the occurrence and antimicrobial susceptibility of methicillin-resistant Staphylococcus aureus (MRSA) uropathogens isolated for 5 consecutive years at University Hospital Waterford between 2010 and 2014. We created 4 clinically relevant subdivisions, based on urine source: hospital inpatients, patients from the Emergency Department, patients referred from their General Practitioner, and Nursing Home patients. We performed a retrospective review from the hospital's electronic microbiological system and calculated resistance rates for each of the standard antimicrobial agents. During the 5-year study period, we studied 151 urine isolates obtained from 128 patients who had an MRSA cultured in their urine sample. There was 100% resistance of all MRSA isolates to Flucloxacillin and Coamoxiclav. Ninety-eight percent of isolates were resistant to Ciprofloxacin. The resistance rate for Trimethoprim was 7.4% and there was only 2.7% resistance for Nitrofurantoin. For a clinical subset of patients, we also demonstrated 100% sensitivity for samples tested against Teicoplanin and Vancomycin. Urinary MRSA is an infrequently studied phenomenon, but with the rising trend of hospital superbugs nationally, its management is of critical importance. Suitable agents to address this within our population include Nitrofurantoin in the well patient requiring urinary MRSA eradication or Vancomycin/Teicoplanin in the unwell patient requiring intravenous therapy. In all groups, fluoroquinolones should be avoided due to significant resistance rates.
Keywords: colonization, methicillin-resistant Staphylococcus aureus, superbugs, urinary, urology, uropathogen
1. Introduction
Urinary tract infection (UTI) is a frequent cause of morbidity both in the community and in the hospital setting.[1,2] The causative pathogen can vary greatly geographically, and so it is prudent to identify those with resistant strains and have current data on the appropriate empirical therapy within a region. The most frequently encountered organisms associated with UTIs include enteric Gram-negative bacteria (with Escherichia coli being the most predominant), coagulase negative Staphylococcus saprophyticus along with Proteus mirabilis, Klebsiella, and Enterococcus, which account for less than 5%.[1,3] However, recent studies have reported the increasing prevalence of Staphylococcus aureus (SA) in UTIs.[4–6]
SA is an opportunistic pathogen affecting both immune competent and immunocompromised individuals, frequently resulting in significant morbidity. Many strains of SA carry a wide variety of multidrug-resistant genes on plasmids, which aid the spread of resistance among species.[7]
Methicillin-resistant SA (MRSA) is widespread in many Irish hospitals and is increasingly seen in community health care units such as nursing homes.[8] Globally, it is considered that there has been an epidemic of MRSA within health care institutions.[9,10] Bacteriuria with SA is hypothesized to occur through a number of mechanisms that includes catheterization, urologic procedures, or seeding of the genitourinary tract—including nephrologically excreted bacteria in overt bacteremia. Bacteremia itself is associated with bacteriuria in patients infected with SA, which suggests that bacteremia is an important precursor for bacteriuria in some patient groups.[11,12]
However, little is published on the features of MRSA-positive urine cultures. Herein, we chronologically assessed the source, patient demographics, and antimicrobial susceptibilities of MRSA-positive isolates from the hospital and community setting over a 5-year period in University Hospital Waterford—a tertiary referral hospital with the primary microbiology laboratory for 4 acute hospitals, serving a total catchment area of almost 500,000 people.
2. Methods
The microbiology laboratory in University Hospital Waterford processes all inpatient urines in the catchment area of almost 500,000, excluding 2 small private elective inpatient institutions. It also processes all urine samples sent from the community (i.e., all samples from General Practice and Nursing Homes) and all specimens from the catchments’ Emergency Departments.
Laboratory diagnosis of MRSA bacteriuria was performed using accredited microbiological microscopy analysis with culture identification criteria and standardized susceptibility testing protocols. Our laboratory utilized Clinical and Laboratory Standards Institute (CLSI; 2010–2014) and European Committee on Antimicrobial Susceptibility Testing (EUCAST; 2014) methodologies.
A retrospective data extraction was performed, from the hospital's electronic microbiological system “Cognos” and de-duplication was executed.
De-duplication analysis identified 128 patients with 151 separate episodes of bacteriuria. We considered a “separate episode” as a urine specimen culturing MRSA at least 6 months following a previous positive culture. Further urine specimens sent within a 6-month period following an initial laboratory diagnosis MRSA bacteriuria were excluded.
Resistance rates were then calculated for the pathogen's susceptibility to 5 commonly used antimicrobial agents—Ciprofloxacin, Coamoxiclav, Flucloxacillin, Nitrofurantoin, and Trimethoprim. For unwell patients, that is, those in whom there was clinical suspicion of sepsis or invasive infection, Teicoplanin and Vancomycin were also tested for sensitivity profiling.
In order to obtain further clinical information from our patient group, we also assessed those patients who had an MRSA-positive swab (usually from groin, nose, or perineum), those who had documented MRSA bacteremia, and we analyzed their demographic details.
Ethical approval was waived for this retrospective observational study in light of its non-interventional nature.
3. Results
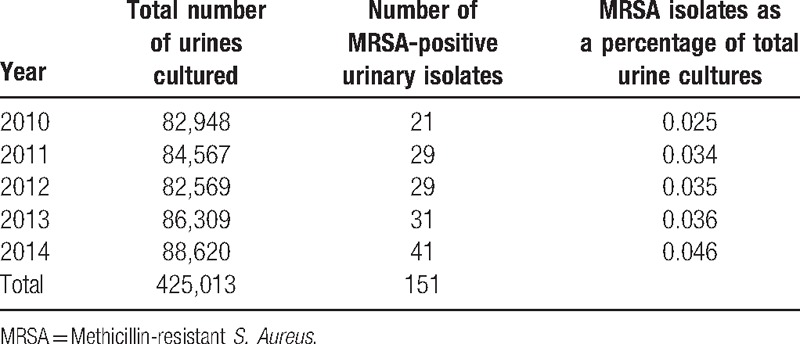
Over a consecutive 5-year period, the laboratory cultured 425,013 urine samples from all sources, with a mean number of 85,003 specimens per year received (Table 1). A total of 542 unique urine isolates cultured SA and 151 of species (27.9%) were methicillin resistant, meaning that 0.04% of all urine samples tested cultured MRSA.
Table 1.
Number of urine cultures by year.
Of these 151 samples that tested positive for MRSA, 92 (61%) were from mid-stream urine (MSU) samples, 50 (33%) from catheter specimen urine (CSU) samples, and 9 (6%) were from an unspecified source (Fig. 1).
Figure 1.
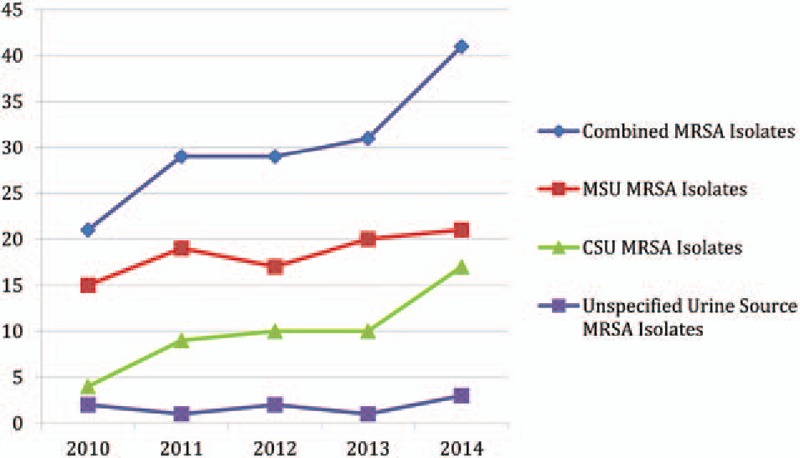
Numbers of MRSA-positive isolates by year and source. CSU = Catheter specimen urine, MRSA = Methicillin Resistant Staphylococcus Aureus, MSU = Mid-stream urine.
MRSA was slightly more common in MSU samples; however, the findings were not statistically significant (18.2% vs 13.9%, P = 0.388).
Forty-nine (32.5%) specimens were from an inpatient cohort, 9 (6%) were from the Emergency Department, 79 (52.3%) from General Practitioners, 12 (7.9%) were from Nursing Homes, with 2 (1.3%) from an unrecorded source (Fig. 2). MRSA was seen in a similar proportion of inpatient and outpatient samples indicating that this is not solely a hospital-acquired phenomenon (29.1% vs 26.9%, P = 0.587).
Figure 2.
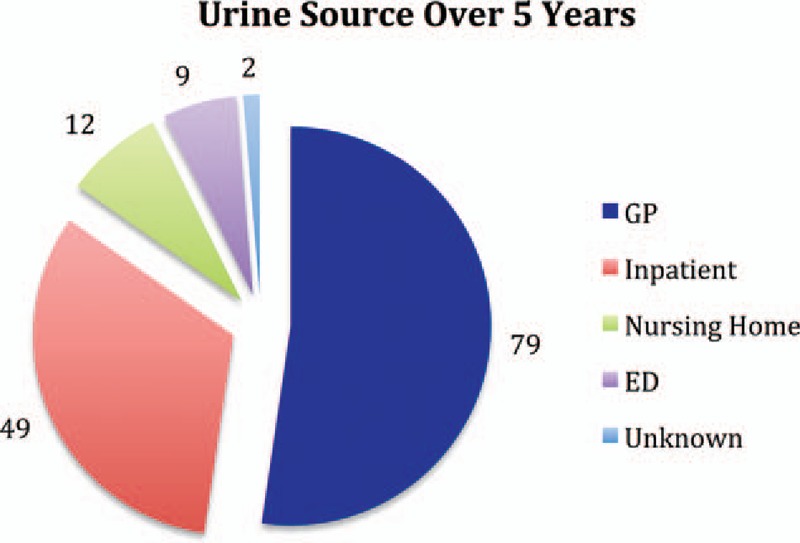
MRSA urines by source. MRSA = Methicillin Resistant Staphylococcus Aureus.
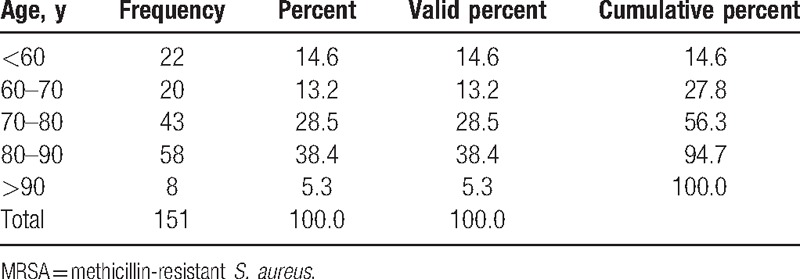
The mean age of our patients who cultured MRSA within their urine was 72.7 years (range 10.97–90.2) and most patients were aged between 70 and 90 years (Table 2). Patients who cultured an MRSA bacterium were older than patients with MSSA, and this was statistically significant (53 vs 73 years, P ≤ 0.001).
Table 2.
Age demographics of MRSA bacteriuric patients.
The number of SA isolates grown over the 5-year study period doubled from 77 in 2010 to 161 in 2014. The number of MRSA isolates had also increased; however, the percentage of SA isolates that were methicillin resistant has decreased (P = 0.145). This is true for both inpatient and outpatient samples (P = 0.313, P = 0.254, Figs. 3–5).
Figure 3.
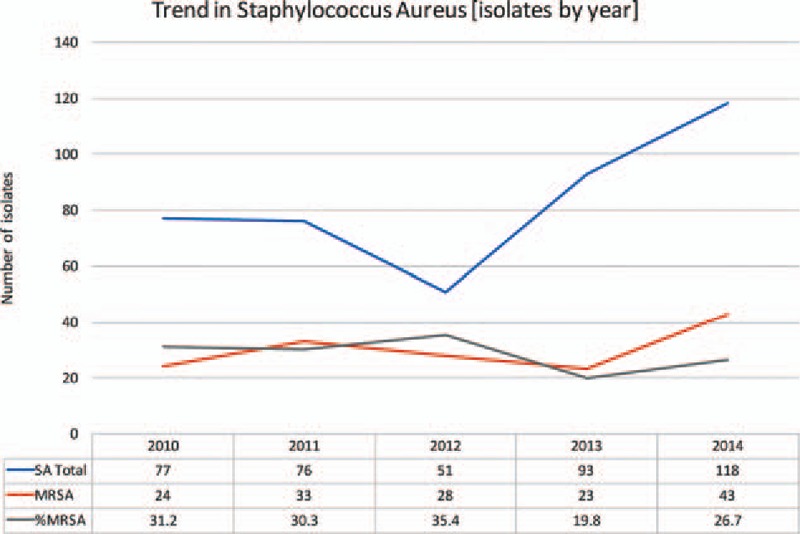
Overall trends in SA isolates. MRSA = Methicillin Resistant Staphylococcus Aureus, MSSA = Methicillin Sensitive Staphylococcus Aureus, SA = Staphylococcus Aureus.
Figure 5.
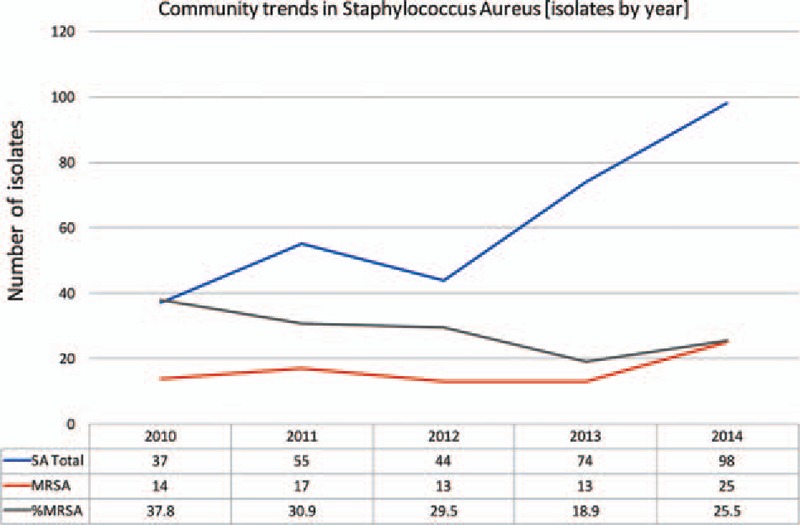
Community trends in SA isolates. MRSA = Methicillin Resistant Staphylococcus Aureus, MSSA = Methicillin Sensitive Staphylococcus Aureus, SA = Staphylococcus Aureus.
Figure 4.
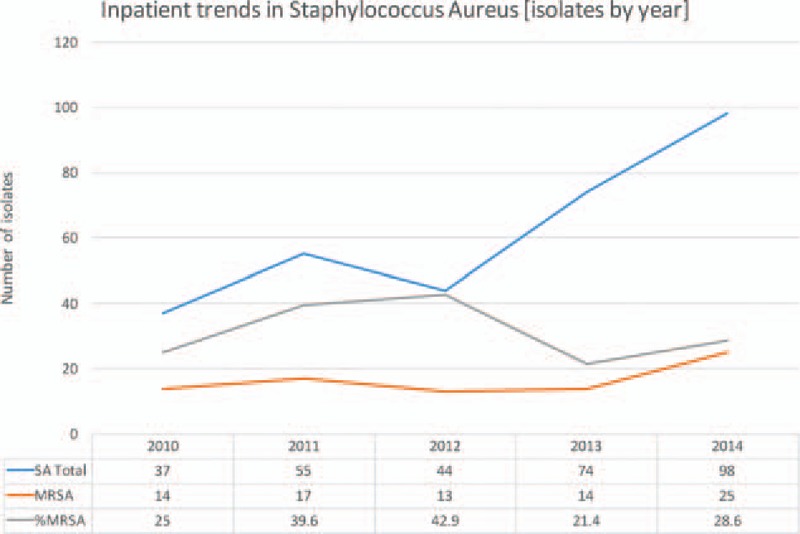
Inpatient trends in SA isolates. MRSA = Methicillin Resistant Staphylococcus Aureus, MSSA = Methicillin Sensitive Staphylococcus Aureus, SA = Staphylococcus Aureus.
Regarding the clinical stance of patients within the cohort, 9 patients (7%) with MRSA-positive urine cultures had a documented history of MRSA bacteremia at that time. Each of these patients cultured MRSA isolates that were sensitive to Nitrofurantoin, and 8 of the 9 patients (88.9%) were sensitive to Trimethoprim. All 9 were resistant to Ciprofloxacin and Flucloxacillin. It was noted that all 9 patients in this cohort were aged 70 years or above (P = 0.079), although this did not reach statistical significance.
Further antimicrobials agents were introduced to the testing panel in 59 (39%) samples, where there was a clinical concern regarding invasive infection or sepsis—including the 9 patients with MRSA bacteremia. All 59 samples (100%) were sensitive to both Vancomycin and Teicoplanin.
Eighty-eight (66.8%) of our 128 patients cultured MRSA-positive isolates on routine swabs from either mucosal or skin sites. All 3 patients with resistance to Nitrofurantoin in their urine had a positive swab from either groin or nose. Eight of the 10 patients (80%) who had Trimethoprim-resistant MRSA bacteriuria and 87 of the 125 (69.6%) of the Ciprofloxacin-resistant MRSA bacteriuria also had MRSA-positive mucosal or skin swabs. There was no association between age and having an MRSA-positive swab in our cohort.
Throughout the study period, there was 100% resistance of all MRSA isolates to Flucloxacillin and Coamoxiclav, as expected. Ninety-eight percent of isolates were resistant to Ciprofloxacin, but of these samples, there was 97.2% sensitivity for Nitrofurantoin and 92.4% sensitivity for Trimethoprim (Fig. 6).
Figure 6.
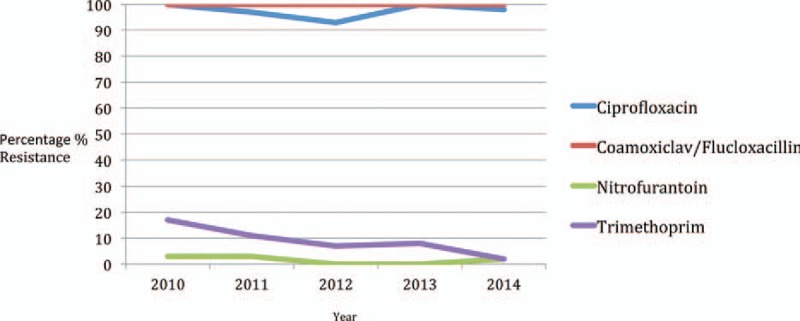
Resistance profiles of MRSA-positive urines by antibiotic and year. MRSA = Methicillin Resistant Staphylococcus Aureus.
Overall, there was only 2.7% resistance for Nitrofurantoin, but all of these samples were sensitive to Trimethoprim. Trimethoprim itself had a 7.4% resistance level, but all of these samples were sensitive to Nitrofurantoin, meaning that no sample was resistant to both oral agents, that is, Nitrofurantoin and Trimethoprim.
There was a statistically significant association between Nitrofurantoin resistance and younger patients, in that 75% of patients with Nitrofurantoin resistance were younger than 70 years (P = 0.025). There was no statistically significant association between age and resistance profile in any other of the antimicrobial panel.
4. Discussion
The impact of MRSA is considerable; in Ireland, approximately 40% to 50% of isolates SA recovered from bloodstream infections are methicillin resistant.[8] Despite available publications and guidelines on the management of MRSA skin-colonized patients, little has been published on the colonization of urine from patients with indwelling urinary catheters.
The identification of an MRSA isolate in a urine culture has important ramifications for patients, both in the community and in the hospital setting. A recent study demonstrated that 22% of patients with MRSA bacteriuria went on to develop invasive MRSA infection within 12 months.[13] MRSA in urine clearly warrants treatment in symptomatic patients, but even in asymptomatic patients, it may require eradication before certain elective procedures—such as endourological surgery. It also has consequences for patients and health care providers when it comes to providing isolation and other barrier precautions that should be administered in a nosocomial setting.
The European Association of Urology Guidelines outlines that colonization with microorganisms is a special risk factor for urological procedures (http://uroweb.org/guideline/urological-infections/), and furthermore, that an indwelling catheter is one of the most important risk factors for complications.[14] Patients with asymptomatic bacteriuria who undergo traumatic genitourinary procedures associated with mucosal bleeding have a high rate of post-procedure bacteremia and sepsis. Bacteremia occurs in up to 60% of bacteriuric patients who undergo transurethral prostatic resection, and sepsis is clinically confirmed in 6% to 10% of these patients.[15] Retrospective analysis[16] and prospective, randomized clinical trials[17–19] support the effectiveness of antimicrobial treatment in preventing these complications in bacteriuric men undergoing transurethral resection of the prostate. There is little information relevant to other genitourinary procedures, but any intervention with a high probability of mucosal bleeding should be considered as a risk for post-procedure sepsis—and treatment of MRSA bacteriuria should be considered.
Some studies have documented that eradication of asymptomatic bacteriuria is not required before nonurologic procedures, such as arthroplasty[20,21]—but S. aureus is the most pathogenic of all Staphylococci[22] and this study did not include patients with MRSA bacteriuria.
Our study would suggest that when it comes to urine-colonizing MRSA eradication, or for treating a patient who is not critically unwell, oral therapy with Nitrofurantoin or Trimethoprim would be a suitable first-line agent, as none of the patients in our cohort were resistant to both Trimethoprim and Nitrofurantoin. In the unwell or septic patient, Vancomycin or Teicoplanin would be a suitable alternative.
Interestingly, less than one-third (32.5%) of our MRSA urine samples came from hospital inpatient sources, implying that MRSA bacteriuria diagnosis is more frequently a community-based phenomenon. National recommendations[8] dictate that hospital patients colonized with MRSA should be isolated in single rooms—and expert opinion would surmise that there is an increased risk of spread from MRSA-colonized catheterized patients—due to increased interventions and manipulations required from staff (e.g. changing catheter drainage devices). However, within the community, these patients are not recommended to be isolated as would happen within the nosocomial environment and this could contribute to further bacterial spread.
The limitations of our study include its retrospective nature—which meant that causation was not addressed, and not all key information was available to our research group. Also, acting as a potential confounder is the fact that MRSA bacteriuria is not a common pathology—meaning we had only small number of urine isolates (167 from 106 patients) despite the large sample size (425,013). To this author's knowledge, there are no prospective MRSA bacteriuria studies underway—and a supra-regional or national study of this type could provide further relevant data in this field.
Footnotes
Abbreviations: CSU = Catheter specimen urine, ED = Emergency Department, GP = General Practitioner, MRSA = Methicillin-resistant Staphylococcus aureus, MSSA = Methicillin-sensitive Staphylococcus aureus, MSU = Mid-stream urine, SA = Staphylococcus aureus, UTI = Urinary Tract Infection.
The authors report no conflicts of interest.
References
- [1].Orenstein R, Wong ES. Urinary tract infections in adults. Am Fam Physician 1999;59:1225–34. [PubMed] [Google Scholar]
- [2].Stamm WE. The epidemiology of urinary tract infections: risks factors reconsidered. Inter Sci Conf Antimicrob Agents Chemother 1999;39:769. [Google Scholar]
- [3].Khan SW, Ahmed A. Uropathogens and their susceptibility pattern: a retrospective analysis. J Pak Med Assoc 2001;51:98–100. [PubMed] [Google Scholar]
- [4].Nwanze PI, Nwaru LM, Oranusi S, et al. Urinary tract infection in Okada village: prevalence and antimicrobial susceptibility pattern. Sci Res Essay 2007;2:112–6. [Google Scholar]
- [5].Akortha EE, Ibadin OK. Incidence and antibiotic susceptibility pattern of Staphylococcus aureus amongst patients with urinary tract infection (UTIS) in UBTH Benin City, Nigeria. Afr J Biotechnol 2008;7:1637–40. [Google Scholar]
- [6].Aboderin OA, Abdu A, Odetoyin BW, et al. Antimicrobial resistance in Escherichia coli strains from urinary tract infections. J Natl Med Assoc 2009;101:1268–73. [DOI] [PubMed] [Google Scholar]
- [7].Todar K. Bacterial resistance to antibiotics. Todar's online textbook of bacteriology. 2011. [Last accessed on 2011 Jul 21]. Available from: http://www.textbookofbacteriology.net/resantimicrobial.html. [Google Scholar]
- [8].The Control and Prevention of MRSA in Hospitals and the Community SARI Infection Control Subcommittee; Strategy for the Control of Antimicrobial Resistance in Ireland (SARI). HSE, Health Protection Surveillance Centre (HPSC); 2005. Available from: http://www.lenus.ie/hse/handle/10147/76918. [Google Scholar]
- [9].Mehndiratta PL, Gur R, Saini S, et al. Staphylococcus aureus phage types and their correlation to antibiotic resistance. Indian J Pathol Microbiol 2010;53:738–41. [DOI] [PubMed] [Google Scholar]
- [10].Fridkin SK, Hageman JC, Morrison M, et al. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med 2005;352:1436–44. [DOI] [PubMed] [Google Scholar]
- [11].Chihara S, Popovich KJ, Weinstein RA, et al. Staphylococcus aureus bacteriuria as a prognosticator for outcome of Staphylo- coccus aureus bacteremia: a case-control study. BMC Infect Dis 2010;10:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lee BK, Crossley K, Gerding DN. The association between Staphylococcus aureus bacteremia and bacteriuria. Am J Med 1978;65:303–6. [DOI] [PubMed] [Google Scholar]
- [13].Al Mohajer M, Musher DM, Minard CG, et al. Clinical significance of Staphylococcus aureus bacteriuria at a tertiary care hospital. Scand J Infect Dis 2013;45:688–95. [DOI] [PubMed] [Google Scholar]
- [14].Bjerklund-Johansen TE, Naber K, Tenke P. The Paneuropean prevalence study on nosocomial urinary tract infections. European Association of Urology; March 24–27, 2004; Vienna, Austria. Available at: www.uroweb.org/peap. [Google Scholar]
- [15].Grabe M. Antimicrobial agents in transurethral prostatic resection. J Urol 1987;138:245–52. [DOI] [PubMed] [Google Scholar]
- [16].Cafferkey MT, Falkiner FR, Gillespie DM, et al. Antibiotics for the prevention of septicaemia in urology. J Antimicrob Chemother 1982;9:471–7. [DOI] [PubMed] [Google Scholar]
- [17].Grabe M, Forsgren A, Bjork T, et al. Controlled trial of a short and a prolonged course with ciprofloxacin in transurethral prostatic surgery. Eur J Clin Microbiol 1987;6:11–7. [DOI] [PubMed] [Google Scholar]
- [18].Olsen JH, Friis-Moller A, Jensen SK, et al. Cefotaxime for prevention of infectious complications in bacteriuric men undergoing transurethral prostatic resection: a controlled comparison with methenamine. Scand J Urol Nephrol 1983;17:299–301. [DOI] [PubMed] [Google Scholar]
- [19].Grabe M, Forsgren A, Hellsten S. The effect of a short antibiotic course in transurethral prostatic resection. Scand J Urol Nephrol 1984;18:37–42. [DOI] [PubMed] [Google Scholar]
- [20].Cordero-Ampuero J, González-Fernández E, Martínez-Vélez D, et al. Are antibiotics necessary in hip arthroplasty with asymptomatic bacteriuria? Seeding risk with/without treatment. Clin Orthop Relat Res 2013;471:3822–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sousa R, Muñoz-Mahamud E, Quayle J, et al. Is asymptomatic bacteriuria a risk factor for prosthetic joint infection? Clin Infect Dis 2014;59:41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Baron S. Medical Microbiology. 4th ed. Univ of Texas Medical Branch; (January 1996). [PubMed] [Google Scholar]


