Abstract
Periodontal disease (PD) is associated with various systemic diseases. We investigated the association between PD and age-related macular degeneration (AMD).
For this population-based, cross-sectional study, we enrolled 13,072 adults at least 40 years of age with gradable retinal fundus photographs and community periodontal index (CPI) data from the Korean National Health and Nutrition Examination Survey (KNHANES) (2008–2010 and 2012). Participants were divided into a middle age group (age ≤62 years) and old age group (age >62 years). PD was divided into 2 categories of mild and severe. Logistic regression analysis was used to evaluate the association between PD and AMD (early and late).
The prevalence of PD and AMD in the study population was 37.4% ± 0.8% and 5.6% ± 0.2%, respectively. Overall, there was no significant difference in the proportion of participants with PD between those with and without AMD. Only participants with AMD in the middle age group had more any PD than those without AMD (P = 0.031). Multivariate logistic regression model after adjusting for all confounding factors showed that PD was not significantly associated with AMD (odds ratio [OR] 1.03, 95% confidence interval [CI] 0.86–1.22). However, according to degree of PD, participants with severe PD in the middle age group were 1.61 times more likely to have AMD (OR 1.61, 95% CI 1.02–2.54).
Our data, collected from an Asian population, showed that only severe PD is independently associated with AMD in individuals aged 62 years or younger.
Keywords: age-related macular degeneration, epidemiology, inflammation, Korean, periodontal disease
1. Introduction
Age-related macular degeneration (AMD) is the major cause of blindness among the elderly in industrialized countries; however, the exact pathogenesis of AMD remains unknown.[1,2] Epidemiologic studies have suggested that factors such as old age, hypertension, low antioxidant levels, obesity, and systemic inflammation are risk factors of AMD.[3–7] In addition, 2 recent Korean-population based studies reported that hepatitis B surface antigen (HBsAg), anemia, and high-density cholesterol levels are also associated with AMD.[8,9] Various pathogenic mechanisms of AMD have been proposed such as oxidative stress, genetic susceptibility, and systemic inflammatory process. Different clinical characteristics of AMD between Asian and Caucasian populations have also been documented regarding prevalence, risk factors, type of disease, and genetic variability.
Periodontal disease (PD) is a common, chronic, and multifactorial inflammatory disease that frequently develops in middle age and is characterized by alveolar bone loss and the destruction of supporting tissue, leading to permanent tooth loss.[10] Recently, many researchers have reported that PD is associated with various systemic diseases such as cardiovascular disease (CVD), diabetes, albuminuria, preterm birth, and chronic obstructive pulmonary disease.[11–15] It has been hypothesized that oral pathogens infiltrate damaged tissue in the setting of periodontitis, subsequently entering systemic circulation and leading to an inflammatory response in other organs.[12] Especially, the association between atherosclerosis and PD is well known, and multiple reports have identified oral pathogens in atherosclerotic plaques.[12,16] According to previous studies, AMD and CVD share risk factors such as aging, smoking, and hypertension.[17] Considering the associations among CVD, AMD, and PD, Wagley et al[18] recently reported that PD is independently associated with AMD in patients aged 40 to 60 years based on the National Health and Nutrition Examination Survey (NHANES) III in the US; however, a similar study has not yet been performed in an Asian population. In this study, we investigated the association between PD and AMD in a representative Korean population using data from the Korea National Health and Nutrition Examination Survey (KNHANES).
2. Methods
2.1. Study population
The KNHANES is an ongoing population-based cross-sectional survey conducted in South Korea by the Division of Chronic Disease Surveillance, a division of the Korea Center for Disease Control and Prevention (KCDC) and the Korean Ministry of Health and Welfare. Initiated in 1998, KHANES has been completed every year since 2007, and ophthalmologic examinations have been included since the 2nd half of 2008. The KNHANES uses a complex, multistage, stratified, and probability clustered sampling method to analyze a representative, civilian, and noninstitutionalized South Korean population. The details of the KNHANES have been described previously.[19] The present study analyzed the data of the 2008 to 2010 and 2012 using the 4th and 5th KNHANES (2007–2009, KNHANES-IV[20]; 2010–2012, KNHANES-V[21]). Data from 2011 were excluded because the raw data from periodontal examinations were not disclosed at the initiation of research.
The KNHANES consists of 3 parts: a health interview survey, a health examination survey including ophthalmologic and periodontal examinations, and a nutritional survey. Without exception, all included participants underwent same examinations. All the tests, including the ophthalmic and periodontal examinations, were performed simultaneously in a same mobile examination unit. Ophthalmologic examinations were performed in a mobile examination unit by a trained ophthalmologist or ophthalmology resident. For retinal examination, nonmydriatic 45° color fundus photographs were obtained for each subject in a dark room using a digital fundus camera (TRC-NW6S, Topcon, Tokyo, Japan). Oral examinations of periodontal health were conducted by trained dentists using a dental mirror and a specially designed, lightweight community periodontal index (CPI) probe with 0.5-mm ball tip that met WHO guidelines.[22] For the present study, we obtained data regarding medical history and socioeconomic status using a set of structured questionnaires, as well as anthropometry investigations, blood tests, oral examinations, and ophthalmic surveys. Among 30,191 individuals that participated in the KNHANES between 2008 and 2010 and in 2012, participants who met the following inclusion criteria were included: age 40 years or older, underwent periodontal examinations, completed the ophthalmology survey, and had a gradable fundus photograph for at least 1 eye. Participants with missing data or unreliable examination results were excluded. This study was based on the same cohort and same dataset of previous studies.[8,9] Written informed consent was obtained from all KNHANES participants. The survey protocol was approved by the Institutional Review Board of the KCDC. The survey adhered to the tenets of the Declaration of Helsinki.
2.2. Diagnosis and grading of AMD
Detailed grading process was described in previous studies.[8,9] In summary, ocular fundus photographs were preliminarily graded for AMD by trained ophthalmologists using standardized protocols as defined by the Age-related Maculopathy Epidemiological Study Group grading system.[23] Detailed grading was later performed by 9 retina specialists with experience in grading AMD, who were verified by the Korean Ophthalmological Society and were masked to the patients’ characteristics. One retinal specialist resolved any interpreting discrepancy between the preliminary and detailed grades. According to the grading system, patients were defined as having early AMD if they met one of the following criteria: presence of soft indistinct drusen or reticular drusen and presence of hard or soft distinct drusen with pigmentary abnormalities in the absence of late AMD.[23] Drusen were classified on the basis of size, appearance, and edge sharpness.[23] Retinal pigmentary abnormalities were graded as hyper- or hypopigmentation of the retinal pigment epithelium (RPE).[23] Late AMD was defined as the presence of neovascularization or geographic atrophy.[23] Neovascular AMD was identified by the detachment of the RPE, serous detachment of the neurosensory retina, subretinal or sub-RPE hemorrhage, or subretinal fibrous scars.[23] Geographic atrophy was identified by a circular discrete area (≥175 μm in diameter) of retinal hypopigmentation with visible choroidal vessels in the absence of signs of neovascular AMD.[23] In this study, because of the low prevalence of late AMD, we defined “any AMD” as the presence of either early or late AMD for analysis, as described previously.[18] The quality of grading was verified by the Korean Ophthalmological Society, and the interrater reliability for AMD grading ranged from 90.2 to 96.6% (available at: https://knhanes.cdc.go.kr/knhanes/sub04/sub04_03_02.do?classType=8; accessed January 6, 2014).
2.3. Definition of periodontal disease
To ensure reliability of the periodontal health survey, an oral health examination was performed by public health dentists who were trained twice per year.[24] The WHO CPI was used to assess periodontal conditions[22] and defined PD as a CPI greater than or equal to a score of 3. The mouth was divided into sextants, 3 each in the maxillary and mandibular arches (maxillary right posterior, maxillary anterior, maxillary left posterior, mandibular right posterior, mandibular anterior, and mandibular left posterior). Periodontal tissues of permanent index teeth in each sextant (#18–14, #13–23, #24–28, #48–44, #43–33, and #34–38) were evaluated and included in the examination of bleeding upon the application of 20 g of pressure using a CPI probe, the presence of dental plaque, and the presence of periodontal pockets with measurable depths.[22] The CPI was scored on a scale of 0 to 4 as follows: 0 points for healthy periodontal tissue (no bleeding, calculus, or a pocket depth ≥4 mm); 1 point for bleeding on probing only (bleeding on probing but no calculus or pocket depth ≥4 mm); 2 points for periodontal tissue with plaques (supra- or subgingival calculus, no pocket depth ≥4 mm); 3 points for periodontal tissue with shallow periodontal pockets (pocket depth of 4–5 mm); and 4 points for periodontal tissue with deep periodontal pockets (pocket depth of ≥6 mm).[24] After assigning scores, the highest CPI score for the 6 sextants was selected. A score of 3 or 4 points was defined as presence of PD, while a score of 0 to 2 points was defined as absence of PD.[24] In the present study, a CPI score of 3 points was defined as mild PD and a score of 4 points was defined as severe PD.
2.4. Covariables
Demographic, socioeconomic, behavioral, and medical data were collected and selected as covariates. Demographic data included age and sex. Participants were divided into 2 age groups: a middle age group for patients 62 years or younger and an old age group for patients older than 62 years. Generally, 60 or 65 years are arbitrarily selected as the threshold for old age, although there is no exact definition. In the age range of 60 to 65 years old, we chose 62 years close to a median value between 60 and 65 as the threshold for old age. Socioeconomic data included education status and house income status. Education status was divided into 2 groups: participants with greater than a high school degree and those who had graduated from high school or less. Household income status was divided into upper and lower half groups. Participants were categorized into 1 of 2 smoking statuses: current smoker (a lifetime history of smoking more than 5 packs of cigarettes or a current smoker at the time of the interview) or nonsmoker (all categories of smoking other than current smoker). Medical data were obtained from the questionnaire or physical examination, which were performed by trained investigators following standardized procedures. Body mass index (BMI) was calculated as the ratio of weight/height2 (kg/m2), with body weight and height measured in light indoor clothing without shoes to the nearest 0.1 kg and 0.1 cm, respectively. Hypertension was defined as having a current prescription for antihypertensive medication or a systolic blood pressure >140 mm Hg and diastolic blood pressure >90 mm Hg measured on the right arm using a standard mercury sphygmomanometer (Baumanometer, Baum, Copiague, NY). Comorbid CVD status was assessed by combining self-reported physician diagnosis of myocardial infarction, stroke, or congestive heart failure. For laboratory tests, blood samples were collected after at least an 8-hour fasting period and were analyzed within 24 hours after transport to a certified laboratory. Hemoglobin and white blood cell counts were measured using an XE-2100D (Sysmex, Kobe, Japan), and participants with a hemoglobin level less than 13 g/dL in men and less than 12 g/dL in women were designated as anemic. Serum high-density lipoprotein (HDL) level (mg/dL) was measured using a Hitachi Automatic Analyzer 7600 (Hitachi, Tokyo, Japan). Hepatitis B antigen (HBsAg) was detected using an E-170 electrochemiluminescence immunoassay (Roche, Germany). Serum ferritin level was measured with a 1470 WIZARD gamma-Counter Immunoradiometric Assay (PerkinElmer, Finland).
2.5. Statistical analysis
Statistical analyses for the complex sampling design were performed by applying stratum variance estimates, stratification variables, and sampling weights in SPSS 21.0 Version (IBM, Armonk, NY). According to the statistical guidelines from the KCDC, survey sample weights were used in all analyses to produce a new integrated dataset from the 4-year data that were representative of the noninstitutionalized civilian Korean population. The baseline characteristics of the study participants were expressed as either weighted mean ± standard error (SE) for continuous variables or number and percentage (%) ± SE for categorical variables as appropriate according to age group and AMD status and were compared using Student t test or the Chi-square test, respectively. Stratified univariate and multivariate logistic regression analyses were used to estimate odds ratios (ORs) and 95% confidence intervals (CIs). The associations between AMD and PD status and other covariables were investigated by univariate logistic regression analysis. In multivariate regression models, the overall and age-stratified analyses were adjusted for all confounding factors (age, sex, education level, house income, smoking, hypertension, CVD, anemia, hepatitis B infection, serum HDL level, BMI, serum ferritin level, and white blood cell count) in order to examine the association between PD and AMD in each age group. Factors that yielded a P-value <0.05 were considered statistically significant.
3. Results
3.1. Baseline characteristics of enrolled participants
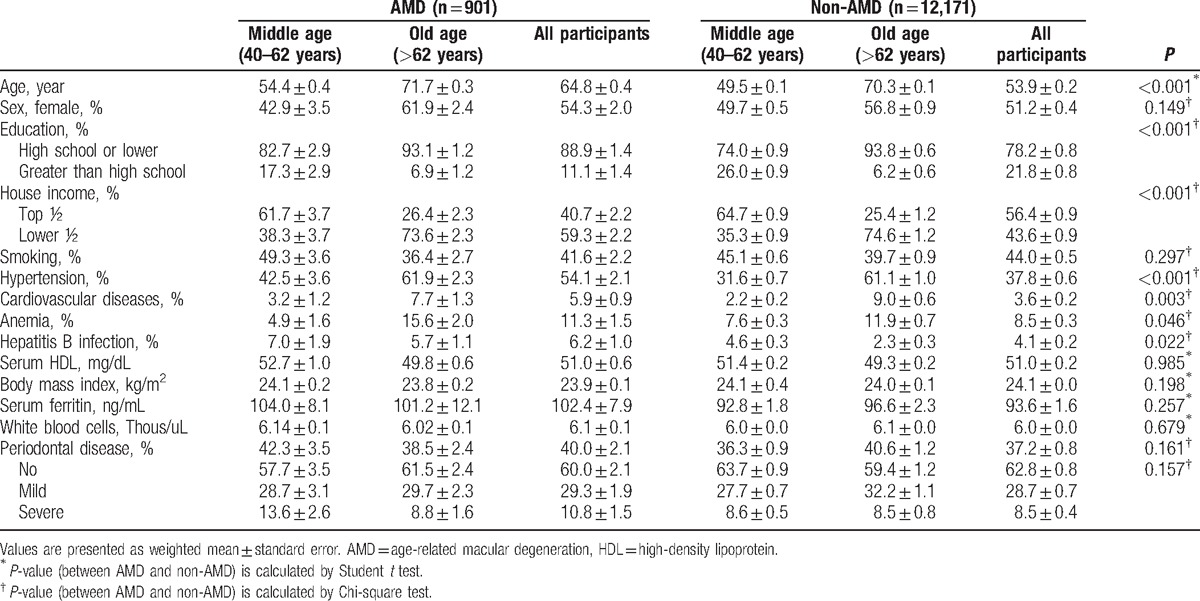
Of all the participants (30,191 participants) who completed the ophthalmic survey, 13,702 were eligible for inclusion in the present study (8616 participants aged ≤62 years and 4456 participants aged >62 years). We excluded 12,223 participants because they were younger than 40 years; had an unreadable fundus image of both eyes; or were missing interviews, dental exams, or laboratory data (Fig. 1). Table 1 shows the detailed demographic data of the enrolled participants. Overall, the mean age of all participants was 54.5 ± 0.2 years, and the prevalence of any AMD and PD was 5.6% ± 0.2% and 37.4% ± 0.8%, respectively. The mean age of participants with AMD was significantly higher (64.8 ± 0.4 years) than that of participants without AMD (53.9 ± 0.2 years) (P < 0.001), but no sex difference was observed (P = 0.149). Participants with AMD were more likely to have a lower household income (59.3% ± 2.2% vs 43.6% ± 0.9% in proportion of lower half, P < 0.001) and a lower education level (88.9% ± 1.4% vs 78.2% ± 0.4% in proportion of high school or less, P < 0.001) than participants without AMD. General medical conditions of presence of hypertension (54.1% ± 2.1% vs 37.8% ± 0.6%, P < 0.001), CVD (5.9% ± 0.9% vs 3.6% ± 0.2%, P = 0.003), anemia (11.3% ± 1.5% vs 8.5% ± 0.3%, P = 0.046), and hepatitis B infection (6.2% ± 1.0% vs 4.1% ± 0.2%, P = 0.022) were more frequent among participants with AMD than in those without. Other factors such as smoking, BMI, serum HDL level, WBC count, and serum ferritin level showed no difference between participants with or without AMD. There was also no significant difference in the presence of PD (40.0% ± 2.1% in participants with AMD vs 37.2% ± 0.8% in participants without AMD) between the 2 groups (P = 0.161). Table 2 shows demographic data stratified by age.
Figure 1.
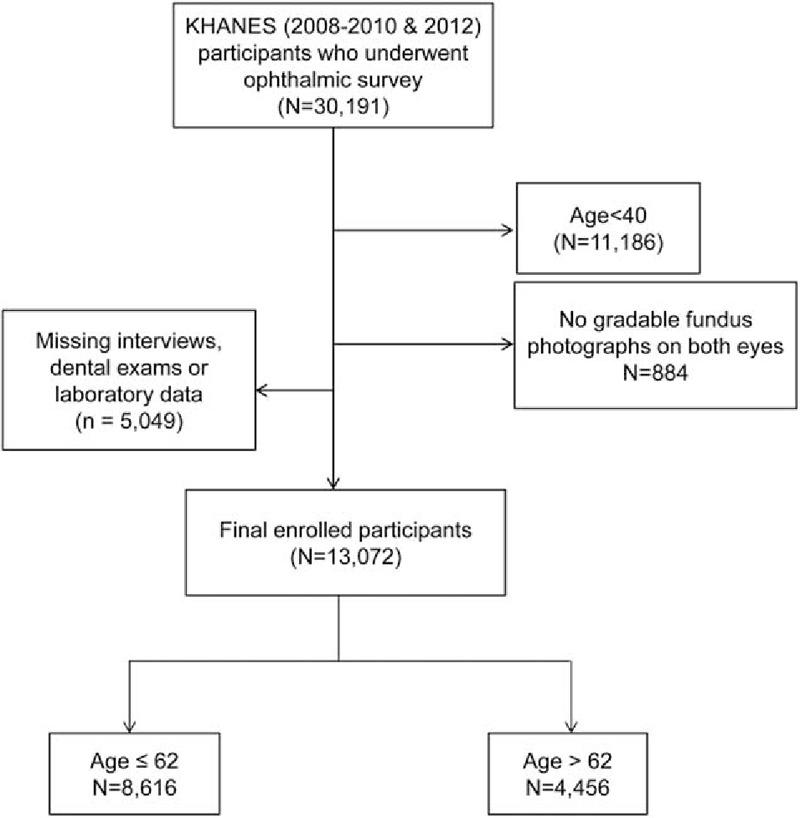
Flow chart of study participant selection. A total of 17,119 participants were excluded due to age <40 years, no gradable fundus image for both eyes, or missing survey data.
Table 1.
Demographics and clinical characteristics of all participants and according to age group.
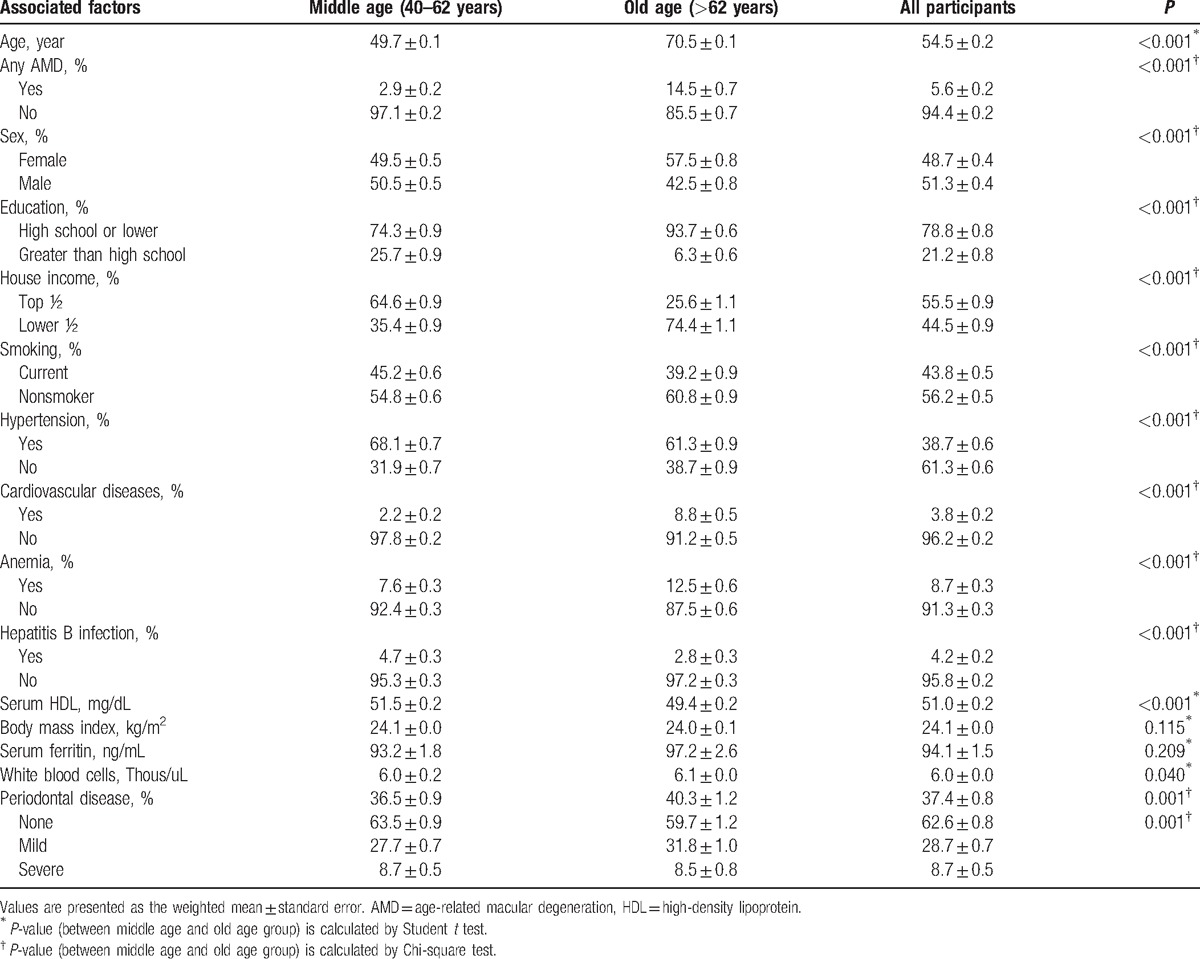
Table 2.
Comparison of demographic data between participants with and without age-related macular degeneration.
3.2. Association of periodontal diseases with age-related macular degeneration
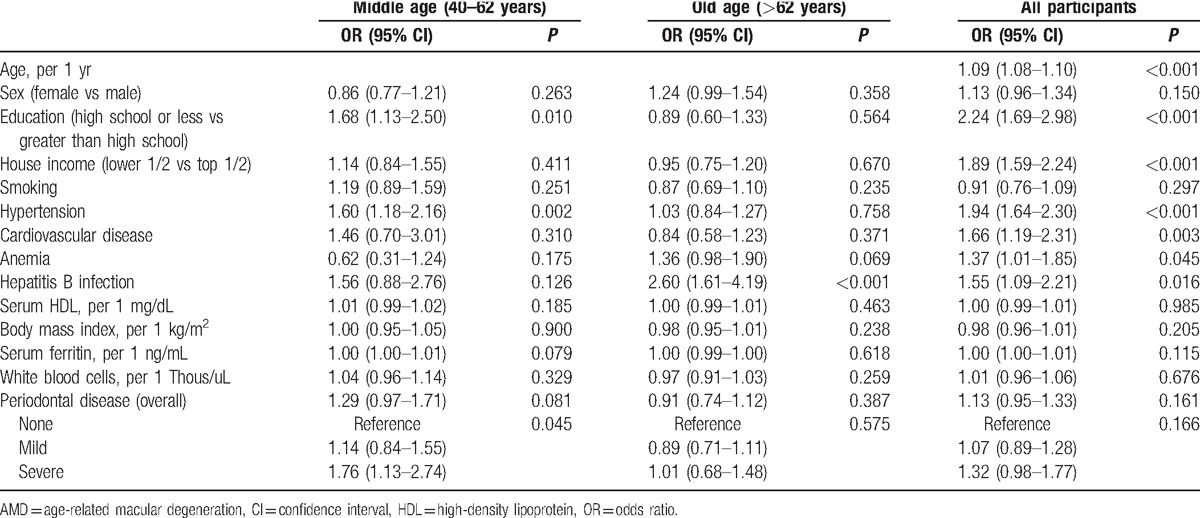
The proportion of any AMD in participants with PD is shown in Fig. 2. Univariate logistic regression analysis showed that old age (OR 1.09, 95% CI 1.08–1.10), low education level (OR 2.24, 95% CI 1.69–2.98), low income level (OR 1.89, 95% CI 1.59–2.24), presence of hypertension (OR 1.94, 95% CI 1.64–2.30), CVD (OR 1.66, 95% CI 1.19–2.31), and HBsAg (OR 1.55, 95% CI 1.09–2.21) were significantly associated with any AMD in all participants. In the middle age group (40–62 years), low education level (OR 1.68, 95% CI 1.13–2.50), hypertension (OR 1.60, 95% CI 1.18–2.16), and severe PD (OR 1.76, 95% CI 1.13–2.74) were significantly associated with any AMD. However, in the old age group (>62 years), only the presence of HBsAg was significantly associated with any AMD (OR 2.60, 95% CI 1.61–4.19) (Table 3).
Figure 2.
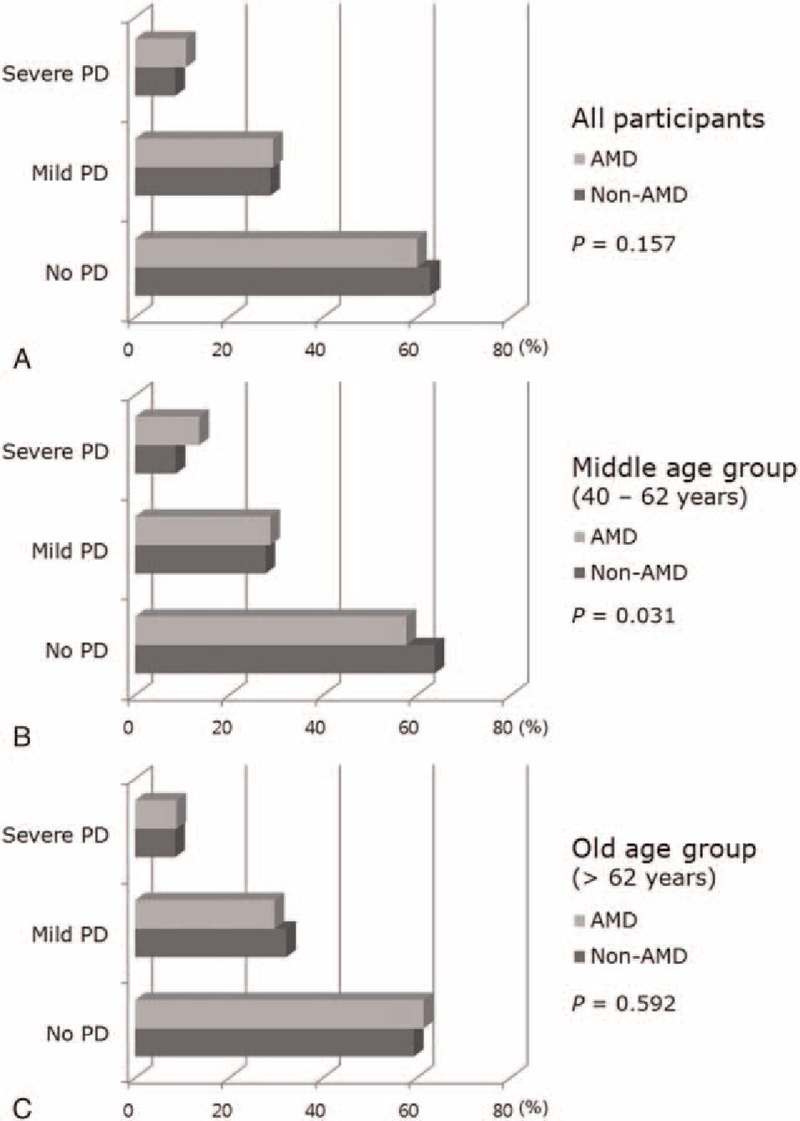
The proportion of periodontal diseases in participants with or without age-related macular degeneration (AMD) in all participants (A), in the middle-age group (40–62 years, B), and in the old-age group (>62 years, C).
Table 3.
Univariate logistic regression analyses for the association between factors and AMD in all participants and according to age group.
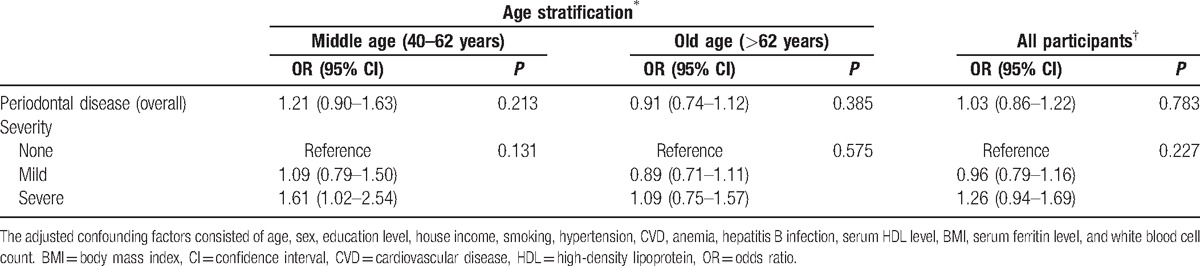
A multiple logistic regression analysis performed after adjusting for all confounding factors showed that overall PD was not significantly associated with any AMD (OR 1.03 95% CI 0.86–1.22). However, in the middle age group, severe PD was significantly associated with any AMD (OR 1.61, 95% CI 1.02–2.54) (Table 4).
Table 4.
Multivariate logistic regression analyses for the association between age-related macular degeneration and periodontal disease after adjusting for all confounding factors.
4. Discussion
In this population-based study, we assessed various risk factors of AMD in Korea. After adjusting for potential associated factors, we found that participants aged 40 to 62 years with severe PD were 1.6 times more likely to have any AMD compared to patients without PD, while no association between PD (both mild and severe) and any AMD was observed in participants over the age of 62.
Although the literature is limited, several studies have reported an association between PD and AMD, all of which were performed in Caucasian populations. Brzozowska and Puchalska-Niedbał[25] performed a small study in which they evaluated the relationship between oral health status and AMD and found that most patients with AMD had inflammatory conditions in the oral cavity, which were most commonly located in the periodontium. Karesvuo et al[26] also assessed the relationship between oral health conditions and AMD using a population-based survey data in Finland. They reported that alveolar bone loss was significantly more common in participants with AMD than in those without AMD, and that alveolar loss was with AMD in males after adjusting for confounding factors (OR 4.3, 95% CI 1.3–14.6). Their study had some limitations including a self-reported diagnosis of AMD, a small number of enrolled participants (1751 individuals ≥30 years old), and insufficient adjustment for confounding factors (age, smoking, and diabetes) associated with AMD. Recently, Wagley et al[18] performed a related research study using the NHANES III (US data, 5887 individuals ≥40 years old) and reported that PD is independently associated with AMD in patients aged 40 to 60 years (OR 1.96, 95% CI 1.22–3.14) after adjusting for potential risk factors, while the same association was not found in patients older than 60 years.
Unlike Wagley et al's study performed using NHANES data,[18] PD was not associated with AMD overall or according to age in our study. However, by classifying PD as mild and severe according to disease severity, we found a significant association between PD and AMD in participants aged 40 to 62 years. This difference may be due to the following reasons. First, the definition of PD was different between our study and that of Wagley et al. Specifically, Wagley et al[18] defined PD as >10% of sites with >3 mm of loss of attachment, which differed from our definition based on the presence of periodontal pockets with measurable depth. Second, the study by Wagley et al[18] used risk factors of demographic data (age, sex, and race), education and income level, smoking status, BMI, hypertension, history of CVD, and serum C-reactive protein (CRP) level. We included additional AMD risk factors, namely, hepatitis B antigen, anemia, and serum HDL level, for statistical analysis based on previous KHANES studies[8,19] in the present study. In contrast to the findings of Western epidemiological studies,[3–7] in the present study, being a hepatitis B carrier was associated with AMD in the KHANES cohort. As the prevalence of hepatitis B infection is relatively high in Asian countries, including Korea, previous researchers conjectured that cross-reactivity between the hepatitis B antigen and retinal S-antigen contributed the development of AMD.[8,9,27] Such cross-reactivity may partially explain the pathogenesis of AMD in Asian populations. Third, ethnic variations may have contributed to the difference in results. Indeed, a recent meta-analysis reported that the prevalence of early AMD in Asian persons is lower than in Caucasian persons, while the prevalence of late AMD is comparable.[28] Consistent with this report, the prevalence of early and late AMD in Wagley et al's study was 10.78% and 0.67%, respectively, whereas the prevalence of early and late AMD in our study was 5.2% and 0.5%, respectively, after applying our study criteria. Lending further support to the possibility of ethnic differences, another study suggested that polypoidal choroidal vasculopathy, a subtype of late AMD, is more common in Asian persons than in Caucasian persons (50% of wet AMD in an Asian population vs 8%–13% in a Caucasian population).[29]
PD, which is used as a synonym for chronic periodontitis, is a chronic inflammatory infectious disorder initiated by an infection (microbial biofilm of plaque) of the tissues that surround and support the teeth. Ongoing inflammation causes periodontal pockets between gums and teeth to become filled with plaque and bacteria, which then release cell wall products (endotoxins) to further activate inflammation. As this inflammation process progresses, deeper pockets can develop, eventually leading to permanent alveolar bone and tooth loss.[10]
Numerous published reports have suggested that PD is a risk factor of various systemic diseases.[11–16,30] There are currently 2 plausible biological mechanisms to explain the possible link between PD and systemic disease.[16,31] The 1st is a metastatic infection characterized by systemic spread of bacteria from a focal infection around periodontal tissue. In this way, bacteria can infiltrate into the systemic circulation via ulcerated gingival pockets and eventually adhere to extra-oral sites, leading to systemic events. The 2nd mechanism consists of systemic damage caused by an inflammatory cascade initiated in the oral cavity. Specifically, immunologic cells release proinflammatory cytokines against bacterial antigen entering into the systemic blood stream, resulting in low-grade chronic systemic inflammation that develops into nonoral organ diseases such as atherosclerosis and preterm birth. Lockhart et al[12] reported that oral pathogens have been identified in human atheromatous plaques, supporting the role of PD in the pathogenesis of atherosclerotic vascular diseases.
For the present study, we considered that PD may play a role in the development of AMD through similar mechanisms as described above. Specifically, inflammation initiated in the oral cavity may spread to systemic circulation, leading to a chronic inflammatory state that contributes to the development of AMD. Although the exact mechanism of AMD remains unknown, there has been increasing evidence that inflammation is one of the causative factors of AMD. Drusen formation, the hallmark of AMD, is reportedly induced through an inflammatory process, and according to histologic studies, several immune-related cells and proteins can be observed in drusen.[32] Inflammation is also involved in the development of choroidal neovascularization (CNV). Specifically, chronic inflammatory cells such as monocytes have been found in eyes with late AMD,[33] and these cell-mediated inflammatory processes are thought to attack Bruch membrane, leading to CNV. An abnormal complement system, such as dysfunction of complement regulatory molecules like complement factor H, has also been shown to cause AMD by increasing the immune response.[34] Furthermore, C-reactive protein (CRP), a marker of systemic inflammation, is associated with AMD.[35] Last, Kalayoglu et al[36] identified Chlamydia pneumoniae, an oral pathogen, within human choroidal neovascular membranes excised from patients with AMD.
Similar to previous studies,[18] we found that only patients aged 40 to 62 years exhibited a significant association between severe PD and AMD, indicating that inflammation may play a central role in the development of AMD in middle-aged patients. On the other hand, the absence of a significant relationship between severe PD and AMD in patients older than 62 may be due to the increasing influence of other age-associated factors diluting the effect of PD on AMD. Indeed, various factors such as oxidative stress, age-related degenerative changes, ischemia, and inflammation may be significantly involved in older patients. Thus, further research is warranted to clarify the role of inflammation in the development of AMD according to age.
A strength of our study was its status as the 1st population-based study performed in Asia to investigate the association between PD and AMD. The sample size was also relatively larger compared to a previous similar study.[18] In addition, the KNHANES is a government-initiated study, and all aspects of the survey were performed using a standardized protocol and well-trained examiners, which produced qualified and validated data from a representative Korean population. However, several issues and limitations should be considered when interpreting our data. First, because of the cross-sectional study design, we could not determine a cause-and-effect relationship between PD and AMD. Second, because serum CRP level was not collected as part of the KNHANES data, we used white blood cell count and serum ferritin level rather than CRP as markers of systemic inflammation.[37] Third, because of the prevalence of late AMD, we analyzed all cases of AMD (ie, early + late AMD) together rather than early and late AMD cases separately. Finally, in this study, PD was defined by CPI only. CPI has been used as an index for PD based on the association between PD and systemic diseases,[38,39] but this method can over- or under-estimate the prevalence of PD due to the use of representative teeth.[40] However, a validated method for defining PD is lacking in epidemiological studies; thus, the definition of PD in our study differed from that of the previous study.[18]
In this study, we found that only severe PD was associated with AMD in middle-aged patients. The differences between our results and those from Western reports may reflect ethnic differences in the pathogenesis of AMD. Our results support the possibility that poor oral heath can influence the development of AMD in an Asian population. Future longitudinal cohort studies and investigations regarding the relationship between periodontal care and reduced risk of AMD may provide more definitive evidence.
Acknowledgments
The authors thank the Epidemiologic Survey Committee of the Korean Ophthalmologic Society for conducting the KNHANES and supplying data for this study.
Footnotes
Abbreviations: AMD = age-related macular degeneration, BMI = body mass index, CPI = community periodontal index, CVD = cardiovascular disease, HBsAg = hepatitis B surface antigen, HDL = high-density lipoprotein, KCDC = Korea Center for Disease Control and Prevention, KNHANES = Korea National Health and Nutrition Examination Survey, KOS = Korean Ophthalmological Society, NHANES = National Health and Nutrition Examination Survey, PD = periodontal disease.
YUS and HWL contributed equally to this work.
Funding/support: This work was supported by the research fund of Hanyang University (201400000003108).
The authors have no conflicts of interest to disclose.
References
- [1].Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med 2008;358:2606–17. [DOI] [PubMed] [Google Scholar]
- [2].Pascolini D, Mariotti SP, Pokharel GP, et al. 2002 global update of available data on visual impairment: a compilation of population-based prevalence studies. Ophthalmic Epidemiol 2004;11:67–115. [DOI] [PubMed] [Google Scholar]
- [3].van Leeuwen R, Boekhoorn S, Vingerling JR, et al. Dietary intake of antioxidants and risk of age-related macular degeneration. JAMA 2005;294:3101–7. [DOI] [PubMed] [Google Scholar]
- [4].Seddon JM, George S, Rosner B, et al. Progression of age-related macular degeneration: prospective assessment of C-reactive protein, interleukin 6, and other cardiovascular biomarkers. Arch Ophthalmol 2005;123:774–82. [DOI] [PubMed] [Google Scholar]
- [5].Shankar A, Mitchell P, Rochtchina E, et al. Association between circulating white blood cell count and long-term incidence of age-related macular degeneration: the Blue Mountains Eye Study. Am J Epidemiol 2007;165:375–82. [DOI] [PubMed] [Google Scholar]
- [6].Tomany SC, Wang JJ, Van Leeuwen R, et al. Risk factors for incident age-related macular degeneration: pooled findings from 3 continents. Ophthalmology 2004;111:1280–7. [DOI] [PubMed] [Google Scholar]
- [7].Chakravarthy U, Wong TY, Fletcher A, et al. Clinical risk factors for age-related macular degeneration: a systematic review and meta-analysis. BMC Ophthalmol 2010;10:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Park SJ, Lee JH, Woo SJ, et al. Age-related macular degeneration: prevalence and risk factors from Korean National Health and Nutrition Examination Survey, 2008 through 2011. Ophthalmology 2014;121:1756–65. [DOI] [PubMed] [Google Scholar]
- [9].Cho BJ, Heo JW, Kim TW, et al. Prevalence and risk factors of age-related macular degeneration in Korea: the Korea National Health and Nutrition Examination Survey 2010–2011. Invest Ophthalmol Vis Sci 2014;55:1101–8. [DOI] [PubMed] [Google Scholar]
- [10].Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet 2005;366:1809–20. [DOI] [PubMed] [Google Scholar]
- [11].Chung JH, Hwang HJ, Kim SH, et al. Associations between periodontitis and chronic obstructive pulmonary disease; the 2010–2012 Korean National Health and Nutrition Examination Survey (KNHANES). J Periodontol 2016. 1–1. [DOI] [PubMed] [Google Scholar]
- [12].Lockhart PB, Bolger AF, Papapanou PN, et al. Periodontal disease and atherosclerotic vascular disease: does the evidence support an independent association?: a scientific statement from the American Heart Association. Circulation 2012;125:2520–44. [DOI] [PubMed] [Google Scholar]
- [13].Borgnakke WS, Ylostalo PV, Taylor GW, et al. Effect of periodontal disease on diabetes: systematic review of epidemiologic observational evidence. J Periodontol 2013;84:S135–52. [DOI] [PubMed] [Google Scholar]
- [14].Boutin A, Demers S, Roberge S, et al. Treatment of periodontal disease and prevention of preterm birth: systematic review and meta-analysis. Am J Perinatol 2013;30:537–44. [DOI] [PubMed] [Google Scholar]
- [15].Han K, Nam GE, Kim do H, et al. Association of periodontitis with urinary albumin excretion in Korean adults with diabetes: The 2012 Korea National Health and Nutrition Examination Survey. Medicine (Baltimore) 2015;94:e1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Nguyen CM, Kim JW, Quan VH, et al. Periodontal associations in cardiovascular diseases: the latest evidence and understanding. J Oral Biol Craniofac Res 2015;5:203–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Snow KK, Seddon JM. Do age-related macular degeneration and cardiovascular disease share common antecedents? Ophthalmic Epidemiol 1999;6:125–43. [DOI] [PubMed] [Google Scholar]
- [18].Wagley S, Marra KV, Salhi RA, et al. Periodontal disease and age-related macular degeneration: results from the National Health and Nutrition Examination Survey III. Retina 2015;35:982–8. [DOI] [PubMed] [Google Scholar]
- [19].Kweon S, Kim Y, Jang MJ, et al. Data resource profile: the Korea National Health and Nutrition Examination Survey (KNHANES). Int J Epidemiol 2014;43:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Uncu G, Avci R, Uncu Y, et al. The effects of different hormone replacement therapy regimens on tear function, intraocular pressure and lens opacity. Gynecol Endocrinol 2006;22:501–5. [DOI] [PubMed] [Google Scholar]
- [21].Altintaş Ö, Caglar Y, Yüksel N, et al. The effects of menopause and hormone replacement therapy on quality and quantity of tear, intraocular pressure and ocular blood flow. Ophthalmologica 2004;218:120–9. [DOI] [PubMed] [Google Scholar]
- [22].World Health Organization. Oral Health Surveys-Basic Methods. 4th ed.Geneva: World Health Organization; 1997. [Google Scholar]
- [23].Bird AC, Bressler NM, Bressler SB, et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group. Surv Ophthalmol 1995;39:367–74. [DOI] [PubMed] [Google Scholar]
- [24].Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980;288:373–6. [DOI] [PubMed] [Google Scholar]
- [25].Brzozowska A, Puchalska-Niedbal L. [Oral status as a potential source of infection in AMD patients – introduction]. Klin Oczna 2012;114:29–32. [PubMed] [Google Scholar]
- [26].Karesvuo P, Gursoy UK, Pussinen PJ, et al. Alveolar bone loss associated with age-related macular degeneration in males. J Periodontol 2013;84:58–67. [DOI] [PubMed] [Google Scholar]
- [27].Roh MI, Kim JH, Byeon SH, et al. Estimated prevalence and risk factor for age-related maculopathy. Yonsei Med J 2008;49:931–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kawasaki R, Yasuda M, Song SJ, et al. The prevalence of age-related macular degeneration in Asians: a systematic review and meta-analysis. Ophthalmology 2010;117:921–7. [DOI] [PubMed] [Google Scholar]
- [29].Laude A, Cackett PD, Vithana EN, et al. Polypoidal choroidal vasculopathy and neovascular age-related macular degeneration: same or different disease? Prog Retin Eye Res 2010;29:19–29. [DOI] [PubMed] [Google Scholar]
- [30].Marques CP, Maor Y, de Andrade MS, et al. Possible evidence of systemic lupus erythematosus and periodontal disease association mediated by Toll-like receptors 2 and 4. Clin Exp Immunol 2016;183:187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mawardi HH, Elbadawi LS, Sonis ST. Current understanding of the relationship between periodontal and systemic diseases. Saudi Med J 2015;36:150–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Klein R, Klein BE, Tomany SC, et al. Association of emphysema, gout, and inflammatory markers with long-term incidence of age-related maculopathy. Arch Ophthalmol 2003;121:674–8. [DOI] [PubMed] [Google Scholar]
- [33].Cousins SW, Espinosa-Heidmann DG, Csaky KG. Monocyte activation in patients with age-related macular degeneration: a biomarker of risk for choroidal neovascularization? Arch Ophthalmol 2004;122:1013–8. [DOI] [PubMed] [Google Scholar]
- [34].Thakkinstian A, Han P, McEvoy M, et al. Systematic review and meta-analysis of the association between complement factor H Y402H polymorphisms and age-related macular degeneration. Hum Mol Genet 2006;15:2784–90. [DOI] [PubMed] [Google Scholar]
- [35].Seddon JM, Gensler G, Milton RC, et al. Association between C-reactive protein and age-related macular degeneration. JAMA 2004;291:704–10. [DOI] [PubMed] [Google Scholar]
- [36].Kalayoglu MV, Bula D, Arroyo J, et al. Identification of Chlamydia pneumoniae within human choroidal neovascular membranes secondary to age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 2005;243:1080–90. [DOI] [PubMed] [Google Scholar]
- [37].Oh IH, Choi EY, Park JS, et al. Association of serum ferritin and kidney function with age-related macular degeneration in the general population. PLoS One 2016;11:e0153624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kwon YE, Ha JE, Paik DI, et al. The relationship between periodontitis and metabolic syndrome among a Korean nationally representative sample of adults. J Clin Periodontol 2011;38:781–6. [DOI] [PubMed] [Google Scholar]
- [39].Sharma P, Dietrich T, Ferro CJ, et al. Association between periodontitis and mortality in stages 3–5 chronic kidney disease: NHANES III and linked mortality study. J Clin Periodontol 2016;43:104–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kingman A, Albandar JM. Methodological aspects of epidemiological studies of periodontal diseases. Periodontol 20002002;29:11–30. [DOI] [PubMed] [Google Scholar]


