Abstract
Rationale:
Melanoma metastases to the pituitary adenoma (MMPA) are extremely rare, with only 1 reported case. To date, the melanoma metastasis to the existing prolactinoma has not been reported in literatures.
Patient concerns:
We report a case of 62-year-old woman presented with progressive visual disturbance and hyperprolactinemia. Magnetic resonance imaging demonstrated the presence of a round sellar mass.
Diagnoses:
Melanoma metastasis to the pituitary adenoma.
Interventions:
Surgery was performed and intraoperative frozensection examination found melanin granules and histopathological examination confirmed melanoma metastasis to the pituitary adenoma.
Outcomes:
After surgery, the patient developed widespread melanoma metastasis to lower limbs. Twenty-two months later, the patient was alive with worse symptoms.
Lessons:
We reviewed and analyzed the clinical data, imaging features, and treatment methods of other reported cases of metastases to pituitary adenoma (MPA). This study provides clinical information for the diagnosis and management of MMPA.
Keywords: diagnosis, melanoma metastasis to the pituitary adenoma, metastases to pituitary adenoma, treatment
1. Introduction
Melanoma has been the fastest-growing malignant tumor in incidence, annual rate of increase of which was 3% to 5%, but the mortality rate did not rise accordingly.[1] Many melanoma patients died of deterioration of multiple system metastases. It is known that metastatic melanoma has a high affinity to the brain. Thirty-nine percent of patients who died of melanoma showed brain metastasis, while the number who showed pituitary metastasis was less than 5%.[2] Involvement of the existing pituitary adenoma is distinctly rare and intractable. To date, the melanoma metastasis to the existing prolactinoma has not been reported in literatures. Therefore, little information is available regarding the clinical and imageology characteristics of melanoma metastasis to the pituitary adenoma (MMPA).
In this study, we present the first reported case of melanoma metastasis to the existing prolactinoma in a 62-year-old woman, who presented with progressive visual disturbance, headache, and hyperprolactinemia. In addition, we reviewed other known cases of metastases to pituitary adenoma (MPA). Our study provides important clinical information for diagnosis and management of MMPA.
2. Case report
In March 2015, a 62-year-old woman was admitted to our hospital. She complained of progressive visual disturbance, which began about 4 years ago and was treated as cataract in local hospital, but no relief was seen. On the contrary, the symptoms aggravated half a year ago, together with headache, left eye pain, tearing and increased secretions, and the computed tomography (CT) scan of the brain in local hospital showed a sellar region lesion. Besides, 2 years earlier, the patient underwent resection of melanoma in the left heel (T2N0M0, ki67 3–5%, Stage II), followed by resection of the recurred melanoma nearby the primary site 15 months later (T3N3M0, Stage III), without lymphadenectomy. She had no family history of melanoma.
On physical examination, the patient had bilateral temporal hemianopsia, the right finger counting was 1 m, and the left finger counting was no more than 0.5 m. Enlarged lymph nodes were palpable in the right groin. On ophthalmologic examination, the patient had right vision of 0.4 and left vision of 0.08, with the same intraocular pressure 15 mm Hg bilaterally. The optometry found the right eye of +6.00DS/+0.25DC∗65° and the left eye of +6.25DS/+0.50DC∗20°. The patient had maculopathy of both eyes and optic atrophy of the left eye. Light reflex and eye movement of both eyes were normal. CT scans of the brain parenchyma, orbital, and chest were unremarkable. CT scan and ultrasound examination of the abdomen showed hepatic portal and retroperitoneal lymphadenectasis and enlarged left lobe of the liver with substantial placeholder lesions. Ultrasound examination of bilateral inguinal lymph nodes discovered multiple low echo light groups, the largest of which was 31 mm in diameter, with hilus of the echo and asymmetrical thickening of the skin. CT scan of sellar region revealed a crumby mass, protruding out of the sphenoid sinus, with obscure boundary and bone destruction. And the average CT value of the mass was 46 HU. Sellar region magnetic resonance imaging (MRI) revealed a round mass of 30 mm in diameter in the enlarged sellae (Fig. 1A, B). The mass showed isointense in T1-weighted images (T1-WI) and T2-weighted images (T2-WI), with homogeneous enhancement after Gadolinium-DTPA injection, and dural tail sign was seen. Small foci inside the tumor showed hyperintense signals in T1-WI and hypointense signals in T2-WI, without enhancement. And it was seen that the mass penetrated meninges, surrounded the left internal carotid artery, and was blurred with the left optic nerve. Pituitary stalk became shorter with a right displacement. Laboratory findings revealed increased levels of prolactin (119.08 μg/L, normal range 5.99–30.04 μg/L) and cortisol (677.10 nmol/L, normal range 118.60–618.00 nmol/L) and decreased levels of free thyroxine (FT4) (6.04 pmol/L, normal range 12.00–22.00 pmol/L) and free triiodothyronine (FT3) (2.09 pmol/L, normal range 3.50–6.50 pmol/L). The patient was diagnosed with a giant prolactinoma.
Figure 1.
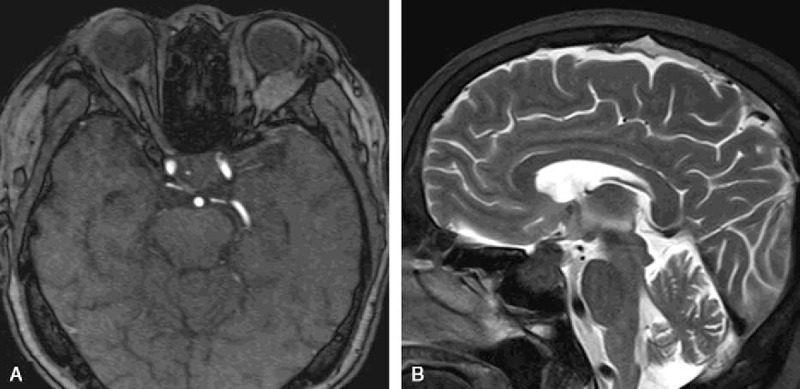
MRI findings: coronal T1-WI (A) and sagittal T2-WI (B) of MRI revealed a round mass in the enlarged sellae.
The patient underwent transnasal transsphenoidal surgery to remove the tumor and relieve the compression of the optic nerve. Intraoperatively, it was seen that the tumor invaded and filled the left interval of the sphenoid sinus, and part of bone in sellar floor and left side parasellar was destroyed and absorbed. A little normal pituitary tissue was seen in the top right of tumor in the sellar turcica. The tumor was reddish black with extremely rich blood supply and had close adhesion to the surrounding structure. The texture in the center of the tumor was soft and much tougher over the rim. Intraoperative frozen-section examination found melanin granules, and it was considered to be malignant melanoma or meningioma. The tumor cells were composed of eosinophilic staining epithelial cells. Most of cell nuclei were round, a few were reniform and hippocrepiform with evident nucleoli, and nuclear fission was seen. The tumor showed no evidence of necrosis (Fig. 2). The tumor was immunopositive focally for melanoma-specific markers such as S-100, HMB45, and Vimentin, and immunopositive for neuroendocrine tumor markers such as CgA and Syn (Fig. 3). The Ki67 index was 3% to 5%; it was higher in metastatic melanoma than in the adenomatous component. Taken the melanoma history and suspected lymph node and hepatic metastasis into consideration, the patient was diagnosised with MMPA. After surgery, significant relief was seen in visual field and headache, and the level of prolactin, cortisol, and FT4 returned to normal with hormone replacement therapy. Because the focal liver lesions and lymphadenectasis did not cause much discomfort, the patient refused any further surgical intervention or other treatment. She was discharged from the hospital immediately and was disease free until 2 months after the third surgery. The patient successively found new melanoma metastatic sites in the skin of lower left leg, knees, the upper left leg, the left groin and the right groin, and the right leg. At the follow-up in late January 2016, the patient could not walk and live by herself and was depressed. At the latest follow-up, in late January, 2017, the patient was alive with worse symptoms, she had sensory deficits of both legs, which could not move, hyperalgesia of hands and mouth, impaired intelligence, but she lived well with the disease by careful nursing of her daughter.
Figure 2.
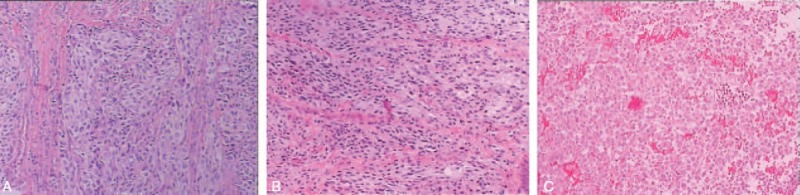
Hematoxylin and eosin (H&E, 100×) staining of resected tumor in the first (A), second (B), and third (C) surgery.
Figure 3.
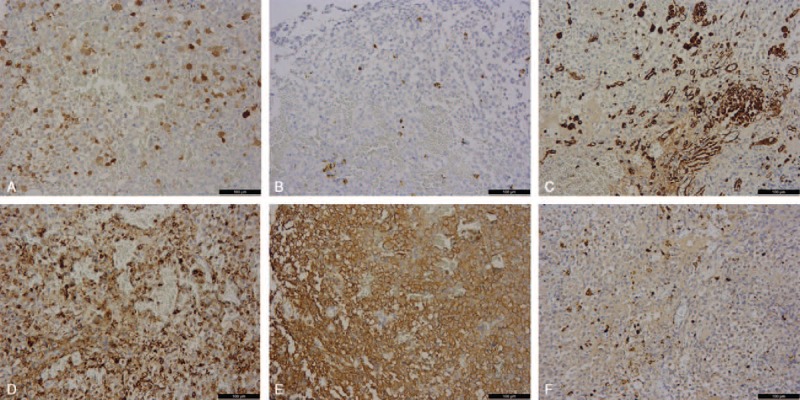
Immunohistochemical staining of the pituitary tumor showed both melanoma biomarker of S-100 (A), HMB45 (B), and Vimentin (C), and pituitary adenoma biomarkers of CgA (D) and Syn (E). The Ki67 (F) index was shown as 3% to 5%.
This study was approved by the Second Affiliated Hospital of Dalian Medical University. The patient provided informed consent.
3. Discussion
Melanoma metastasis to the pituitary gland (MMP) is rare, and MMPA is much rarer. It is known that metastasis is one of the properties of malignant melanoma. Metastatic melanoma cells almost can reach any human organ by lymphatic or blood circulation system. The most common organ involved is lung, followed by liver, small intestine, pancreas, adrenal, heart, kidney, thyroid, and brain. Melanoma metastasis to the brain is not uncommon, while pituitary involvement is rare. To our knowledge, there are no more than 20 cases of pituitary metastatic melanoma reported. The mean age of the patients was 46 years.[3,4] The common presentations of pituitary metastatic melanoma include headache, extraocular cranial nerve palsy, and diabetes insipidus. The majority of the cases suggested metastatic melanoma in the posterior pituitary, while a small part of cases in the anterior pituitary. This involves 3 different metastatic ways. First, melanoma cells metastasize to the posterior pituitary via the inferior hypophyseal artery, which provide direct blood supply to the posterior pituitary, and then invade the anterior pituitary. Second, melanoma cells pass through the unsound blood–brain barrier of the adenohypophysis and trigonum paraplexus, and settle in the pituitary. Third, melanoma cells disseminate through lymphatic microvessels and flourish in the pituitary.[5,6] The reason why the pituitary has an affinity for melanoma cells may be that both the pituitary and the melanoma cells produce proopiomelanocortin (POMC), alpha-melanocyte stimulating hormone (α-MSH), and α-MSH receptor, the levels of which in melanoma patients are much higher than healthy people.[7,8] POMC gives rise to α-MSH, and α-MSH regulates the production of melanin. POMC expressed in pituitary adenoma is much higher than the pituitary.[9,10] Accordingly, it is presumed to be that tumors have a propensity to metastasize to pituitary adenoma.
Metastases of circulating tumor cells to remote organs have to overcome many obstacles, including infiltrating the distal tissue, avoiding the immune defense, and adapting to the local environment, and then settle down and flourish and take the place of the host cells. Because of this, efficiency of metastases is not high.[11] To date, there is only 1 case reported regarding melanoma metastasis to pituitary adenoma (Table 1)[12] and 26 cases of different tumors metastatic to pituitary adenoma (Table 2).[13–34] Tumor metastasis to tumor is rare in clinical practice and much more intractable, because it is usually accompanied by multiple metastases. The mean age of the MPA and MMPA patients was 67 and 68.5 years, respectively, much older than MMP. The common aggressive high-grade seed tumors[35] most often metastasize to pituitary adenoma are lung cancer in man, breast cancer in woman, followed by tumor from kidney, stomach, pancreas, colorectum, and pelvis. And the common receptor pituitary adenomas are nonfunctional adenoma, prolactinoma, and FSH/LH adenoma (Tables 1 and 2). Pituitary adenomas usually possess direct arterial supply from carotid and capsular vessels, which promote the development of metastasis. As in our case, the tumor surrounded the internal carotid and had rich blood supply. To the best of our knowledge, ours was the first reported case of melanoma metastasis to an already existing enlarged prolactinoma. It was suggested that the prolactinoma began to develop since 4 years ago, when the visual disturbance began to deteriorate. Together with lymph nodes metastases, melanoma cells settled in the prolactinoma via direct blood supply or via blood and lymph circulation in the meninges nearby the sella.
Table 1.
Clinical characteristics of 2 cases of MMPA.
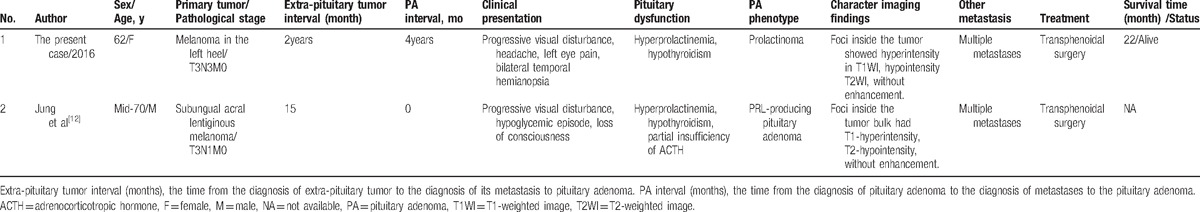
Table 2.
Clinical characteristics of 26 cases of MPA.
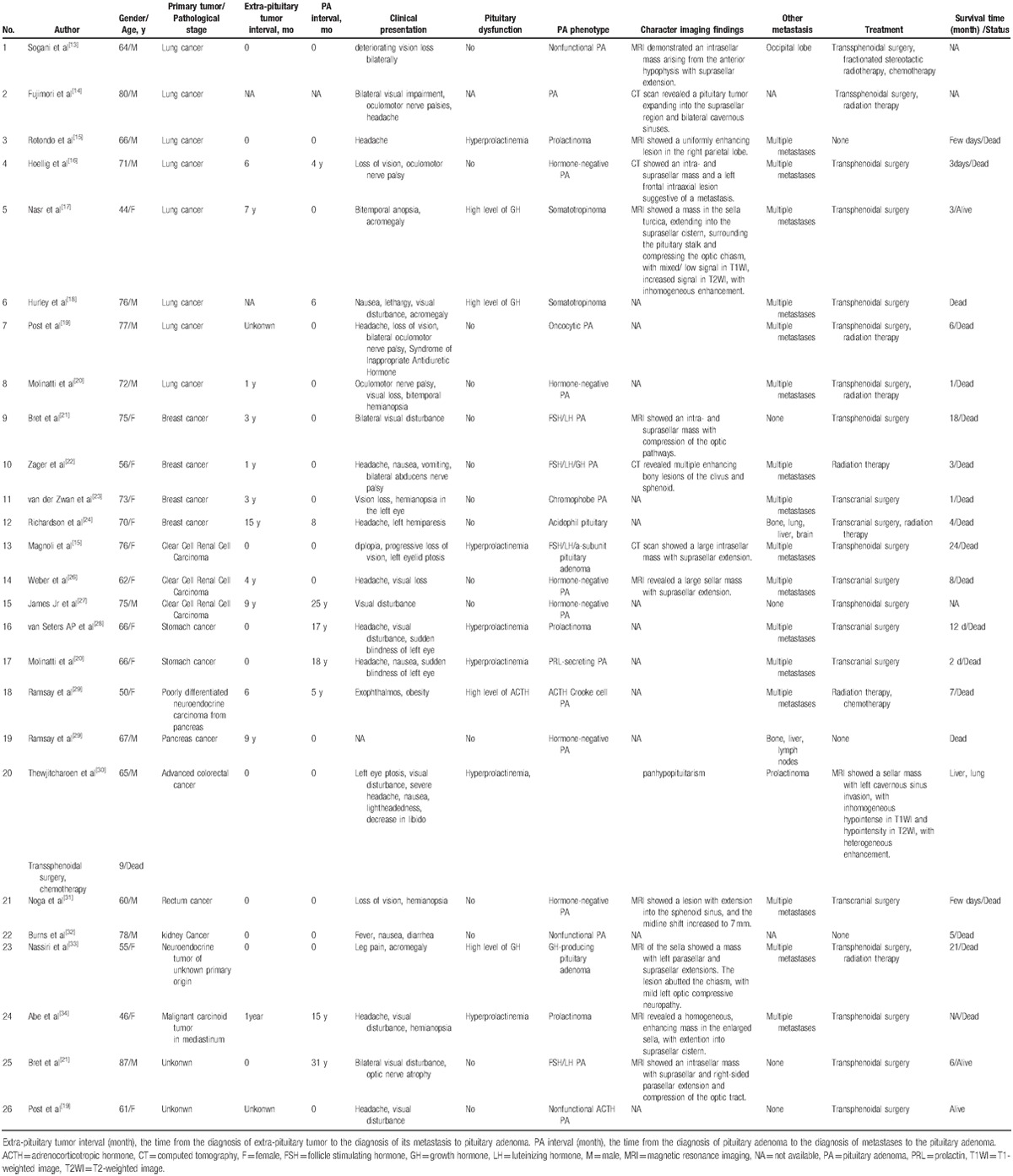
Visual disturbance, headache, cranial nerves palsy were most common seen in metastases to pituitary adenoma (MPA). Most of the pituitary adenomas in the cases were clinically nonfunctioning. Functional pituitary adenomas included prolactinoma, somatotropinoma. Pituitary metastases and MPA usually cause hypopituitarism, as we can conclude that the anterior pituitary and posterior pituitary are usually involved in MPA. In the present case and the case of melanoma metastasis to the pituitary oncocytoma, both patients presented progressive visual disturbance, hyperprolactinemia, and slight hypothyroidism (Table 1). MMPA led to the rapid enlarging sella mass and deterioration of the situation, probably because the melanoma cells and the products could irritate the enlargement of the prolactinoma and deteriorate the optic compression.
Imaginology examinations are helpful in preoperative discovery of MMPA. Intracranial melanoma metastasis is usually present in a single site. CT scan mostly shows round, homogeneous, high-density signal, usually with adjacent bone destruction. Heterogeneous and low-density signal can also be seen. The MRI findings generally change with the content of melanin and intratumoral hemorrhage. First, melanotic metastasis usually presents as hyperintense in T1-weighted images (T1-WI) and hypointense in T2-weighted images (T2-WI). Second, nonmelanotic metastasis usually presents as hypo- or iso-intense in T1-WI and hyper- or iso-intense in T2-WI. Third, the mixed type usually presents as heterogeneous signal. Fourth, the hematoma type usually has complex presentation as the hematoma develop.[36] Notably, as shown in our case, the hyperintense signals of posterior pituitary disappeared in T1-WI and pituitary stalk became thicker with a displacement, probably due to the invasion of the posterior pituitary by melanoma. As in our case, the T1-WI revealed iso-intense signal and focal hyperintense signal and T2-WI revealed isointense and focal hypointense signals, and dural tail sign was also seen. Melanin in MRI usually shows hyperintense signal in T1WI and hypointense in T2WI[37] (Table 1). However, in the early disease progression, the melanin in the metastatic sites could be very little and ignored by doctors. The differential diagnosis includes invasive pituitary adenoma, craniopharyngioma, and meningioma. Combination of imaginology findings with clinical findings and disease history can help to diagnose MMPA preoperatively.
Traditional treatment, including surgery, radiotherapy, chemotherapy, has an important role in the management of MPA patients, but they are suggested to be palliative.[3] Total resection of the tumor is often very difficult because of the invasive growth, close adhesion with optic nerves, and rich blood supply. Besides, the metastatic melanoma is not sensitive to radiation therapy and chemotherapy.[38] The prognosis is poor in reality. The median survival time after the diagnosis of MPA was 8.2 months. Patients with sole metastasis limited to the pituitary adenoma had a median survival of 12 months versus 7.7 months of patients with multiple metastases (Tables 1 and 2). As shown in our case, the survival time after surgery is more than 22 months; we believe that early diagnosis and total resection as much as possible may slow down the disease progression and have an influence on survival. Nowadays, some new adjuvant therapies and drugs have been introduced to treat advanced melanoma. For example, high-dose interferon therapy, ipilimumab in targeted immunotherapy, BRAFv600 inhibitor vemurafenib, KIT inhibitor imatinib, and T-EC and PV-10 can induce local tumor ablation and tumor cell immune.[1,39] It is suggested that patients with metastatic melanoma who received adjuvant therapies had better survival than those who did not. Notably, emotional support, social support, and stress management have a positive effect on patients’ body and mind and help provide better survival.[40] As metastasis is the key of the intractable situation, it is advisable that cell track which can capture and isolate circulating tumor cells from peripheral blood will play a great role in future in routine monitoring of cancer development and therapy,[41] including the management of MMP, MPA, and MMPA.
In conclusion, we present a rare case of MMPA. Together with the increasing number of patients with melanoma and longer survival time, the number of patients with melanoma metastases and MMPA is likely to increase. The presentation of MMPA usually mimics pituitary adenoma, but MMPA should be taken into consideration in patients over 60 years old, with a history of pituitary adenoma and melanoma, especially the multiple metastatic melanoma, and had rapidly aggravated visual disturbance, and characteristic manifestation of melanin in MRI. Combination of imaginology findings with clinical findings and disease history is of great importance for preoperative diagnose of MMPA. Traditional treatments followed by adjuvant therapies including high-dose interferon therapy, targeted immunotherapy, mutated gene inhibitors may be helpful for improving the survival of patients with MMPA.
Footnotes
Abbreviations: CT = computed tomography, MMPA = melanoma metastasis to the pituitary adenoma, MPA = metastasis to pituitary adenoma, MRI = magnetic resonance imaging.
CY and LL, and BZ and XL contributed equally.
Funding/support: This study was funded by The National Natural Science Foundation of China (NSFC) (Grant No. 81372714); the Natural Science Fund Project in Liaoning Province (Grant No. 2015020571).
The authors declare no conflict of interest with this work.
References
- [1].Guo J, Qin S, Liang J, et al. Chinese Guidelines on the Diagnosis and Treatment of Melanoma (2015 Edition). Ann Transl Med 2015;3:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dasgupta T, Brasfield R. Metastatic melanoma: a clinicopathological study. Cancer 1964;17:1323–39. [DOI] [PubMed] [Google Scholar]
- [3].McCutcheon IE, Waguespack SG, Fuller GN, et al. Metastatic melanoma to the pituitary gland. Can J Neurol Sci 2007;34:322–7. [DOI] [PubMed] [Google Scholar]
- [4].Leung GK, Chow WS, Tan KC, et al. Metastatic melanoma of the pituitary gland. Case report. J Neurosurg 2003;99:913–5. [DOI] [PubMed] [Google Scholar]
- [5].Yang C, Zhang H, Zhang S, et al. Oculomotor paralysis, postorbital pain, and hypopituitarism as first presentations of metastatic gastric cancer in the pituitary flourished by internal carotid aneurysm: a case report. Medicine (Baltimore) 2015;94:e2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Louveau A, Smirnov I, Keyes TJ, et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015;523:337–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Funasaka Y, Sato H, Chakraborty AK, et al. Expression of proopiomelanocortin, corticotropin-releasing hormone (CRH), and CRH receptor in melanoma cells, nevus cells, and normal human melanocytes. J Investig Dermatol Symp Proc 1999;4:105–9. [DOI] [PubMed] [Google Scholar]
- [8].Williams RR. Breast and thyroid cancer and malignant melanoma promoted by alcohol-induced pituitary secretion of prolactin, T.S.H and M. S. H. Lancet 1976;1:996–9. [DOI] [PubMed] [Google Scholar]
- [9].Chan JS, Gaspar L, Iguchi H, et al. Synergistical effects of ovine corticotropin-releasing factor (CRF) and arginine vasopressin (AVP) on the release of pro-opiomelanocortin (POMC) related peptides by pituitary adenoma of a patient with Nelson's syndrome in vitro. Clin Invest Med 1984;7:205–8. [PubMed] [Google Scholar]
- [10].Cassarino MF, Sesta A, Pagliardini L, et al. AZA-deoxycytidine stimulates proopiomelanocortin gene expression and ACTH secretion in human pituitary ACTH-secreting tumors. Pituitary 2014;17:464–9. [DOI] [PubMed] [Google Scholar]
- [11].Massagué J, Obenauf AC. Metastatic colonization by circulating tumour cells. Nature 2016;529:298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jung SM, Hsu YY, Chuang CC, et al. A man in his mid-70 s with a sellar mass. Brain Pathol 2007;17:115–6. 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sogani J, Yang W, Lavi E, et al. Sellar collision tumor involving metastatic lung cancer and pituitary adenoma: radiologic-pathologic correlation and review of the literature. Clin Imaging 2014;38:318–21. [DOI] [PubMed] [Google Scholar]
- [14].Fujimori T, Okauchi M, Shindo A, et al. Intrapituitary adenoma metastasis from lung cancer with progressive cranial nerve palsies: a case report and literature review. No Shinkei Geka 2014;42:943–9. [DOI] [PubMed] [Google Scholar]
- [15].Rotondo F, Kovacs K, Macdonald RL, et al. Non-small cell bronchial carcinoma metastasizing into a prolactin producing pituitary adenoma. Int J Surg Pathol 2013;21:68–71. [DOI] [PubMed] [Google Scholar]
- [16].Hoellig A, Niehusmann P, Flacke S, et al. Metastasis to pituitary adenoma: case-report and review of the literature. Cent Eur Neurosurg 2009;70:149–53. [DOI] [PubMed] [Google Scholar]
- [17].Nasr C, Mason A, Mayberg M, et al. Acromegaly and somatotroph hyperplasia with adenomatous transformation due to pituitary metastasis of a growth hormone-releasing hormone-secreting pulmonary endocrine carcinoma. J Clin Endocrinol Metab 2006;91:4776–80. [DOI] [PubMed] [Google Scholar]
- [18].Hurley TR, D’Angelo CM, Clasen RA, et al. Adenocarcinoma metastatic to a growth-hormone-secreting pituitary adenoma: case report. Surg Neurol 1992;37:361–5. [DOI] [PubMed] [Google Scholar]
- [19].Post KD, McCormick PC, Hays AP, et al. Metastatic carcinoma to pituitary adenoma. Report of two cases. Surg Neurol 1988;30:286–92. [DOI] [PubMed] [Google Scholar]
- [20].Molinatti PA, Scheithauer BW, Randall RV, et al. Metastasis to pituitary adenoma. Arch Pathol Lab Med 1985;109:287–9. [PubMed] [Google Scholar]
- [21].Bret P, Jouvet A, Madarassy G, et al. Visceral cancer metastasis to pituitary adenoma: report of two cases. Surg Neurol 2001;55:284–90. [DOI] [PubMed] [Google Scholar]
- [22].Zager EL, Hedley-Whyte ET. Metastasis within a pituitary adenoma presenting with bilateral abducens palsies: case report and review of the literature. Neurosurgery 1987;21:383–6. [DOI] [PubMed] [Google Scholar]
- [23].van der Zwan A, Luyendijk W, Bots GT. Metastasis of mammary carcinoma in a chromophobe adenoma of the hypophysis. Psychiatr Neurol Neurochir 1971;74:369–77. [PubMed] [Google Scholar]
- [24].Richardson JF, Katayama I. Neoplasm to neoplasm metastasis. An acidophil adenoma harbouring metastatic carcinoma: a case report. Arch Pathol 1971;91:135–9. [PubMed] [Google Scholar]
- [25].Magnoli F, Finzi G, Riva C, et al. Renal cell carcinoma metastatic to a pituitary FSH/LH adenoma: case report and review of the literature. Ultrastruct Pathol 2014;38:430–7. [DOI] [PubMed] [Google Scholar]
- [26].Weber J, Gassel AM, Hoch A, et al. Concomitant renal cell carcinoma with pituitary adenoma. Acta Neurochir (Wien) 2003;145:227–31. [DOI] [PubMed] [Google Scholar]
- [27].James RL, Jr, Arsenis G, Stoler M, et al. Hypophyseal metastatic renal cell carcinoma and pituitary adenoma. Case report and review of the literature. Am J Med 1984;76:337–40. [DOI] [PubMed] [Google Scholar]
- [28].van Seters AP, Bots GT, van Dulken H, et al. Metastasis of an occult gastric carcinoma suggesting growth of a prolactinoma during bromocriptine therapy: a case report with a review of the literature. Neurosurgery 1985;16:813–7. [DOI] [PubMed] [Google Scholar]
- [29].Ramsay JA, Kovacs K, Scheithauer BW, et al. Metastatic carcinoma to pituitary adenomas: a report of two cases. Exp Clin Endocrinol 1988;92:69–76. [DOI] [PubMed] [Google Scholar]
- [30].Thewjitcharoen Y, Shuangshoti S, Lerdlum S, et al. Colorectal cancer manifesting with metastasis to prolactinoma: report of a case involving symptoms mimicking pituitary apoplexy. Intern Med 2014;53:1965–9. [DOI] [PubMed] [Google Scholar]
- [31].Noga C, Prayson RA, Kowalski R, et al. Metastatic adenocarcinoma to a pituitary adenoma. Ann Diagn Pathol 2001;5:354–60. [DOI] [PubMed] [Google Scholar]
- [32].Burns WA, Kadar AT. Unusual metastases from a transitional-cell carcinoma of the renal pelvis and ureter. Med Ann Dist Columbia 1973;42:65–6. [PubMed] [Google Scholar]
- [33].Nassiri F, Cusimano M, Rotondo F, et al. Neuroendocrine tumor of unknown origin metastasizing to a growth hormone-secreting pituitary adenoma. World Neurosurg 2012;77:201.e9–12. [DOI] [PubMed] [Google Scholar]
- [34].Abe T, Matsumoto K, Iida M, et al. Malignant carcinoid tumor of the anterior mediastinum metastasis to a prolactin-secreting pituitary adenoma: a case report. Surg Neurol 1997;48:389–94. [DOI] [PubMed] [Google Scholar]
- [35].Brown HM, McCutcheon IE, Leeds NE, et al. Melanoma metastatic to central neurocytoma: a novel case of tumor-to-tumor metastasis. J Neurooncol 2003;61:209–14. [DOI] [PubMed] [Google Scholar]
- [36].Yamamoto J1, Kakeda S, Shimajiri S, et al. Tumor consistency of pituitary macroadenomas: predictive analysis on the basis of imaging features with contrast-enhanced 3D FIESTA at 3T. AJNR Am J Neuroradiol 2014;35:297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Atlas SW, Grossman RI, Gomori JM, et al. MR imaging of intracranial metastatic melanoma. J Comput Assist Tomogr 1987;11:577–82. [DOI] [PubMed] [Google Scholar]
- [38].Gerber NK, Young RJ, Barker CA, et al. Ipilimumab and whole brain radiation therapy for melanoma brain metastases. J Neurooncol 2015;121:159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Mackiewicz-Wysocka M, Krokowicz L, Kocur J, et al. Resistance to vemurafenib can be reversible after treatment interruption: a case report of a metastatic melanoma patient. Medicine (Baltimore) 2014;93:e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Spiegel D. Mind matters in cancer survival. JAMA 2011;305:502–3. [DOI] [PubMed] [Google Scholar]
- [41].Fan X, Jia C, Yang J, et al. A microfluidic chip integrated with a high-density PDMS-based microfiltration membrane for rapid isolation and detection of circulating tumor cells. Biosens Bioelectron 2015;71:380–6. [DOI] [PubMed] [Google Scholar]


