Abstract
Urethral catheterization is a predictor of agitation during the general anesthesia recovery period. The aim of this study was to determine the effect of intraurethral 5% lidocaine and 25 mg/g prilocaine cream in reducing catheter-related bladder discomfort (CRBD) in male patients during the general anesthesia recovery period. Adult male patients undergoing elective operations that required urinary catheterization under general anesthesia were enrolled and assigned randomly to 2 groups. In the lidocaine-prilocaine cream group (n = 72), approximately 5 g of topical cream was spread in the preputial sac, the glans, the meatus, and on the urinary catheter surface before urinary catheterization. In the control group (n = 74), the urinary catheter was lubricated with lidocaine gel. The incidence and severity of CRBD were assessed 15, 30, 45, and 60 minutes postoperatively. We found that the incidence of CRBD in the lidocaine-prilocaine cream group was significantly lower than in the control group. Multivariate logistic regression analysis showed that lidocaine-prilocaine cream applications reduced moderate or severe CRBD. Thirty minutes postoperation was the most frequent time point for the incidence of CRBD. Application of lidocaine-prilocaine cream on the surface of the urinary catheter is an efficient and safe method to reduce the incidence and severity of CRBD.
Keywords: catheter-related bladder discomfort, topical anesthesia, urinary catheter
Key Points
Methods should be investigated to prevent or reduce the severity of CRBD.
Urinary topical anesthesia may be helpful to enhance patient quality of life and reduce postoperative emergency agitation.
Spreading lidocaine-prilocaine cream in the preputial sac, the glans, the meatus and on the surface of the urinary catheter before urinary catheterization reduces distress in male patients during general anesthesia recovery period in postanesthesia care unit.
1. Introduction
Urethral catheterization is commonly performed during general anesthesia to relief of urinary retention and monitor urinary output, but it often leads to pain and discomfort. Urinary catheters are important causes of postoperative agitation.[1–4] The pain and discomfort associated with urethral catheterization are collectively known as catheter-related bladder discomfort (CRBD). CRBD can involve a burning sensation with an urge to void or discomfort in the suprapubic area caused by irritation of the mucosa of the urinary tract and bladder due to the urinary catheter. CRBD is resistant to conventional analgesic therapy, such as opioids and antismuscarinic drugs like tolterodine or oxybutynin, and postoperative administration of ketamine or tramadol has been reported to effectively treat CRBD. Nevertheless, side effects, such as sedation, dry mouth, facial flushing, and blurred vision, may occur.[1,2,5,6]
The use of lubricants in urethral catheterization can minimize urethral trauma, reduce the risk of infection, and minimize pain and discomfort during the operation, especially when using a lubricant containing local anesthetic. In male patients, pain is experienced higher up in the urethra, particularly around the prostate area.[1,7] If the gel is not instilled into the urethra or the gel is wiped off the catheter at the urethral introitus, there will be no anesthetic effect.[8] Intraurethral topical anesthesia has been widely used and is effective in urological surgery,[5] but the incidence and treatment of CRBD after other types of surgery in the postanesthesia care unit (PACU) have been seldom reported. The PACU is one of the most important areas for clinical nursing. We wanted to find an effective, convenient, and economical method to address the problem of CRBD. Therefore, we investigated the preventive effect of a topical anesthetic cream containing 5% lidocaine and 25 mg/g prilocaine on CRBD in adult male patients in the PACU.
2. Methods
This trial was conducted from August to November 2015 in the Tianjin Union Medical Center, which performs approximately 29,800 surgical operations per year. After obtaining approval from the Institutional Review Board of Tianjin Union Medical Center and written informed consent from each patient, we performed this prospective, randomized, case-controlled study. The protocol for this clinical trial was registered in the Chinese Clinical Trial Registry (ChiCTR-ICR-15007017).
Patients aged 23 to 83 years with American Society of Anesthesiologists physical status I–III and scheduled to undergo elective surgery were included in this study. These patients were scheduled to undergo a urinary catheterization after anesthesia induction.
Patients were excluded if they had a history of hypersensitivity to lidocaine or prilocaine, conduction disturbance, cognitive deficit, intellectual disability, long-term use of analgesics or sedatives medication, chronic renal insufficiency (creatinine >1.5 mg/dL) or liver insufficiency (bilirubin >34.2 μmol/L), benign prostatic hyperplasia, overactive bladder, neurogenic bladder, or had a history of allergy to opiates, or any other drug used in the study.
Patients were educated on the symptoms of CRBD during a preoperative visit. They fasted after midnight, and no prior medication was administered. Anesthesia was induced with 0.05 mg/kg midazolam, 2.0 μg/kg fentanyl, and 2 mg/kg propofol. Tracheal intubation was performed after administration of 2 mg/kg cis-atracurium (all intravenously [IV]). Patients were randomized to the lidocaine-prilocaine or control groups using computer-generated random numbers. A sealed envelope containing a computer-generated random number was opened immediately after induction by a nurse, who was responsible for preparing the study drug. Each lidocaine-prilocaine cream or gel is designed for single use.
In the cream group, topical anesthesia was initially achieved by applying approximately 5 g of lidocaine-prilocaine cream (#1311141, Ziguang, China, Eutectic Mixture of Local Anesthetics, a 5% lidocaine 25 mg and 25 mg/g prilocaine cream) in the preputial sac, the glans, the meatus, and on the surface of the urinary catheter 5 minutes before urinary catheterization. In the control group, the urinary catheter was lubricated with traditional 2% lidocaine gel. The urethra was filled with 10 mL lidocaine gel. The glans was clamped for 5 minutes with a penile clamp. Urinary catheterization was performed using 16-Fr Foley catheters, and their balloons were filled with 10 mL normal saline after induction of anesthesia. The catheter was fixed without traction and was not clamped during the follow-up period. Catheterization was undertaken with a strict aseptic technique and using only sterile equipment.
After intubation, general anesthesia was maintained with the effect-site model of target control infusion with propofol and remifentanil. The target concentrations were maintained at 2 to 3 μg/mL propofol and 2 to 6 ng/mL remifentanil. Dexmedetomidine was infused 10 minutes before induction at an initial dose of 0.5 μg/kg/h for 10 minutes and then adjusted to 0.2 to 0.5 μg/kg/h from tracheal intubation to skin closure. The propofol dose was titrated according to the depth of anesthesia. The bispectral index (Narcotrend, Germany) was maintained within 45 to 60. Fentanyl 1 μg/kg was administered before the end of surgery. Intravenous patient-controlled analgesia mixed with fentanyl, ketamine, and azasetron was initiated in all patients at the end of the operation. The trachea was extubated after the patient regained adequate spontaneous ventilation and responded to all commands. The period from the cessation of propofol and remifentanil to removing the endotracheal tube was defined as the extubation time. The period from the cessation of propofol and remifentanil to arousing patient was defined as open eyes time. All patients were transferred to the PACU after recovery of consciousness.
The primary outcomes were differences in the incidence and severity of CRBD 15, 30, 45, and 60 minutes postoperatively between the 2 groups. The secondary outcomes included the incidence of postoperative nausea and vomiting (PONV), sedation, respiratory depression, and dizziness, as well as use of propofol and tramadol. Patients with a Ramsay sedation score ≥4 were considered sedated. The oxygen saturation <90% was considered respiratory depression.
Patients were questioned by blinded investigators about the presence and severity of CRBD while in the PACU. CRBD was evaluated on a 4-point scale: no pain, mild pain (revealed only by interviewing the patient), moderate (a spontaneous complaint by the patient of a burning sensation in the urethra, an urge to urinate, and/or sensation of a urethral foreign body without any emotional agitation), and severe discomfort (agitation, loud complaints, and attempts to remove the bladder catheter associated with a burning sensation in the urethra). Mild, moderate, or severe discomfort was considered CRBD. When moderate or severe CRBD was reported, tramadol (1 mg/kg IV) was administrated as a rescue therapy. In the event of agitation, propofol (0.5–1 mg/kg IV) was administered as a rescue therapy.
According to previous studies, 70% of patients complain of CRBD postoperatively.[3] Assuming that the incidence would decrease to 40% after treatment with lidocaine-prilocaine cream, we calculated that 48 patients would be needed in each group to achieve statistical significance (Type I error rate of 0.05 and Type II error rate of 0.20).
2.1. Statistical analysis
All statistical analyses were performed using SPSS version 18.0 statistical software (SPSS Inc, Chicago, IL). Normally distributed variables are described as the mean (standard deviation) and were compared using unpaired 2-sample independent t tests. Categorical variables are described using a number (%) and were compared using Pearson's χ2 test or Fisher exact test. Variables that were not normally distributed were compared by using the Mann–Whitney U test. Factors of moderate and severe CRBD were analyzed by multivariate logistic regression. Statistical significance was accepted as P < 0.05.
3. Results
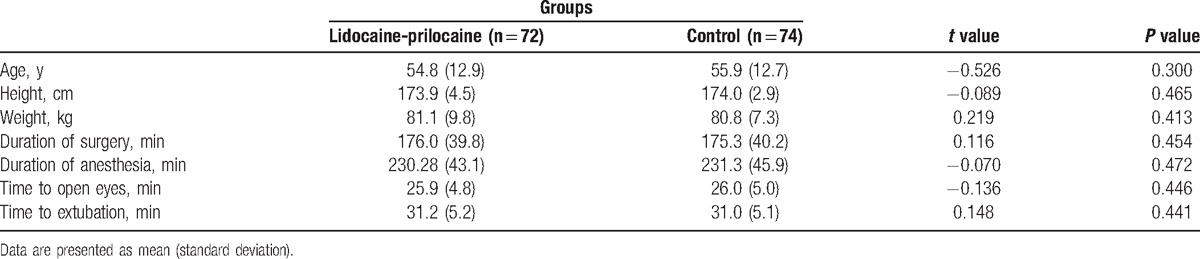
One hundred eighty-two patients were screened for inclusion in the study. Thirty-six patients were excluded (refusal [n = 22] or cancelled the operation [n = 14]), leaving 146 patients for analysis (Fig. 1). No differences in the group demographic characteristics were observed (Table 1).
Figure 1.
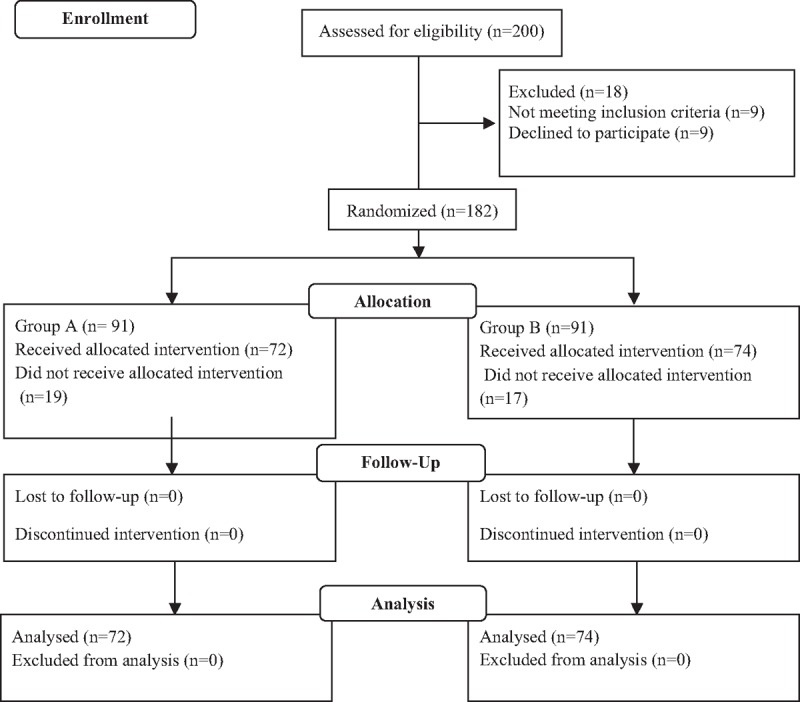
Flow diagram.
Table 1.
Demographic data of study participants.
Multivariate logistic regression analysis showed lidocaine-prilocaine decreased moderate and severe CRBD (odds ratio [OR]: 0.055, 95% confidence interval [CI]:0.021–0.144, P = 0.01). Lidocaine-prilocaine also decreased the CRBD incidence at 15, 30, 45, and 60 minutes from 43% to 14%, 70% to 21%, 45% to 7%, and 27% to 4%, respectively. Thirty minutes postoperation was the peak time point for CRBD (OR: 5.889, 95% confidence interval: 3.247–10.680, P = 0.001). In total, CRBD including mild, moderate, and severe CRBD occurred in 15 patients (20.8%) in the lidocaine-prilocaine group, which was significantly lower than the 52 patients (70.3%) in the control group at the 30-minute time point (P = 0.000). Moderate and severe CRBD occurred in the lidocaine-prilocaine group for 6 patients (8.3%) and no patients (0%) respectively, which was lower than the 10 patients (13.5%) and 13 patients (17.6%) in the control group (P = 0.000) (Table 2 and Fig. 2). No patient required treatment with propofol for sedation in the lidocaine-prilocaine group, while 13 patients (18%) were administered 30 to 50 mg propofol to control agitation in the control group (P = 0.000). Six patients in the lidocaine-prilocaine group and 10 patients in control group were administered 1 mg/kg tramadol (P = 0.316). After treatment, the incidences of moderate CRBD were 14% at 45 minutes and 12% at 60 minutes in the control group, respectively (P = 0.001 and P = 0.003).
Table 2.
Incidence and severity of catheter-related bladder discomfort.
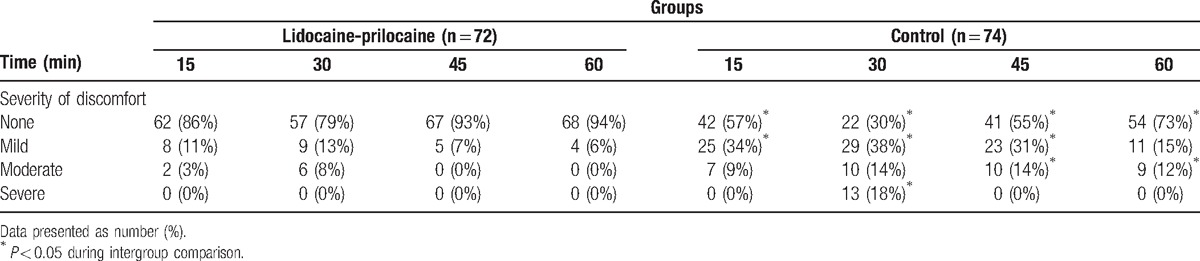
Figure 2.
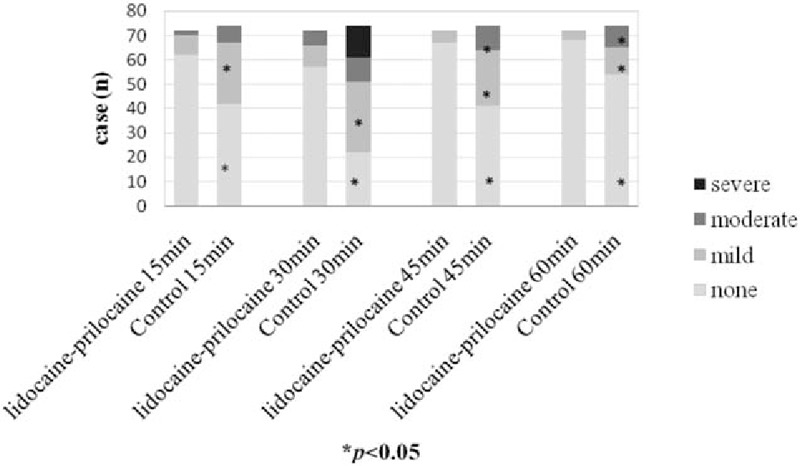
CRBD incidence and severity. CRBD = catheter-related bladder discomfort.
No patients in either group became desaturated with oxygen (oxygen saturation <90%) or became too sedated (Ramsay sedation score ≥4) during the study period. Adverse effects, such as postoperative sedation, respiratory depression, and drowsiness, were not different between groups.
4. Discussion
We have demonstrated that spreading topical anesthetic lidocaine-prilocaine cream in the preputial sac, the glans, the meatus, and on the surface of the urinary catheter before urinary catheterization reduces the incidence and severity of postoperative CRBD in the PACU.
CRBD is caused by an indwelling urinary catheter and is a common, painful, and distressing complication particularly for patients awakening from anesthesia.[2] The symptoms of CRBD are similar to those of an overactive bladder, that is, urgency, discomfort, and frequency. However, CRBD is frequently neglected and left untreated, despite it being a recognized risk factor for emergence agitation.[1–4,9] Therefore, preventing or decreasing the severity of CRBD may be helpful to enhance patient quality of life and reduce postoperative emergency agitation. Muscarinic antagonists (butylscopolamine, oxybutynin, and tolterodine), anesthetics (ketamine and tramadol), antiepileptics (gabapentin and pregabalin), and paracetamol appear to provide the greatest improvement in clinical symptoms and reduce CRBD incidence. However, high incidences of intervention-related side effects, such as dry mouth, facial flushing, blurred vision, sedation, PONV, respiratory depression, somnolence, dizziness, ataxia, and fatigue, were observed.
Local anesthesia using topical anesthetic cream is a patient-friendly approach because the anxiety and the distress of patient are alleviated. Lidocaine-prilocaine cream is an oil-in-water emulsion of 5% lidocaine and 25 mg/g prilocaine. The eutectic mixture contains a thickener, an emulsifier, and distilled water and is adjusted to a pH level of 9.4.[10] Application of lidocaine-prilocaine cream as a local anesthetic is simple and straightforward. When applied to a mucous membrane, absorption is rapid, occlusive dressing is not necessary, and the absorption from the mucosa occurs in 5 to 7 minutes.[11–13] The overall efficacy of lidocaine-prilocaine applied on urethral mucosal surfaces in our series was excellent, and provided adequate analgesia for the completion of the procedure. Penetration of the anesthetic was possibly easier through the thinner mucosal epithelium and lamina propria of the male urethra. In our study, lidocaine-prilocaine reduced the incidence and severity of CRBD within 60 minutes in the PACU.
Safety issues have always been a concern with topical anesthetics. No significant adverse effects were recorded in our study. Every effort should be made to cover the smallest mucosal area necessary. A single application of a topical lidocaine preparation does not generally cause systemic side-effects. The pharmacodynamics profile of topical lidocaine demonstrates that peak absorption is not reached until 15 to 60 minutes after application. Methemoglobinemia is the most important systemic concern regarding the use of lidocaine-prilocaine cream, and it is a complication of prilocaine. A history of congenital or idiopathic methemoglobinemia is an absolute contraindication. Patients with glucose-6-phosphate dehydrogenase deficiency and those who require treatment with methemoglobin-inducing drugs are more susceptible to acquired methemoglobinemia.[14] Since the total amount of prilocaine used (i.e., 125 mg) in our study was much less than the 250 mg that is normally used, we did not measure methemoglobin concentrations. Intravenous administration of 250 mg prilocaine was shown to give rise to a low concentration of methemoglobin in the blood with few side effects.[15] We used the low dose (5 g) lidocaine-prilocaine cream in our study to anesthetize the adult urethra, no systemic toxicity was observed. The topical application of 5 g lidocaine-prilocaine cream appeared to be safe and effective.[16] The dose and plasma concentrations of lidocaine-prilocaine cream after topical application to the urethra area need to be measured because the mucosa of urethra may absorb medication faster than the skin.
Dexmedetomidine is used as an anesthetic adjuvant during general anesthesia and has an antimuscarinic effect, which may be beneficial to prevent and treat CRBD.[17,18] Kim et al reported that dexmedetomidine administered intraoperatively decreased the incidence and severity of early postoperative CRBD.[17,18] Dexmedetomidine inhibits muscarinic receptor subtype 3.[8,17] In our study, general anesthesia was maintained with propofol, remifentanil, and dexmedetomidine from the time of tracheal intubation to skin closure.
The total incidence of CRBD in the lidocaine-prilocaine group, which was less than 21% in our study, was lower than those of other investigations that used dexmedetomidine. The result highlights the topical anesthetic and analgesic effect of lidocaine-prilocaine cream on the urethral mucosa. Tramadol (1 mg/kg IV) was administered as a rescue therapy in our study because it could prevent or treat CRBD.[19] Its antimuscarinic actions on type-1 muscarinic and type-3 muscarinic receptors are responsible for the effective management of postoperative CRBD.[20–22]
Fewer patients in lidocaine-prilocaine group needed tramadol or propofol to treat CRBD and agitation. The sequential use of intraurethral lidocaine-prilocaine produced the greatest cost benefit due to the decrease in pharmaceutical sedation costs.
The main limitation of our study is that it was not double-blinded. The appearance and smell of the 2 lubricants are indistinguishable. Subjects and the investigator who collected the data in the PACU did not know the drug had been applied. Second, urinary catheterization was performed using unified 16-Fr Foley catheters, and we did not compare the differently sized Foley catheters. A large diameter Foley catheter is a known risk factor for CRBD.[4] Binhas et al have reported that the diameters of Foley catheters >18 G Fr and male gender were independent predictors of moderate or severe CRBD in the PACU.[1] Third, we did not observe pelvic procedures, such as urogenital surgery and proctectomy, because these surgeries may affect the occurrence of postoperative CRBD. Therefore, limitations exist in the application of lidocaine-prilocaine to patients who undergo urologic operations requiring catheterization. Finally, patients usually stayed in PACU for less than 1 hour. Therefore, we did not evaluate the duration of the beneficial effect.
5. Conclusion
Topical anesthetic lidocaine-prilocaine cream applied on the urethral mucosal surfaces is an efficient and safe tool for reducing the incidence and severity of CRBD. It is a patient-friendly method of local anesthesia that provides excellent results via mucosal applications. Additionally, it is a low-cost intervention for a key indicator of patient comfort and satisfaction. Side effects can be minimized when the suggested doses are given during urinary mucosal application.
Acknowledgments
The authors thank all staff and participants in this study.
Footnotes
Abbreviations: CRBD = catheter-related bladder discomfort, IV = intravenously, OR = odds ratio, PACU = postanesthesia care unit, PONV = postoperative nausea and vomiting.
LM helped conduct the study and analyze the data. Attestation: LM has conducted the study, reviewed the analysis of the data, and approved the final manuscript.
L-cG helped design the study and write the manuscript. Attestation: L-cG has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
HX helped conduct the study. Attestation: HX has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
ML helped conduct the study and write the manuscript. Attestation: ML has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
J-mG helped conduct the study and analyze the data. Attestation: J-mG has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
LL helped design the study, conduct the study, and write the manuscript. Attestation: LL has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files
Institution: the Department of Anesthesia, Tianjin Union Medical Center.
This study was conducted with written informed consent from the study subjects. The study was registered prior to patient enrolment.
Registry Url: https://www.clinicaltrials.gov/ct2/contact-org-admin#wrapper Identifier: Chi CTR-ICR-15007017.
The authors have no conflicts of interest to disclose.
References
- [1].Bai Y, Wang X, Li X, et al. Management of catheter-related bladder discomfort in patients who underwent elective surgery. J Endourol 2015;29:640–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Binhas M, Motamed C, Hawajri N, et al. Predictors of catheter related bladder discomfort in the post-anaesthedis care unit. Ann Fr Anesth Reanim 2011;30:122–5. [DOI] [PubMed] [Google Scholar]
- [3].Li C, Liu Z, Yang F. Predictors of catheter-related bladder discomfort after urological surgery. J Huazhong Univ Sci Technolog Med Sci 2014;34:559–62. [DOI] [PubMed] [Google Scholar]
- [4].Kim HC, Kim E, Jeon YT, et al. Postanaesthetic emergence agitation in adult patients after general anesthesia for urological surgery. J Int Med Res 2015;43:226–35. [DOI] [PubMed] [Google Scholar]
- [5].Gordetsky J, Bendana E, O’Brien J, et al. (Almost) painless surgery: a historical review of the evolution of intraurethral anesthesia in urology. Urology 2011;77:12–6. [DOI] [PubMed] [Google Scholar]
- [6].Iirola T, Ihmsen H, Laitio R, et al. Population Pharmacokinetics of dexmedetomidine during long-term sedation in intensive care patients. Br J Anaesth 2012;108:460–8. [DOI] [PubMed] [Google Scholar]
- [7].Li JY, Yi ML, Liao R. Dorsal penile nerve block with ropivacaine-reduced postoperative catheter-related bladder discomfort in male patients after emergence of general anesthesia: a prospective, randomized, controlled study. Medicine (Baltimore) 2016;95:e3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bardsley A. Use of lubricant gels in urinary catheterization. Nurs Stand 2005;20:41–5. [DOI] [PubMed] [Google Scholar]
- [9].Burk RS, Grap MJ, Munro CL, et al. Predictors of agitation in critically ill adults. Am J Crit Care 2014;23:414–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Priyadarshi V, Puri A, Singh JP, et al. Meatotomy using topical anesthesia: a painless option. Urol Ann 2015;7:67–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kundu S, Achar S. Principles of office anesthesia: Part II. Topical anesthesia. Am Fam Physician 2002;66:99–102. [PubMed] [Google Scholar]
- [12].Ljunghall K, Lillieborg S. Local anesthesia with a lidocaine/prilocaine cream (EMLA) for cautery of condylomata acuminata on the vulval mucosa. The effect of timing of application of the cream. Acta Derm Venereol 1989;69:362–5. [PubMed] [Google Scholar]
- [13].Arnau B, Jovell E, Redón S, et al. Lidocaine-prilocaine (EMLA®) cream as analgesia in hysteroscopy practice: a prospective, randomized, non-blinded, controlled study. Acta Obstet Gynecol Scand 2013;92:978–81. [DOI] [PubMed] [Google Scholar]
- [14].Wright RO, Lewander WJ, Woolf AD. Methemoglobinemia: etiology, pharmacology, and clinical management. Ann Emerg Med 1999;34:646–56. [DOI] [PubMed] [Google Scholar]
- [15].Arthur GR, Scott DH, Boyes RN, et al. Pharmacokinetic and clinical pharmacological studies with mepivacaine and prilocaine. Br J Anaesth 1979;51:481–5. [DOI] [PubMed] [Google Scholar]
- [16].Shiau JM, Su HP, Chen HS, et al. Use of a topical anesthetic cream (EMLA) to reduce pain after hemorrhoidectomy. Reg Anesth Pain Med 2008;33:30–5. [DOI] [PubMed] [Google Scholar]
- [17].Kim HC, Lee YH, Jeon YT, et al. The effect of intraoperative dexmedetomidine on postoperative catheter-related bladder discomfort in patients undergoing transurethral bladder tumour resection. Eur J Anaesthesiol 2014;31:1–6. [DOI] [PubMed] [Google Scholar]
- [18].Kim HC, Lee YH, Jeon YT, et al. The effect of intraoperative dexmedetomidine on postoperative catheter-related bladder discomfort in patients undergoing transurethral bladder tumor resection: a double-blind randomized study. Eur J Anasthesiol 2015;32:596–601. [DOI] [PubMed] [Google Scholar]
- [19].Agarwal A, Yaday G, Gupta D, et al. Evaluation of intra-operative tramadol for prevention of catheter-related bladder discomfort: a prospective, randomized, double-blind study. Br J Anaesth 2008;101:506–10. [DOI] [PubMed] [Google Scholar]
- [20].Hu B, Li C, Pan M, et al. strategies for the prevention of catheter-related bladder discomfort: a PRISMA-compliant systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2015;95:e4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Shiga Y, Minami K, Shiraishi M, et al. The inhibitory effects of tramadol on muscarinic receptor-induced responses in Xenopus oocytes expressing cloned M (3) receptors. Anesth Analg 2002;95:1269–73. [DOI] [PubMed] [Google Scholar]
- [22].Shiraishi M, Minami K, Uezono Y, et al. Inhibition by tramadol of muscarinic receptor-induced responses in cultured adrenal medullary cells and in Xenopus laevis oocytes expressing cloned M1 receptors. J Pharmacol Exp Ther 2001;299:255–60. [PubMed] [Google Scholar]


