Supplemental Digital Content is available in the text
Keywords: magnetic resonance spectroscopy, metabolic biomarkers, multiple sclerosis, normal appearing white matter
Abstract
Demyelination and axonal degeneration caused by multiple sclerosis (MS) exist in the white matter and not only in the lesion area. Magnetic resonance spectroscopy (MRS) could provide a unique insight into metabolic changes in the normal appearing white matter (NAWM). To evaluate the subtle axonal degeneration and delineate the spatial distribution of metabolite abnormalities in the NAWM in patients with MS. A total of 17 clinically definite relapsing-remitting MS (RRMS) patients and 21 healthy controls were enrolled in this study. 2D 1H magnetic resonance spectroscopic imaging (MRSI) performed at 3 Tesla was used to measure metabolite concentrations in the frontal-parietal-occipital NAWM. Ratios of N-acetyl-aspartate (NAA) and choline (Cho) to creatine (Cr) and Cho to NAA were calculated in each voxel. MS patients showed decreased NAA/Cr and increased Cho/NAA ratios in the NAWM compared to healthy controls. In the parietal NAWM, the extent of NAA/Cr decrease was significantly higher than that in the frontal and parietal-occipital NAWM. Decreased NAA in the NAWM would provide useful metabolic information for evaluation of disease progression in MS. The high extent of NAA decrease in the parietal NAWM helps improve the accuracy of the prediction.
1. Introduction
Multiple sclerosis (MS) is a typical serious central nervous system (CNS) disease that is characterized by white matter lesions.[1–3] Worldwide, more than 1,000,000 people suffer from physical disabilities caused by MS, and the incidence rate is increasing.[4] Inflammation and demyelination are typical neuropathological features of MS.[5,6] The pathogenesis of MS is rather complex and still remains unclear. The varied accompanying histopathological changes, such as axonal damage or loss, have been considered as the important indicators of MS.[7,8]
Over the last several decades, conventional magnetic resonance imaging (cMRI), an in vivo, noninvasive technique, has been using for the diagnosis of MS in combination with characteristic clinical symptoms.[9,10–12] cMRI also plays an important role in assessing the therapeutic efficacy of MS patients.[9,10] However, in parallel with advancements in MRI technology, some limitations of cMRI in studies on MS have become increasingly evident. First, although MS focal lesions could manifest as hyperintensity on T2-weighted MR images, it is difficult to distinguish them from other neurological abnormalities or white matter lesions caused by aging.[3] Second, cMRI cannot help in visualizing subtle changes that occur in the normal appearing white matter (NAWM) in MS patients.[11,13] In recent years, proton magnetic resonance spectroscopy (MRS), a noninvasive quantitative MRI technique, has received wide attention in MS studies.[11,12] Using the MRS technique, many types of metabolite concentrations can be observed that could directly reflect brain metabolism.[14] Compared to cMRI, MRS is more specific and is capable of detecting subtle metabolic alterations in the white matter of the human brain.[15] For example, N-acetyl-aspartate (NAA), an MRS-observed metabolite, has been widely accepted as a biomarker of neuroaxonal integrity.[16] According to previous MRS studies, a decreased NAA level can be found not only in MS focal lesions but also in the NAWM, which can be an indicator of wide axonal dysfunction or loss in the brains of MS patients.[17–19]
To date, MRS mainly includes single-voxel spectroscopy (SVS) and multivoxel spectroscopy.[20] SVS has been employed in many previous MS studies.[21,22] However, abnormalities and impairments are believed to distribute discrepantly in the brain tissue of MS patients.[23] Therefore, SVS is not specific enough to provide a full-scale observation of tissue metabolic changes caused by MS. By contrast, multivoxel spectroscopy techniques, such as proton 2D magnetic resonance spectroscopic imaging (MRSI), have an advantage in that they provide simultaneous metabolome data across the brain regions, which is critical in the assessment of pathological effects of MS.[24] Therefore, multivoxel MRSI is increasingly being used in MS studies. In addition, it is clear that the quality of the spectrum, particularly the signal-to-noise ratio (SNR) that determines spectral resolution, is largely influenced by magnetic field strength. At higher field strengths, such as 3 Tesla, the improved spectral resolution could increase the sensitivity and accuracy of MRS in evaluating subtle changes in the tissue.[25,26]
The metabolite changes in the NAWM now still remain under investigation, and few available MRS studies on MS have focused on the spatial distribution of metabolite abnormalities. The major goal of our study was to investigate specific metabolite changes, particularly NAA alterations, in the different areas of NAWM of MS patients using the multivoxel MRSI technique performed at 3 Tesla. We hypothesize that the changes of metabolites are region related, namely different regions in the NAWM in MS patients may have different degrees of abnormal alterations.
2. Materials and methods
2.1. Subjects
In total, 17 patients with relapsing-remitting MS were recruited from the First Affiliated Hospital of Henan University of Science and Technology, Luoyang, China. The MS diagnoses were determined based on the McDonald criteria[27] and traditional clinical MRI examinations (T2 and FLAIR). The level of disability was assessed with the Extended Disability Status Scale (EDSS).[28] For comparison, 21 healthy controls were enrolled in this study who were matched for age, sex, and handedness. The demographic and clinical information for the 38 participants are illustrated in Table 1. All subjects were fully informed of the nature of the study and provided written consent for participation prior to enrollment. The local institutional ethics committee on human research of the First Affiliated Hospital, and College of clinical medicine of Henan University of Science and Technology approved this study and all procedures. The IRB number is 2016-0007.
Table 1.
Demographic information of study subjects.
2.2. Conventional magnetic resonance imaging acquisition
MRI scans were performed with a Philips Achieva 3.0-Tesla TX scanner (Philips, The Netherlands) equipped with a 16-channel SENSE head-spine coil. MR images comprised axial T1- and T2-weighted images as well as fluid-attenuated-inversion recovery (FLAIR) images. The sequence parameters were TR/TE = 7.6/3.7 ms and flip angle (FA) = 8° for T1-weighted images, 190/80 ms for T2-weighted images, and TR/TI/TE = 6000/2000/140 ms for FLAIR images; field of view (FOV) = 250 × 250 mm; slice thickness = 7.5 mm; and intersection gap = 1 mm. Prior to proton MRS examinations, cMRI was performed to exclude other pathologic changes.
2.3. Proton magnetic resonance spectroscopy acquisition
Automatic shimming was employed to compensate for the inhomogeneity of the magnetic field. The linewidth was less than 10 Hz. The signal of water was suppressed using a chemical shift selective imaging sequence (CHESS). An outer-volume suppression pulse sequence was used to avoid signal contamination from outside the volume of interest (VOI). The point resolve spectroscopy sequence (PRESS) technique was used for VOI selection, as shown in Fig. 1. The sequence parameters were TR/TE = 2000/53 ms, FOV = 150 × 195 mm, slice thickness = 15 mm, in-plane resolution 15 × 15 mm2, and number of averages = 1024. The total acquisition time of the spectroscopic sequences was less than 20 minutes.
Figure 1.
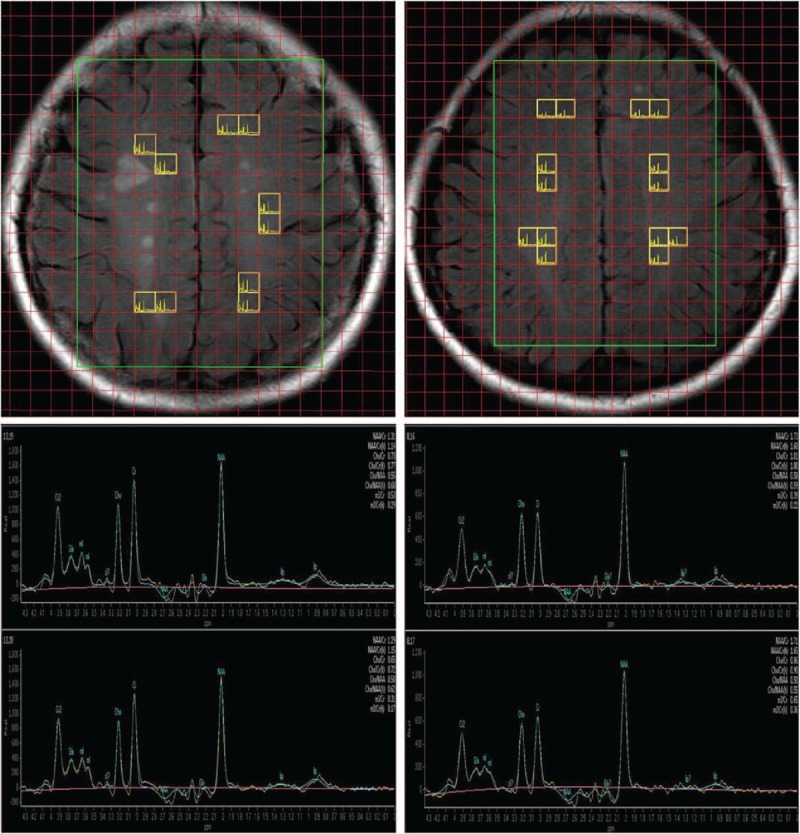
The volume of interest superimposed on a fluid-attenuated inversion recovery image, including an MS patient (top left) and a control subject (top right). Representative spectra maps of an MS patient (lower left) and a healthy control (lower right). All spectra used for analysis were selected from voxels located in the NAWM. MS = multiple sclerosis, NAWM = normal appearing white matter.
A standard software package (Spectra view, Philips, The Netherlands) was used for postprocessing the MR spectroscopic data. Spectral signals from voxels matching exclusively frontal-parietal-occipital NAWM without any signs of lesion load manifested on T2-weighted MR images were selected. We measured metabolite concentrations within individual voxel by calculating the area under the curve. All metabolite concentrations were expressed as ratios of signal intensities, including NAA/Cr, Cho/NAA, and Cho/Cr.
2.4. Statistical analysis
The statistical analysis of acquired data was performed using the Statistical Package for Social Science version 16.0 (SPSS Inc., Chicago, IL). The means and standard deviations (SDs) of the NAA/Cr, Cho/NAA, and Cho/Cr ratios of MRS were calculated. Data analyses were performed to determine statistically significant differences. The Kolmogorov–Smirnov (K-S) test was used to study the differences in metabolites ratios between the patient group and the control group. In addition, Spearman correlations were used to assess the relationship between the NAA/Cr ratio from the global NAWM and those from the frontal, parietal, and parietal-occipital NAWM within the patient group. P values were calculated, and all tests were considered to be statistically significant if the P value was <0.05.
3. Results
Our study compared the ratios of metabolites in the NAWM between patients and healthy controls, and the results are shown in Fig. 2. Significant differences in metabolite ratios were found between MS patients and healthy controls. Compared with the control group, the NAA/Cr ratio in the NAWM in MS patients was significantly lower (P < 0.0001 according to the K–S test), and the Cho/NAA ratio was significantly higher (P < 0.001 according to the K–S test). However, we did not observe a significant difference in the Cho/Cr ratio between groups. The degrees of metabolite alteration in the frontal, parietal, and parietal-occipital NAWM in the MS group compared to the control group are shown in Fig. 3. Our group results found that the NAA/Cr decrease in the NAWM was closely related to the region. In the frontal NAWM, the NAA/Cr = 1.54 ± 0.14 in MS patients was 11% lower than in the control group 1.72 ± 0.08 (P < 0.001). In the parietal NAWM, the NAA/Cr = 1.35 ± 0.12 in the patient group was 23% lower than in the control group 1.65 ± 0.07 (P < 0.0001). In the parietal-occipital NAWM, the NAA/Cr = 1.37 ± 0.13 in the patient group was 12% lower than in the control group 1.54 ± 0.08 (P < 0.001). The correlations between the NAA/Cr ratios from the global and local NAWM are shown in Fig. 4. In the patient group, we found a strong correlation between the NAA/Cr ratios from the global and parietal NAWM (r = 0.6094, P < 0.05). However, the NAA/Cr ratios from the frontal and parietal-occipital NAWM did not correlate significantly with that from the global NAWM (r = 0.0376, P > 0.05; r = 0.4062, P > 0.05). The validity of our approach can be observed in supplementary document and supplementary Table S1 showing all of the metabolic results for different brain region.
Figure 2.
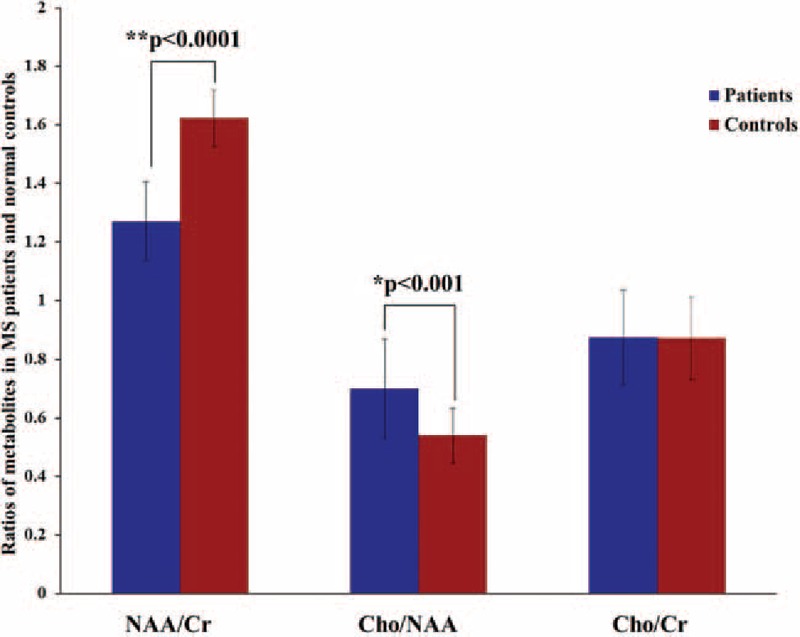
Values of the metabolite ratios of NAA/Cr, Cho/NAA, and Cho/Cr. The NAA/Cr and Cho/NAA ratios in the patient group showed significant changes compared to the control group (P < 0.0001 for NAA/Cr, P < 0.001 for Cho/NAA). Cho = choline, Cr = creatine, NAA = N-acetyl-aspartate.
Figure 3.
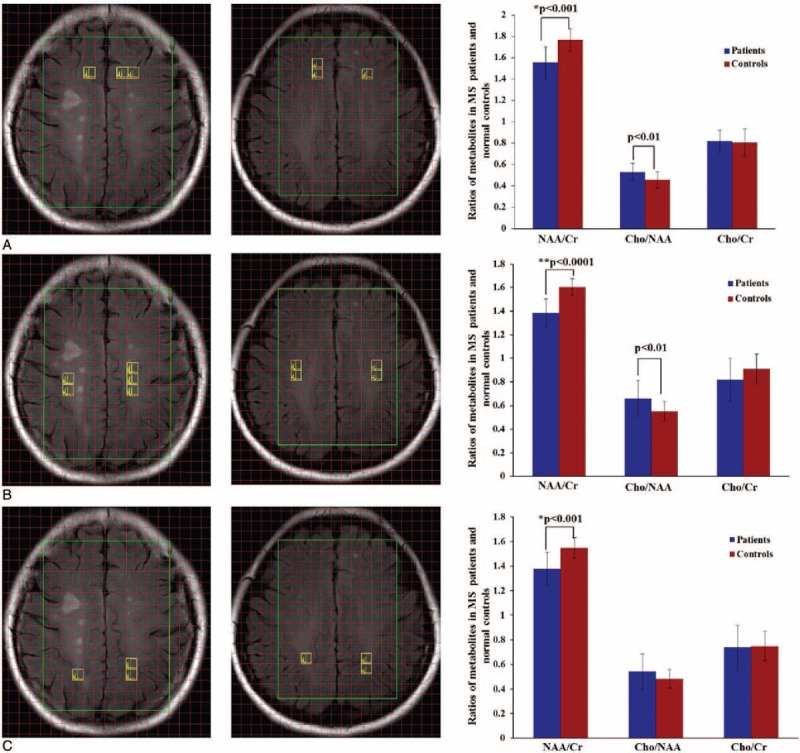
Voxels selected from 3 different regions in the normal appearing white matter (NAWM), including the frontal NAWM (A), parietal NAWM (B), and parietal-occipital NAWM (C) (2 left columns). Comparisons of values of metabolites ratios between patients and healthy controls from the 3 NAWM regions (right column).
Figure 4.
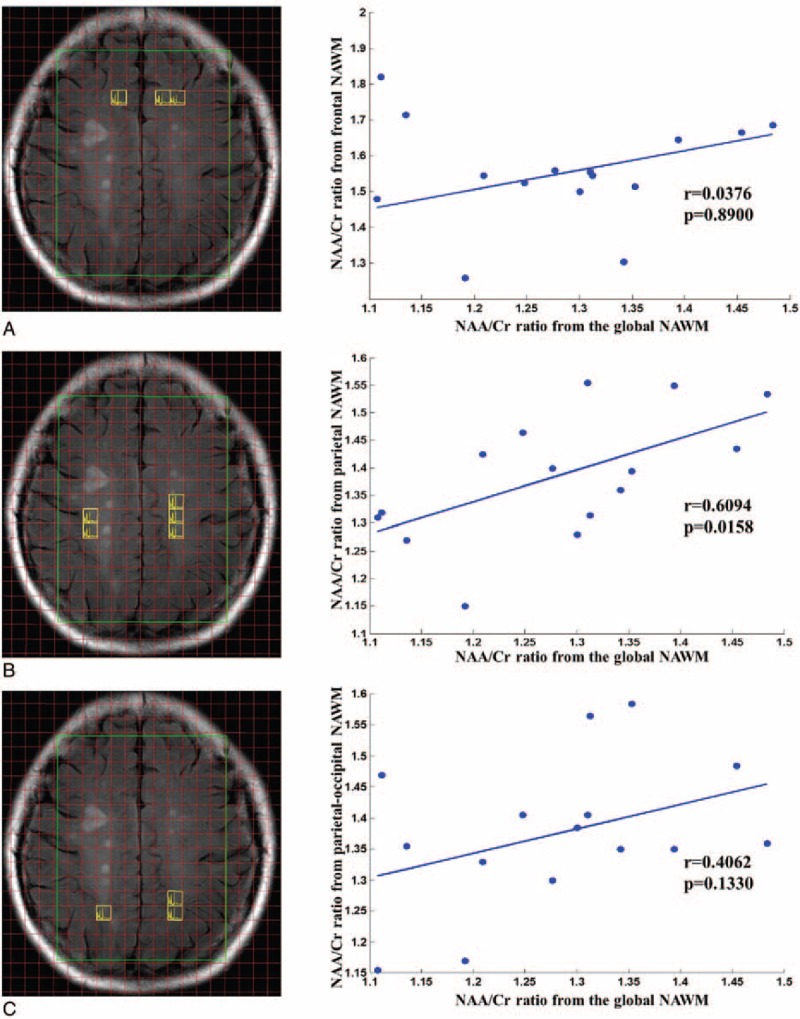
(A) Correlation of the NAA/Cr ratios from the frontal NAWM and the global NAWM. (B) Correlation of the NAA/Cr ratios from the parietal NAWM and the global NAWM. (C) Correlation of NAA/Cr ratios from the parietal-occipital NAWM and the global NAWM. Cr = creatine, NAA = N-acetyl-aspartate, NAWM = normal appearing white matter.
4. Discussion
In this study, we first analyzed the changes of several primary metabolite ratios, including NAA/Cr, Cho/NAA, and Cho/Cr, from the NAWM in 17 established MS patients and 21 healthy controls using the multivoxel MRSI technique performed at 3 Tesla. Through the analysis of MRS measurements, we found a significant decrease in the NAA/Cr ratio and a significant increase in the Cho/NAA ratio in the NAWM in the patient group compared to the control group, whereas no significant difference in the Cho/Cr ratio was found between the 2 groups. We then respectively analyzed the local changes in the metabolite ratios in the frontal, parietal, and parietal-occipital NAWM in MS patients, and we found that metabolite alterations were differently distributed in regional white matter. In other words, the degree of abnormalities in the NAWM was region related. Our results show that the region closest to the parietal NAWM had significantly higher abnormal degree than the regions closer to the frontal or parietal-occipital NAWM.
Many studies have showed that investigating abnormal metabolites in NAWM plays an important role in understanding the pathophysiological mechanism of MS.[29,30] In our study, a significant decrease in NAA/Cr ratio was found in the NAWM in MS patients compared to healthy controls. Our results indicated widespread axonal dysfunction or loss in the white matter rather than only in the focal lesions. Recently, MRS studies have reported that metabolic changes in the brain not only in focal lesions but also in NAWM in MS patients.[29] To our knowledge, NAA is mainly synthesized in neuronal mitochondria and exits almost exclusively in the neurons of the mature brain, which makes it a cogent surrogate biomarker of brain neuronal integrity.[31,32] A decrease in the NAA concentration often suggests axonal damage or loss.[33] For example, in Brex's study, the NAA level decreased significantly in chronic MS lesions compared to healthy tissue.[34] Therefore, alterations in the NAA concentration can be indicator of axonal viability. A significant decreased NAA/Cr ratio may be associated with nerve fiber loss in NAWM may make a significant contribution to MS-related clinical disability. An increase in the Cho/NAA ratio in the NAWM relative to that in healthy volunteers in NAWM has previously been reported.[35] The possible explain is that an abundance of Cho containing compounds in myelin and inflammatory cells during in the demyelination process. Thus, Cho/NAA ratio might be related to changes within the surrounding NAWM during the MS patients. We did not found the significant difference in Cho/Cr ratio between healthy and MS patients. Here, Cho/Cr ratio seems to less sensitive change within NAWM. Our results are in agreement with the previous studies.[29] In addition, according to Tartaglia et al's[30] investigation of MS, voxels with metabolite abnormalities in the NAWM more easily evolve into future visible MRI lesion loads compared to normal NAWM voxels. Therefore, determining the hidden pathological changes in NAWM may offer important insights into both the degenerative pathology and the lesion formation mechanism of MS, which could have great value in the early diagnosis and treatment of MS.
More importantly, to the best of our knowledge, there are few available MRS studies on MS that focused on the spatial distribution of metabolite abnormalities. In our study, we investigated metabolite alterations in different regions of the NAWM, including the frontal, parietal, and parietal-occipital NAWM, in MS patients. Our results showed that the NAA/Cr ratios in the frontal, parietal, and parietal-occipital NAWM in the patient group were all significantly lower than in the control group, which is in accordance with the results of the global investigation. In particular, the degree of decrease in the NAA/Cr ratio in the parietal NAWM was found to be significantly higher than in the other regions (frontal and parietal-occipital). In addition, the results of a correlation analysis clearly showed that only the NAA/Cr ratio from the parietal NAWM had a strong correlation with the NAA/Cr ratio from the global NAWM in the patient group. These results indicated that the decrease in NAA/Cr ratio in the parietal NAWM has significantly associated with global metabolic alterations. Therefore, the axonal integrity or function in the region closest to the parietal white matter may be more severely affected by MS pathology than other white matter regions. Taken together, the results here strongly indicate that the parietal NAWM has the highest sensitivity of metabolic alterations compared to the frontal and parietal-occipital NAWM.
It is well known that the brain's frontal lobe participates in the regulation of cognitive function. The abnormal metabolic in frontal lobe was associated with cognitive deficits in MS. Here, the significant decrease in the NAA/Cr ratio in the frontal NAWM may in part account for the phenomenon of cognitive deficits that occurs in most MS patients, and this has been demonstrated by previous studies.[36–39] For example, a global memory disorder involving indices for short-term memory, long-term memory, and working memory was found in MS patients in Staffen et al's study,[40] and deficits in decision-making under risk conditions, which might be related to deficits in processing speed or visuospatial learning, were found in MS patients in a study by Rovaris M and colleagues[41] Regarding NAA/Cr abnormalities in the parietal-occipital NAWM, Kotov et al's[42] work showed that they may be associated with vision impairment caused by MS.
In addition, many previous MS studies have confirmed the close relationship between motor dysfunction and MS.[43,44] Tur et al,[44] for example, investigated the metabolic changes in MS patients along the cortical-spinal tract (CST), which is also known as the primary motor pathway. Their work found that metabolic abnormalities are more common in MS patients than in healthy controls and in the patient group as a whole, lower CST Cho concentrations were found to be associated with a poorer walking ability.[44] In our study, we measured voxels close to the motor-related fiber located in the parietal NAWM to investigate the differences in metabolite levels between the 2 groups. As expected, a significant decrease in the NAA/Cr value was found in the patient group compared to the control group (see supplementary Fig. S1), indicating the close links between motor impairment and the pathological characteristics of MS. Therefore, a potential explanation for the high degree of the NAA/Cr decrease in the parietal lobe NAWM within MS patients might be that the functional brain region related to motion control is located in the parietal lobe of the human brain. It is already known that metabolic disorders in the NAWM, which are an indicator of MS progression, tend to be subtle and hidden, which makes it rather difficult to accurately detect them.[45] Our work may provide good evidence that focusing more attention on the area around the parietal white matter would make it easier to detect specific metabolic alterations and obtain a more accurate evaluation of both pathological progression and the response to clinical treatment.
5. Limitations
There were a number of limitations in our study. First, our current study is limited by the small sample size in adult MS group of relapsing-remitting MS type. Future work should endeavor to increase the sample size so that different pathological types of MS may be examined. On the other hand, the Cr concentration decrease in MS lesions has been reported and that metabolite ratios using Cr concentration as an internal reference are questionable. Third, the investigation of metabolic changes in (NAA, Cho, and Cr) in NAWM may not be sufficient to understand the complex pathogenesis of MS. Further investigations should consider more metabolites, such as mI, glutamate, and lipids, and include a combination of other tissues, such as the gray matter or even the cortex, to obtain a deeper understanding of the altered metabolism affected by MS. The 4th limitation of the current study is that we used MRSI in 3T clinical MRI scanner. Considering 7T scanner is higher signal-to-noise and better separation of the metabolite peaks. So, the usage of 7T scanner would have been more desirable.
6. Conclusions
Metabolic changes in the NAWM are associated with demyelination process within MS patients. MRS could provide important information in metabolic changes in the NAWM. In this study, we evaluated multivoxel MRS imaging for measuring metabolic changes in the NAWM in patients with MS. Our results showed that the NAA/Cr ratio significantly reduced in the NAWM of patients with MS compared to healthy controls. We also found Cho/NAA ratio significantly increased in the NAWM within MS patients. We also demonstrated that the abnormal metabolic changes in the frontal, parietal, and parietal-occipital NAWM in the patients group, separately. Furthermore, the NAWM region closest to the parietal lobe was found to have the highest degree for NAA/Cr alterations. The multivoxel MRS imaging approach has the potential to play a critical role in diagnosing of MS and assessing the therapeutic efficacy of MS patients.
Acknowledgments
The authors thank the National Natural Science Foundation of China (Project No. 61473221, 61401363, 61462031), by Nature Science Foundation of Shaan Xi Province of China (2015JM3105), and by the Fundamental Research Funds for the Central Universities of China (1191320118) for the support.
Supplementary Material
Footnotes
Abbreviations: MRS = magnetic resonance spectroscopy, MRSI = magnetic resonance spectroscopic imaging, MS = multiple sclerosis, NAA = N-acetyl-aspartate, NAWM = normal appearing white matter.
JS and HS contributed equally to this work.
This study was supported by the National Natural Science Foundation of China (Project No. 61473221, 61401363, 61462031), by Nature Science Foundation of Shaan Xi Province of China (2015JM3105), and by the Fundamental Research Funds for the Central Universities of China (1191320118).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Compston A, Coles A. Multiple sclerosis. Lancet (Lond, Engl) 2008;372:1502–17. [DOI] [PubMed] [Google Scholar]
- [2].Mader I, Roser W, Kappos L, et al. Serial proton MR spectroscopy of contrast-enhancing multiple sclerosis plaques: absolute metabolic values over 2 years during a clinical pharmacological study. AJNR Am J Neuroradiol 2000;21:1220–7. [PMC free article] [PubMed] [Google Scholar]
- [3].Traboulsee AL, Li DK. The role of MRI in the diagnosis of multiple. Adv Neurol 2006;98:125–46. [PubMed] [Google Scholar]
- [4].Roschger C, Cabrele C. The Id-protein family in developmental and cancer-associated pathways. Cell Commun Signal 2017;15:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rodriguez M. Multiple sclerosis: basic concepts and hypothesis. Mayo Clinic Proc 1989;64:570–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lassmann H. Comparative neuropathology of chronic experimental allergic encephalomyelitis and multiple sclerosis. Schriftenr Neurol 1983;25:1–35. [PubMed] [Google Scholar]
- [7].Dedeoglu SS, Imren Y, Cabuk H, et al. Results of minimal invasive coracoclavicular fixation by double button lift-up system in Neer type II distal clavicle fractures. J Orthop Surg 2017;25:2309499016684722. [DOI] [PubMed] [Google Scholar]
- [8].Ferguson B, Matyszak MK, Esiri MM, et al. Axonal damage in acute multiple sclerosis lesions. Brain 1997;120(pt 3):393–9. [DOI] [PubMed] [Google Scholar]
- [9].Napoli S, Bakshi R. Magnetic resonance imaging in multiple sclerosis. Rev Neurol Dis 2004;2:109–16. [PubMed] [Google Scholar]
- [10].Sahraian MA, Eshaghi A. Role of MRI in diagnosis and treatment of multiple sclerosis. Clin Neurol Neurosurg 2010;112:609–15. [DOI] [PubMed] [Google Scholar]
- [11].Filippi M. Non-conventional MR techniques to monitor the evolution of multiple sclerosis. Neurol Sci 2001;22:195–200. [DOI] [PubMed] [Google Scholar]
- [12].Simone IL, Tortorella C, Federico F, et al. Axonal damage in multiple sclerosis plaques: a combined magnetic resonance imaging and 1H-magnetic resonance spectroscopy study. J Neurol Sci 2001;182:143–50. [DOI] [PubMed] [Google Scholar]
- [13].Rovaris M, Comi G, Filippi M. The role of non-conventional MR techniques to study multiple sclerosis patients. J Neurol Sci 2001;186(suppl 1):S3–9. [DOI] [PubMed] [Google Scholar]
- [14].De Stefano N, Filippi M, Miller D, et al. Guidelines for using proton MR spectroscopy in multicenter clinical MS studies. Neurology 2007;69:1942–52. [DOI] [PubMed] [Google Scholar]
- [15].Lin A, Ross BD, Harris K, et al. Efficacy of proton magnetic resonance spectroscopy in neurological diagnosis and neurotherapeutic decision making. NeuroRx 2005;2:197–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Duarte JM, Lei H, Mlynarik V, et al. The neurochemical profile quantified by in vivo 1H NMR spectroscopy. NeuroImage 2012;61:342–62. [DOI] [PubMed] [Google Scholar]
- [17].Fu L, Matthews P, De Stefano N, et al. Imaging axonal damage of normal-appearing white matter in multiple sclerosis. Brain 1998;121:103–13. [DOI] [PubMed] [Google Scholar]
- [18].Sarchielli P, Presciutti O, Pelliccioli GP, et al. Absolute quantification of brain metabolites by proton magnetic resonance spectroscopy in normal-appearing white matter of multiple sclerosis patients. Brain 1999;122(pt 3):513–21. [DOI] [PubMed] [Google Scholar]
- [19].Narayanan S, Fu L, Pioro E, et al. Imaging of axonal damage in multiple sclerosis: spatial distribution of magnetic resonance imaging lesions. Ann Neurol 1997;41:385–91. [DOI] [PubMed] [Google Scholar]
- [20].Ross B, Bluml S. Magnetic resonance spectroscopy of the human brain. Anat Rec 2001;265:54–84. [DOI] [PubMed] [Google Scholar]
- [21].Bellenberg B, Busch M, Trampe N, et al. 1H-magnetic resonance spectroscopy in diffuse and focal cervical cord lesions in multiple sclerosis. Eur Radiol 2013;23:3379–92. [DOI] [PubMed] [Google Scholar]
- [22].Pokryszko-Dragan A, Bladowska J, Zimny A, et al. Magnetic resonance spectroscopy findings as related to fatigue and cognitive performance in multiple sclerosis patients with mild disability. J Neurol Sci 2014;339:35–40. [DOI] [PubMed] [Google Scholar]
- [23].Wegner C. Recent insights into the pathology of multiple sclerosis and neuromyelitis optica. Clin Neurol Neurosurg 2013;115(suppl 1):S38–41. [DOI] [PubMed] [Google Scholar]
- [24].Hsu SH, Chou MC, Ko CW, et al. Proton MR spectroscopy in patients with pyogenic brain abscess: MR spectroscopic imaging versus single-voxel spectroscopy. Eur J Radiol 2013;82:1299–307. [DOI] [PubMed] [Google Scholar]
- [25].Kolind SH, Madler B, Fischer S, et al. Myelin water imaging: implementation and development at 3.0T and comparison to 1.5T measurements. Magn Reson Med 2009;62:106–15. [DOI] [PubMed] [Google Scholar]
- [26].Wattjes MP, Lutterbey GG, Harzheim M, et al. Higher sensitivity in the detection of inflammatory brain lesions in patients with clinically isolated syndromes suggestive of multiple sclerosis using high field MRI: an intraindividual comparison of 1.5 T with 3.0 T. Eur Radiol 2006;16:2067–73. [DOI] [PubMed] [Google Scholar]
- [27].Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kurtzke JF. Rating neurologic impairment in multiple sclerosis an expanded disability status scale (EDSS). Neurology 1983;33:1444–1444. [DOI] [PubMed] [Google Scholar]
- [29].Aboul-Enein F, Krssak M, Hoftberger R, et al. Reduced NAA-levels in the NAWM of patients with MS is a feature of progression. A study with quantitative magnetic resonance spectroscopy at 3 Tesla. PLoS One 2010;5:e11625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Tartaglia MC, Narayanan S, De Stefano N, et al. Choline is increased in pre-lesional normal appearing white matter in multiple sclerosis. J Neurol 2002;249:1382–90. [DOI] [PubMed] [Google Scholar]
- [31].Birken DL, Oldendorf WH. N-acetyl-L-aspartic acid: a literature review of a compound prominent in 1H-NMR spectroscopic studies of brain. Neurosci Biobehav Rev 1989;13:23–31. [DOI] [PubMed] [Google Scholar]
- [32].Baslow MH. N-acetylaspartate in the vertebrate brain: metabolism and function. Neurochem Res 2003;28:941–53. [DOI] [PubMed] [Google Scholar]
- [33].Arnold DL. Magnetic resonance spectroscopy: imaging axonal damage in MS. J Neuroimmunol 1999;98:2–6. [DOI] [PubMed] [Google Scholar]
- [34].Brex PA, Gomez-Anson B, Parker GJ, et al. Proton MR spectroscopy in clinically isolated syndromes suggestive of multiple sclerosis. J Neurol Sci 1999;166:16–22. [DOI] [PubMed] [Google Scholar]
- [35].Mader I, Roser W, Kappos L, et al. Serial proton MR spectroscopy of contrast-enhancing multiple sclerosis plaques: absolute metabolic values over 2 years during a clinical pharmacological study. Am J Neuroradiol 2000;21:1220–7. [PMC free article] [PubMed] [Google Scholar]
- [36].Beatty WW, Goodkin DE, Beatty PA, et al. Frontal lobe dysfunction and memory impairment in patients with chronic progressive multiple sclerosis. Brain Cogn 1989;11:73–86. [DOI] [PubMed] [Google Scholar]
- [37].Foong J, Rozewicz L, Quaghebeur G, et al. Executive function in multiple sclerosis. The role of frontal lobe pathology. Brain 1997;120(Pt 1):15–26. [DOI] [PubMed] [Google Scholar]
- [38].Bobholz JA, Rao SM. Cognitive dysfunction in multiple sclerosis: a review of recent developments. Curr Opin Neurol 2003;16:283–8. [DOI] [PubMed] [Google Scholar]
- [39].Farez MF, Crivelli L, Leiguarda R, et al. Decision-making impairment in patients with multiple sclerosis: a case-control study. BMJ Open 2014;4:e004918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Staffen W, Zauner H, Mair A, et al. Magnetic resonance spectroscopy of memory and frontal brain region in early multiple sclerosis. J Neuropsychiatry Clin Neurosci 2005;17:357–63. [DOI] [PubMed] [Google Scholar]
- [41].Rovaris M, Gambini A, Gallo A, et al. Axonal injury in early multiple sclerosis is irreversible and independent of the short-term disease evolution. Neurology 2005;65:1626–30. [DOI] [PubMed] [Google Scholar]
- [42].Kotov SV, Kuchina NV, Lapitan DG, et al. Low contrast non-color vision in patients with multiple sclerosis. Zh Nevrol Psikhiatr Im S S Korsakova 2015;115(2 pt 2):16–20. [DOI] [PubMed] [Google Scholar]
- [43].Bjartmar C, Kidd G, Mork S, et al. Neurological disability correlates with spinal cord axonal loss and reduced N-acetyl aspartate in chronic multiple sclerosis patients. Ann Neurol 2000;48:893–901. [PubMed] [Google Scholar]
- [44].Tur C, Wheeler-Kingshott CA, Altmann DR, et al. Spatial variability and changes of metabolite concentrations in the cortico-spinal tract in multiple sclerosis using coronal CSI. Hum Brain Mapp 2014;35:993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Griffin CM, Chard DT, Ciccarelli O, et al. Diffusion tensor imaging in early relapsing-remitting multiple sclerosis. Mult Scler 2001;7:290–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


