Abstract
Although acyclovir prophylaxis against varicella zoster virus (VZV) infection for ≥1 year is recommended after allogeneic hematopoietic cell transplantation (HCT), the emergence of acyclovir-resistant viruses and adverse drug effects cannot be ignored. We investigated the cumulative incidence of VZV infection after allogeneic HCT in children receiving a shorter duration of acyclovir prophylaxis than recommended and evaluated the appropriateness of the short duration of acyclovir prophylaxis.
Medical records of 217 children who received allogeneic HCT were retrospectively reviewed until a median of 25 months (range = 1–59 months) after HCT. Acyclovir prophylaxis was given for a median of 9 weeks (range = 3–24 weeks) after HCT.
VZV infection was diagnosed in 33 (15.2%) children at a median time of 5 months (range = 2–41 months) after HCT. The 1-year and 2-year cumulative incidences of VZV infection after allogeneic HCT were 11.2% and 15.5%, respectively. These incidences were between the previously reported 1-year incidence of 25% to 30% in patients not receiving prophylaxis and 1-year incidence of 4% to 5% in patients receiving ≥1 year duration of prophylaxis. Male sex and older age were significantly associated with VZV infection after allogeneic HCT. Only 1 chickenpox patient experienced severe complications because of VZV infection, and there were no deaths attributable to VZV infection.
In conclusion, a shorter duration of acyclovir prophylaxis may be appropriate for children receiving allogeneic HCT, based on the rare occurrence of severe complications because of VZV infection and the expected discomfort because of daily oral medication for a long time.
Keywords: child, prophylaxis, stem cell transplantation, varicella zoster virus
1. Introduction
The Herpesviridae family, including herpes simplex virus (HSV), varicella zoster virus (VZV), Epstein–Barr virus (EBV), and cytomegalovirus (CMV), maintains latency in the host after its primary infection,[1] and the reactivation of the latent virus under the immunosuppressed state after hematopoietic cell transplantation (HCT) may increase morbidity and mortality in the HCT recipients.[2–5] Accordingly, a recent guideline recommends prophylaxis against HSV and prophylaxis or pre-emptive therapy against CMV,[6] and the effect of this strategy on decreasing HSV and CMV diseases after HCT has been demonstrated in previous studies.[7–9]
VZV infection was found to occur in 33% to 41% of HCT recipients who did not receive acyclovir prophylaxis,[10–12] whereas the occurrence rate decreased to 20% to 32% in HCT recipients treated with acyclovir prophylaxis.[12–15] Although proper administration route, dose, and duration of prophylactic acyclovir have not been defined, a recent guideline recommends prophylaxis for ≥1 year against VZV infection after allogeneic HCT for VZV-seropositive recipients.[6] However, some investigators reported a rising incidence of VZV infection after discontinuation of long-term acyclovir prophylaxis.[15,16] In addition, acyclovir-resistant HSV was reported in human immunodeficiency virus (HIV)-infected patients who received long-term acyclovir therapy and in allogeneic HCT recipients.[17–19] Another consideration is that the children and their parents may be distressed by taking medicine everyday for a long period of time and adverse effects of acyclovir may occur in HCT recipients receiving various drugs concurrently.
In our hospital where long-term acyclovir prophylaxis has not been performed, the incidence of VZV infection after allogeneic HCT seemed to be lower than that previously reported and severe complications of VZV infection were rare even in allogeneic HCT recipients. This retrospective study was conducted to investigate the incidence and clinical characteristics of VZV infection after pediatric allogeneic HCT in a hospital where relatively short-term acyclovir prophylaxis has been performed and to evaluate the necessity of long-term acyclovir prophylaxis after allogeneic HCT.
2. Patients and methods
2.1. Patients and study design
Medical records of patients <20 years who received allogeneic HCT in the Pediatric division of the Catholic Blood and Marrow Transplantation Center, College of Medicine, the Catholic University of Korea, Seoul, Republic of Korea between April 2009 and March 2013 were retrospectively reviewed. April 1, 2014, which was when ≥1 year had passed after HCT in all of the subjects, was defined as the study endpoint because most cases of VZV infection after allogeneic HCT occur <1 year after HCT.[3,13,20,21] If the recipient died or was transferred to another hospital before the defined endpoint, the event day was decided to be an endpoint. The development of VZV infection from HCT to the endpoint was observed in the enrolled subjects and they were divided into 2 groups: a VZV group and a non-VZV group. VZV infection included both chickenpox and herpes zoster. Clinical and laboratory characteristics of patients who experienced VZV infection after allogeneic HCT were investigated, and demographic and clinical characteristics were compared between the 2 patient groups to determine risk factors for VZV infection. This study was approved by the Institutional Review Board of Seoul St. Mary's Hospital, College of Medicine, the Catholic University of Korea with an exemption for acquiring informed consent (approval no.: KC14RISI0353).
2.2. Transplantation and antibacterial, antiviral, and antifungal prophylaxis
Pre-transplant conditioning and graft-versus-host disease (GvHD) prevention were performed as has been introduced in a previously reported study.[22] In brief, nonmyeloablative conditioning based on fludarabine (30 mg/m2/day for 4 days) and cyclophosphamide (25 mg/kg/day for 4 days) was given to recipients with several types of anemia including severe aplastic anemia (SAA). All of the other recipients received myeloablative conditioning based on total body irradiation (TBI, 200 cGy twice daily for 3 days) and cyclophosphamide (60 mg/kg/day for 2 days), busulphan (130 mg/m2/day for 4 days), and fludarabine (40 mg/m2/day for 4 days). Rabbit anti-thymocyte globulin (2.5 mg/kg/day for 3 days) was additionally given to all SAA patients to prevent acute rejection and those who received unrelated donor transplants to prevent severe acute GvHD. For GvHD prevention, cyclosporine (3 mg/kg/day) was administered from day-1, and mini-dose methotrexate (5 mg/m2/day at days 1, 3, 6, and 11) instead of standard dose methotrexate was administered to reduce methotrexate-related toxicities. Glucocorticoids or methotrexate were added according to the degree of GvHD. Fluoroquinolone prophylaxis was not performed because almost all recipients were <18 years. Oral trimethoprim/sulfamethoxazole (150 mg trimethoprim/m2/day, 3 times weekly) was administered from engraftment to the discontinuation of immune suppressants to prevent Pneumocystis jirovecii infection. As an antifungal prophylaxis, parenteral micafungin (1 mg/kg/day) was administered from conditioning to engraftment, and then oral fluconazole (3 mg/kg/day) was administered until the discontinuation of immune suppressants. CMV reactivation was monitored after HCT using real-time quantitative polymerase chain reaction weekly until 3 months, biweekly until 6 months, and monthly until 1 year after HCT. Pre-emptive ganciclovir therapy was performed when the CMV DNA titer was >10,000 copies/mL in recipients who received matched-related donor transplants or > 1,000 copies/mL in the other recipients. Oral acyclovir (20 mg/kg 4 times daily, maximum 800 mg/day) was administered from conditioning to 6 weeks after HCT for prophylaxis against HSV and VZV, and the prophylaxis was individualized and could be extended based on the degree of acute GvHD and glucocorticoid therapy in each recipient. Intravenous acyclovir (5 mg/kg 3 times daily) was substituted for oral acyclovir if the recipient could not swallow oral medications because of severe mucositis. Acyclovir was suspended during ganciclovir, foscarnet, or cidofovir administration to treat CMV reactivation as these antiviral agents also have inhibitory effects on VZV as well as CMV.[23]
2.3. Definitions
VZV infection was diagnosed by at least 2 pediatricians independently based on clinical symptoms and signs of the patient. Chickenpox was diagnosed when typical vesicular skin lesions were observed in a scattered pattern, not showing a dermatomal distribution, and herpes zoster was diagnosed when the skin lesions were restricted to a unilateral dermatome.[24] Cutaneous dissemination of herpes zoster was diagnosed when ≥2 dermatomes were involved. If clinical and laboratory findings suggesting damage to internal organs were observed and other specific causes of them were not defined, a visceral involvement of VZV infection was defined.
2.4. Statistical analysis
Demographic and clinical characteristics including age, sex, underlying hematologic/oncologic disease and the status of the underlying disease, types of pretransplant conditioning and transplant, acute and chronic GvHD, duration of acyclovir prophylaxis, and CMV and EBV reactivation after HCT were compared between the VZV and non-VZV groups to determine risk factors for VZV infection after HCT. In a univariate analysis, categorical factors were compared using a chi-square test and numerical factors were compared using a Mann–Whitney test. A multivariate analysis was performed using a binary logistic regression test with the factors that exhibited a significant difference in the univariate analysis. The incidence of VZV infection was calculated using the cumulative incidence (CI) function, with death without experiencing VZV infection as a competing risk. Statistical analyses were performed using SPSS Statistics 17.0 (SPSS Inc., Chicago, IL), and the statistical significance was defined as a 2-tailed P value <0.05. The CI of chickenpox and herpes zoster was calculated using R package version 2.14.2 (R foundation for Statistical Computing, Vienna, Austria).
3. Results
During the study period, a total of 223 allogeneic HCTs were performed, and 217 of them were included in this study, excluding 6 children with engraftment failure or who died before engraftment. Among the enrolled children, 11 (5.1%) children were transferred to other hospitals or lost to follow-up and 51 (23.5%) children died before the defined endpoint, April 1, 2014. The median follow-up time was 25 months (range = 1–59 months), and acyclovir prophylaxis was performed for a median of 9 weeks after HCT (range = 3–24 weeks). VZV infection was diagnosed in 33 (15.2%) children including 8 children with chickenpox and 25 children with herpes zoster. VZV infection occurred at a median of 5 months (range = 2–41 months) after HCT, and 23 (69.7%) of the infection occurred <1 year after HCT and 32 (97.0%) of them occurred <2 years after HCT. The 1-year and 2-year CIs of VZV infection after allogeneic HCT were 11.2% (standard error [SE] = 2.9) and 15.5% (SE = 3.6), respectively (Fig. 1).
Figure 1.
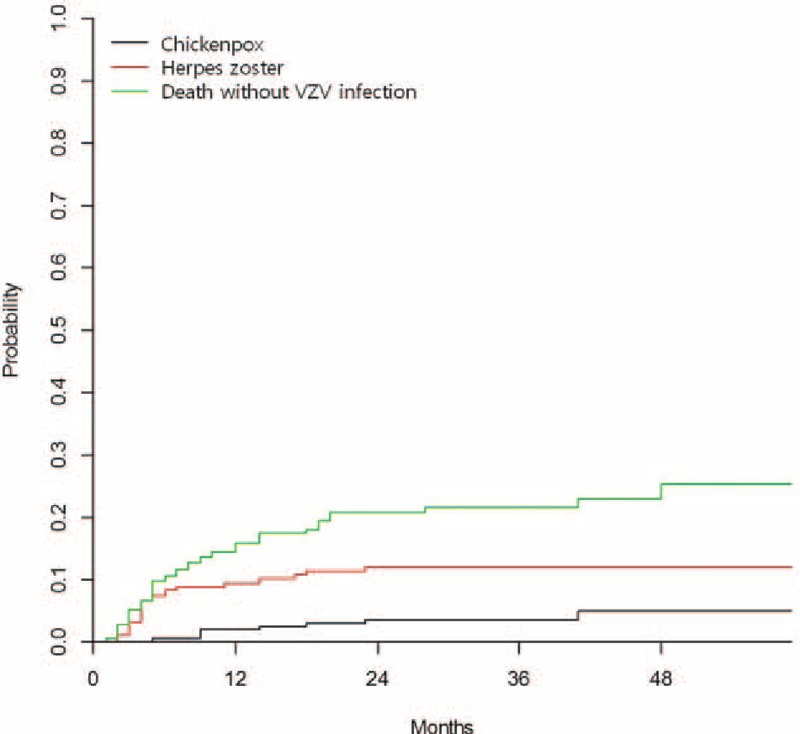
The cumulative incidence of chickenpox and herpes zoster after pediatric allogeneic hematopoietic cell transplantation.
3.1. Comparison of demographic and clinical characteristics between children with and without VZV infection after allogeneic HCT
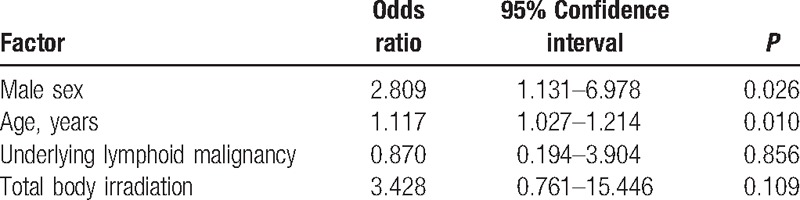
Demographic and clinical characteristics were compared between 33 children who experienced VZV infection after allogeneic HCT and 184 children who did not (Table 1). Significantly more males were included in the VZV group than in the non-VZV group, and the median age of the children of the VZV group was significantly higher than those of the non-VZV group (Table 1). VZV infection occurred more frequently in children aged ≥ 10 years compared to children <10 years (P = 0.02), and no children <5 years experienced VZV infection after allogeneic HCT (Fig. 2). In a univariate analysis, significantly more children suffered from lymphoid diseases, such as acute lymphoblastic leukemia, and malignant lymphoma, and received TBI during pretransplant conditioning in the VZV group compared to the non-VZV group (Table 1). The duration of follow-up and acyclovir prophylaxis after allogeneic HCT was not significantly different between the 2 groups (Table 1). Overall mortality was also not significantly different between the 2 groups and there was no death attributable to VZV infection (Table 1). A multivariate analysis was performed with significant factors in the univariate analysis including sex, age, underlying disease, and TBI during conditioning (Table 2). Male sex and older age were significant risk factors for VZV infection after pediatric allogeneic HCT in the multivariate analysis.
Table 1.
Comparison of characteristics between children with and without varicella zoster virus infection after allogeneic hematopoietic cell transplantation.
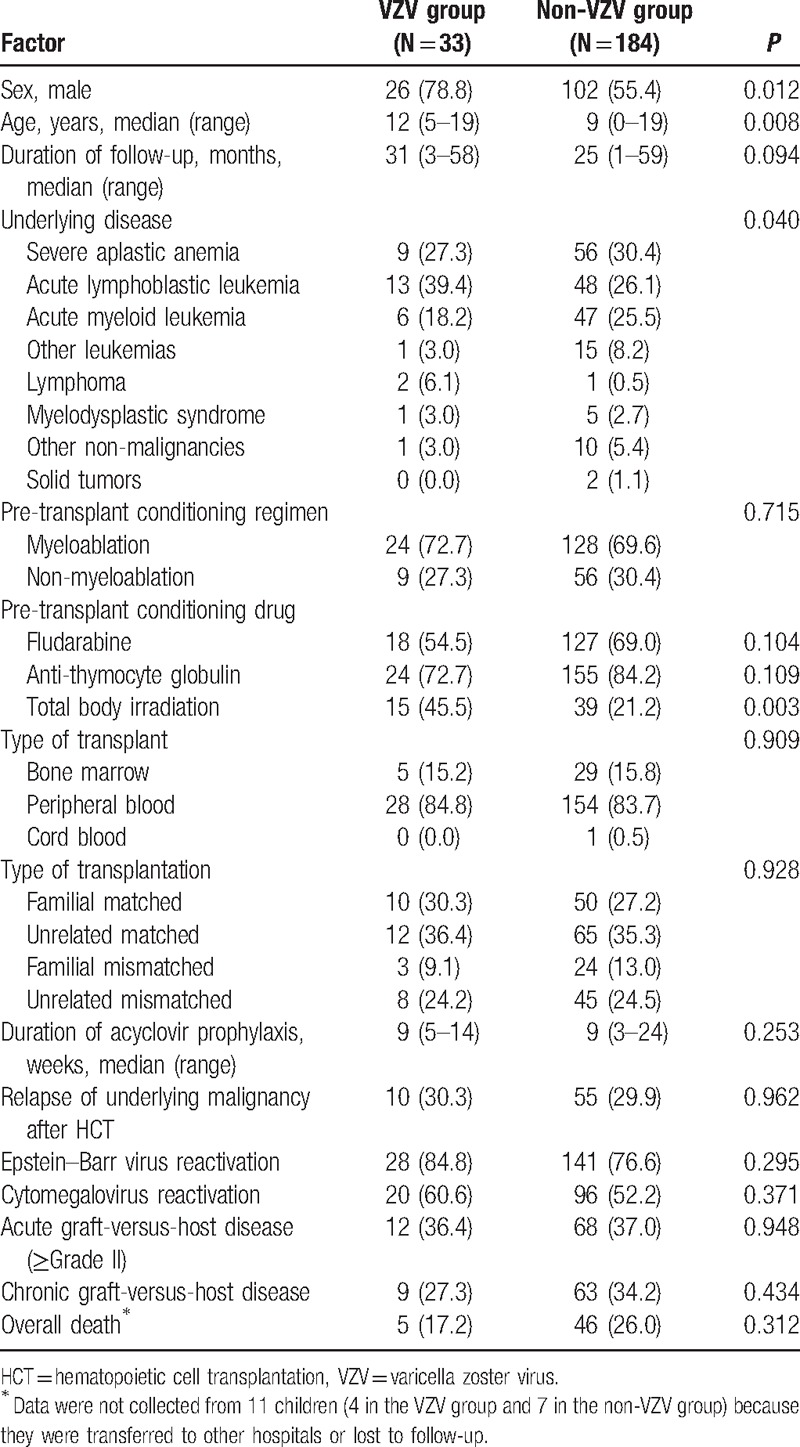
Figure 2.
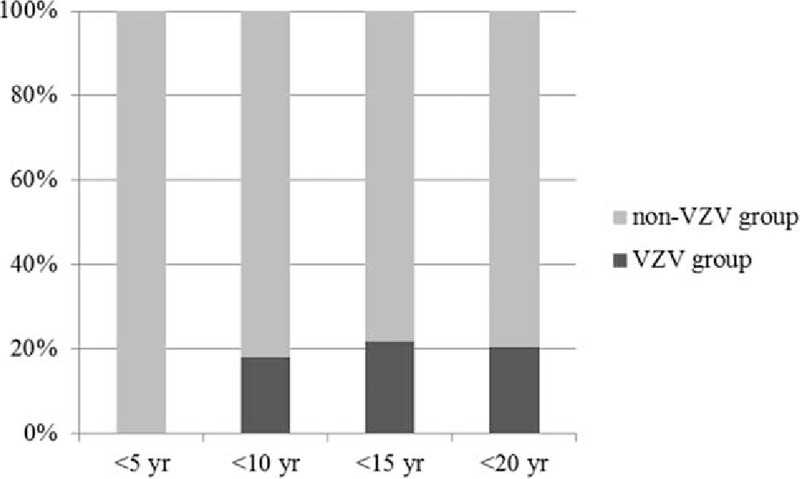
Age distribution of varicella zoster virus infection after pediatric allogeneic hematopoietic cell transplantation (P <0.01).
Table 2.
Multivariate analysis for risk factors of varicella zoster virus infection after pediatric allogeneic hematopoietic cell transplantation.
3.2. Characteristics of children who experienced VZV infection after allogeneic HCT
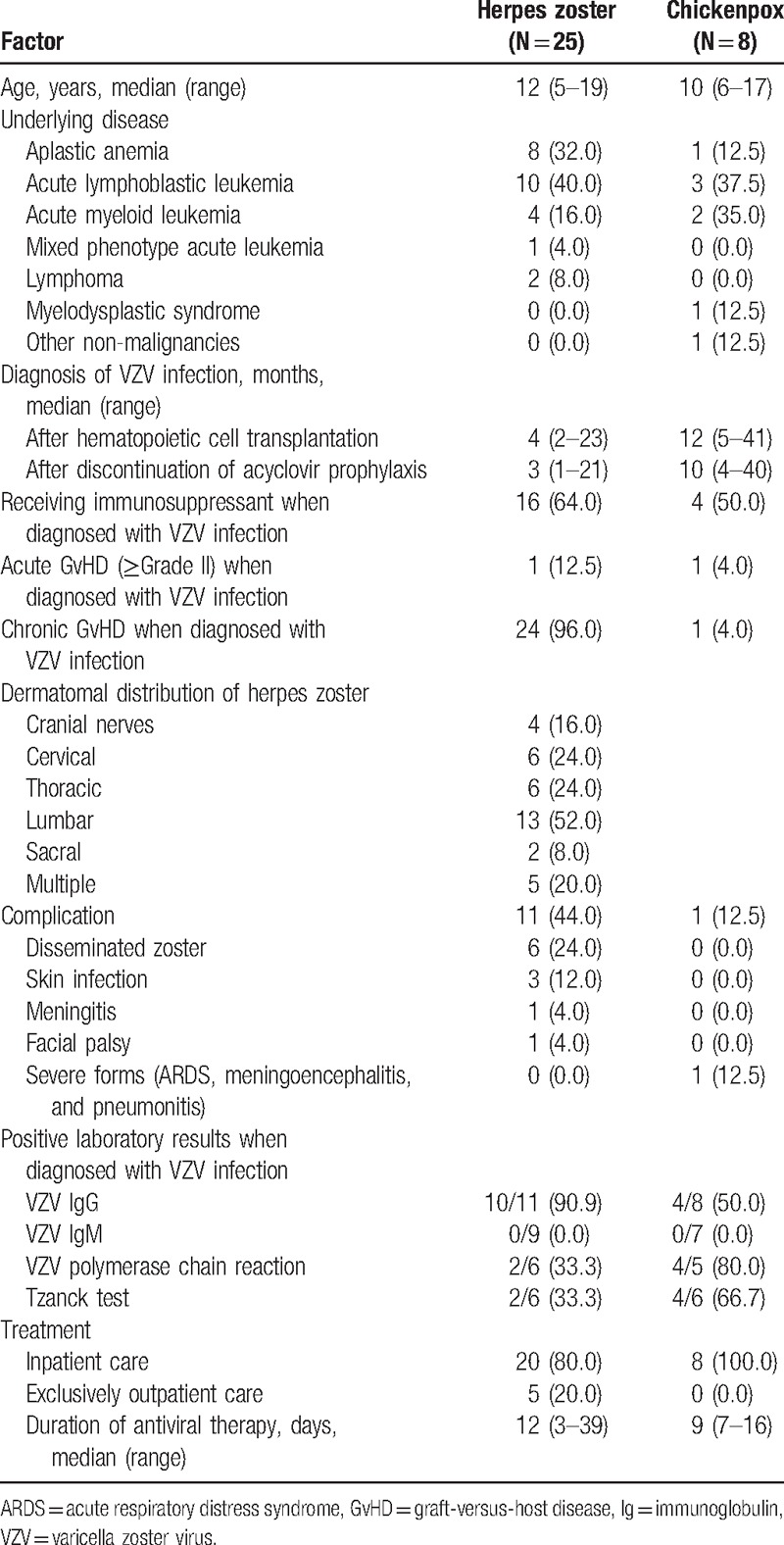
A total of 33 children were diagnosed with VZV infection after allogeneic HCT: 8 with chickenpox and 25 with herpes zoster (Table 3). No breakthrough infection occurred during acyclovir prophylaxis, and VZV infection occurred at a median time of 4 months (range = 1–40 months) after the discontinuation of acyclovir prophylaxis. The proportion of children receiving immunosuppressant treatment when diagnosed with VZV infection and the frequencies of acute and chronic GvHD were higher in the children with herpes zoster than those with chickenpox. One child diagnosed with chickenpox experienced severe complications including pneumonitis, meningoencephalitis, and acute respiratory distress syndrome. He survived after intensive care and intravenous acyclovir and high-dose intravenous immunoglobulin therapy, and has been reported as a single case.[25] The remaining 7 children diagnosed with chickenpox recovered without any severe complications. Herpes zoster occurred most frequently in the lumbar dermatome (Table 3). The most common complication of herpes zoster was cutaneous dissemination in 6 (24.0%) children, and no child experienced postherpetic neuralgia lasting >1 month, which was dissimilar to what is often observed in adults. All the children with VZV infection recovered after antiviral therapy and 28 (84.8%) children were hospitalized, with 5 (15.2%) children with herpes zoster being treated in the outpatient clinic. The 28 hospitalized children received intravenous acyclovir (10 mg/kg or 500 mg/m2 3 times daily) for a median of 9 days (range = 2–16 days), and the median duration of the entire antiviral therapy was 12 days (range = 3–39 days). The 5 non-hospitalized children received oral acyclovir or famciclovir therapy for a median of 12 days (range = 7–15 days). Serologic tests for VZV were performed in 19 children when diagnosed with chickenpox or herpes zoster. VZV IgG was positive in 14 (73.3%) of the 19 children, and VZV IgM was negative in all of the 16 children tested (Table 3). Blood VZV polymerase chain reaction on the diagnosis of VZV infection was positive in 6 (54.5%) of 11 tested children, and the Tzanck test was positive in 6 (50.0%) of 12 tested children.
Table 3.
Characteristics of children diagnosed with varicella zoster virus infection after allogeneic hematopoietic cell transplantation.
4. Discussion
In the present study, male sex and older age were significant risk factors for VZV infection after allogeneic HCT. It was assumed that VZV reactivation was infrequent in children <10 years because of the low seroprevalence against VZV before HCT in this age group.[3] In Korea, the seroprevalence against VZV gradually increases from 1 year of age and it is maintained at >94% in teenagers,[26] and therefore, the seroprevalence against VZV according to the recipient's age would be expected to affect the incidence of VZV infection after HCT in the present study, although we could not determine the exact seropositivity of recipients. Although several previous studies reported that recipients with VZV infection after HCT were more frequently males compared to those without VZV infection,[11,13,16] differences between the 2 sexes exhibited no statistical significance. The significance of sex in VZV infection after HCT should be further evaluated in future studies that include more subjects.
The CIs of VZV infection at 1 year and 2 years after pediatric allogeneic HCT in the present study were 11.2% and 15.5%, respectively, and these were lower than previously reported 1-year CIs of 14% to 34%,[13,15,27] and were also lower than the 1-year CI in adult recipients of our hospital, 22%.[5] VZV infection occurred at a median of 2 to 3 months after HCT without acyclovir prophylaxis in previous studies,[10,27] and a median of 5 months after HCT with a median of 9 weeks of prophylaxis in the present study. These findings indicate that acyclovir prophylaxis can shift the development of VZV infection to a later time after HCT, and that acyclovir prophylaxis is effective in preventing VZV infection during the early period after HCT. Previous studies reported that the development of VZV infection was delayed to 1 year after HCT with 1 year of prophylaxis,[15,16] and some investigators reported lower incidence rates of VZV infection after HCT with a prolonged duration of prophylaxis. Kim et al[28] reported that the 2-year CI of VZV infection in adults who received allogeneic peripheral blood stem cell transplantation was 5.8% under a median of 11 months of acyclovir prophylaxis. Erard et al[2] reported that the 2-year CIs of VZV infection in adults and children who received allogeneic HCT were 7.4% and 3.6% under 1-year and over 1-year of acyclovir prophylaxis, respectively. These results may support the necessity of a long-term acyclovir prophylaxis after allogeneic HCT. However, pediatric studies on the effect of long-term acyclovir prophylaxis are scarce, and the emergence of acyclovir-resistant viruses and adverse effects of long-term acyclovir prophylaxis should be considered. Considering the correlation between older patient age and VZV infection, the low rate of severe VZV-related complications in our study, and the patient and caregiver distress related to prolonged medication administration, a relatively short duration of acyclovir prophylaxis of <1 year may be feasible for pediatric allogeneic HCT recipients. Future studies should determine an appropriate duration of acyclovir prophylaxis, one that achieves a lower incidence of VZV infection and simultaneously minimizes concern for the emergence of acyclovir-resistant viruses and the discomfort of daily oral medication. The appropriate endpoint of acyclovir prophylaxis should be the time of the restoration of VZV-specific cellular immunity after HCT, and VZV-specific cellular immune responses were reported to recover between 6 weeks and 18 months after allogeneic HCT.[1,29] In addition, a comparison of cost-effectiveness between a long-term and short-term acyclovir prophylaxis, taking into account the incidence of VZV infection and the discomfort of daily oral medication, should be performed in children receiving allogeneic HCT.
All the children diagnosed with VZV infection after allogeneic HCT recovered with acyclovir or famciclovir therapy in the present study. Previous studies also reported that most VZV-infected patients, including breakthrough infection cases, responded to acyclovir therapy,[9,15,20,30] and therefore, VZV resistant to acyclovir is considered to be very rare. Herpes zoster in the present study occurred most frequently in lumbar dermatomes rather than thoracic dermatomes, which were previously reported as those most frequently involved.[5,10,11,15,20,30] However, a different study reported that cervical dermatomes were the most frequently involved dermatome,[13] and lumber dermatomes were usually the second most frequently involved area in most previous reports.[3,15,20,31]
Limitations of the present study arose from the retrospective nature of the study. We could not evaluate enough clinical factors that are suspected of being associated with VZV infection after HCT. The presence of anti-VZV antibodies before HCT in the HCT recipients was significantly associated with increasing incidence of VZV infection after HCT.[20] However, we could not evaluate the effect of recipients’ anti-VZV antibodies in the present study because recipients’ serologic status for VZV had not been evaluated before HCT in our institute. The present study could not evaluate the varicella vaccine effect in HCT recipients because previous histories of chickenpox, herpes zoster, and varicella vaccination in recipients could not be collected, although a single dose of varicella vaccination at 12 to 15 months of age has been recommended in Korea since 2005. Although the occurrence of herpes zoster was reduced with the introduction of varicella vaccination in a community,[32–34] it has not been defined whether the occurrence of VZV infection in HCT recipients who have been vaccinated against VZV before HCT decreases or not.
In conclusion, the incidence of VZV infection after pediatric allogeneic HCT under a median of 9 weeks of acyclovir prophylaxis was higher than that under 1 year or longer duration of prophylaxis. However, a shorter duration of acyclovir prophylaxis may be appropriate for children receiving allogeneic HCT compared to adults, based on the rare occurrence of severe complications because of VZV infection and the expected discomfort because of daily oral medication for a long time. Further studies to determine the appropriate duration, administration route, and dose of acyclovir prophylaxis are necessary. In addition, individualized prophylaxis strategies based on a previous history of chickenpox and varicella vaccination in HCT recipients may be needed as varicella and zoster vaccination is expanding.
Footnotes
Abbreviations: CI = cumulative incidence, CMV = cytomegalovirus, EBV = Epstein–Barr virus, GvHD = graft-versus-host disease, HCT = hematopoietic cell transplantation, HIV = human immunodeficiency virus, HSV = herpes simplex virus, SAA = severe aplastic anemia, SE = standard error, TBI = total body irradiation, VZV = varicella zoster virus.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Arvin AM. Varicella–Zoster virus: pathogenesis, immunity, and clinical management in hematopoietic cell transplant recipients. Biol Blood Marrow Transplant 2000;6:219–30. [DOI] [PubMed] [Google Scholar]
- [2].Erard V, Guthrie KA, Varley C, et al. One-year acyclovir prophylaxis for preventing varicella–zoster virus disease after hematopoietic cell transplantation: no evidence of rebound varicella–zoster virus disease after drug discontinuation. Blood 2007;110:3071–7. [DOI] [PubMed] [Google Scholar]
- [3].Locksley RM, Flournoy N, Sullivan KM, et al. Infection with varicella–zoster virus after marrow transplantation. J Infect Dis 1985;152:1172–81. [DOI] [PubMed] [Google Scholar]
- [4].Umezawa Y, Kakihana K, Oshikawa G, et al. Clinical features and risk factors for developing varicella zoster virus dissemination following hematopoietic stem cell transplantation. Transpl Infect Dis 2014;16:195–202. [DOI] [PubMed] [Google Scholar]
- [5].Lee DG. Common infectious diseases in hematopoietic stem cell transplant recipients. Korean J Med 2013;84:158–67. [Google Scholar]
- [6].Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant 2009;15:1143–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wade JC, Newton B, Flournoy N, et al. Oral acyclovir for prevention of herpes simplex virus reactivation after marrow transplantation. Ann Intern Med 1984;100:823–8. [DOI] [PubMed] [Google Scholar]
- [8].Erard V, Wald A, Corey L, et al. Use of long-term suppressive acyclovir after hematopoietic stem-cell transplantation: impact on herpes simplex virus (HSV) disease and drug-resistant HSV disease. J Infect Dis 2007;196:266–70. [DOI] [PubMed] [Google Scholar]
- [9].Gluckman E, Lotsberg J, Devergie A, et al. Prophylaxis of herpes infections after bone-marrow transplantation by oral acyclovir. Lancet 1983;2:706–8. [DOI] [PubMed] [Google Scholar]
- [10].Kawasaki H, Takayama J, Ohira M. Herpes zoster infection after bone marrow transplantation in children. J Pediatr 1996;128:353–6. [DOI] [PubMed] [Google Scholar]
- [11].Koc Y, Miller KB, Schenkein DP, et al. Varicella zoster virus infections following allogeneic bone marrow transplantation: frequency, risk factors, and clinical outcome. Biol Blood Marrow Transplant 2000;6:44–9. [DOI] [PubMed] [Google Scholar]
- [12].Masaoka T, Hiraoka A, Teshima H, et al. Varicella–zoster virus infection in immunocompromised patients. J Med Virol 1993;(suppl 1):82–4. [DOI] [PubMed] [Google Scholar]
- [13].Aytaç S, Yalçin SS, Küçükbayrak O, et al. Dynamics in children and adolescents who experience varicella zoster virus infections after haematopoietic stem cell transplantation: a case-control study. Epidemiol Infect 2011;139:1701–9. [DOI] [PubMed] [Google Scholar]
- [14].Berman JN, Wang M, Berry W, et al. Herpes zoster infection in the post-hematopoietic stem cell transplant pediatric population may be preceded by transaminitis: an institutional experience. Bone Marrow Transplant 2006;37:73–80. [DOI] [PubMed] [Google Scholar]
- [15].Asano-Mori Y, Kanda Y, Oshima K, et al. Long-term ultra-low-dose acyclovir against varicella–zoster virus reactivation after allogeneic hematopoietic stem cell transplantation. Am J Hematol 2008;83:472–6. [DOI] [PubMed] [Google Scholar]
- [16].Boeckh M, Kim HW, Flowers ME, et al. Long-term acyclovir for prevention of varicella zoster virus disease after allogeneic hematopoietic cell transplantation—a randomized double-blind placebo-controlled study. Blood 2006;107:1800–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chakrabarti S, Pillay D, Ratcliffe D, et al. Resistance to antiviral drugs in herpes simplex virus infections among allogeneic stem cell transplant recipients: risk factors and prognostic significance. J Infect Dis 2000;181:2055–8. [DOI] [PubMed] [Google Scholar]
- [18].Langston AA, Redei I, Caliendo AM, et al. Development of drug-resistant herpes simplex virus infection after haploidentical hematopoietic progenitor cell transplantation. Blood 2002;99:1085–8. [DOI] [PubMed] [Google Scholar]
- [19].Jacobson MA, Berger TG, Fikrig S, et al. Acyclovir-resistant varicella zoster virus infection after chronic oral acyclovir therapy in patients with the acquired immunodeficiency syndrome (AIDS). Ann Intern Med 1990;112:187–91. [DOI] [PubMed] [Google Scholar]
- [20].Leung TF, Chik KW, Li CK, et al. Incidence, risk factors and outcome of varicella–zoster virus infection in children after haematopoietic stem cell transplantation. Bone Marrow Transplant 2000;25:167–72. [DOI] [PubMed] [Google Scholar]
- [21].Styczynski J, Reusser P, Einsele H, et al. Management of HSV, VZV and EBV infections in patients with hematological malignancies and after SCT: guidelines from the Second European Conference on Infections in Leukemia. Bone Marrow Transplant 2009;43:757–70. [DOI] [PubMed] [Google Scholar]
- [22].Han SB, Bae EY, Lee JW, et al. Features of Epstein-Barr virus reactivation after allogeneic hematopoietic cell transplantation in Korean children living in an area of high seroprevalence against Epstein-Barr virus. Int J Hematol 2014;100:188–99. [DOI] [PubMed] [Google Scholar]
- [23].De SK, Hart JC, Breuer J. Herpes simplex virus and varicella zoster virus: recent advances in therapy. Curr Opin Infect Dis 2015;28:589–95. [DOI] [PubMed] [Google Scholar]
- [24].Gershon AA. Varicella–zoster virus infections. Pediatr Rev 2008;29:5–10. [DOI] [PubMed] [Google Scholar]
- [25].Kim JH, Kwon DH, Bae EY, et al. Use of intravenous immunoglobulin in a disseminated varicella infection in an immunocompromised child. Korean J Pediatr 2014;57:370–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lee H, Cho HK, Kim KH. Seroepidemiology of varicella–zoster virus in Korea. J Korean Med Sci 2013;28:195–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Vermont CL, Jol-van der Zijde EC, Hissink Muller P, et al. Varicella zoster reactivation after hematopoietic stem cell transplant in children is strongly correlated with leukemia treatment and suppression of host T-lymphocyte immunity. Transpl Infect Dis 2014;16:188–94. [DOI] [PubMed] [Google Scholar]
- [28].Kim DH, Kumar D, Messner HA, et al. Clinical efficacy of prophylactic strategy of long-term low-dose acyclovir for Varicella–Zoster virus infection after allogeneic peripheral blood stem cell transplantation. Clin Transplant 2008;22:770–9. [DOI] [PubMed] [Google Scholar]
- [29].Ljungman P, Lönnqvist B, Gahrton G, et al. Clinical and subclinical reactivations of varicella–zoster virus in immunocompromised patients. J Infect Dis 1986;153:840–7. [DOI] [PubMed] [Google Scholar]
- [30].Steer CB, Szer J, Sasadeusz J, et al. Varicella–zoster infection after allogeneic bone marrow transplantation: incidence, risk factors and prevention with low-dose aciclovir and ganciclovir. Bone Marrow Transplant 2000;25:657–64. [DOI] [PubMed] [Google Scholar]
- [31].Takayama N, Yamada H, Kaku H, et al. Herpes zoster in immunocompetent and immunocompromised Japanese children. Pediatr Int 2000;42:275–9. [DOI] [PubMed] [Google Scholar]
- [32].Hardy I, Gershon AA, Steinberg SP, et al. The incidence of zoster after immunization with live attenuated varicella vaccine. A study in children with leukemia. Varicella Vaccine Collaborative Study Group. N Engl J Med 1991;325:1545–50. [DOI] [PubMed] [Google Scholar]
- [33].Marin M, Güris D, Chaves SS, et al. Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC). Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007;56(RR–4):1–40. [PubMed] [Google Scholar]
- [34].Brunell PA, Taylor-Wiedeman J, Geiser CF, et al. Risk of herpes zoster in children with leukemia: varicella vaccine compared with history of chickenpox. Pediatrics 1986;77:53–6. [PubMed] [Google Scholar]


