Abstract
Background:
Previous studies investigating the association between BRAF mutations and nonsmall cell lung cancer (NSCLC) remain controversial. To address the issue, we performed an updated meta-analysis of related articles.
Methods:
We conducted a comprehensive literature search in the electronic databases including ISI Science Citation Index, EMBASE, PubMed, and CNKI (up to January 2016). The odds ratios (ORs) and 95% confidence interval (CI) were assessed based on random-effects or fixed-effects models according to the heterogeneity of eligible studies.
Results:
A total of 16 studies enrolled 11,711 patients with NSCLC were involved in the meta-analysis. The overall BRAF mutation rate was 2.6% (303/11,711). There was a significant association between BRAF mutations and adenocarcinomas (ADCs) in NSCLC compared with non-ADCs (OR = 3.96, 95% CI = 2.13–7.34, P < 0.0001). No significant difference was observed in smoking and stage in patients with BRAF mutations. However, a significant difference of BRAF mutation rate was observed between women and men (OR = 0.72, 95% CI = 0.55–0.95, P = 0.02). In addition, the BRAFV600E mutations were more frequent in women (OR = 0.45, 95% CI = 0.26–0.77, P = 0.004) and never smokers (OR = 0.12, 95% CI = 0.05–0.29, P < 0.00001).
Conclusions:
BRAF mutations in ADCS and female significantly increased the risk of NSCLC compared to non-ADCS and male, respectively. BRAFV600E mutation in NSCLC patients was significantly associated with female and nonsmokers.
Keywords: BRAF, meta-analysis, mutation, nonsmall cell lung cancer
1. Introduction
Lung carcinoma is one of the leading causes of cancer-related death universally, and patients with lung carcinoma are often diagnosed at advanced stage with poor prognosis.[1] Nonsmall cell lung cancers (NSCLCs) occupies most of lung carcinoma and 5-year survival rate remain very low.[2,3] Traditional therapeutic methods for lung carcinoma are mainly based on cancer histology. Classifying NSCLC into clinically related molecular subclasses may be helpful for the cure of NSCLC. The classification was constructed depending on mutations of genes, including HER2, KRAS, PIK3CA, BRAF, and others in frequencies more than 1%.[4–6] In addition, the NSCLC subtypes have been successfully established by using an overlap of genomic molecular markers and immunohistochemistry.[7]
BRAF is one of RAF kinase family member and plays a vital role in the mitogen-activated protein kinase signal pathways.[8] BRAF variations have been investigated in multiple cancers, such as colorectal cancer, papillary thyroid cancer, melanoma, and ovarian cancer.[9–12] Moreover, BRAF variations were also observed in NSCLCs.[13]
Gene variations are closely correlated to specific clinic pathologic characteristics, including gender, smoking, tumor histology, and clinical stage.[14,15] Although BRAF mutations have been found in NSCLC for quite a few years, associations between BRAF mutations and NSCLC are still disputable due to the lack of patient cases explored.[16,17] Therefore, we performed a meta-analysis from much more related studies to accurately evaluate the association of BRAF mutations with NSCLC.
2. Materials and methods
2.1. Search strategy and study selection
A computerized literature search was conducted in the PubMed, EMBASE, ISI, and CNKI databases to screen eligible articles. The deadline of retrieval period was up to January 2016. The terms and keywords used for search were as follows: “BRAF or B-RAF,” “large cell lung cancer or carcinoma,” “squamous-cell lung carcinoma or cancer,” “non-small cell lung cancer or carcinoma,” “lung adenocarcinoma,” and “NSCLC.”
Studies were selected if they met the following criteria: studies on the relationship between BRAF mutations and NSCLC; studies available as a full text; the patient number with BRAF mutations was more than one. The excluded standards were as follows: duplicate publications; studies without valuable data; reviews, letters, case reports, and expert opinions. The institutional review board was approved by the First Affiliated Hospital of Zhengzhou University.
2.2. Data extraction
The following information was extracted by 2 investigators (GC and DL) independently from the eligible studies: author's last name, publication year, number of patient cases, country of origin, gene detection method, number of BRAF mutations, number of BRAFV600E mutations, gender, smoking, histology, and clinical stage of patients. Any disagreement was resolved by discussion among all investigators.
2.3. Statistical analysis
Odds ratios (ORs) and 95% confidence interval (95% CI) were used to estimate the association between BRAF mutations and NSCLC risk by RevMan (version 5.2). The degree of heterogeneity between the studies was evaluated by the Chi-square value and I2 value. A Chi-square test P < 0.10 or an I2 value >50% indicated a significant heterogeneity.[18] If I2 ≤ 50%, a fixed-effects model was chosen for analysis. In contrast, a random-effects model was applied. Potential publication bias was assessed using Begg test. Sensitivity analysis was explored to ensure the reliability of the results.
3. Results
3.1. Search results and study characteristics
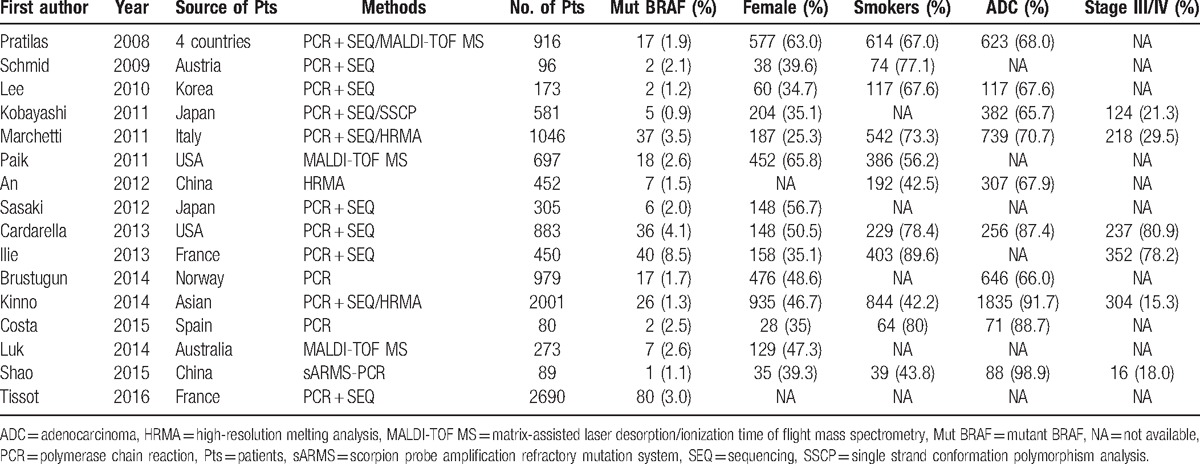
The steps of retrieving eligible articles for the meta-analysis are represented in Fig. 1. The main characteristics of 16 eligible studies with 11,711 patients included in the meta-analysis are shown in Table 1.[16,17,19–32] The articles were published from November 2008[22] to January 2016.[31] Overall, 6 studies were performed in Asian, 6 studies from Europe, 2 from USA, 1 from Australia, and 1 from a mixed 4 countries. Different methods, including PCR, PCR + SEQ, PCR + SEQ/MALDI-TOF MS, PCR + SEQ/SSCP, PCR + SEQ/HRMA, MALDI-TOF MS, HRMA, sAMS-PCR, were used to detect BRAF mutation. The number of patient cases ranged from 80 to 2690. BRAF mutation rate in distinct studies was from 0.9% to 8.9%. Only 303 (2.6%) of these patients with NSCLC harbored BRAF mutations.
Figure 1.
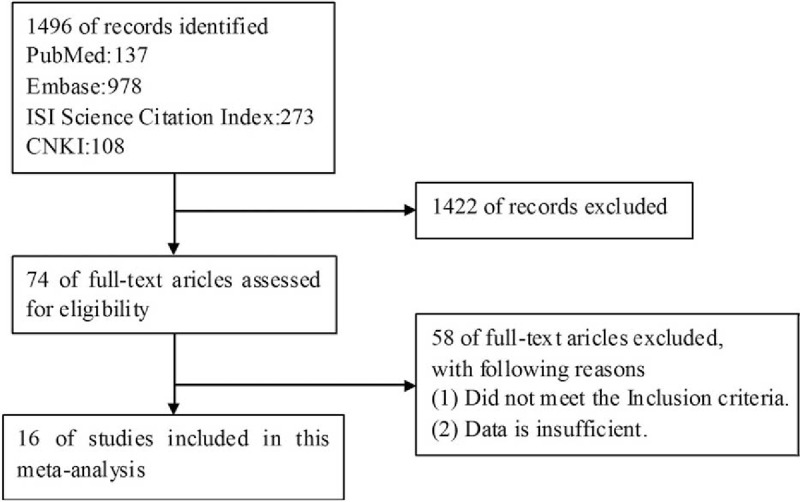
The flow chart for retrieving eligible articles used in the meta-analysis.
Table 1.
Characteristics of the studies included in the meta-analysis.
3.2. Correlation between BRAF mutations and clinic pathologic characteristics in NSCLC
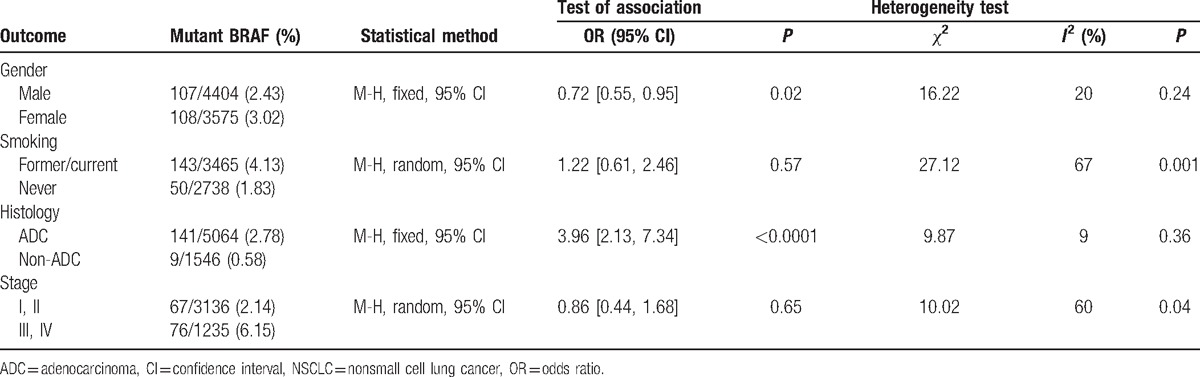
The associations of BRAF mutations with clinic pathologic characteristics in NSCLC are shown in Fig. 2 and Table 2. Fourteen studies including 7979 patients were analyzed for associations between the mutations of BRAF and gender. The results showed that 107 of 4404 male patients (2.43%) were BRAF mutations positive and 108 (3.02%) of 3575 female patients were BRAF mutations positive, indicating a significant difference of BRAF mutations between female and male (OR = 0.72, 95% CI = 0.55–0.95, P = 0.02). Eleven studies reported the association of BRAF gene mutations with smoking. The results suggested that 143 (4.13%) of 3465 smokers were detected harboring BRAF mutations and 50 (1.83%) of 2738 never smokers were detected harboring BRAF mutations, indicating that there were no significant differences in BRAF mutations between smokers and never smokers (OR = 1.22, 95% CI = 0.61–2.46, P = 0.57). Ten studies informed the association of BRAF mutation with cancer histology. One hundred forty-one of 5064 ADCs (2.78%) harbored BRAF mutations and 9 (0.58%) of 1546 non-ADCs had BRAF mutations, indicating a significant difference in BRAF mutations between ADCs and non-ADCs (OR = 3.96, 95% CI = 2.13–7.34, P < 0.0001). Only 5 studies contained the related data on mutations of BRAF and the stage of NSCLC. The results demonstrated that 67 of 3136 stage I/II NSCLCs (2.14%) harbored BRAF mutations and 76 (6.15%) of 1235 stage III/IV NSCLCs had BRAF mutations, indicating there were no significant differences in BRAF mutation between stage I/II and stage III/IV (OR = 0.86, 95% CI = 0.44–1.68, P = 0.65).
Figure 2.
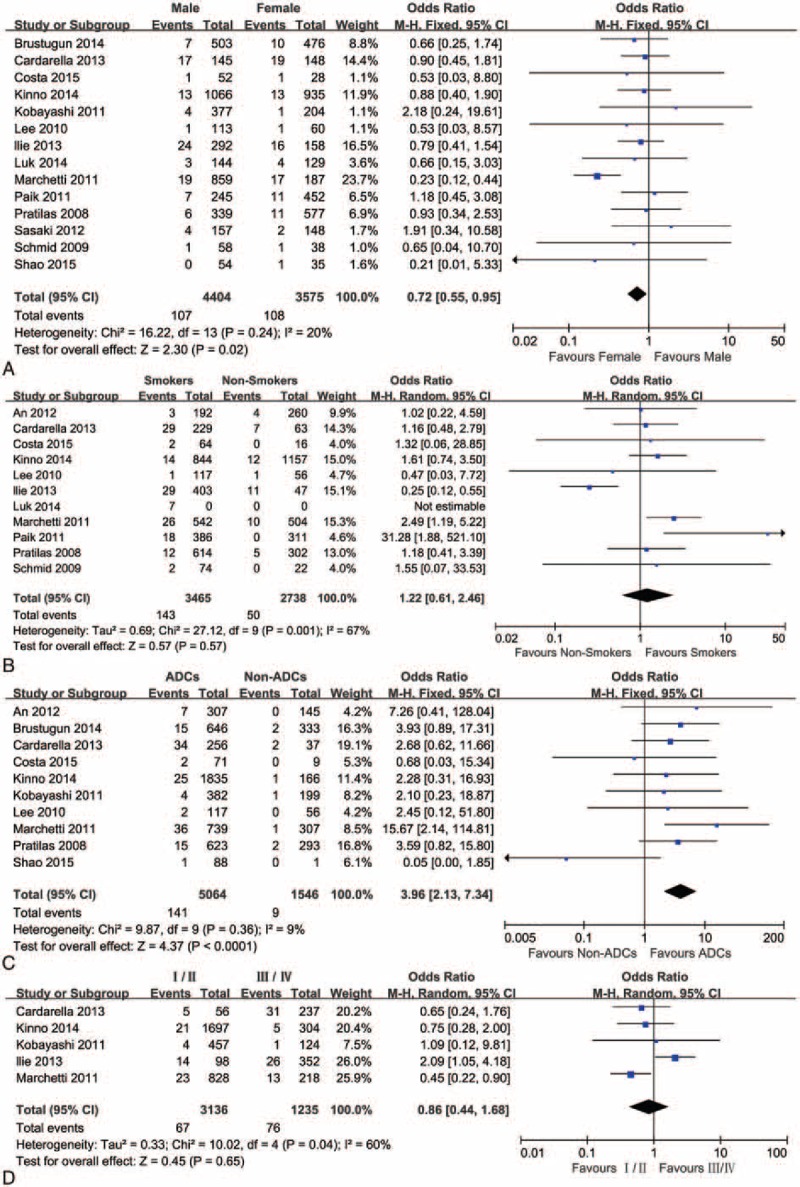
A forest plot for the association between BRAF mutations and gender (A), smoking (B), histology (C), and stage (D).
Table 2.
Association between BRAF mutation and gender, smoking, histology, and stage in NSCLC.
3.3. Correlation between BRAFV600E mutation and clinic pathologic characteristics in NSCLC
The association between BRAFV600E mutation and clinic pathologic characteristics in NSCLC is evaluated in Fig. 3. A fixed-effects model was used to systematically analyze the BRAFV600E mutations in NSCLC reported in 6 studies. 51.0% (107/210) were BRAFV600E mutations in all of the BRAF mutations. Forty-seven (32.6%) of 144 male patients were BRAFV600E mutations positive and 60 (62.5%) of 96 female patients were BRAFV600E mutations positive, indicating a significant difference in BRAFV600E mutations between female and male (OR = 0.45, 95% CI = 0.26–0.77, P = 0.004). Sixty-nine (45.1%) of 153 former or current smokers had BRAFV600E mutations and 40 of 46 never smokers (87.0%) harbored BRAFV600E mutation, indicating that there was a significant difference in BRAFV600E mutation between never smokers and smokers (OR = 0.12, 95% CI = 0.05–0.29, P < 0.00001). Twenty-eight (48.3%) of 58 stage I or II patients harbored BRAFV600E mutations and 74 (55.2%) of 134 stage III or IV patients harbored BRAFV600E mutation, indicating no significant difference in BRAFV600E mutation between stage I/II and stage III/IV (OR = 0.65, 95% CI = 0.33–1.28, P = 0.21).
Figure 3.
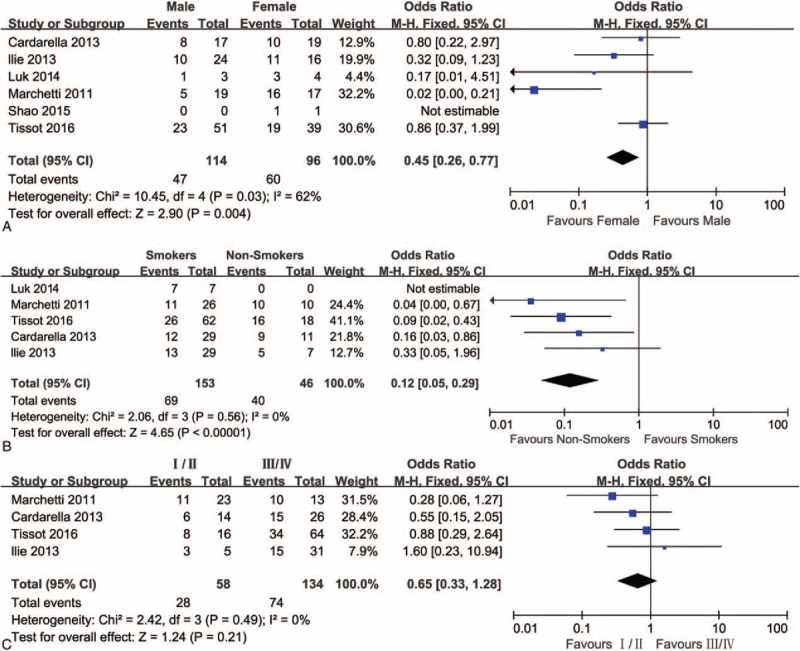
A forest plot for the association between BRAFV600E mutations and gender (A), smoking (B), and stage (C).
3.4. Sensitivity analysis and publication bias
Sensitivity analysis was performed to assess the stability of the results and Begg funnel plot was conducted to evaluate the reliability of the results. The sensitivity analysis indicated that removal of any one from the eligible articles had no effect on the final results. The funnel plots (Fig. 4) with low asymmetry suggested that there was no strong publication bias in the meta-analysis of BRAF mutations and clinic pathologic characteristics in NSCLC.
Figure 4.
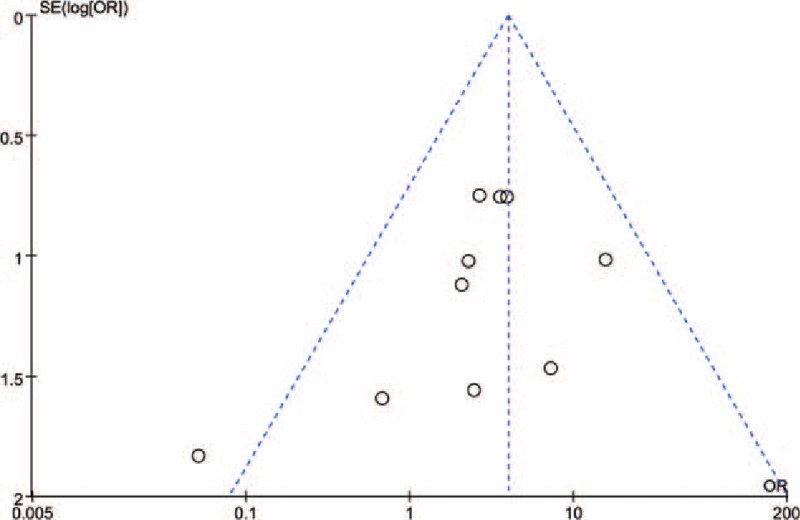
Begg funnel plot for publication bias of BRAF mutations and ADCs risk.
4. Discussion
BRAF mutations may be related to BRAF-mediated downstream signaling pathways, which have acquired extensive interest.[33] Several meta-analyses have been performed to study BRAF mutations in papillary thyroid cancer, colorectal cancer, and melanoma.[34–36] BRAF mutations were closely associated with specific pathologic characteristics, including tumor histology, clinical stage, gender, and smoking. Some studies have reported that BRAF mutations played a significant role in the clinical features of NSCLC. However, due to lack of estimated patient cases, a consensus has not been reached. Though one meta-analysis reported the association of BRAF mutations with NSCLC,[37] the number of NSCLC patients was not enough. Therefore, our updated meta-analysis was performed to explore the characteristics of NSCLC patients harboring BRAF mutations.
Sixteen studies, including 11,711 NSCLC patients, were used to systematically review the association between mutations of BRAF and pathologic features of NSCLC. The BRAF mutation rate was about 2.6% (303/11711), consistent with previous published studies.[24,38] In the studies of Marchetti et al and Pratilas et al, BRAF gene mutation rates were reported to be 2% and 3.5%, respectively,[21,22] which were close to the mutation rates found in our study.
In order to improve tumor mutation analysis, the clinical features of NSCLC were investigated in our study. The meta-analysis was performed to evaluate the association of BRAF mutations with four clinic pathologic features. A significant association was found between gender and BRAF mutation rate (OR = 0.72, 95% CI = 0.55–0.95, P = 0.02), which was opposite to previous meta-analysis in Chen's study.[37] An association of BRAF mutations with female was reported in colorectal cancer patients.[35,39] Further, we detected the associations between mutations of BRAF and smoking status. Our analysis showed that no association was observed between BRAF mutations and smoking status, which was the same with the results of previous studies.[32,37]
NSCLC covers three diverse histologic types, including ADC, large-cell carcinoma, and squamous-cell carcinoma, of which more than 50% are ADC. Our meta-analysis indicated that BRAF mutations were more common in ADCs than those in other histology (OR = 3.96, 95% CI = 2.13–7.34, P < 0.0001), which was consistent with a previous study.[21] Clinical stage, as a vital factor, plays an important role in the prognosis of NSCLC. However, no significant associations between BRAF mutations and stage of NSCLC were demonstrated in our meta-analysis. This result may be caused by lack of a number of patient cases.
BRAFV600E is the most common mutation occurring in the BRAF gene.[40] Up to now, 6 studies have involved the association between clinic pathologic features and BRAFV600E mutation.[19,21,27,30–32] Significant differences were found in these studies between the clinical features of NSCLC patients with BRAFV600E and ones with non-BRAFV600E mutations.[19,21,27] Several reports suggested that BRAFV600E mutations were more frequent in females and never smokers, however, the phenomenon was not observed in other clinic pathologic features.[21,27] Our meta-analysis also indicated that the BRAFV600E mutation was significantly more frequent in females than in males. Compared to smokers, the BRAFV600E mutation was also significantly frequent in never-smokers (OR = 0.12, 95% CI = 0.05–0.29, P < 0.00001). Ilie et al[27] demonstrated that there was a significant association between early-stage tumors and non-BRAFV600E mutations. We also observed a tendency for earlier-stage disease but there was no statistical significance, which may result from lack of cases.
Several limitations in our study should be taken into consideration when construing the findings. The number of involved studies on BRAFV600E mutations was not enough. Therefore, additional studies on BRAFV600E mutations are required to extend and approve our results. Moreover, we did not well describe the association of BRAF mutation with clinical stage due to the lack of data.
In conclusion, we found that BRAF mutations in NSCLC patients were significantly associated with ADCs and tend to occur in female. Moreover, BRAFV600E mutation was significantly associated with NSCLC in female and nonsmokers. This may provide a theoretical basis for the diagnosis of NSCLC.
Footnotes
Abbreviations: ADCs = adenocarcinomas, CI = confidence interval, NSCLC = nonsmall cell lung cancer, ORs = odds ratios.
The authors have no conflicts of interest to disclose.
References
- [1].Bach PB, Mirkin JN, Oliver TK, et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA 2012;307:2418–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Yamadori T, Ishii Y, Homma S, et al. Molecular mechanisms for the regulation of Nrf2-mediated cell proliferation in non-small-cell lung cancers. Oncogene 2012;31:4768–77. [DOI] [PubMed] [Google Scholar]
- [3].Chung C, Christianson M. Predictive and prognostic biomarkers with therapeutic targets in breast, colorectal, and non-small cell lung cancers: a systemic review of current development, evidence, and recommendation. J Oncol Pharm Pract 2014;20:11–28. [DOI] [PubMed] [Google Scholar]
- [4].Jin G, Kim MJ, Jeon H-S, et al. PTEN mutations and relationship to EGFR, ERBB2, KRAS, and TP53 mutations in non-small cell lung cancers. Lung Cancer 2010;69:279–83. [DOI] [PubMed] [Google Scholar]
- [5].Pao W, Chmielecki J. Rational, biologically based treatment of EGFR-mutant non-small-cell lung cancer. Nat Rev Cancer 2010;10:760–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735–42. [DOI] [PubMed] [Google Scholar]
- [7].Clinical Lung Cancer Genome Project (CLCGP); Network Genomic Medicine (NGM). A genomics-based classification of human lung tumors. Sci Transl Med 2013;5: 209ra153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Pendharkar D, Ausekar B, Gupta S. Molecular biology of lung cancer—a review. Indian J Surg Oncol 2013;4:120–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Weisenberger DJ, Siegmund KD, Campan M, et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet 2006;38:787–93. [DOI] [PubMed] [Google Scholar]
- [10].Tufano RP, Teixeira GV, Bishop J, et al. BRAF mutation in papillary thyroid cancer and its value in tailoring initial treatment: a systematic review and meta-analysis. Medicine 2012;91:274–86. [DOI] [PubMed] [Google Scholar]
- [11].Lee JH, Choi JW, Kim YS. Frequencies of BRAF and NRAS mutations are different in histological types and sites of origin of cutaneous melanoma: a meta-analysis. Br J Dermatol 2011;164:776–84. [DOI] [PubMed] [Google Scholar]
- [12].Nakayama N, Nakayama K, Yeasmin S, et al. KRAS or BRAF mutation status is a useful predictor of sensitivity to MEK inhibition in ovarian cancer. Br J Cancer 2008;99:2020–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Planchard D, Mazieres J, Riely GJ, et al. Interim results of phase II study BRF113928 of dabrafenib in BRAF V600E mutation-positive non-small cell lung cancer (NSCLC) patients. ASCO Annual Meeting Proceedings. 2013; 31: 8009. [Google Scholar]
- [14].Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121–8. [DOI] [PubMed] [Google Scholar]
- [15].Yasuda H, Kobayashi S, Costa DB. EGFR exon 20 insertion mutations in non-small-cell lung cancer: preclinical data and clinical implications. Lancet Oncol 2012;13:e23–31. [DOI] [PubMed] [Google Scholar]
- [16].Schmid K, Oehl N, Wrba F, et al. EGFR/KRAS/BRAF mutations in primary lung adenocarcinomas and corresponding locoregional lymph node metastases. Clin Cancer Res 2009;15:4554–60. [DOI] [PubMed] [Google Scholar]
- [17].Kinno T, Tsuta K, Shiraishi K, et al. Clinicopathological features of nonsmall cell lung carcinomas with BRAF mutations. Ann Oncol 2014;25:138–42. [DOI] [PubMed] [Google Scholar]
- [18].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cardarella S, Ogino A, Nishino M, et al. Clinical, pathologic, and biologic features associated with BRAF mutations in non–small cell lung cancer. Clinical Cancer Res 2013;19:4532–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lee SY, Kim MJ, Jin G, et al. Somatic mutations in epidermal growth factor receptor signaling pathway genes in non-small cell lung cancers. J Thorac Oncol 2010;5:1734–40. [DOI] [PubMed] [Google Scholar]
- [21].Marchetti A, Felicioni L, Malatesta S, et al. Clinical features and outcome of patients with non-small-cell lung cancer harboring BRAF mutations. J Clin Oncol 2011;29:3574–9. [DOI] [PubMed] [Google Scholar]
- [22].Pratilas CA, Hanrahan AJ, Halilovic E, et al. Genetic predictors of MEK dependence in non-small cell lung cancer. Cancer Res 2008;68:9375–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kobayashi M, Sonobe M, Takahashi T, et al. Clinical significance of BRAF gene mutations in patients with non-small cell lung cancer. Anticancer Res 2011;31:4619–23. [PubMed] [Google Scholar]
- [24].Paik PK, Arcila ME, Fara M, et al. Clinical characteristics of patients with lung adenocarcinomas harboring BRAF mutations. J Clin Oncol 2011;29:2046–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].An SJ, Chen ZH, Su J, et al. Identification of enriched driver gene alterations in subgroups of non-small cell lung cancer patients based on histology and smoking status. PLoS ONE 2012;7:e40109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sasaki H, Shitara M, Yokota K, et al. Braf and erbB2 mutations correlate with smoking status in lung cancer patients. Exp Ther Med 2012;3:771–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ilie M, Long E, Hofman V, et al. Diagnostic value of immunohistochemistry for the detection of the BRAFV600E mutation in primary lung adenocarcinoma Caucasian patients. Ann Oncol 2013;24:742–8. [DOI] [PubMed] [Google Scholar]
- [28].Brustugun OT, Khattak AM, Trømborg AK, et al. BRAF-mutations in non-small cell lung cancer. Lung Cancer 2014;84:36–8. [DOI] [PubMed] [Google Scholar]
- [29].Costa EC, Estival A, Martinez LV, et al. , editors. Prevalence of ROS1 translocation, HER2, and BRAF mutations in a cohort of advanced non-small cell lung cancer (NSCLC) patients (p) triple negative (TN). ASCO Annual Meeting Proceedings. 2015; 33: e22139. [Google Scholar]
- [30].Luk PP, Yu B, Ng CC, et al. BRAF mutations in non-small cell lung cancer. Transl Lung Cancer Res 2014;4:142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Tissot C, Couraud S, Tanguy R, et al. Clinical characteristics and outcome of patients with lung cancer harboring BRAF mutations. Lung Cancer 2016;91:23–8. [DOI] [PubMed] [Google Scholar]
- [32].Shao L, Hou DY, Leng ZJ, et al. ARMS-PCR detected KRAS, BRAF gene mutations in non-small cell lung cancer. J Anhui Med Univ 2015;11:1669–73. [Google Scholar]
- [33].Zhang C, Spevak W, Zhang Y, et al. RAF inhibitors that evade paradoxical MAPK pathway activation. Nature 2015;526:583–6. [DOI] [PubMed] [Google Scholar]
- [34].Ardekani GS, Jafarnejad SM, Tan L, et al. The prognostic value of BRAF mutation in colorectal cancer and melanoma: a systematic review and meta-analysis. PLoS ONE 2012;7:e47054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kalady MF, DeJulius KL, Sanchez JA, et al. BRAF mutations in colorectal cancer are associated with distinct clinical characteristics and worse prognosis. Dis Colon Rectum 2012;55:128–33. [DOI] [PubMed] [Google Scholar]
- [36].Li C, Lee KC, Schneider EB, et al. BRAF V600E mutation and its association with clinicopathological features of papillary thyroid cancer: a meta-analysis. J Clin Endocrinol Metab 2012;97:4559–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Chen D, Zhang LQ, Huang JF, et al. BRAF mutations in patients with non-small cell lung cancer: a systematic review and meta-analysis. PLoS ONE 2014;9:e101354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Pao W, Girard N. New driver mutations in non-small-cell lung cancer. Lancet Oncol 2011;12:175–80. [DOI] [PubMed] [Google Scholar]
- [39].Roth AD, Tejpar S, Delorenzi M, et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol 2010;28:466–74. [DOI] [PubMed] [Google Scholar]
- [40].Sclafani F, Gullo G, Sheahan K, et al. BRAF mutations in melanoma and colorectal cancer: a single oncogenic mutation with different tumour phenotypes and clinical implications. Crit Rev Oncol Hematol 2013;87:55–68. [DOI] [PubMed] [Google Scholar]


