Abstract
Rationale:
Aorto-esophageal fistula (AEF), a postoperative complication of esophagectomy, constitutes a very small percentage of all cases presenting with AEF; however, it is associated with a high mortality rate. Acute massive hemorrhage is the single largest cause of death in patients developing AEF. There is a lack of consensus on the optimal treatment of AEF.
Patient concerns:
We present 3 cases secondary to esophagectomy due to lower thoracic esophageal carcinoma. All 3 patients presented with similar acute symptoms including a critical and life-threatening course with dead feeling, thoracic pain, and projectile hematemesis, and also hypovolemic shock.
Diagnoses:
Digital subtraction angiography identified AEF as the diagnosis of these 3 cases.
Interventions:
All patients were treated with emergency thoracic endovascular aortic repair, the aortic fistulas were repaired.
Outcomes:
All patients successfully survived the perioperative period.
Lessons:
Thoracic endovascular aortic repair, a minimally invasive technique, is a better method to achieve faster and safer hemodynamic stability in patients with AEF compared with open thoracic aortic repair.
Keywords: aorto-esophageal fistula, endovascular treatment, esophagectomy
1. Introduction
Aorto-esophageal fistula (AEF) is a rare but usually fatal disorder.[1,2] AEFs specifically resulting from esophagectomy constitute a very small percentage of all cases. We report the management of 3 patients who developed AEF after undergoing an esophagectomy. Thoracic endovascular aortic repair (TEVAR) was performed emergently to control fatal bleeding.
2. Case report
Patient 1 was a 50-year-old man without a pertinent medical history. Patient 2 was a 69-year-old woman with a 7-year history of chronic bronchitis. Patient 3 was a 62-year-old man suffering from diabetes and a history of myocardial infarction more than 10 years before.
Each patient underwent a standardized Sweet operation (esophagectomy and esophagogastric stapled anastomosis via a left thoracotomy) to treat lower thoracic esophageal carcinoma. The Commission of Medical Ethics at the Shandong Provincial Hospital approved our study protocol. Written informed consent was obtained from all participants before enrollment in the study.
All 3 patients had persistent mild fever after the esophagectomy. Chest radiographs in all 3 patients showed a small amount of pleural effusion and pneumonia, which were treated with thoracentesis and antibiotics. Upper gastrointestinal barium examination showed the anastomotic stomas were located above the aortic arch in all 3 patients (Fig. 1).
Figure 1.
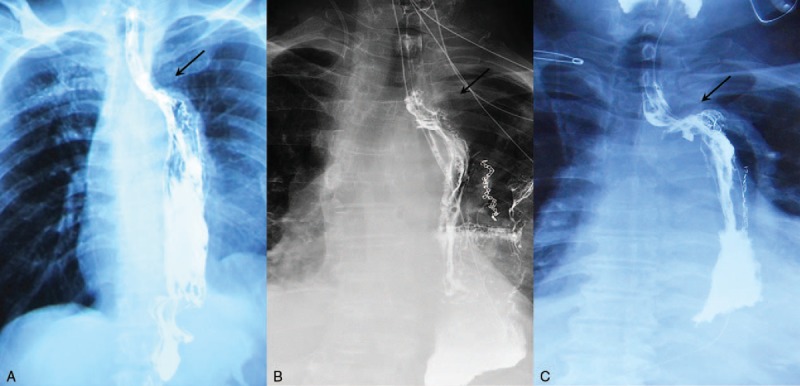
Upper gastrointestinal barium examination showed the positions of anastomotic stomas (arrows). A, patient 1; B, patient 2; C, patient 3.
All patients showed similar acute symptoms including dead feeling, thoracic pain, and projectile hematemesis of arterial blood, and hypovolemic shock on the 12th, 20th, and 10th postoperative day in patients 1, 2, and 3, respectively. Hematemesis spontaneously stopped within 10 minutes. They were all treated with fluid replacement, blood transfusion, and control of systolic blood pressure to about 100 mm Hg. Digital subtraction angiography (DSA) was used to confirm the initial bedside diagnosis of AEF.
Percutaneous right femoral artery or left brachial artery access under general anesthesia was used to insert the endovascular device into the ascending aorta. All aortic fistulas were located in the aortic arch, 2 to 2.5 cm distal to the left subclavian artery and near the esophagogastric junction (Fig. 2). The right femoral artery was then exposed surgically. A 28 mm × 160 mm covered endovascular stent graft (LifeTech Scientific Corporation, Shenzhen, China) was deployed via the right femoral artery route. The aortic arch was covered just distal to the origin of the left subclavian artery. The TEVAR achieved repair of the fistula without aortic leakage (Fig. 3). We chose a conservative treatment approach for esophagogastric anastomotic leakage, including broad-spectrum antibiotics, decompression with a nasogastric tube, thoracentesis or thoracic drainage, enteral and parenteral nutrition, and no oral feeding. Patients 1 and 3 recovered well after the TEVAR with the esophagogastric anastomotic leakages seen to heal on the 17th and 14th postoperative day, respectively, after which they were discharged from the hospital. Antibiotics were continued for 1 month postoperatively, and then stopped. The anastomotic leakage in patient 2 did not heal; she refused reoperation and died of recurrent hematemesis 88 days later.
Figure 2.
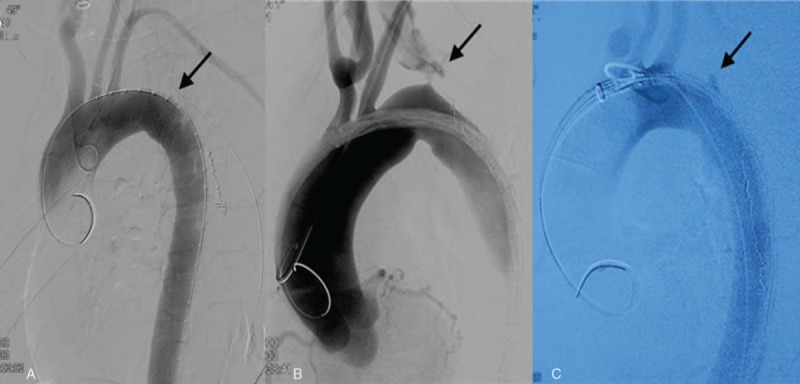
Digital subtraction angiography demonstrated the aorto-esophageal fistula (arrows). A, patient 1; B, patient 2; C, patient 3.
Figure 3.
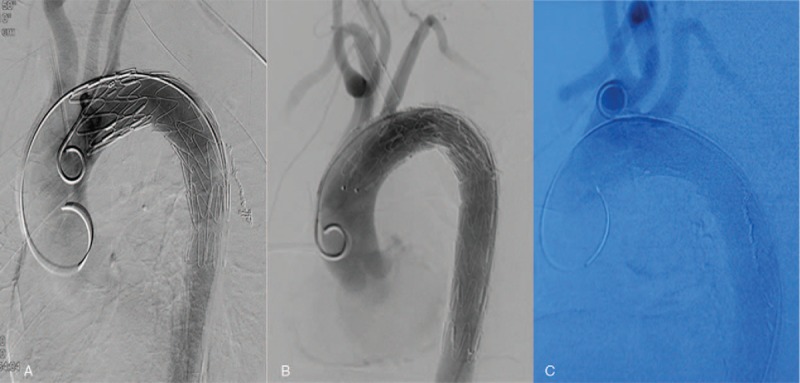
Images of digital subtraction angiography after TEVAR. A, patient 1; B, patient 2; C, patient 3. TEVAR = thoracic endovascular aortic repair.
3. Discussion
Aorto-esophageal fistula is a rare postoperative complication of esophagectomy.[1] In our case series, all 3 patients underwent the Sweet operation. The position of the anastomotic stoma was near the thoracic aorta in this operative method. We concluded that inflammation and corrosion induced by anastomotic leakage, and mechanical damage from the stapled anastomosis might be the chief contributors to the AEFs seen in our patients.
Chiari first described a triad of symptoms for the diagnosis of AEF.[3] Projectile hematemesis of arterial blood, thoracic pain, and a near-death experience were the main symptoms of AEF in all 3 patients we diagnosed and treated. We believe projectile hematemesis should be considered a warning of “signal hemorrhage.” The development of an AEF should be suspected in any patient manifesting with hematemesis after Sweet surgery. The projectile hematemesis of arterial blood characteristically distinguishes AEF from other sources of massive hematemesis. Anastomotic bleeding, although sometimes arterial, is lesser in volume and more likely to occur at an early postoperative stage. These typical clinical characteristics help to make a presumptive bedside diagnosis.
In regard to the initial conservative management, we believe it is important to control the systolic blood pressure in a patient presenting with such a clinical picture. We noted that patients 2 and 3 experienced recurrent hematemesis when the blood pressure returned to a normal level. Unrelenting and repeated hemorrhage could be fatal and must receive immediate attention.
The primary aim of management in a patient having AEF is to maintain hemodynamic stability. TEVAR was first introduced for the treatment of a fistula between the aorta and an adjacent organ in 1996 by Chuter et al.[4] TEVAR has proved to be efficacious in the rapid control of aortic bleeding compared with the traditional surgical treatment, and is a less invasive and safer approach.
However, with the growing number of patients undergoing this operation, this technique presents some limitations in treating AEF.[5,6] Late infection and associated mortality rates are higher when TEVAR is used as the sole therapeutic strategy.[7] Thus, a staged approach for AEF treatment has been proposed in recent years. This approach includes a variety of combinations of TEVAR and sequential radical surgeries, involving surgical aortic replacement, surgical repair of the esophagus, and mediastinal drainage. The evidence, however, is not strong enough to justify the advantages of surgical aortic replacement.[8] Based on a review conducted on patient 2, we preferred surgical aortic replacement after esophagectomy, because a foreign body might affect healing after anastomotic leakage.
To conclude, AEF may be the outcome of an anastomotic leakage and local infection after an esophagectomy, especially the Sweet operation. We consider TEVAR to be an effective option for the management of a secondary AEF after esophagectomy and to maintain hemodynamic stability in a faster and safer way. Although it presents challenges for improvement because of some limitations that need to be overcome, TEVAR can provide a path for the future treatment of AEFs.
Footnotes
Abbreviations: AEF = aorto-esophageal fistula, DSA = digital subtraction angiography, TEVAR = thoracic endovascular aortic repair.
The authors declare that they have no conflict of interest.
References
- [1].Hollander JE, Quick G. Aortoesophageal fistula: a comprehensive review of the literature. Am J Med 1991;91:279–87. [DOI] [PubMed] [Google Scholar]
- [2].Kieffer E, Chiche L, Gomes D. Aortoesophageal fistula: value of in situ aortic allograft replacement. Ann Surg 2003;238:283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].H. C. Ueber Fremdkorperverletzung des Oesophagus mit Aortenperforation. Berlin Klein Wschr 1914;51:7–9. [Google Scholar]
- [4].Chuter TAM, Ivancev K, Lindblad B, et al. Endovascular stent-graft exclusion of an aortobronchial fistula. J Vasc Intervent Radiol 1996;7:357–9. [DOI] [PubMed] [Google Scholar]
- [5].Jonker FH, Heijmen R, Trimarchi S, et al. Acute management of aortobronchial and aortoesophageal fistulas using thoracic endovascular aortic repair. J Vasc Surg 2009;50:999–1004. [DOI] [PubMed] [Google Scholar]
- [6].Akashi H, Kawamoto S, Saiki Y, et al. Therapeutic strategy for treating aortoesophageal fistulas. General Thorac Cardiovasc Surg 2014;62:573–80. [DOI] [PubMed] [Google Scholar]
- [7].Chiesa R, Melissano G, Marone EM, et al. Endovascular treatment of aortoesophageal and aortobronchial fistulae. J Vasc Surg 2010;51:1195–202. [DOI] [PubMed] [Google Scholar]
- [8].Vallabhajosyula P, Komlo C, Wallen T, et al. Two-stage surgical strategy for aortoesophageal fistula: emergent thoracic endovascular aortic repair followed by definitive open aortic and esophageal reconstruction. J Thorac Cardiovasc Surg 2012;144:1266–8. [DOI] [PubMed] [Google Scholar]


