Supplemental Digital Content is available in the text
Keywords: cholelithiasis, exogenous estrogen, meta-analysis, relative risk
Abstract
Background:
Association between exogenous estrogen intake and cholelithiasis risk has been reported in several epidemiological studies, including oral contraceptive (OC) and hormone replacement therapy (HRT), while the results were controversial. This study aimed to perform a comprehensive meta-analysis of this issue.
Methods:
PUBMED, EMBASE, and Cochrane library database were searched up to October 2016. Two reviewers independently extracted data from eligible studies, relative risks (RRs), and/or odds ratios (ORs) with 95% confidence intervals (95% CIs) for the highest versus lowest categories of intake were adopted. Either a fixed- or a random-effects model was adopted to estimate overall RRs or ORs. Besides, subgroup and publication bias analyses were applied to explain the heterogeneity. An original study was also conducted to verify our conclusion.
Results:
A total of 19 studies with approximately 556,620 participants were included in this meta-analysis. The pooled RR of cholelithiasis for the highest versus the lowest categories was 1.59 (95% CI: 1.44–1.75), indicating that exogenous estrogen was positive associated with the intake of exogenous estrogen. However, the pooled RR of OC intake and cholelithiasis risk was 1.19 (95% CI: 0.97–1.45), and the RR for HRT was 1.79 (95% CI: 1.61–2.00).
Conclusion:
The HRT was positively associated with the cholelithiasis risk, and the OC will not increase the risk of cholelithiasis.
1. Introduction
The gender difference in the prevalence of gallstones is presumed to be caused by endogenous female sex hormones, particularly estrogen.[1] So the exogenous estrogen may be a subversive, mainly including the oral contraceptive (OC) and hormone replacement therapy (HRT) for women. What is more, the estrogen was also used in the treatment of prostate cancer.[2] However, due to the gap in the subgroup analysis, the association between OC/HRT and cholelithiasis was specifically illustrated in the present study.
The OC was commonly used in daily life, which was the main source of exogenous estrogen for young women. There has been a series of studies that investigated the relationship between OC and the incidence of gallstones; however, the results were inconsistent. For example, some studies[3–5] showed positive relationship between the OC and gallstones, while others showed[6–10] that the OC had no influence on the incidence of gallstones.
Postmenopausal HRT has been widely used to improve symptoms and protect the bone and vessel for menopause women. However, HRT is a double-edged sword for increasing the incidence of cancer of breast, thyroid, and endometrium, which has come into our notice. However, the relationship between HRT and gallstone was controversial: most of the studies indicated that HRT was a risk factor of gallstone,[1,6,10–14] while others did not.[9,15,16]
Thus, the aim of this article is to systemically evaluate the association of exogenous estrogen (OC and HRT) and incidence of gallstone by a meta-analysis approach, and to provide evidences for clinical decision.
2. Method
2.1. The meta-analysis
2.1.1. Data sources, search strategy, and selection criteria
PUBMED, EMBASE, and Cochrane library database (up to October 2016) were searched to identify eligible studies. The following terms were applied: “oral contraceptive” OR “OC” OR “HRT” OR “Postmenopausal hormone replacement therapy” OR “estrogen”; “gallstone” OR “cholelithiasis” OR “cholecystectomy.” The detailed search strategy can be found in Supplementary table S1.
In the study selection process, the first-round search was based on the titles and abstracts, and then full papers of potential eligible studies were reviewed. This meta-analysis was designed, conducted, and reported according to PRISMA and MOOSE statements.[17,18] Articles were included if they met all the following criteria: studies evaluated the association between the estrogen or OC or HRT and incidence of gallstone; relative risk (RR) or odds ratio (OR) estimates and their 95% confidence intervals (95% CI) were given or could be calculated; articles as full papers in English or Chinese were retrieved. In addition, only the case–control study and cohort study were included. Studies that assessed the changes of bile composition and gene expression level were excluded. Reviews, meeting abstracts, letters, comments, editorials, and case reports were also excluded because of the limited data.
2.1.2. Data extraction and quality assessment
Two reviewers (CYX and WSQ) independently extracted the following information of each study: the first author, year of publication, country, study design (case–control or cohort), characteristics of patients (including sample size, gender, and mean age), RRs/ORs with 95% CIs, and the discrepancies were resolved by a third investigator (WYQ). The quality of each study was assessed according to NEWCASTLE-OTTAWA quality assessment,[19] as shown in Supplementary table S3.
2.1.3. Data synthesis and statistical analysis
According to the extent of heterogeneity, either a fixed- or random-effect model was adopted to pool these study-specific RRs (ORs). The significance of the pooled RR was determined by Z test (P < 0.05 was considered to be significant). Heterogeneities across studies were checked by the chi-square test and I2 test (I2 test quantifies the proportion of total variation across studies due to heterogeneity rather than chance). P < 0.10 and or I2 > 50% indicates significant heterogeneity, and the random-effect model was used. Otherwise, a fixed-effect model was applied. Subgroup analyses were applied to explore source of heterogeneity and to evaluate potential effect modification of variables including estrogen type, study quality, and design. Funnel plots were constructed and Begg and Egger tests were performed to assess the publication bias, and P ≤ 0.10 was considered to be significant. All analyses were conducted using Stata software (version 12.0; StatCorp, College Station, TX).
2.1.4. The original study
An original study from our center was also conducted to further evaluate the association between estrogen intake and gall stone risk in Chinese population. A total of 540 patients who were diagnosed as gallstone and underwent surgery between May 2010 and December 2016 in QiLu hospital were admitted. Meanwhile, 540 healthy people were matched in Center of physical examination of Qilu hospital. The information of the 2 groups was shown in the Supplementary table S4. Questionnaires by a telephone follow-up was conducted, which mainly included the following information: the history of estrogen (OC and HRT) intake (yes or no); if it is yes, then the time for drug intake (less than 1 year or more than 1 year); for people who were treated with HRT, the of time for drug withdrawal was inquired (within 2 years or beyond 2 years). Then the RR value and 95% CI were calculated by SPSS software, version 18.0,Chicago, IL, USA.
The research was approved by Ethics Committee of Shandong University Qilu Hospital.
3. Results
3.1. Study characteristic and data quality
PUBMED, EMBASE, and Cochrane library databases were searched, and finally 1020 articles were included. After removing 223 duplicate articles, we reviewed the titles and abstracts to exclude 709 irrelevant studies. The full text of the remaining 88 relevant articles were evaluated to make further exclusion: without insufficient data (n = 62), and not original articles (n = 7). Finally 19 articles were included for meta-analysis. The detailed screening and selection process was shown in Fig. 1.
Figure 1.
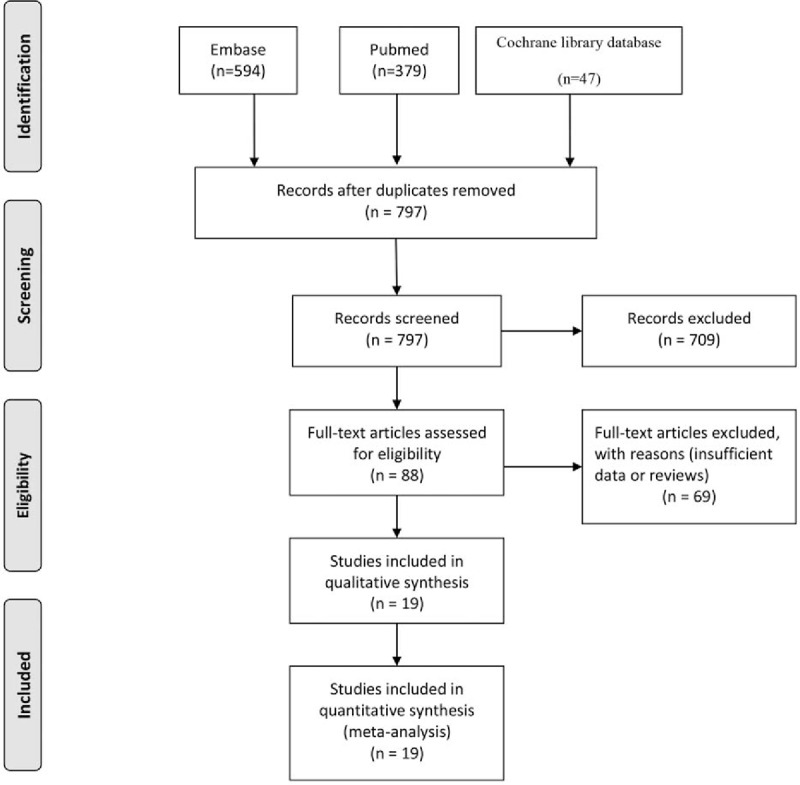
Flow diagram of the literature search and trials selection process.
Totally, 19 articles, including 9 case–control studies and 10 cohort studies, with 21,476 patients and 556,620 participants were included. The age of participants ranged from 14 to 80 years old, and most of them are women by focusing on the OC and HRT; only Henriksson et al[2] reported the estrogen use in male with prostatic cancer. The characteristics of each article were presented in Supplementary table S2.
3.2. Association between exogenous estrogen intake and cholelithiasis
To assess the influence of exogenous estrogen on the cholelithiasis risk, we used random-effect model to pool the study-specific RRs. Compared with the lowest categories of estrogen intake, the pooled RR of cholelithiasis for the highest categories was 1.59 (95% CI: 1.44–1.75), indicating that exogenous estrogen was positive associated with the intake of exogenous estrogen (Fig. 2). Heterogeneity was found to be significant (I2 = 85.5%, P = 0.000) (Fig. 2).
Figure 2.
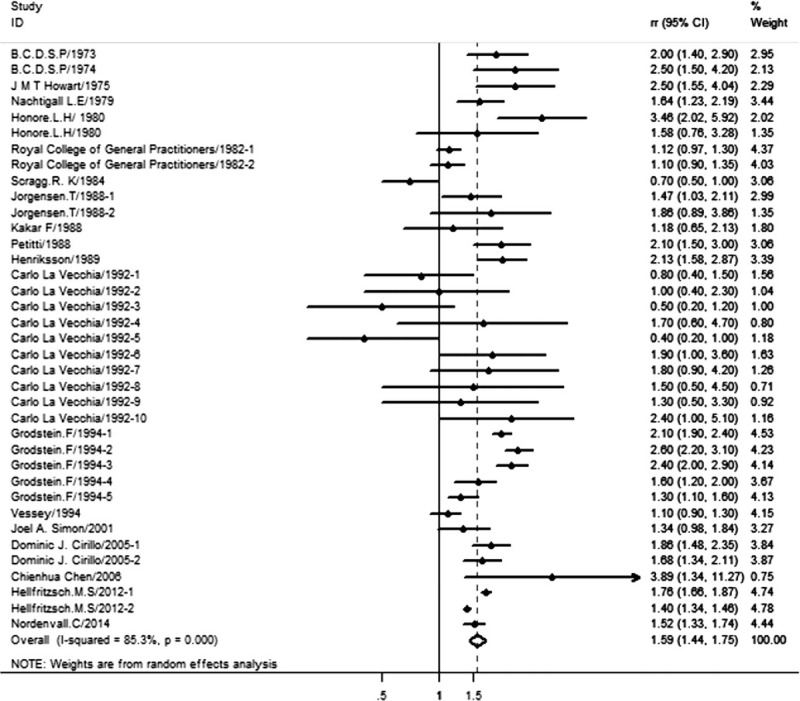
Association between exogenous estrogen intake and cholelithiasis risk; Jorgensen/1988-1: risk of gallstone with oral contraceptives (OC) use; Jorgensen/1988-2: gallstone and hormone replacement therapy (HRT). Carlo/1992:1–5 risk of gallstone with OC: the OC used at any time; duration of OC use <2 years; duration of OC ≥ 2 years; times since last use <5years; times since last use >5years; 6–10 risk of gallstone with estrogen replacement therapy (ERT): the ERT used at any time; duration of ERT use <2 years; duration of ERT ≥ 2 years; times since last use <10 years; times since last use ≥10 years. Dominic J. Cirillo/2005-1: risk of gallstone with conjugated equine estrogens (CEE); Dominic J. Cirillo/2005-2: risk of gallstone with estrogen plus progestin (E + P). The CEE and E + P are different clinical treatment of menopause HRT. Hellfritzsch. M.S/2012-1: relative risk (RR) for current HRT users; Hellfritzsch. M.S/2012-2: RR for former HRT user.
3.3. Association between OC and cholelithiasis
Due to the different type and dose between the OC and HRT, we detected the RR of them respectively. The results showed that there was no signification relationship between OC intake and cholelithiasis risk with the RR = 1.15 (95% CI: 0.94–1.40). Thus, the OC did not significantly increase the risk of cholelithiasis (Table 1).
Table 1.
Subgroup analysis about the association between exogenous estrogen intake and cholelithiasis risk.
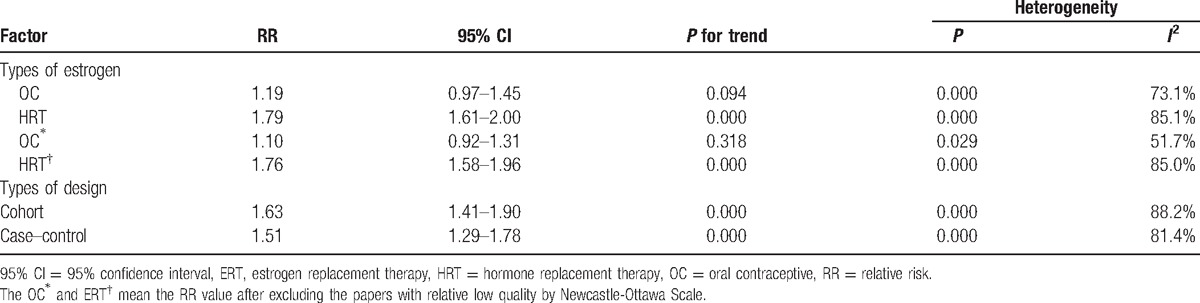
3.4. Association between HRT and cholelithiasis
HRT is significantly associated with the risk of cholelithiasis according to the pooled RR = 1.79 (95% CI: 1.61–2.00); thus, HRT can increase the incidence of cholelithiasis (Table 1).
3.5. Original study
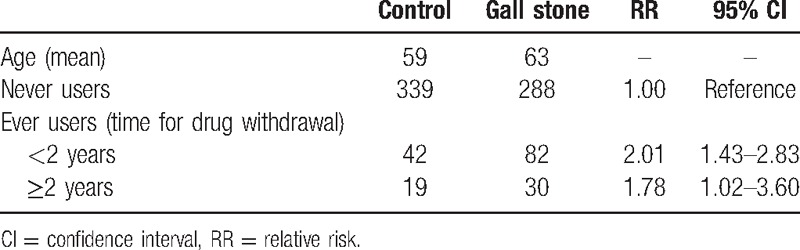
As shown in Table 2, 140 patients and 140 healthy people were included in our original study to investigate the association between exogenous estrogen intake and risk of cholelithiasis. When OC intake lasted for less than 1 year, the RR was 1.23 (95% CI: 0.90–1.68), and it was 1.52 (95% CI: 0.64–3.60) for use of OC more than 1 year. As for the HRT, we chose patients and control groups who were postmenopausal. After HRT for less than 2 years, the RR was 2.01 (95% CI: 1.43–2.83), and after HRT for more than 2 years, the RR was 1.78 (95% CI: 1.02–3.60) (Table 3).
Table 2.
Original study—association between OC and cholelithiasis risk in Chinese population.
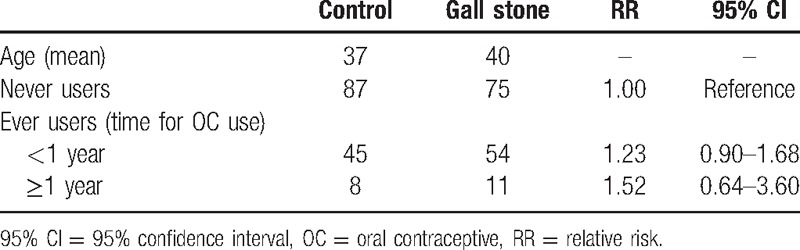
Table 3.
Original study—association between HRT and cholelithiasis risk in Chinese population.
3.6. Publication bias
Begg funnel plot and Egger-weighted regression indicated that there was no significant publication bias (Pegger = 0.689 > 0.05) (Supplementary Fig. S1).
4. Discussion
Our work firstly assessed the association between exogenous estrogen intake and cholelithiasis by a meta-analysis approach, which will be useful to guide clinical decision. We found that exogenous estrogen can significantly improve the risk of cholelithiasis (RR: 1.59 (95% CI: 1.44–1.75). The main exogenous estrogen include the OC and HRT; however, the pooled RR values were different: RR of OC is 1.19 (95% CI: 0.97–1.45), RR of HRT is 1.79 (95% CI: 1.61–2.00). It indicates that OC is not the risk factor for cholelithiasis, which will be beneficial to eliminate the bias for the OC. As for the HRT, our results proved it can significantly increase the incidence of cholelithiasis. The risk of breast cancer, ovarian cancer, and endometrial cancer were the well known side effects of exogenous estrogen, but its effect on cholelithiasis did not gain much attention. Several suggestions might be driven on the basis of the current meta-analysis results: First, women who ever underwent diseases in biliary tract should apply the HRT deliberative. Second, besides the test for breast, ovarian and uterus, the healthy station of bile duct before and during the HRT should be examined. And 1 report indicated that estrogen therapy can promote incidence of gallstone in male with prostatic cancer.[2] Thus, it could be speculated that estrogen maybe a risk factor for male either; however, due to the lack of eligible clinical trials, we cannot calculate the pooled RR to support this conception, and more studies are needed to further investigate this issue. What is more, our research would benefit to understand why women and overweight people are easier to undergo gallstone.
In addition, an original research was also conducted to verify the effects of exogenous estrogen in Chinese population, and similar results were validated.
Why the exogenous estrogen can lead to gallstone? There have been many researches focusing on the molecule mechanism of estrogen in bile duct and gall bladder. First, estrogen impacts the lipid metabolism[20–23] by G protein-coupled receptor 30,[22] estrogen receptor α[24] that increases burden of gall bladder and leading to formation of gall stone. Second, estrogen can cause relaxation of human gallbladder via G protein-coupled estrogen receptors.[25,26]
The work exists heterogeneity as shown above; so it is important to analyze the heterogeneity. As shown in Table 1, after eliminating the low-quality articles (less than 8 points) according the Newcastle–Ottawa Scale, the heterogeneity of OC group was significant decreased (I2 = 51.7%, P = 0.029), so the heterogeneity of OC group mainly resulted from the low-quality study. As for the HRT subgroup analyses, geographic region, design type, article quality, and number of sample were analyzed, whereas only part of heterogeneity was explained. So more high quality researches are needed to verify our conclusion.
Our study has several strengths. First, we demonstrate that the HRT is a risk factor for cholelithiasis, whereas OC is not, by a meta-analysis approach for the first time. Second, we provided an original study to verify our conclusion, which strengthened the current analysis. Finally, most of the included studies were of high methodological quality, and no publication bias was observed.
There also exist some limitations. First, due to the lack of data, dose–response analysis of estrogen intake could not be conducted. Second, although subgroup analysis was conducted, heterogeneity among the included studies could not be completely explained, and more high quality and well designed studies may be needed to validate this issue and reduce heterogeneity.
5. Conclusion
HRT is positively associated with cholelithiasis risk; however, OC has no significant association with cholelithiasis.
Supplementary Material
Footnotes
Abbreviations: 95% CI = 95% confidence interval, HRT = hormone replacement therapy, OC = oral contraceptive, OR = odds ratio, RR = relative risk.
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Simonsen MH, Erichsen R, Frøslev T, et al. Postmenopausal estrogen therapy and risk of gallstone disease: a population-based case–control study. Drug Saf 2013;36:1189–97. [DOI] [PubMed] [Google Scholar]
- [2].Henriksson P, Einarsson K, Eriksson A, et al. Estrogen-induced gallstone formation in males. Relation to changes in serum and biliary lipids during hormonal treatment of prostatic carcinoma. J Clin Invest 1989;84:811–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].[No authors listed]. Oral contraceptives and venous thromboembolic disease, surgically confirmed gallbladder disease, and breast tumours. Report from the Boston Collaborative Drug Surveillance Programme. Lancet 1973;1:1399–404. [PubMed] [Google Scholar]
- [4].Howat JMT, Jones CB, Schofield PF. Gall stones and oral contraceptives. J Int Med Res 1975;3:59–62. [DOI] [PubMed] [Google Scholar]
- [5].Kakar F, Weiss NS, Strite SA. Non-contraceptive estrogen use and the risk of gallstone disease in women. Am J Public Health 1988;78:564–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Honore LH. Increased incidence of symptomatic cholesterol cholelithiasis in perimenopausal women receiving estrogen replacement therapy: a retrospective study. J Reprod Med 1980;25:187–90. [PubMed] [Google Scholar]
- [7].[No authors listed]. Oral contraceptives and gallbladder disease. Royal College of General Practitioners’ oral contraception study. Lancet 1982;2:957–9. [PubMed] [Google Scholar]
- [8].Scragg RKR, McMichael AJ, Seamark RF. Oral contraceptives, pregnancy, and endogenous oestrogen in gall stone disease—a case–control study. Br Med J 1984;288:1795–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].La Vecchia C, Negri E, D’Avanzo B, et al. Oral contraceptives and non-contraceptive oestrogens in the risk of gallstone disease requiring surgery. J Epidemiol Community Health 1992;46:234–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Grodstein F, Colditz GA, Stampfer MJ. Postmenopausal hormone use and cholecystectomy in a large prospective study. Obst Gynecol 1994;83:5–11. [PubMed] [Google Scholar]
- [11].[No authors listed]. Surgically confirmed gallbladder disease, venous thromboembolism, and breast tumors in relation to postmenopausal estrogen therapy. A report from the Boston Collaborative Drug Surveillance Program, Boston University Medical Center. N Engl J Med 1974;290:15–9. [DOI] [PubMed] [Google Scholar]
- [12].Nachtigall LE, Nachtigall RH, Nachtigall RD, et al. Estrogen replacement therapy II: a prospective study in the relationship to carcinoma and cardiovascular and metabolic problems. Obstet Gynecol 1979;54:74–9. [DOI] [PubMed] [Google Scholar]
- [13].Cirillo DJ, Wallace RB, Rodabough RJ, et al. Effect of estrogen therapy on gallbladder disease. JAMA 2005;293:330–9. [DOI] [PubMed] [Google Scholar]
- [14].Nordenvall C, Oskarsson V, Sadr-Azodi O, et al. Postmenopausal hormone replacement therapy and risk of cholecystectomy: a prospective cohort study. Scand J Gastroenterol 2014;49:109–13. [DOI] [PubMed] [Google Scholar]
- [15].Jorgensen T. Gall stones in a Danish population: fertility period, pregnancies, and exogenous female sex hormones. Gut 1988;29:433–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Simon JA, Hunninghake DB, Agarwal SK, et al. Effect of estrogen plus progestin on risk for biliary tract surgery in postmenopausal women with coronary artery disease. The Heart and Estrogen/progestin Replacement Study. Ann Intern Med 2001;135:493–501. [DOI] [PubMed] [Google Scholar]
- [17].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- [19].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [20].Torresquevedo R, Grajeda JM. Gallbladder metastasis of lobular breast carcinoma. HPB 2016;18:e527. [Google Scholar]
- [21].de Bari O, Wang HH, Portincasa P, et al. The deletion of the estrogen receptor α gene reduces susceptibility to estrogen-induced cholesterol cholelithiasis in female mice. Biochim Biophys Acta 2015;1852:2161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].De Bari O, Wang HH, Portincasa P, et al. Molecular mechanisms underlying the critical role of the G protein-coupled receptor 30 (GPR30), a novel estrogen receptor, in the formation of lithogenic bile through a non-transcriptional regulatory mode in 17b-estradiol (E2)-treated mice. Gastroenterology 2015;148:S295. [Google Scholar]
- [23].Van der Werf SDJ, van Berge Henegouwen GP, Ruben AT, et al. Biliary lipids, bile acid metabolism, gallbladder motor function and small intestinal transit during ingestion of a sub-fifty oral contraceptive. J Hepatol 1987;4:318–26. [DOI] [PubMed] [Google Scholar]
- [24].De Bari O, Wang TY, Liu M, et al. Estrogen induces two distinct cholesterol crystallization pathways by activating ERα and GPR30 in female mice. J Lipid Res 2015;56:1691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lee MC, Yang YC, Chen YC, et al. Estrogen causes relaxation of human gallbladder via G protein-coupled estrogen receptors. Gastroenterology 2014;146:S-363. [Google Scholar]
- [26].Kline LW, Karpinski E. Gallbladder motility and the sex of the guinea pig. FASEB J 2016;30:e12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


